Abstract
Cognitive function is an important component of aging and predicts quality of life, functional independence, and risk of institutionalization. Advances in our understanding of the role of cardiovascular risks have shown them to be closely associated with cognitive impairment and dementia. Because many cardiovascular risks are modifiable, it may be possible to maintain brain health and to prevent dementia in later life. The purpose of this American Heart Association (AHA)/American Stroke Association presidential advisory is to provide an initial definition of optimal brain health in adults and guidance on how to maintain brain health. We identify metrics to define optimal brain health in adults based on inclusion of factors that could be measured, monitored, and modified. From these practical considerations, we identified 7 metrics to define optimal brain health in adults that originated from AHA’s Life’s Simple 7: 4 ideal health behaviors (nonsmoking, physical activity at goal levels, healthy diet consistent with current guideline levels, and body mass index <25 kg/m2) and 3 ideal health factors (untreated blood pressure <120/<80 mm Hg, untreated total cholesterol <200 mg/dL, and fasting blood glucose <100 mg/dL). In addition, in relation to maintenance of cognitive health, we recommend following previously published guidance from the AHA/American Stroke Association, Institute of Medicine, and Alzheimer’s Association that incorporates control of cardiovascular risks and suggest social engagement and other related strategies. We define optimal brain health but recognize that the truly ideal circumstance may be uncommon because there is a continuum of brain health as demonstrated by AHA’s Life’s Simple 7. Therefore, there is opportunity to improve brain health through primordial prevention and other interventions. Furthermore, although cardiovascular risks align well with brain health, we acknowledge that other factors differing from those related to cardiovascular health may drive cognitive health. Defining optimal brain health in adults and its maintenance is consistent with the AHA’s Strategic Impact Goal to improve cardiovascular health of all Americans by 20% and to reduce deaths resulting from cardiovascular disease and stroke by 20% by the year 2020. This work in defining optimal brain health in adults serves to provide the AHA/American Stroke Association with a foundation for a new strategic direction going forward in cardiovascular health promotion and disease prevention.
Keywords: AHA Scientific Statements, aging, brain, cognitive dysfunction, prevention and control, risk factors, stroke
Life expectancy is projected to continue to increase in developed countries in the Americas, Australasia, central and western Europe, and Asia-Pacific and is expected to be highest in South Korea, western European countries, and some emerging economies.1 Anticipated gains in longevity will occur in older age groups, particularly among women. By 2030, it is estimated there will be a >50% probability that women in some of the aforementioned regions will break the 90-year survival barrier.1 These gains may be explained by improvements in social status, education, and childhood and adolescent nutrition; expanded primary and secondary health care; a rapid scale-up of new medical technologies; and advances in public health and hygiene.
An increase in life expectancy creates an important public health challenge to plan for health and social services, housing, and pensions for the elderly and to care for those at risk for cognitive impairment and dementia.2 Another major challenge is that increased longevity is likely to be associated with an increase in the prevalence of cognitive impairment and dementia. It is estimated that there are 47 million people with dementia worldwide and that this will increase to 75 million in 2030 and 131 million by 2050.3 These public health projections suggest the need for the prevention of cognitive impairment and treatments to delay cognitive decline.4 Cardiovascular risks have been viewed as important targets for strategies to prevent or delay cognitive impairment.5,6 Thus, there is a linkage between brain health and cardiovascular health.
Cognitive function is an important feature of successful aging, affecting quality of life, functional independence, and risk of institutionalization.7 According to the US Centers for Disease Control and Prevention, a healthy brain is one that can perform all the mental processes that encompass cognition such as the ability to learn and judge, use language, and remember.8 The Centers for Disease Control and Prevention has concluded, however, that there is limited consumer information about cognitive health and maintenance of cognition, existent messaging is inadequate, and the perception of brain health varies by ethnic, cultural, and geographic group.8 In this American Heart Association/American Stroke Association (AHA/ASA) presidential advisory, we aim to expand knowledge in this area by defining optimal brain health in adults and suggesting ways to maintain it. Such information will provide the AHA/ASA with the foundation for a new strategic direction going forward in cardiovascular health promotion and disease prevention.9
Methods
The writing committee was commissioned in November 2016 to review the literature on brain health and maintenance and to develop a definition of optimal brain health in adults. With guidance from the officers of the AHA and members of the AHA Stroke Council, a task force developed an outline for the establishment of a manuscript on optimal brain health in adults. The outline focused on 3 key topics: public health impact of cognitive impairment, definition of optimal brain health, and recommendations for maintenance of brain health. In December 2016, the writing committee members were selected by the chair and co-chairs of this writing group. Writing committee members were chosen on the basis of expert knowledge in the area and experience with guideline development. Discretion was taken to achieve a balance of early-career and later-career investigators, as well as a balance in relation to sex, race, and ethnicity. All writing committee members were required to declare conflicts of interest.
The first writing committee conference call was held in March 2017. During the call, the purpose and topics of the statement were discussed and finalized, and a literature strategy was established. Each of the major sections of the manuscript was assigned a lead, and the chair and co-chairs were responsible for drafting the introduction, methods, and concluding sections and editing all subsections of the manuscript. Relevant literature was identified through a systematic review complemented by hand searching of reference lists and expert knowledge of relevant articles focused primarily on topics relating to brain health and maintenance of brain health. Literature searches were conducted centrally. Full details of the search strategy are available in the Data Supplement (see Systematic Review: Supplement to Defining Brain Health). Section writing leads and members reviewed abstracts and titles for relevance and abstracted key information from the articles. Detailed evidence tables, however, were not required.
The statement was independently reviewed by the AHA Science Advisory and Coordinating Committee. The writing committee responded point by point to each comment made by the reviewers. Final approval of the manuscript was provided by the AHA Science Advisory and Coordinating Committee.
Public Health Impact of Cognitive Impairment, Dementia, Stroke, and Cardiovascular and Stroke Risks
Cognitive Impairment and Dementia
A healthy brain is essential for living a longer and fuller life. Brain health enables thought, planned action, and emotional connections that affect the daily lives and progress of individuals, families, and communities. Sustaining brain health over the course of a lifetime is important to allow one to maximize one’s overall ability and independence. Maintenance of brain health may also curtail the need for diversion of economic and healthcare resources for care and treatments that may have limited the allocation of resources to efforts aimed at maintaining and restoring one to a healthy brain state. Thus, the impact of maximizing and maintaining brain health has the potential to benefit individuals, family and friends, healthcare providers and systems, and society.
Poor brain health may eventually manifest as cognitive impairment or dementia via underlying disorders that include but are not limited to Alzheimer disease (AD), stroke and other causes of vascular cognitive impairment, brain trauma, and other neurodegenerative disorders. In 2010, it was estimated that the second largest number of people living with dementia resided in the United States (3.9 million people),10 with the largest number living in China (5.4 million people). Modifiable risks associated with poor cardiovascular health such as uncontrolled hypertension, diabetes mellitus, obesity, physical inactivity, smoking, and depression are associated with compromised brain health.4
Worldwide, >7 million new dementia cases are diagnosed annually.10 By 2050, the prevalence of dementia is expected to increase by 116% in high-income countries and 264% in low-income countries. From self-report, it is estimated that 1 in 8 adults >60 years of age has memory loss and ≈35% of those report functional difficulties.11 In addition, an estimated 5.1 million individuals in the United States who are ≥65 years of age have AD, which in the United States is predicted to rise to 13.2 million by 2050.10,12
Cognitive impairment and dementia exact a high economic cost. In the United States, AD and related dementias are among the most expensive diseases to treat, with direct care expenses being greater than those for cancer and equal to those for heart disease.13 In 2013, direct payments for health care, long-term care, and hospice care were $203 billion, and among chronic diseases, dementia is the largest single contributor to disability and to the need for longer-term care among older individuals. However, direct care costs of cognitive impairment represent only part of the total financial costs. In 2011, >15 million Americans spent an average of 21.9 h/wk caring for family members with dementia, and the estimated monetary cost may be as high as $215 billion annually.12 This does not take into account, however, stress and related factors placed on caregiv-ers and other family members. The toll on caregivers’ emotional state, for example, is just as striking, with an increased risk of anxiety, depression, and poorer quality of life. Although some studies have shown a benefit of caregiving,14 up to 50% of care-givers have been shown to suffer from depression compared with 15% of noncaregiving individuals.15 Finally, dementia and cognitive impairment challenge the effective treatment of many concurrent illnesses, complicating consent and compliance.
Impact of Stroke
Stroke is especially common among those who are older, and it is estimated that 1 in 3 individuals will have a stroke, dementia, or both.16 Subclinical or silent stroke occurs at least 5 times as commonly as symptomatic stroke and may not be silent because subclinical events are associated with impairment of cognition and disturbances of mood and personality.17 Furthermore, macroscopic infarcts occur in about one third to one half of elderly individuals, and of all major cognitively impairing disorders, including AD, there is a vascular component in up to 80%.4,18,19 Thus, stroke and its antecedents play a pivotal role in the processes underlying cognitive impairment and dementia.
Cardiovascular and Stroke Risks
Cardiovascular and stroke risks are common in the United States and worldwide. In the United States, there are 75 million people (1 of every 3 adults) with hypertension, and 54% of those have uncontrolled blood pressure (BP). When race and ethnicity are taken into account, there are higher rates of hypertension in blacks, followed by US non-Hispanic whites and Mexican Americans.20 In addition, diabetes mellitus and obesity are increasing in frequency across the United States. In 2014, there were an estimated 22 million Americans living with diabetes mellitus, a 4-fold increase from the 5.5 million with diabetes mellitus in 1980 and nearly double the 12.1 million in 2000.18 Obesity rates are also high in the United States. Currently, 36.5% of US adults are categorized as being obese, with the prevalence of obesity being higher in women (38.3%) than men (34.3%) and highest in non-Hispanic blacks and Hispanics followed by non-Hispanic whites and non-Hispanic Asians.21 Among the 20 most populous countries, the highest level of age-standardized childhood obesity occurs in the United States.22
Because hypertension, obesity, smoking, and diabetes mellitus contribute to cognitive impairment and dementia23,24 and there are interventions that successfully prevent or modify these risk factors, a strategy using AHA’s Life’s Simple 7 to decrease cardiovascular risk, stroke, and heart attack should also contribute to maintenance of brain health.25
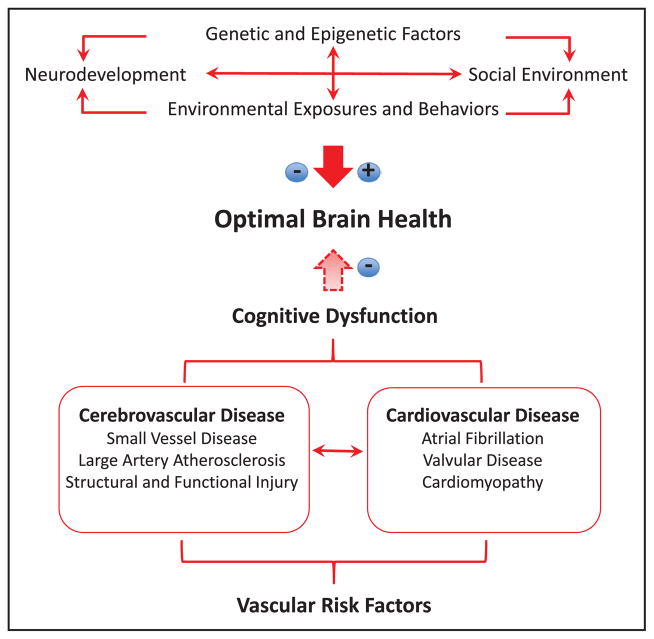
Potential factors that may or may not favor optimal brain health and a causal chain of factors mediated by cardiovascular risks that lead to stroke, dementia, and cognitive dysfunction are depicted in Figure 1. Cardiovascular risks may mediate subclinical and clinical brain injury, the antecedents of cognitive impairment.
Figure 1.
Major determinants of optimal brain health. Genetic, environmental, and behavioral factors can either promote or hinder optimal brain health. Vascular risk factors negatively influence optimal brain health through cardiovascular and cerebrovascular alterations that produce brain dysfunction and damage, leading to cognitive dysfunction.
Summary
Cognitive impairment and dementia are common in the population, especially among older people, and exact a substantial economic and personal toll. As the population in the United States ages, cardiovascular risk factors such as obesity, hypertension, and diabetes mellitus are expected to continue to significantly increase in frequency. Subclinical vascular brain injury (eg, brain white matter hyperintensities, microinfarcts, cerebral microbleeds) and symptomatic stroke, antecedent risks, and associated factors are frequently observed and linked to cognitive impairment and dementia in both clinical and neuropathologic studies. Thus, risks for stroke and cardiovascular disease are well positioned to be targets of preventive strategies for cognitive impairment and dementia. In addition, maintaining brain health is paramount because many public health sectors such as individual health care, healthcare finance, maintenance of employment, and family and community health may be affected.
Optimal Brain Health
Basic Mechanisms and Milieu
Optimal brain function depends on many energy-intensive activities ranging from synaptic activity and subsequent restoration of resting ionic gradients and chemical milieu to protein synthesis and axonal transport.26 With minimal or no energy reserves and the exquisite sensitivity of neurons and glia to chemical changes in the internal milieu, normal brain function is highly dependent on adequate delivery of energy substrates, mainly oxygen and glucose. These are delivered by cerebral blood flow, which, in turn, depends on cardiovascular and cerebrovascular health.
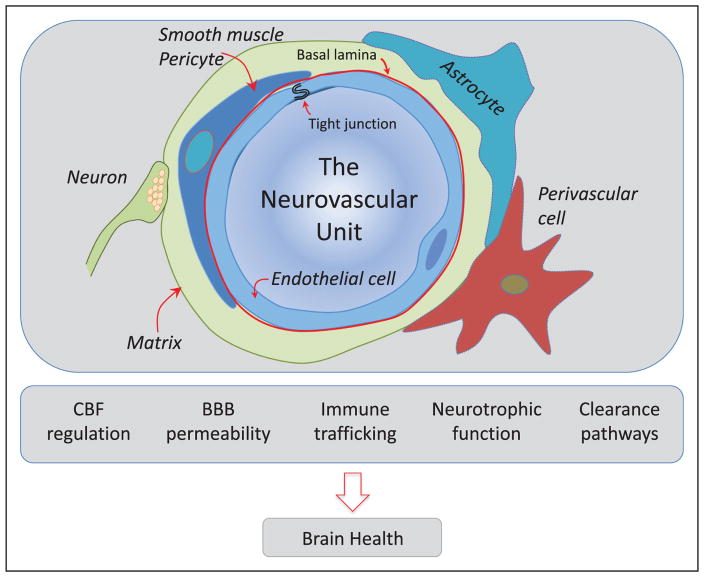
Vital cerebrovascular regulatory mechanisms ensure that the brain is adequately perfused. Neurovascular coupling ensures that focal increases in brain activity are associated with corresponding increases in blood flow to the activated areas.27 Cerebrovascular autoregulation maintains cerebral blood flow relatively independently of changes in arterial pressure to protect the brain from damaging fluctuations in cerebral perfusion,28 whereas cerebral endothelial cells regulate microvascular flow by releasing vasoactive agents such as nitric oxide and prostanoids.29 These fundamental properties of the cerebral microcirculation depend on the coordinated interaction of neurons, astrocytes, vascular endothelial cells, smooth muscle cells, pericytes, perivascular cells, and the cerebrovascular matrix, which collectively constitute a functional ensemble called the neurovascular unit (NVU; Figure 2).
Figure 2.
The neurovascular unit (NVU): the guardian of the homeostasis of the brain microenvironment. Schematic representation of the NVU at the level of the cerebral microvasculature (arterioles, capillaries). The NVU is constituted by endothelial cells, smooth muscle cells (replaced by pericytes in capillaries), astrocytes, neurons, perivascular cells, and the extracellular matrix, a complex meshwork of proteins and carbohydrates that provides support to the different cellular constituents. The NVU is responsible for maintaining the homeostasis of the cerebral microenvironment, ensuring an optimal milieu for the function of neurons and other brain cells. The NVU regulates the delivery of blood flow to the brain, ensuring that it is well matched to its energetic needs; is the site of the blood-brain barrier (BBB), which controls the bidirectional trafficking of molecules into and out of the brain; is involved in the trafficking of immune cells, promoting immunosurveillance in the normal state and innate and adaptive immune responses after brain injury; produces neuronal, glial, and vascular growth factors that support the reciprocal survival of its cellular constituents and the adjacent brain parenchyma; and is a major conduit for the clearance of potentially deleterious byproducts of brain activity such as β-amyloid and tau through transvascular, perivascular, and paravascular (glymphatic) pathways. CBF indicates cerebral blood flow.
In addition to regulating cerebral perfusion, the NVU is responsible for the blood-brain barrier, which finely controls the molecular exchange between blood and brain and vice versa through an intricate system of transporters located on the endothelial cell membrane.30 Another important function of the NVU is to dispose of unwanted products of brain activity and metabolism, such as β-amyloid and tau to prevent their accumulation in the brain tissue.31 To this end, clearance pathways efficiently remove these potentially deleterious molecules through transport across the vessel wall (transvascular pathway), retrograde convective flow in the perivascular space or vessel wall (perivascular pathway), and paravascular pathways involving astrocytic end-feet (glymphatic system).32 The NVU is also involved in the immune surveillance of the brain and in producing growth factors that support the survival of brain cells and blood vessels.33 Therefore, the NVU is critical for maintaining the homeostasis of the cerebral microenvironment, which is essential for brain health (Figure 2).
Increasing evidence indicates that cumulative exposure to vascular risk factors throughout life, perhaps starting as early as in utero, and certainly from the fourth decade onward, affects the risk of common neurological diseases, such as stroke and dementia, as well as the burden of covert (or silent) brain lesions that may diminish optimal brain function, although not sufficiently to be recognized clinically until later in the course of disease. Vascular risk factors, for example, hypertension, diabetes mellitus, and dyslipidemia, have deleterious effects on the structure and function of cerebral blood vessels, leading to vascular remodeling and stiffness, impaired cerebral blood flow autoregulation, endothelial dysfunction, altered neurovascular coupling, and failure of clearance systems, resulting in neurovascular dysfunction and suboptimal brain health.33,34
Parallels Between Cardiovascular and Cerebrovascular Health and Brain Health
Aging affects all organ systems through common underlying mechanisms such as changes in the immune system, oxidative stress, and DNA damage and replication errors, as well as epigenetic changes and accumulation of abnormal proteins across multiple organ systems.35–38 Aging has profound effects on the structure and function of the cerebrovascular system,34,39 which may act in concert with vascular risk factors to further compromise brain health. Furthermore, age-related alterations in systemic organs such as the liver, kidney, lung, and endocrine and immune systems may also produce secondary deleterious effects on the brain. In turn, brain dysfunction and damage caused by age, age-related systemic diseases, and vascular risk factors may lead to deleterious effects on the cardiovascular system by altering neurohumoral mechanisms controlling the heart, blood vessels, and metabolism and promoting cardiac damage and hypertension.40,41 Therefore, the health of the brain is inextricably related to cardiovascular and cerebrovascular health, and interventions aimed at promoting cardiovascular health would be expected to promote brain health and vice versa.
How Do We Define Optimal Brain Health?
Most existing definitions of brain health emphasize an absence of overt vascular or neurodegenerative injury such as from stroke and AD. Some definitions additionally emphasize the absence of subclinical injury on brain imaging such as white matter hyperintensities, silent infarcts, microbleeds on brain magnetic resonance imaging (MRI), or cognitive function assessment values above the age- and education-dependent normative thresholds. More recently, molecular interrogation of brain parenchyma through cerebrospinal fluid studies and positron emission tomography imaging of amyloid and tau burden and of brain network connectivity patterns through functional and diffusion-weighted MRI has been added as a marker of subtle brain injury. There is a need for a broader perspective of the definition of brain health that does not depend on the mere absence of anatomic and physiological disease and encompasses the potential measurement, monitoring, and modification in individuals and in the population.
Optimal brain health can theoretically be defined as an optimal capacity to function adaptively in the environment. This could be assessed in terms of competencies across the domains of “thinking, moving, and feeling,” encompassing, for example, the abilities pay attention, perceive, and recognize sensory input; to learn and remember; to communicate; to problem solve and make decisions; to have mobility; and to regulate emotional status. These domains are largely attributable to the functions of the brain (except for aspects of mobility); can be operationally defined and measured; are affected by environment, behaviors, and disease; and are potentially modifiable if changes are detected early enough. Bodily functions such as sleep, continence, and appetite are also affected by the brain. These constructs can be readily understood by patients and their primary care providers and should be used as the equivalent of vital signs of the brain, that is, early warning indexes of brain health to be monitored and addressed at an at-risk stage for the brain.
Maintenance of Brain Health Requires Consideration of the Life Continuum
Many brain disorders manifest later in life but, in fact, are life-course illnesses. It has been shown that even during the period of brain development, subclinical injury can begin to accrue. Thus, the risk of stroke in the fifth and sixth decades of life depends not only on the BP at the time that risk is being assessed but also on BP patterns experienced in the preceding 1 or 2 decades. Although this advisory statement is focused on adults, to best prevent late-life disease, interventions focused on modifiable risk and protective factors may ideally need to be applied in young adulthood, possibly as far back as childhood. Optimal brain health may be defined at any life stage as average performance levels among all people at that age who are free of known brain or other organ system disease in terms of decline from previously documented levels of function or as adequacy to perform all activities that the individual wishes to undertake.
Pragmatic Criteria to Evaluate and Promote Optimal Brain Health Throughout the Life Span
Actionable criteria need to focus on age-appropriate sensitive measures and modifiable risk factors. Screening tests for brain health range from structured or semistructured questionnaires that score self-assessments or close family member assessments of daily function (Everyday Cognition,42 AD8,43 Informant Questionnaire on Cognitive Decline in the Elderly,44 Clinical Dementia Rating Scale,45 Lawton-Brody Instrumental Activities of Daily Living46) to brief objective assessments of the abilities to attend, perceive, learn, remember, communicate, problem solve, and decide (Folstein Mini-Mental State Examination,47 Montreal Cognitive Assessment,48 Telephone Interview of Cognitive Status,49 Modified Mini-Mental State Examination,50 Clock Drawing,51 7-minute screen,52 Canadian Stroke Network–National Institute for Neurological Disorders and Stroke 5-minute screen).53 In addition, there are assessments of mood (Center for Epidemiological Studies Depression Scale,54 Geriatric Depression Scale,55 Physician Health Questionnaire,56 Neuropsychiatric Inventory57) and mobility (timed walk,58 Short Physical Performance Battery,59 Get Up and Go60). Such indexes have been shown to be adversely affected by simple composite indexes of vascular risk factor exposure such as AHA’s Life’s Simple 7, which includes 4 ideal health behaviors and 3 ideal health factors.9
In addition to such subjective or objective assessments, interpretation of future risk depends on an accurate assessment of a priori risks in the specific sample. Various simple clinical tools have been proposed to assess such a priori risks in an office or community setting (Barnes index).61 They also depend on consideration of future disease risks not just in terms of annual, 5-year, or 10-year risks but in relation to risk over the remaining lifetime, assuming an average life expectancy. These predictor models are the so-called lifetime risks of stroke and dementia described within cohorts, such as the Framingham Heart Study, and estimated for more diverse populations within the United States and worldwide.62 Such lifetime risk estimates may be used to encourage young and middle-aged people to assume personal responsibility for preservation of brain health. Educational models to promote risk factor awareness among these younger cohorts need to be developed.
Race-, ethnicity-, and geography-specific variations in disease-incidence risks associated with specific risk factors need to be better quantified, as do gene-environment interactions. Furthermore, interactions between risk factors should be explored so that primary risk prevention prescriptions to prevent subclinical injury and clinical disease can be tailored to emphasize changes most likely to benefit the individual. The parameters of the risk or protective factors most correlated with brain health often remain unclear. For instance, it is unknown whether light physical activity is sufficient to maintain brain health and whether optimal physical activity levels vary across each decade. In addition, the role of physical fitness in brain health needs to be further clarified.
Risk Factors
Background
There has been substantial interest in the promotion of strategies to achieve healthy brain aging and to reduce the risk of stroke and cognitive decline. Research has convincingly demonstrated that cardiovascular risk factors are major contributors to late-life cognitive health and risk of stroke and AD. For example, having high fasting glucose or diabetes mellitus has been associated with cognitive impairment and dementia.63–65 Potential mechanisms include vascular and neuronal damage,66 as well as changes in cerebral blood flow and alterations in β-amyloid processing and deposition.67 Smoking has been implicated in the risk of cognitive decline68,69 and dementia70 through atherosclerosis, inflammation, and oxidative stress pathways.68,69 Obesity,71–73 dyslipidemia,74–77 and high BP78,79 are also major contributors to vascular brain health. Finally, adherence to a Mediterranean or DASH (Dietary Approach to Stop Hypertension) diet80 has been associated with reduced cognitive decline, potentially and controversially a result of homocysteine reduction, and through antioxidant and anti-inflammatory pathways.81–83 Despite being well-established key risk factors of cognitive decline, AD, and other dementias, much less is known about how cardiovascular risk factor exposure affects brain health in midlife or earlier in the life course.
The specific critical periods of influence of some key cardiovascular risk factors such as high body mass index (BMI) and hypertension on cognitive decline remain uncertain. For example, studies have shown that there may be a U-shaped association between BMI and dementia such that individuals with either low or high BMI have the greatest risk.84 In addition, the timing of obesity (midlife versus late life) and the type of obesity (overall versus central) may have different effects on cognitive outcomes in late life. Studies suggest that midlife and late-life central obesity increases the risk of cognitive impairment but that high late-life obesity (as assessed by BMI) may be protective.71–73 Further study is needed to clarify these risk relationships, which may be explained away, at least in part, by reverse causation.
Hypertension is another risk factor with an influence on cognition and dementia that may depend on age, severity, and cumulative time of exposure. Several population-based studies have found that midlife hypertension or high systolic BP is associated with cognitive dysfunction and decline85–88 and dementia.65,79 A J- or U-shaped association between systolic BP and cognitive decline has also been suggested in old age.79 Inconsistencies in these associations may reflect the timing of exposure measurement relative to the age of study subjects, reverse causation, potential survivor bias, and the possibility of either a cumulative effect or more specific window of opportunity for the exposure to exert a deleterious effect during the life course. The CARDIA study (Coronary Artery Risk Development in Young Adults), for example, showed that cumulative systolic and diastolic BPs and fasting glucose were consistently associated with worse cognition.89 The reader is referred to additional informative studies that detail the effect of various BP metrics in young adults on cognitive function in midlife.90–92
Prospective observational studies have generally found a positive association between physical activity and cognitive health outcomes. A meta-analysis of these studies concluded that physically active adults had a 35% lower risk of cognitive decline (relative risk, 0.65; 95% confidence interval, 0.55–0.76) compared with those who were physically inactive.93 In another recent systematic review, 21 of 24 prospective studies showed a positive association between physical activity and cognitive outcomes.94
A number of intervention studies have reported a benefit of physical activity and exercise on cognition. For example, in a randomized trial, older women assigned to aerobic exercise for 4 months had better performance on cognitive tasks than women randomized to strength and flexibility training or no exercise.95 Other interventional studies have often, but not always, found beneficial effects of aerobic exercise on cognition.96–99 Several meta-analyses100–102 have reported modest positive effect sizes for the exercise-cognition relationship. Some studies have shown larger benefits on executive tasks than other cognitive domains.97,99,102,103
Finally, 2 other factors merit mention. Atrial fibrillation is an important risk in that it is causally linked to stroke and subclinical stroke, is increased by certain cardiovascular risks, is associated with cognitive impairment, and is modifiable or preventable.104–106 In addition, a number of studies have suggested that various aspects of sleep (eg, short or long sleep duration, excessive daytime sleepiness, greater number of night awakenings) may be associated with poorer cognition or cognitive decline.107–111 Because the findings have been inconsistent, additional studies are needed to determine whether and which sleep characteristics are causal in relation to cognitive impairment.
AHA’s Life’s Simple 7
A recent study examined the association between overall cardiovascular health defined by AHA’s Life’s Simple 7 in early life to midlife and cognition in midlife. The authors investigated 7 components of cardiovascular health (BMI, diet, smoking status, physical activity, total cholesterol, BP, and fasting glucose) and reported that having a greater number of cardiovascular health metrics at ideal levels in early life was associated with better cognition in midlife.112 Furthermore, in another study, cumulative exposure to cardiovascular risk factors from early to middle adulthood, especially above recommended guideline levels, has been associated with worse cognition in midlife.89 In REGARDS (Reasons for Geographic and Racial Differences in Stroke), a US national study of 17 761 blacks and whites >45 years of age and followed up for an average of 4 years, those with intermediate or high cardiovascular health defined by AHA’s Life’s Simple 7 had a significantly lower incidence of cognitive impairment compared with those with low indexes of cardiovascular health.25
Other Factors
Education and literacy are well-established predictors of later-life cognitive function.113–115 This protective effect may be mediated by higher socioeconomic conditions that influence health behaviors and lifestyle choices, as well as improved access to health care and quality health care.116 Higher socioeconomic conditions also provide more opportunities for cognitive enrichment through more complex occupational exposure. Aside from education level, quality of education in early life, which is known to vary greatly by geographic region,117,118 also contributes to cognitive outcomes later in life. Regional disparities in cognitive decline have been shown to be similar to regional disparities in stroke mortality; residents of the Stroke Belt in the southeastern United States had greater adjusted odds of incident cognitive impairment than non–Stroke Belt residents,119 suggesting shared risk factors for these 2 outcomes. Less is known about other geographic and environmental factors that may contribute to vascular health. Air pollution is the factor that has been studied the most, but the results have not been consistent. Thus, air pollution was not included as a metric for optimal brain health in this AHA statement.120–122
Rationale for Incorporation of the Ability to Monitor, Measure, and Modify as a Consideration for Defining Metrics of Brain Health and the Distinction Between Metrics and Outcomes
To efficiently translate knowledge into action and to achieve the desired goal of optimal brain health, practitioners, individuals, and policy makers should be provided with metrics that are measurable, modifiable, and easily monitored. For instance, although age and genetic factors are among the substantial determinants of brain health, they are not modifiable and thus not well suited as metrics. To achieve the highest possible gain, metrics for optimal brain health should meet the following requirements: have a strong basis of evidence for affecting brain health; be measurable by simple methods that are broadly available and can be performed at reasonable cost; have a sufficient basis of evidence that modifying the metric will result in improved brain health; be applicable to all subsets of the population; and have face validity to ensure acceptance by relevant parties and can be acted on by individuals, practitioners, and policy makers. For instance, public health messages about a diet rich in fruits, vegetables, and fiber-rich whole grains is better suited for health communication, translation, and action by practitioners, policy makers, and individuals than details on specific nutrients, which may be difficult to communicate and monitor.
Criteria for metrics to achieve optimal brain health (eg, ability to monitor, measure, and modify) are to be distinguished from outcomes such as cognitive function, stroke, and dementia. The latter outcomes are strongly influenced by metrics that act as antecedents for optimal brain health and serve as ongoing indicators for brain health and the potential for maintenance of optimal brain health. Outcomes should meet the following criteria: be clearly attributable to brain health, have a documented impact on quality of life, and be measurable by simple methods. The primary outcome should be a robust measure consisting of items that are easy to assess (eg absence of both stroke and dementia). However, additional metrics to define secondary outcomes, including both clinical (eg, cognitive decline) and neuroimaging (eg, brain infarcts) outcomes, are needed.
Metrics for Defining Optimal Brain Health: AHA’s Life’s Simple 7
The health-related behaviors and health factors listed below have been chosen as metrics to define optimal brain health. These factors are derived from AHA’s Life’s Simple 7.9 Available evidence from study of AHA’s Life’s Simple 7 indicates that these factors play a role in the preservation of cognition and can be measured, modified, and monitored.25,112 These factors have been reviewed in the Risk Factors section above, and other study information on the factors is found elsewhere for nonsmoking status,4,123,124 physical activity,4,99,123–125 BMI <25 kg/m2,71,126–133 healthy diet consistent with current guidelines,4 untreated BP <120/<80 mmHg,4,5 untreated total cholesterol <200 mg/dL,5,86,134–142 and fasting blood glucose <100 mg/dL.4,123,124
Health-Related Behaviors9
-
1
Nonsmoking status
-
2
Physical activity at goal levels
-
3
BMI <25 kg/m2
-
4
Healthy diet consistent with current guidelines
Health-Related Factors
-
5
Untreated BP <120/<80 mm Hg
-
6
Untreated total cholesterol <200 mg/dL
-
7
Fasting blood glucose <100 mg/dL
Of note, the thresholds for definition of ideal levels of BP, glucose, and cholesterol are consistent with current guidelines and expert recommendations.9
Other Factors
Besides AHA’s Life’s Simple 7, a number of additional factors have an established impact on brain health either by influencing stroke or dementia risk or by independent mechanisms. Examples include but are not limited to atrial fibrillation, which can be prevented or efficiently monitored and treated to prevent cardioembolism; low cardiac output, leading to hypoperfusion dementia; acute or chronic brain diseases distinct from stroke or neurodegeneration; and head injury, which can in part be influenced by behavior. Although the importance of these factors with respect to optimal brain health is acknowledged, AHA’s Life’s Simple 7 was chosen to be the backbone of the definition of the metrics because of their relevance on individual and population levels, life-course perspective, and fit with the ability to monitor, measure, and modify, and because they further affect other established risk factors for brain health, including atrial fibrillation.
The relationship between education or social engagement across the life span and brain health is complex. For instance, education may affect brain health by enhancing the capacity to compensate for the effects of brain pathology on brain function (eg, cognitive reserve). A potential benefit of interventions to enhance social or cognitive lifestyle has thus far not convincingly been demonstrated. Nevertheless, this remains an important area for further investigation. A more detailed appraisal of other factors beyond AHA’s Life’s Simple 7 will be the focus of future brain health publications.
Primary and Secondary Outcomes for Brain Health and Monitoring
Outcomes for brain health can be broadly classified into 3 categories: clinical diagnoses coded in International Classification of Diseases, 10th Revision (eg, stroke or dementia); competencies across the domains of thinking, moving, or feeling; and metrics of brain structure or function as assessed by instrumental investigations (eg, brain imaging). The last category includes subclinical injury on brain imaging (eg, presence or volume of MRI-defined white matter hyperintensities, covert infarcts, microbleeds, and microstructural change [quantifiable by diffusion tensor imaging]), molecular markers (eg, amyloid measured by positron emission tomography or cerebrospinal fluid analysis), and both structural and functional connectivity, as assessed by diffusion tensor imaging and functional MRI, respectively.143,144
On a population level, International Classification of Diseases, 10th Revision–coded clinical diagnoses are more practical to assess than competencies or metrics of brain structure. In addition, surveillance of certain outcomes such as stroke, dementia, and mortality from these causes is more reliable than the surveillance of milder clinical disease expressions such as transient ischemic attack (defined as a transient focal neurological deficit of vascular cause) or subjective cognitive impairment. Hence, defining stroke and dementia as the primary outcomes of interest and transient ischemic attack and subjective or mild cognitive impairment as secondary outcomes seems most logical given that the secondary outcomes correlate with less severe clinical expressions, competencies, and manifestations of brain dysfunction. Primary and secondary outcomes for brain health monitoring are listed in Table 1.
Table 1.
Primary and Secondary Outcomes for Brain Health Monitoring
| Primary outcomes |
| Stroke |
| Dementia |
| Secondary outcomes |
| Transient ischemic attack |
| Subjective or mild cognitive impairment |
Maintenance of Brain Health
Evidence for Maintenance of Brain Health
There is robust observational epidemiological evidence associating young adult and midlife vascular risk factors with later development of cognitive impairment. However, the randomized clinical trials (RCTs) investigating the effect of vascular risk factor modification on the prevention of cognitive decline have not shown consistent results (Table 2). Observational epidemiological study shows that individuals with hypertension treated to a BP <120/<80 mm Hg have less risk of stroke and cardiovascular events, but that risk is not as low as those the risk for those who have maintained untreated BP <120/<80 mmHg.159 Thus, primordial prevention may be a powerful means to prevent key primary and secondary cardiovascular outcomes because once risk factors are present, one may not be able to optimally reduce stroke and cardiovascular disease risk.
Table 2.
Summary of Trial Data on Maintenance of Cognitive Function
| Study | n | Age at Inclusion (mean), y | Follow-Up, y | Intervention | Cognitive Outcome | Results |
|---|---|---|---|---|---|---|
| Syst-Eur trial145 | 2418 | ≥60 (69.9) | 2 | Nitrendipine±enalapril±hydrochlorothiazide to reduce systolic BP by at least 20 mm Hg to reach a value <150 mm Hg | Dementia (DSM-III, Revised, criteria) and Mini-Mental State Examination | Active treatment was associated with a lower incidence of dementia by 50% (P=0.05) |
| ONTARGET146 | 22 629 | ≥55 (66) | 4.6 | Ramipril vs telmisartan vs a combination of both drugs | Mini-Mental State Examination | No effect of treatment on cognition |
| TRANSCEND146 | 5231 | ≥55 (67) | … | Telmisartan vs placebo | Mini-Mental State Examination | No effect of treatment on cognition |
| HYVET147 | 3336 | ≥80 (84) | 2.2 | Indapamide±perindopril vs placebo | Dementia (DSM-IV criteria) and Mini-Mental State Examination | No effect of treatment on dementia |
| SCOPE148 | 4694 | 70–89 (76) | 3.7 | Candesartan vs placebo | Mini-Mental State Examination | No effect of treatment on cognition |
| PRoFESS149 | 20 332 | ≥55 (66) | 2.4 | 2×2 Factorial allocation | Mini-Mental State Examination | No effect of treatment on cognition |
| Aspirin+extended-release dipyridamole vs clopidogrel | ||||||
| Telmisartan vs placebo | ||||||
| PROGRESS150 | 6105 | No set age criteria (64) | 3.9 | Perindopril±indapamide vs placebo | Dementia (DSM-IV criteria) and Mini-Mental State Examination | Active treatment reduced cognitive decline by 19% (P =0.01) but had no effect on dementia |
| SHEP151 | 4736 | ≥60 (72) | 5 | Chlorthalidone±atenolol vs placebo | Short-Comprehensive Assessment and Referral evaluation | No effect of treatment on cognition |
| ACCORD-MIND152 | 2977 | No set age criteria (62) | 3.3 | Diabetic patients randomized to systolic BP <120 vs <140 mm Hg and to fibrate vs placebo | Digit Symbol Substitution Test | No effect of intensive blood pressure and fibrate treatment on cognition |
| HOPE-3153 | 1626 | Cognition assessed in individuals ≥70 only (74) | 5.6 | 2×2 Factorial allocation | Digit Symbol Substitution Test | No effect of treatment on cognition |
| Rosuvastatin vs placebo and candesartan+hydrochlorothiazide vs placebo | ||||||
| SPS3154 | 2916 | No set age criteria (63) | 3 | 2×2 Factorial allocation | Cognitive Abilities Screening Instrument | No effect of treatment on cognition |
| Aspirin vs clopidogrel; systolic BP <130 vs 130–149 mm Hg | ||||||
| preDIVA155 | 3526 | 70–78 (75) | 6.7 | Usual care vs multidomain intervention (smoking habits, diet, physical activity, weight, BP, diabetes mellitus, and dyslipidemia) | Dementia (DSM-IV criteria) | No effect on dementia |
| FINGER156 | 2654 | 60–77 (69) | 2 | Usual care vs multidomain intervention (diet, physical exercise, cognitive training, and intensive vascular risk factor monitoring) | Neuropsychological test battery | Multidomain intervention reduced mean z score at 2 y from 0.20±0.02 to 0.16±0.51 (P=0.03) |
| MAPT157 | 1680 | ≥60 (75) | 3 | Usual care vs multidomain intervention (cognitive training, diet, nutrition advice, and 3 preventive consultations ± omega 3 polyunsaturated fatty acids) | Free and Cued Selective Reminding test, 10 Mini-Mental State Examination orientation items, Digit Symbol Substitution Test, and Category Naming Test | No effect of treatment on cognition |
| PREDIMED158 | 334 | Men: 55–80 Women: 60–80 (67) |
4.1 | Mediterranean diet supplemented with extravirgin olive oil vs Mediterranean diet supplemented with mixed nuts versus control diet | Mini-Mental State Examination, Rey Auditory Verbal Learning Test, Animals Semantic Fluency, Digit Span Subtest from the Wechsler Adult Intelligence Scale, Verbal Paired Associates from the Wechsler Memory Scale, and the Color Trails Test | Improvement in cognition |
| LIFE99 | 1635 | 70–79 and 80–89 | 2 | Moderate-intensity physical activity program vs educational workshops and upper-extremity stretching | Digit Symbol Coding, Wechsler Adult Intelligence Scale, and Hopkins Verbal Learning Test | Among sedentary adults, a moderate-intensity physical activity program vs a health education program did not improve global or domain-specific cognitive function |
ACCORD-MIND indicates Action to Control Cardiovascular Risk in Diabetes; BP, blood pressure; DSM, Diagnostic and Statistical Manual of Mental Disorders; FINGER, Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; HOPE-3, Heart Outcomes Prevention Evaluation–3; HYVET, Hypertension in the Very Elderly Trial; LIFE, Life Interventions and Independence for Elders; MAPT, Multidomain Alzheimer Preventive Trial; ONTARGET, Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial; PREDIMED, Prevención con Dieta Mediterránea; preDIVA, Prevention of Dementia by Intensive Vascular Care; PRoFESS, Prevention Regimen for Effectively Avoiding Second Strokes; PROGRESS, Perindopril Protection Against Recurrent Stroke Study; SCOPE, Study on Cognition and Prognosis in the Elderly; SHEP, Systolic Hypertension in the Elderly Program; SPS3, Secondary Prevention of Small Subcortical Strokes Trial; Syst-Eur, Systolic Hypertension in Europe; and TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease.
There is some suggestion for clinical benefit from BP control or multidomain interventions, including vascular risk modification combined with lifestyle and behavioral interventions156 (Table 2). For example, in the placebo-controlled Syst-Eur study (Systolic Hypertension in Europe), a BP reduction of 8.3/3.8 mm Hg was associated with a marginally significant drop in the risk of dementia of 50% at 2 years (P<0.05).145 However, several additional randomized trials failed to show a beneficial effect of BP reduction on cognition. 146–149,151–154 Thus, while BP lowering has been shown to substantially reduce the risk of stroke, in individuals with elevation of BP, BP lowering in a clinical trial setting has not been shown conclusively to preserve cognitive function.
Data from longitudinal observational studies indicate that different cardiovascular and lifestyle-related factors such as diabetes mellitus, dyslipidemia, anthropometric measures, and smoking, among others, may influence the trajectory of cognitive health. Encouraging results have been observed in some, but not all,99 RCTs targeting some of these conditions. In the PREDIMED study (Prevención con Dieta Mediterránea), for example, participants randomized to a Mediterranean diet had modestly better cognition at 4 years.158 In the FINGER study (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability), participants randomized to a multidomain intervention, including coaching for vascular risk reduction, exercise, cognitive training, and adherence to a Mediterranean diet, had modestly better cognitive performance at 2 years, with improvements in executive functions and processing speed but not episodic memory.156
On the other hand, several trials have had neutral results for maintenance of cognition155,157 (Table 2). The neutral results or modest beneficial effect on cognition in these studies might be explained by administration of the intervention too late in the disease time course, limited follow-up and thus inadequate statistical power, poor sustainability of healthy lifestyle changes, and relatively small differences in the baseline and longitudinal management of vascular risk factors in the placebo or control groups compared with active or intensive treatment because the control participants were already adhering well to standard care. In addition, none of the trials showed that dementia was prevented; even the positive trials showed only modest improvements in cognitive performance on neuropsychological tests of unclear significance for daily functioning and quality of life. Although the clinical trials showed that implementing a long-term multi-domain lifestyle intervention was feasible and in some cases improved cognition, the applicability and cost-effectiveness of such labor-intensive interventions (ie, often requiring a lifestyle coach, nutritionist, and supervised exercise sessions) in the general population are unclear. Thus, additional studies are needed, especially in younger age groups, with the inclusion of sensitive indicators of trajectories of cognitive function and vascular function.
Summary of Previous Guidance for Maintenance of Cognitive Vitality From the AHA/ASA and Other Organizations
Although the RCT evidence to prevent cognitive decline and dementia is still incomplete and evolving, the observational epidemiological evidence for modifiable risk factors is substantial. This evidence has been previously reviewed by several national organizations, along with guidance on how to translate this information into strategies to improve brain health.
In 2011, the AHA/ASA released a scientific statement on vascular contributions to cognitive impairment and dementia, reviewing clinical considerations for diagnosis and management of symptomatic patients and prospects for the prevention of vascular cognitive impairment and AD.4 The statement did not recommend changes to the current management of cardiovascular risk factors to prevent dementia; however, it should be noted that the statement was released before the publication of the FINGER, LIFE (Life Interventions and Independence for Elders), and PREDIMED trials. The only Class I recommendation was, “In people at risk for vascular cognitive impairment, treatment of hypertension is recommended.”4 A more contemporary scientific statement found inconclusive evidence that BP control preserves cognition in the population at-large.5 Another scientific statement found insufficient evidence on how to prevent or treat silent cerebrovascular disease (including silent strokes) detected on neuroimaging, which has been associated with increased risk of future dementia.143
The Alzheimer’s Association has summarized previous systematic reviews and meta-analyses, consensus statements, and selected articles on modifiable risk factors for cognitive decline and dementia.160 The writing committee concluded that the evidence was sufficiently strong to support promotion of physical activity, management of vascular risk factors, healthy eating, and lifelong learning and cognitive training. The Alzheimer’s Association website lists “10 Ways to Love Your Brain,” consisting of advice to increase physical activity, pursue lifelong learning, cease smoking, manage cardiovascular risk, protect against brain injury, eat a healthy diet, get adequate sleep, take care of mental health, stay socially engaged, and partake in challenging mental activities.161
In 2015, the Institute of Medicine commissioned a report on cognitive aging.162 For individuals and families, the report recommended being physically active, managing cardiovascular risk factors, discussing health conditions and medications that might influence cognition with a healthcare professional, being socially and intellectually engaged, getting adequate sleep, taking steps to avoid delirium if hospitalized, and carefully evaluating products advertised to improve cognition. For society, key recommendations included increasing research on preserving cognitive function, ensuring appropriate policies and guidelines for products that affect cognitive function, developing core competencies and curricula in cognitive aging distinct from dementia for health professionals, and expanding community services to meet the needs of older adults and their families with respect to cognitive health.
Recommendations for Maintenance of Optimal Brain Health
Considering the emerging evidence that now justifies a definition of optimal brain health, prudent actions are warranted to maintain optimal brain health throughout the life span. Thus, this statement endorses the recommendations from the Institute of Medicine and Alzheimer’s Association as means to maintain brain health.160–162 In addition, for primordial, primary, and recurrent stroke prevention and its relationship to brain health, we recommend following AHA/ASA guidance4,5,124,142,143,163–167 (see also Table 3).
Table 3.
Recommendations for Promotion and Maintenance of Optimal Brain Health
| Individual168 |
| Check health status with AHA’s Life’s Simple 7 (http://www.heart.org) |
| Remain physically active |
| Eat a healthy diet; evidence suggests that a Mediterranean-style diet preserves cognitive function better than a low-fat diet |
| Address vascular risk factors, if present, with a primary care practitioner |
| Pursue cognitively stimulating and rewarding activities |
| Address mental health concerns with a primary care practitioner or specialist as needed |
| Healthcare practitioners |
| Apply primordial and primary preventive care for cardiovascular disease and stroke according to AHA/ASA guidelines9,124,142,163,164 |
| Diagnose and treat symptomatic stroke according to AHA/ASA guidelines165–167 |
| Administer brief screens to monitor cognitive status |
| Health systems |
| Support patients by providing access to preventive care and lifestyle modification |
| Support good-quality care for stroke169 and for primary prevention of cardiovascular disease170 |
| Public health, health policy, private sector9,168 |
| Disseminate knowledge of potentially modifiable risk factors for cognitive decline and dementia |
| Provide tools and resources to maintain healthy lifestyles such as the AHA Healthy for Good program171 |
| Provide opportunities for stimulating cognitive, physical, and social activities |
| Maintain a healthy environment, including neighborhoods that promote cognitive and physical activity |
| Fund research on risk factors for cognitive decline and dementia and how to intervene to reduce risk |
AHA indicates American Heart Association; and ASA, American Stroke Association.
Decades of efforts to reduce stroke and cardiovascular risks by the AHA/ASA and other health organizations and, more recently, to promote cardiovascular health are likely to have already contributed to the declining incidence of dementia.172 These efforts should be additionally leveraged for the promotion of optimal brain health. This should include not only prevention of stroke but also treatment of stroke to improve outcomes, including poststroke cognitive impairment.
Addressing cardiovascular and stroke risk is a large part of maintaining optimal brain health, but not the only part. Addressing the many factors that may promote cognitive reserve, including such factors as access to education, meaningful interpersonal and community social engagement, and access to mental health care, is also likely to be a useful strategy. Active engagement by not only healthcare practitioners but also policymakers at all levels of government is needed.
Maintaining optimal brain health requires actions by individuals, healthcare practitioners, public health organizations, policy makers, and the private sector. An overview of approaches to promoting optimal brain health is provided in Table 3 and categorized by target stakeholder.
Discussion
The main aim of this AHA/ASA advisory is to provide an initial definition of optimal brain health and guidance on how to maintain brain health. Our definition of optimal brain health emphasizes the importance of a favorable or ideal cardiovascular risk profile. Such a risk profile earlier in life is associated with compression of morbidity and mortality. This has been shown in a recent publication from the CHA (Chicago Heart Association Detection Project in Industry). CHA included a cohort of men and women employed in 1967 to 1973 who were 90% white, 44 years of age on average at baseline, and followed up for 40 years. Overall, those CHA cohort members who had a favorable health profile in midlife had prolonged survival by ≈4 years and postponed onset of all-cause and cardiovascular mortality of 7 years.173 In addition, all morbidities were reduced, compression of morbidity was observed both absolutely and relatively, and there were lower cumulative and annual healthcare costs during the Medicare-eligibility life span in older ages for those with favorable cardiovascular health profiles in midlife.173 Although cognition was not the primary focus of this publication, several other study results support the belief that favorable cardiovascular health earlier in life leads to maintenance of cognitive vitality.89,174
Although healthy lifestyle, advances in overall medical care, and favorable socioeconomic profile might compress major morbidities of later life, they may be influenced by the age at which the subjects are studied and the time in life when preventives are administered, as suggested by the results of a Chinese longevity study of the oldest old.175 In this study, there was compression of activities of daily living disability, but life-span extension was associated with expansion of disability of physical and cognitive functioning because there were more frail elderly who survived health problems.175
Modifiable cardiovascular risks are prevalent worldwide, may occur across the age continuum, and are associated with adverse consequences later in life.128,176 Furthermore, cardiovascular mortality, including ischemic heart disease and stroke, may vary substantially on the basis of geographic variation.177 Atherosclerosis, a manifestation of nonfavorable cardiovascular health, however, may not be inevitable. Recently, it has been shown that a forager-horticulturalist population, the South American Tsimane of Bolivia, who have few coronary artery disease risk factors, have the lowest reported levels of coronary artery disease of any population,178 suggesting the value of primordial prevention.
Furthermore, the development of vascular arterial stiffness, a measure of vascular aging, may not be inevitable.179 In the Framingham study, lower age, female sex, lower BMI, use of lipid-lowering drugs, and absence of diabetes mellitus in a cross-sectional study were associated with healthy vascular aging. In addition, for every 1-unit higher AHA’s Life’s Simple 7 score (ie, 1 more factor or behavior at ideal levels), there was a 1.55-fold age- and sex-adjusted odds of healthy vascular aging. This observational study provides support for prevention strategies focused on modifiable health factors and behaviors to prevent or delay vascular aging and risk of cardiovascular disease and possibly other health consequences associated with aging such as cognitive impairment.179 This approach may apply to elderly populations.
Prevention of cognitive impairment and dementia may be viewed as a lifelong process predicated on a number of antecedent factors, with cardiovascular risks playing an important role.112 The promotion of healthy living across the life span may allow one to avoid adverse health outcomes such as multimorbidity, including cognitive impairment and other health-related adverse outcomes or events.9,180,181 In addition, favorable cardiovascular health may be associated with lower healthcare expenditures and resource use.182
In this advisory, AHA’s Life’s Simple 7 was chosen as the metric to define optimal brain health. AHA’s Life’s Simple 7 consists of 4 ideal health behaviors (physical activity at goal levels, healthy diet consistent with current guideline recommendations, nonsmoking, and BMI <25 kg/m2) and 3 ideal health factors (untreated total cholesterol <200 mg/dL, fasting blood glucose <100 mg/dL, and untreated BP <120/<80 mm Hg).9 These factors have been shown to improve the health trajectory.9,181 In addition, AHA’s Life’s Simple 7 has been linked to successful cognitive health25,94,95,112 and is an ideal starting point for defining optimal brain health because it is easy to measure, monitor, and modify. Additional study to test the influence of AHA’s Life’s Simple 7 on brain health among different racial and ethnic groups, men and women, and people from different age groups across the life continuum is needed.
Beyond the metrics to initially define optimal brain health, primary and secondary outcomes for brain health have been identified in this advisory. On the basis of practical considerations, 2 main primary outcomes, stroke and dementia, and 2 main secondary outcomes, transient ischemic attack and subjective or mild cognitive impairment, have been designated. Metrics of brain structure and function such as subclinical injury detected on brain imaging (eg, presence or volume of MRI-defined white matter hyperintensities, covert infarcts, microbleeds, and microscopic injury [quantifiable by diffusion tensor imaging]), molecular markers (eg, amyloid measured by positron emission tomography or cerebrospinal fluid analysis), and those of structural and functional connectivity, as assessed by diffusion tensor imaging and functional MRI, are important factors for future consideration as the definition of optimal brain health evolves.
Finally, the available RCT and observational epidemiological data were reviewed to provide guidance on the maintenance of cognitive health. Although the RCT evidence to prevent cognitive decline and dementia is incomplete but evolving, the observational epidemiological evidence for modifiable risk factors was judged to be substantially in favor of modifying key cardiovascular risks. Thus, application of the Institute of Medicine and Alzheimer’s Association guidance for the maintenance of cognitive vitality, which includes both cardiovascular risk factor modification and other strategies,160–162 and primordial, primary, and secondary stroke prevention recommendations from the AHA/ASA as they relate to brain health has been recommended.4,5,124,142,143,163–167
The definition of optimal brain health and its maintenance chosen for this advisory has limitations. We acknowledge the complexity of defining optimal brain health for adults and that a broader definition of optimal brain health is possible when taking into account the various stages of the life continuum spanning from conception to old age and consequent environmental, lifestyle, and genetic exposures. For example, there is a limited but increasing body of knowledge on the importance of childhood exposures affecting cognition in later life.183 Furthermore, intrauterine and early-life exposures may play important roles in neurocognitive development, as may stress in early life. Therefore, we anticipate that future publications from the AHA/ASA brain health writing group will expand the definition of optimal brain health and incorporate factors of interest from, for example, earlier in life. In addition, there were inherent limitations of availability of variables of interest such as those based on demographic and race-ethnic composition of the available RCTs and observational epidemiological studies that were reviewed. Such limitations suggest the need for study of individuals representing groups disproportionately affected by cardiovascular health disparities and those from early stages of life such as childhood.
Future Directions and the AHA Strategic Impact Goal
The AHA/ASA has set a Strategic Impact Goal by the year 2020 to improve the cardiovascular health of Americans by 20% while reducing deaths from cardiovascular diseases and stroke by 20%.9 The AHA/ASA is promoting cardiovascular health through application of Life’s Simple 7. As part of the effort to promote cardiovascular health, the AHA/ASA is adding brain health to its overall strategic initiatives. Brain health is an important component of successful aging and one in which cardiovascular risks seem to play a key role. The main purpose of this document is to serve as the first step in defining optimal brain health and how to maintain it. Future work will serve to refine the definition, metrics, and strategies to maintain cognitive vitality.
Supplementary Material
Table 1. Selection Criteria
Table 1. Classification of Included vs. Excluded Exposure Categories
Appendix 1. Search strategy
Acknowledgments
The authors wish to express their thanks to the following AHA staff for their assistance in the development of this manuscript: Anne Leonard, senior science and medicine advisor, and Connie Land, assistant managing editor, Scientific Publishing.
Disclosures
Writing Group Disclosures
| Writing Group Member |
Employment | Research Grant | Other Research Support |
Speakers’ Bureau/ Honoraria |
Expert Witness |
Ownership Interest |
Consultant Advisory Board |
Other |
|---|---|---|---|---|---|---|---|---|
| Philip B. Gorelick | Michigan State University, Mercy Health Hauenstein Neurosciences | None | None | None | None | None | None | None |
| Karen L. Furie | Rhode Island Hospital and Alpert Medical School of Brown University | None | None | None | None | None | None | None |
| Costantino Iadecola | Weill Cornell Medicine, Feil Family Brain and Mind Research Institute | NIH† | None | None | None | None | Broadview Ventures† | None |
| Hee-Joon Bae | Seoul National University, Bundang Hospital, Republic of Korea | Bayer†; Boehringer Ingelheim†; Daichi Sankyo†; AstraZeneca Korea†; Dong-A Pharmaceutical†; Yuhan Corp†; ESAI- Korea†; BMS Korea† | None | Korean Drug Co, Ltd*; Shire Korea Ltd* | None | None | None | None |
| Mary Ann Bauman | Self-employed | None | None | None | None | None | None | None |
| Martin Dichgans | Klinikum der Universität München, Ludwig- Maximilians-University, Institute for Stroke and Dementia Research, Munich, Germany | EU FP7/Horizon 2020†; DFG/DLR†; DZNE†; BMBF†; Fondation Leducq†; Vascular Dementia Research Foundation† | None | None | None | None | DZNE*; Bayer Vital*; EVER*; Boehringer Ingelheim*; Daiichi Sankyo* | None |
| Pamela W. Duncan | Wake Forest Baptist Medical Center | PCORI (PI of COMPASS Trial)†; PCORI (investigator FALLS Trial)*; NIA (investigator REHAB_HF)* | None | Molec* | None | Care Directions* | None | PCORI/NIH† |
| Meighan Girgus | American Heart Association | None | None | None | None | None | None | None |
| Virginia J. Howard | University of Alabama at Birmingham | NIH-NINDS†; NIH-NIA† | None | None | None | None | None | None |
| Ronald M. Lazar‡ | Columbia University Medical Center Neurological Institute | None | None | None | None | None | None | None |
| Donald M. Lloyd-Jones | Northwestern University Feinberg School of Medicine | None | None | None | None | None | None | None |
| Sudha Seshadri | Boston University School of Medicine, Framingham Heart Study | None | None | None | None | None | None | None |
| Eric E. Smith | University of Calgary, Canada | Canadian Institutes of Health Research†; Brain Canada†; McMaster University†; Ottawa Heart Institute† | None | None | None | None | None | None |
| Fernando D. Testai | University of Illinois at Chicago | None | None | None | None | None | None | None |
| Stephen van Gaal | University of Calgary, Canada | None | None | None | None | None | None | None |
| Salina P. Waddy | NIDDK, NIH, Office of Minority Research | None | None | None | None | None | None | None |
| Hank Wasiak | University of Southern California, Marshall School of Business | None | None | None | None | None | None | None |
| Kristine Yaffe | University of California, San Francisco | NHLBI† | None | None | None | None | None | None |
| Charlotte Zerna | University of Calgary, Canada | None | None | None | None | None | None | None |
This table represents the relationships of writing group members that may be perceived as actual or reasonably perceived conflicts of interest as reported on the Disclosure Questionnaire, which all members of the writing group are required to complete and submit. A relationship is considered to be “significant” if (a) the person receives $10 000 or more during any 12-month period, or 5% or more of the person’s gross income; or (b) the person owns 5% or more of the voting stock or share of the entity, or owns $10 000 or more of the fair market value of the entity. A relationship is considered to be “modest” if it is less than “significant” under the preceding definition.
Modest.
Significant.
Dr Lazar is now at the University of Alabama.
Footnotes
The views in this report are those of the authors and do not necessarily reflect those of the National Institute of Diabetes and Digestive and Kidney Diseases, the Department of Health and Human Services, or the government of the United States.
The American Heart Association makes every effort to avoid any actual or potential conflicts of interest that may arise as a result of an outside relationship or a personal, professional, or business interest of a member of the writing panel. Specifically, all members of the writing group are required to complete and submit a Disclosure Questionnaire showing all such relationships that might be perceived as real or potential conflicts of interest.
This advisory was approved by the American Heart Association Science Advisory and Coordinating Committee on July 28, 2017, and the American Heart Association Executive Committee on August 21, 2017. A copy of the document is available at http://professional.heart.org/statements by using either “Search for Guidelines & Statements” or the “Browse by Topic” area. To purchase additional reprints, call 843-216-2533 , kelle.ramsay@wolterskluwer.com.
The Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STR.0000000000000148/-/DC1.
References
- 1.Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017;389:1323–1335. doi: 10.1016/S0140-6736(16)32381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santosa A. A better world towards convergence of longevity? Lancet. 2017;389:1278–1279. doi: 10.1016/S0140-6736(17)30314-8. [DOI] [PubMed] [Google Scholar]
- 3.Winblad B, Amouyel P, Andrieu S, Ballard C, Brayne C, Brodaty H, Cedazo-Minguez A, Dubois B, Edvardsson D, Feldman H, Fratiglioni L, Frisoni GB, Gauthier S, Georges J, Graff C, Iqbal K, Jessen F, Johansson G, Jönsson L, Kivipelto M, Knapp M, Mangialasche F, Melis R, Nordberg A, Rikkert MO, Qiu C, Sakmar TP, Scheltens P, Schneider LS, Sperling R, Tjernberg LO, Waldemar G, Wimo A, Zetterberg H. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S on behalf of the American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gorelick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A on behalf of the American Heart Association Council on Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leshner AI, Landis S, Stroud C, Downey A, editors. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Committee on Preventing Dementia and Cognitive Impairment. Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press; 2017. [Accessed June 23, 2017]. pp. 1–130. http://www.nap.edu/24782. [PubMed] [Google Scholar]
- 7.Fiocco AJ, Yaffe K. Defining successful aging: the importance of including cognitive function over time. Arch Neurol. 2010;67:876–880. doi: 10.1001/archneurol.2010.130. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Healthy Aging. What is a healthy brain? [Accessed April 11, 2017];New research explores perceptions of cognitive health among diverse older adults. https://www.cdc.gov/aging/pdf/perceptions_of_cog_hlth_factsheet.pdf.
- 9.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD on behalf of the American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s Strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization and Alzheimer’s Disease International. Dementia: a public health priority. Paper presented at: World Health Organization; April 11, 2012; Geneva, Switzerland. [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Self-reported increased confusion or memory loss and associated functional difficulties among adults aged ≥ 60 years - 21 States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62:347–350. [PubMed] [Google Scholar]
- 12.Alzheimer’s Association. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9:208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: a reappraisal from population-based studies. Gerontologist. 2015;55:309–319. doi: 10.1093/geront/gnu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: a meta-analysis. Psychol Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Hachinski V. Stroke and Alzheimer disease: fellow travelers or partners in crime? Arch Neurol. 2011;68:797–798. doi: 10.1001/archneurol.2011.118. [DOI] [PubMed] [Google Scholar]
- 17.Hachinski V World Stroke Organization. Stroke and potentially preventable dementias proclamation: updated World Stroke Day proclamation [published correction appears in Stroke. 2016;47:d18] Stroke. 2015;46:3039–3040. doi: 10.1161/STROKEAHA.115.011237. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. [Accessed May 23, 2017];Diabetes public health resource. https://www.cdc.gov/diabetes/statistics/prev/national/figpersons.htm.
- 19.Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573–591. doi: 10.1161/CIRCRESAHA.116.308426. [DOI] [PubMed] [Google Scholar]
- 20.Merai R, Siegel C, Rakotz M, Basch P, Wright J, Wong B, Thorpe P. CDC grand rounds: a public health approach to detect and control hypertension. MMWR Morb Mortal Wkly Rep. 2016;65:1261–1264. doi: 10.15585/mmwr.mm6545a3. [DOI] [PubMed] [Google Scholar]
- 21.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. NCHS Data Brief. 2015:1–8. [PubMed] [Google Scholar]
- 22.GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unverzagt FW, McClure LA, Wadley VG, Jenny NS, Go RC, Cushman M, Kissela BM, Kelley BJ, Kennedy R, Moy CS, Howard V, Howard G. Vascular risk factors and cognitive impairment in a stroke-free cohort. Neurology. 2011;77:1729–1736. doi: 10.1212/WNL.0b013e318236ef23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life’s Simple 7 and incident cognitive impairment: the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. J Am Heart Assoc. 2014;3:e000635. doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 28.Cipolla MJ. The Cerebral Circulation. San Rafael, CA: Morgan & Claypool Life Sciences; 2009. pp. 1–59. [PubMed] [Google Scholar]
- 29.Andresen J, Shafi NI, Bryan RM., Jr Endothelial influences on cerebro-vascular tone. J Appl Physiol (1985) 2006;100:318–327. doi: 10.1152/japplphysiol.00937.2005. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163:1064–1078. doi: 10.1016/j.cell.2015.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Ménard J, Zetterberg H, Wisniewski T, de Leon MJ. Clearance systems in the brain: implications for Alzheimer disease [published correction appears in Nat Rev Neurol. 2016;12:248] Nat Rev Neurol. 2015;11:457–470. doi: 10.1038/nrneurol.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faraco G, Iadecola C. Hypertension: a harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol. 2011;300:H1566–H1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pal S, Tyler JK. Epigenetics and aging. Sci Adv. 2016;2:e1600584. doi: 10.1126/sciadv.1600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaeberlein M, Rabinovitch PS, Martin GM. Healthy aging: the ultimate preventative medicine. Science. 2015;350:1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahn LM. Aging and variability among immune cells. Science. 2017;355:1386–1387. doi: 10.1126/science.355.6332.1386-f. [DOI] [PubMed] [Google Scholar]
- 38.Labbadia J, Morimoto RI. The biology of proteostasis in aging and disease. Annu Rev Biochem. 2015;84:435–464. doi: 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- 40.Tahsili-Fahadan P, Geocadin RG. Heart-brain axis: effects of neurologic injury on cardiovascular function. Circ Res. 2017;120:559–572. doi: 10.1161/CIRCRESAHA.116.308446. [DOI] [PubMed] [Google Scholar]
- 41.Young CN, Davisson RL. Angiotensin-II, the brain, and hypertension: an update. Hypertension. 2015;66:920–926. doi: 10.1161/HYPERTENSIONAHA.115.03624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Farias ST, Mungas D, Reed BR, Cahn-Weiner D, Jagust W, Baynes K, Decarli C. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22:531–544. doi: 10.1037/0894-4105.22.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galvin JE, Roe CM, Powlishta KK, Coats MA, Muich SJ, Grant E, Miller JP, Storandt M, Morris JC. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. doi: 10.1212/01.wnl.0000172958.95282.2a. [DOI] [PubMed] [Google Scholar]
- 44.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19:1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 45.Berg L. Clinical Dementia Rating. Br J Psychiatry. 1984;145:339. [PubMed] [Google Scholar]
- 46.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 48.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 49.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–110. [Google Scholar]
- 50.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 51.Tuokko H, Hadjistavropoulos T, Miller JA, Beattie BL. The Clock Test: a sensitive measure to differentiate normal elderly from those with Alzheimer disease. J Am Geriatr Soc. 1992;40:579–584. doi: 10.1111/j.1532-5415.1992.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 52.Solomon PR, Hirschoff A, Kelly B, Relin M, Brush M, DeVeaux RD, Pendlebury WW. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349–355. doi: 10.1001/archneur.55.3.349. [DOI] [PubMed] [Google Scholar]
- 53.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards [published correction appears in Stroke. 2007;38:1118] Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 54.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Measure. 1977;1:385–401. [Google Scholar]
- 55.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 58.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 59.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 60.Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387–389. [PubMed] [Google Scholar]
- 61.Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: the late-life dementia risk index. Neurology. 2009;73:173–179. doi: 10.1212/WNL.0b013e3181a81636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 63.Yaffe K, Weston AL, Blackwell T, Krueger KA. The metabolic syndrome and development of cognitive impairment among older women. Arch Neurol. 2009;66:324–328. doi: 10.1001/archneurol.2008.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young SE, Mainous AG, 3rd, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care. 2006;29:2688–2693. doi: 10.2337/dc06-0915. [DOI] [PubMed] [Google Scholar]
- 65.Kalmijn S, Foley D, White L, Burchfiel CM, Curb JD, Petrovitch H, Ross GW, Havlik RJ, Launer LJ. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men: the Honolulu-Asia Aging Study. Arterioscler Thromb Vasc Biol. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 66.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cosentino F, Battista R, Scuteri A, De Sensi F, De Siati L, Di Russo C, Camici GG, Volpe M. Impact of fasting glycemia and regional cerebral perfusion in diabetic subjects: a study with technetium-99m-ethyl cysteinate dimer single photon emission computed tomography. Stroke. 2009;40:306–308. doi: 10.1161/STROKEAHA.108.520627. [DOI] [PubMed] [Google Scholar]
- 68.Anstey KJ, von Sanden C, Salim A, O’Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166:367–378. doi: 10.1093/aje/kwm116. [DOI] [PubMed] [Google Scholar]
- 69.Kalmijn S, van Boxtel MP, Verschuren MW, Jolles J, Launer LJ. Cigarette smoking and alcohol consumption in relation to cognitive performance in middle age. Am J Epidemiol. 2002;156:936–944. doi: 10.1093/aje/kwf135. [DOI] [PubMed] [Google Scholar]
- 70.Yaffe K. Preclinical Alzheimer disease: prevention holy grail or Pandora’s box? Comment on “Heavy smoking in midlife and long-term risk of Alzheimer disease and vascular dementia. ” Arch Intern Med. 2011;171:339–340. doi: 10.1001/archinternmed.2010.547. [DOI] [PubMed] [Google Scholar]
- 71.Whitmer RA, Gunderson EP, Barrett-Connor E, Quesenberry CP, Jr, Yaffe K. Obesity in middle age and future risk of dementia: a 27 year longitudinal population based study. BMJ. 2005;330:1360. doi: 10.1136/bmj.38446.466238.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barrett-Connor E. An introduction to obesity and dementia. Curr Alzheimer Res. 2007;4:97–101. doi: 10.2174/156720507780362074. [DOI] [PubMed] [Google Scholar]
- 73.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: Cardiovascular Health Study. Arch Neurol. 2009;66:336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Solomon A, Kåreholt I, Ngandu T, Wolozin B, Macdonald SW, Winblad B, Nissinen A, Tuomilehto J, Soininen H, Kivipelto M. Serum total cholesterol, statins and cognition in non-demented elderly. Neurobiol Aging. 2009;30:1006–1009. doi: 10.1016/j.neurobiolaging.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 75.Solomon A, Kivipelto M, Wolozin B, Zhou J, Whitmer RA. Midlife serum cholesterol and increased risk of Alzheimer’s and vascular dementia three decades later. Dement Geriatr Cogn Disord. 2009;28:75–80. doi: 10.1159/000231980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mielke MM, Zandi PP, Sjögren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 78.Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia Aging Study. Neurobiol Aging. 2000;21:49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- 79.Birns J, Kalra L. Cognitive function and hypertension. J Hum Hypertens. 2009;23:86–96. doi: 10.1038/jhh.2008.80. [DOI] [PubMed] [Google Scholar]
- 80.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 82.Miller JW, Green R, Ramos MI, Allen LH, Mungas DM, Jagust WJ, Haan MN. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78:441–447. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 83.Elias MF, Sullivan LM, D’Agostino RB, Elias PK, Jacques PF, Selhub J, Seshadri S, Au R, Beiser A, Wolf PA. Homocysteine and cognitive performance in the Framingham Offspring Study: age is important. Am J Epidemiol. 2005;162:644–653. doi: 10.1093/aje/kwi259. [DOI] [PubMed] [Google Scholar]
- 84.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes: a systematic review and meta-analysis [published correction appears in Obes Rev. 2008;9:267] Obes Rev. 2008;9:204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kivipelto M, Helkala EL, Hänninen T, Laakso MP, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and late-life mild cognitive impairment: a population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 86.Reitz C, Tang MX, Manly J, Mayeux R, Luchsinger JA. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, Coresh J, Knopman D, Power MC, Rawlings A, Sharrett AR, Wruck LM, Mosley TH. Midlife hypertension and 20-year cognitive change: the Atherosclerosis Risk in Communities Neurocognitive Study. JAMA Neurol. 2014;71:1218–1227. doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knopman D, Boland LL, Mosley T, Howard G, Liao D, Szklo M, McGovern P, Folsom AR Atherosclerosis Risk in Communities (ARIC) Study Investigators. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 89.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yano Y, Ning H, Allen N, Reis JP, Launer LJ, Liu K, Yaffe K, Greenland P, Lloyd-Jones DM. Long-term blood pressure variability throughout young adulthood and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Hypertension. 2014;64:983–988. doi: 10.1161/HYPERTENSIONAHA.114.03978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yano Y, Ning H, Muntner P, Reis JP, Calhoun DA, Viera AJ, Levine DA, Jacobs DR, Jr, Shimbo D, Liu K, Greenland P, Lloyd-Jones D. Nocturnal blood pressure in young adults and cognitive function in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Hypertens. 2015;28:1240–1247. doi: 10.1093/ajh/hpv028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yano Y, Ning H, Reis JP, Lewis CE, Launer LJ, Bryan RN, Yaffe K, Sidney S, Albanese E, Greenland P, Lloyd-Jones D, Liu K. Blood pressure reactivity to psychological stress in young adults and cognition in midlife: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc. 2016;5:e002718. doi: 10.1161/JAHA.115.002718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Blondell SJ, Hammersley-Mather R, Veerman JL. Does physical activity prevent cognitive decline and dementia? A systematic review and meta-analysis of longitudinal studies. BMC Public Health. 2014;14:510. doi: 10.1186/1471-2458-14-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beydoun MA, Beydoun HA, Gamaldo AA, Teel A, Zonderman Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohn NB, Dustman RE, Bradford DC. Age-related decrements in Stroop Color Test performance. J Clin Psychol. 1984;40:1244–1250. doi: 10.1002/1097-4679(198409)40:5<1244::aid-jclp2270400521>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 96.Blumenthal JA, Siegel WC, Appelbaum M. Failure of exercise to reduce blood pressure in patients with mild hypertension: results of a randomized controlled trial. JAMA. 1991;266:2098–2104. [PubMed] [Google Scholar]
- 97.Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- 98.Madden DJ, Blumenthal JA, Allen PA, Emery CF. Improving aerobic capacity in healthy older adults does not necessarily lead to improved cognitive performance. Psychol Aging. 1989;4:307–320. doi: 10.1037//0882-7974.4.3.307. [DOI] [PubMed] [Google Scholar]
- 99.Sink KM, Espeland MA, Castro CM, Church T, Cohen R, Dodson JA, Guralnik J, Hendrie HC, Jennings J, Katula J, Lopez OL, McDermott MM, Pahor M, Reid KF, Rushing J, Verghese J, Rapp S, Williamson JD LIFE Study Investigators. Effect of a 24-month physical activity intervention vs health education on cognitive outcomes in sedentary older adults: the LIFE randomized trial. JAMA. 2015;314:781–790. doi: 10.1001/jama.2015.9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008:CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed]
- 101.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 102.Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, Browndyke JN, Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72:239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu CL, Best JR, Davis JC, Nagamatsu LS, Wang S, Boyd LA, Hsiung GR, Voss MW, Eng JJ, Liu-Ambrose T. Aerobic exercise promotes executive functions and impacts functional neural activity among older adults with vascular cognitive impairment [published online ahead of print April 21, 2017] [Accessed August 14, 2017];Br J Sports Med. doi: 10.1136/bjsports-2016-096846. http://www.bjsm.bmj.com/content/early/2017/04/21/bjsports-2016-096846.long. [DOI] [PubMed]
- 104.de Bruijn RF, Heeringa J, Wolters FJ, Franco OH, Stricker BH, Hofman A, Koudstaal PJ, Ikram MA. Association between atrial fibrillation and dementia in the general population. JAMA Neurol. 2015;72:1288–1294. doi: 10.1001/jamaneurol.2015.2161. [DOI] [PubMed] [Google Scholar]
- 105.Singh-Manoux A, Fayosse A, Sabia S, Canonico M, Bobak M, Elbaz A, Kivimaki M, Dugravot A. Atrial fibrillation as a risk factor for cognitive decline and dementia [published online ahead of print April 29, 2017] [Accessed August 14, 2017];Eur Heart J. doi: 10.1093/eurheartj/ehx208. https://www.academic.oup.com/eurheartj/article-lookup/doi/10.1093/eurheartj/ehx208. [DOI] [PMC free article] [PubMed]
- 106.Thacker EL, McKnight B, Psaty BM, Longstreth WT, Jr, Sitlani CM, Dublin S, Arnold AM, Fitzpatrick AL, Gottesman RF, Heckbert SR. Atrial fibrillation and cognitive decline: a longitudinal cohort study. Neurology. 2013;81:119–125. doi: 10.1212/WNL.0b013e31829a33d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 108.Wu L, Sun D, Tan Y. A systematic review and dose-response meta-analysis of sleep duration and the occurrence of cognitive disorders [published online ahead of print June 6, 2017] [Accessed August 14, 2017];Sleep Breath. doi: 10.1007/s11325-017-1527-0. https://www.link.springer.com/article/10.1007%2Fs11325-017-1527-0. [DOI] [PubMed]
- 109.Devore EE, Grodstein F, Schernhammer ES. Sleep duration in relation to cognitive function among older adults: a systematic review of observational studies. Neuroepidemiology. 2016;46:57–78. doi: 10.1159/000442418. [DOI] [PubMed] [Google Scholar]
- 110.Keage HA, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–892. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 111.Blackwell T, Yaffe K, Laffan A, Ancoli-Israel S, Redline S, Ensrud KE, Song Y, Stone KL Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: the MrOS sleep study. Sleep. 2014;37:655–663. doi: 10.5665/sleep.3562.AB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Jr, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Alley D, Suthers K, Crimmins E. Education and cognitive decline in older Americans: results from the AHEAD sample. Res Aging. 2007;29:73–94. doi: 10.1177/0164027506294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cagney KA, Lauderdale DS. Education, wealth, and cognitive function in later life. J Gerontol B Psychol Sci Soc Sci. 2002;57:P163–P172. doi: 10.1093/geronb/57.2.p163. [DOI] [PubMed] [Google Scholar]
- 115.Kaup AR, Simonsick EM, Harris TB, Satterfield S, Metti AL, Ayonayon HN, Rubin SM, Yaffe K. Older adults with limited literacy are at increased risk for likely dementia. J Gerontol A Biol Sci Med Sci. 2014;69:900–906. doi: 10.1093/gerona/glt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yaffe K, Falvey C, Harris TB, Newman A, Satterfield S, Koster A, Ayonayon H, Simonsick E Health ABC Study. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. doi: 10.1136/bmj.f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Glymour M. thesis. Cambridge, MA: Harvard University; 2004. Identifying Social Determinants of Old Age Cognitive Function. [Google Scholar]
- 118.Sisco S, Gross AL, Shih RA, Sachs BC, Glymour MM, Bangen KJ, Benitez A, Skinner J, Schneider BC, Manly JJ. The role of early-life educational quality and literacy in explaining racial disparities in cognition in late life. J Gerontol B Psychol Sci Soc Sci. 2015;70:557–567. doi: 10.1093/geronb/gbt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wadley VG, Unverzagt FW, McGuire LC, Moy CS, Go R, Kissela B, McClure LA, Crowe M, Howard VJ, Howard G. Incident cognitive impairment is elevated in the Stroke Belt: the REGARDS study. Ann Neurol. 2011;70:229–236. doi: 10.1002/ana.22432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peters R, Peters J, Booth A, Mudway I. Is air pollution associated with increased risk of cognitive decline? A systematic review. Age Ageing. 2015;44:755–760. doi: 10.1093/ageing/afv087. [DOI] [PubMed] [Google Scholar]
- 121.Jung CR, Lin YT, Hwang BF. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: a population-based cohort study in Taiwan. J Alzheimers Dis. 2015;44:573–584. doi: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- 122.Loop MS, Kent ST, Al-Hamdan MZ, Crosson WL, Estes SM, Estes MG, Jr, Quattrochi DA, Hemmings SN, Wadley VG, McClure LA. Fine particulate matter and incident cognitive impairment in the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort [published correction appears in PLoS One. 2015;10:e0125137] PLoS One. 2013;8:e75001. doi: 10.1371/journal.pone.0075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43:3137–3146. doi: 10.1161/STROKEAHA.112.651778. [DOI] [PubMed] [Google Scholar]
- 124.Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, Creager MA, Eckel RH, Elkind MS, Fornage M, Goldstein LB, Greenberg SM, Horvath SE, Iadecola C, Jauch EC, Moore WS, Wilson JA on behalf of the American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3754–3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Office of Disease Prevention and Health Promotion. [Accessed June 27, 2017];Physical Activity Guidelines for Americans. https://www.health.gov/paguidelines/guidelines/
- 126.Bogers RP, Bemelmans WJ, Hoogenveen RT, Boshuizen HC, Woodward M, Knekt P, van Dam RM, Hu FB, Visscher TL, Menotti A, Thorpe RJ, Jr, Jamrozik K, Calling S, Strand BH, Shipley MJ BMI-CHD Collaboration Investigators. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728. doi: 10.1001/archinte.167.16.1720. [DOI] [PubMed] [Google Scholar]
- 127.Gorospe EC, Dave JK. The risk of dementia with increased body mass index. Age Ageing. 2007;36:23–29. doi: 10.1093/ageing/afl123. [DOI] [PubMed] [Google Scholar]
- 128.Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N, Knopman DS, Mintz A, Rahmim A, Sharrett AR, Wagenknecht LE, Wong DF, Mosley TH. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Horie NC, Serrao VT, Simon SS, Gascon MR, Dos Santos AX, Zambone MA, Del Bigio de Freitas MM, Cunha-Neto E, Marques EL, Halpern A, de Melo ME, Mancini MC, Cercato C. Cognitive effects of intentional weight loss in elderly obese individuals with mild cognitive impairment. J Clin Endocrinol Metab. 2016;101:1104–1112. doi: 10.1210/jc.2015-2315. [DOI] [PubMed] [Google Scholar]
- 130.Murphy RA, Patel KV, Kritchevsky SB, Houston DK, Koster A, Simonsick EM, Tylvasky FA, Cawthon PM, Harris TB. Weight change, body composition, and risk of mobility disability and mortality in older adults: a population-based cohort study. J Am Geriatr Soc. 2014;62:1476–1483. doi: 10.1111/jgs.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1–7. doi: 10.1016/j.gtc.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Body adiposity in later life and the incidence of dementia: the Health in Men Study. PLoS One. 2011;6:e17902. doi: 10.1371/journal.pone.0017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yatsuya H, Yamagishi K, North KE, Brancati FL, Stevens J, Folsom AR ARIC Study Investigators. Associations of obesity measures with subtypes of ischemic stroke in the ARIC Study. J Epidemiol. 2010;20:347–354. doi: 10.2188/jea.JE20090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banach M, Rizzo M, Nikolic D, Howard G, Howard V, Mikhailidis D. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther. 2017;170:181–191. doi: 10.1016/j.pharmthera.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 135.Gómez-Pinilla F. Brain foods: the effects of nutrients on brain function. Nat Rev Neurosci. 2008;9:568–578. doi: 10.1038/nrn2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jones RW, Kivipelto M, Feldman H, Sparks L, Doody R, Waters DD, Hey-Hadavi J, Breazna A, Schindler RJ, Ramos H LEADe Investigators. The Atorvastatin/Donepezil in Alzheimer’s Disease Study (LEADe): design and baseline characteristics. Alzheimers Dement. 2008;4:145–153. doi: 10.1016/j.jalz.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 138.Ott BR, Daiello LA, Dahabreh IJ, Springate BA, Bixby K, Murali M, Trikalinos TA. Do statins impair cognition? A systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2015;30:348–358. doi: 10.1007/s11606-014-3115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reed B, Villeneuve S, Mack W, DeCarli C, Chui HC, Jagust W. Associations between serum cholesterol levels and cerebral amyloidosis. JAMA Neurol. 2014;71:195–200. doi: 10.1001/jamaneurol.2013.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schreurs BG. The effects of cholesterol on learning and memory. Neurosci Biobehav Rev. 2010;34:1366–1379. doi: 10.1016/j.neubiorev.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG PROSPER Study Group; PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 142.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129[suppl 2]:S46–S48 and Circulation. 2015;132:e396] Circulation. 2014;129(suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 143.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, Greenberg SM, Higashida RT, Kasner SE, Seshadri S on behalf of the American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Functional Genomics and Translational Biology; Council on Hypertension. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:e44–e71. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 144.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge Rv, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M STandards for ReportIng Vascular changes on nEuroimaging AB (STRIVE v1) Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhäger WH, Babarskiene MR, Babeanu S, Bossini A, Gil-Extremera B, Girerd X, Laks T, Lilov E, Moisseyev V, Tuomilehto J, Vanhanen H, Webster J, Yodfat Y, Fagard R. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352:1347–1351. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 146.Anderson C, Teo K, Gao P, Arima H, Dans A, Unger T, Commerford P, Dyal L, Schumacher H, Pogue J, Paolasso E, Holwerda N, Chazova I, Binbrek A, Young J, Yusuf S ONTARGET and TRANSCEND Investigators. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011;10:43–53. doi: 10.1016/S1474-4422(10)70250-7. [DOI] [PubMed] [Google Scholar]
- 147.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C HYVET Investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 148.Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, Trenkwalder P, Zanchetti A SCOPE Study Group. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. J Hypertens. 2003;21:875–886. doi: 10.1097/01.hjh.0000059028.82022.89. [DOI] [PubMed] [Google Scholar]
- 149.Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, Palesch Y, Martin RH, Albers GW, Bath P, Bornstein N, Chan BP, Chen ST, Cunha L, Dahlöf B, De Keyser J, Donnan GA, Estol C, Gorelick P, Gu V, Hermansson K, Hilbrich L, Kaste M, Lu C, Machnig T, Pais P, Roberts R, Skvortsova V, Teal P, Toni D, VanderMaelen C, Voigt T, Weber M, Yoon BW Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) Study Group. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study [published correction appears in Lancet Neurol. 2008;7:985] Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, Chalmers J PROGRESS Collaborative Group. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Arch Intern Med. 2003;163:1069–1075. doi: 10.1001/archinte.163.9.1069. [DOI] [PubMed] [Google Scholar]
- 151.Applegate WB, Pressel S, Wittes J, Luhr J, Shekelle RB, Camel GH, Greenlick MR, Hadley E, Moye L, Perry HM, Jr, Schron E, Wegener V. Impact of the treatment of isolated systolic hypertension on behavioral variables: results from the Systolic Hypertension in the Elderly Program. Arch Intern Med. 1994;154:2154–2160. [PubMed] [Google Scholar]
- 152.Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, Murray AM, Sullivan MD, Horowitz KR, Ding J, Marcovina S, Lovato L, Lovato J, Margolis KL, Davatzikos C, Barzilay J, Ginsberg HN, Linz PE, Miller ME Action to Control Cardiovascular Risk in Diabetes Memory in Diabetes Investigators. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA Intern Med. 2014;174:324–333. doi: 10.1001/jamainternmed.2013.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bosch J HOPE Investigators. The effect of blood pressure and cholesterol lowering on cognition. Circulation. 2016;134:e703–e704. [Google Scholar]
- 154.Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, Benavente OR SPS3 Investigators. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014;13:1177–1185. doi: 10.1016/S1474-4422(14)70224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, Hoevenaar-Blom MP, Vermeulen M, van Gool WA. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 156.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 157.Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, Bories L, Cufi MN, Dantoine T, Dartigues JF, Desclaux F, Gabelle A, Gasnier Y, Pesce A, Sudres K, Touchon J, Robert P, Rouaud O, Legrand P, Payoux P, Caubere JP, Weiner M, Carrié I, Ousset PJ, Vellas B MAPT Study Group. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a ran-domised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 158.Valls-Pedret C, Sala-Vila A, Serra-Mir M, Corella D, de la Torre R, Martínez-González MÁ, Martínez-Lapiscina EH, Fitó M, Pérez-Heras A, Salas-Salvadó J, Estruch R, Ros E. Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern Med. 2015;175:1094–1103. doi: 10.1001/jamainternmed.2015.1668. [DOI] [PubMed] [Google Scholar]
- 159.Liu K, Colangelo LA, Daviglus ML, Goff DC, Pletcher M, Schreiner PJ, Sibley CT, Burke GL, Post WS, Michos ED, Lloyd-Jones DM. Can antihypertensive treatment restore the risk of cardiovascular disease to ideal levels? The Coronary Artery Risk Development in Young Adults (CARDIA) Study and the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2015;4:e002275. doi: 10.1161/JAHA.115.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 161.Alzheimer’s Association. [Accessed May 15, 2017];10 Ways to love your brain. http://www.alz.org/brain-health/10_ways-to-love-your-brain.asp.
- 162.IOM (Institute of Medicine) Cognitive Aging: Progress in Understanding and Opportunities for Action. 1. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 163.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Jr, Svetkey LP, Wadden TA, Yanovski SZ, Kendall KA, Morgan LC, Trisolini MG, Velasco G, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published corrections appear in Circulation. 2014;129[suppl 2]:S100–S101 and Circulation. 2015;131:e326] Circulation. 2014;129(suppl 2):S76–S99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 164.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines [published correction appears in Circulation. 2014;129[suppl 2]:S74–S75] Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 165.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D on behalf of the American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 166.Jauch EC, Saver JL, Adams HP, Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW, Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H on behalf of the American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 167.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA on behalf of the American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association [published correction appears in Stroke. 2015;46:e54] Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 168.Spring B, Ockene JK, Gidding SS, Mozaffarian D, Moore S, Rosal MC, Brown MD, Vafiadis DK, Cohen DL, Burke LE, Lloyd-Jones D on behalf of the American Heart Association Behavior Change Committee of the Council on Epidemiology and Prevention, Council on Lifestyle and Cardiometabolic Health, Council for High Blood Pressure Research, Council on Cardiovascular and Stroke Nursing. Better population health through behavior change in adults: a call to action. Circulation. 2013;128:2169–2176. doi: 10.1161/01.cir.0000435173.25936.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith EE, Saver JL, Alexander DN, Furie KL, Hopkins LN, Katzan IL, Mackey JS, Miller EL, Schwamm LH, Williams LS on behalf of the AHA/ASA Stroke Performance Oversight Committee. Clinical performance measures for adults hospitalized with acute ischemic stroke: performance measures for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:3472–3498. doi: 10.1161/STR.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 170.Redberg RF, Benjamin EJ, Bittner V, Braun LT, Goff DC, Jr, Havas S, Labarthe DR, Limacher MC, Lloyd-Jones DM, Mora S, Pearson TA, Radford MJ, Smetana GW, Spertus JA, Swegler EW. AHA/ACCF 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Performance Measures for Primary Prevention of Cardiovascular Disease) [published correction appears in Circulation. 2010;121:e445–e446] Circulation. 2009;120:1296–1336. doi: 10.1161/CIRCULATIONAHA.109.192617. [DOI] [PubMed] [Google Scholar]
- 171.American Heart Association. [Accessed May 15, 2017];Healthy for Good. https://www.healthyforgood.heart.org/
- 172.Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Allen NB, Zhao L, Liu L, Daviglus M, Liu K, Fries J, Shih YT, Garside D, Vu TH, Stamler J, Lloyd-Jones DM. Favorable cardiovascular health, compression of morbidity, and healthcare costs: forty-year follow-up of the CHA Study (Chicago Heart Association Detection Project in Industry) Circulation. 2017;135:1693–1701. doi: 10.1161/CIRCULATIONAHA.116.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gorelick PB, Erkinjuntti T, Hofman A, Rocca WA, Skoog I, Winblad B. Prevention of vascular dementia. Alzheimer Dis Assoc Disord. 1999;13(suppl 3):S131–S139. [PubMed] [Google Scholar]
- 175.Zeng Y, Feng Q, Hesketh T, Christensen K, Vaupel JW. Survival, disabilities in activities of daily living, and physical and cognitive functioning among the oldest-old in China: a cohort study. Lancet. 2017;389:1619–1629. doi: 10.1016/S0140-6736(17)30548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet. 2017;389:1885–1906. doi: 10.1016/S0140-6736(17)30819-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roth GA, Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJL. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. doi: 10.1001/jama.2017.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kaplan H, Thompson RC, Trumble BC, Wann LS, Allam AH, Beheim B, Frohlich B, Sutherland ML, Sutherland JD, Stieglitz J, Rodriguez DE, Michalik DE, Rowan CJ, Lombardi GP, Bedi R, Garcia AR, Min JK, Narula J, Finch CE, Gurven M, Thomas GS. Coronary atherosclerosis in indigenous South American Tsimane: a cross-sectional cohort study. Lancet. 2017;389:1730–1739. doi: 10.1016/S0140-6736(17)30752-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Niiranen TJ, Lyass A, Larson MG, Hamburg NM, Benjamin EJ, Mitchell GF, Vasan RS. Prevalence, correlates, and prognosis of healthy vascular aging in a Western community-dwelling cohort: the Framingham Heart Study. Hypertension. 2017;70:267–274. doi: 10.1161/HYPERTENSIONAHA.117.09026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Younus A, Aneni EC, Spatz ES, Osondu CU, Roberson L, Ogunmoroti O, Malik R, Ali SS, Aziz M, Feldman T, Virani SS, Maziak W, Agatston AS, Veledar E, Nasir K. A systematic review of the prevalence and outcomes of ideal cardiovascular health in US and non-US populations. Mayo Clin Proc. 2016;91:649–670. doi: 10.1016/j.mayocp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 181.Arena R, Lavie CJ. Preventing bad and expensive things from happening by taking the healthy living polypill: everyone needs this medicine [published online ahead of print March 13, 2017] [Accessed August 14, 2017];Mayo Clin Proc. 2017 92:483–487. doi: 10.1016/j.mayocp.2017.02.005. https://www.linkinghub.elsevier.com/retrieve/pii/S0025-6196(17)30121-0. [DOI] [PubMed] [Google Scholar]
- 182.Osondu CU, Aneni EC, Valero-Elizondo J, Salami JA, Rouseff M, Das S, Guzman H, Younus A, Ogunmoroti O, Feldman T, Agatston AS, Veledar E, Katzen B, Calitz C, Sanchez E, Lloyd-Jones DM, Nasir K. Favorable cardiovascular health is associated with lower health care expenditures and resource utilization in a large US employee population: the Baptist Health South Florida Employee Study [published online ahead of print March 13, 2017] [Accessed August 14, 2017];Mayo Clin Proc. 2017 92:512–524. doi: 10.1016/j.mayocp.2016.12.026. https://www.linkinghub.elsevier.com/retrieve/pii/S0025-6196(17)30088-5. [DOI] [PubMed] [Google Scholar]
- 183.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention and care [published online ahead of print on July 19, 2017] [Accessed August 14, 2017];Lancet. 2017 doi: 10.1016/S0140-6736(17)31363-6. http://dx.doi.org/10.1016/S0140-6736(17)31363-6. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Selection Criteria
Table 1. Classification of Included vs. Excluded Exposure Categories
Appendix 1. Search strategy