Abstract
Cancer stem/initiating cells (CSCs) are a subset of tumor cells proposed to play privileged roles in seeding tumors and driving metastasis. CSCs have emerged as an increasingly important target of interest in cancer biology and therapy. Recent work has suggested that CSC maintenance and metastatic potential may be modulated by physical inputs within the tissue microenvironment, including interstitial pressure and extracellular matrix stiffness. Here we review recent progress in our understanding of CSC regulation by biophysical signals within the tumor microenvironment. While the mechanistic basis of this signaling remains incompletely understood, we discuss emerging evidence that mechanical inputs can epigenetically regulate CSC behavior and that some CSCs can evade mechanotransductive signals to more efficiently infiltrate tissue. We also describe efforts to leverage these findings to engineer culture platforms for the characterization of CSC mechanics for discovery and screening.
Graphical Abstract
Introduction
Despite tremendous progress in research and treatment over the past half-century, cancer remains one of the leading causes of mortality worldwide [1,2]. Conventional cancer treatment, which includes tumor resection, chemotherapy, and radiation therapy, is designed to remove or kill rapidly dividing cancer cells, and although this paradigm has had important clinical impact, it is typically based on nonspecifically targeting dividing cells, making no fine distinctions between tumor cells and normal cells, or between subpopulations of cells within the tumor. In particular, these approaches do not specifically target tumor cells most likely to seed metastasis, the process by which cancer cells escape from the primary tumor and seed a new tumor [3] and the main cause of mortality in most cancers [3–5]. In 1855, Rudolf Virchow suggested that stem cells may be involved in the etiology of cancer, a concept that was revisited by Lapidot and colleagues over a century later [6,7]. Over the past decade, the evidence for stem-like cells within tumors has been dramatically expanded and reinforced that not all cancer cells are equally able to maintain the growth of a secondary tumor, and that a specialized subpopulation of cancer stem/initiating cells (CSCs) critically drive tumor formation and metastasis [8–11].
The American Association for Cancer Research defines a CSC as “a cell within a tumor that possesses the capacity to self-renew and to cause the heterogeneous lineages of cancer cells that comprise the tumor” [12]. Although much controversy surrounds the origin and prevalence of CSCs, these cells have been identified in several solid tumors including breast, colon, brain, pancreas, liver, and prostate [13–20]. The emergence of CSCs has ushered in a completely new organizing principle for tumor initiation and progression in which tumors are composed of a hierarchy of cells (e.g., stem cell, progenitor cell, and mature cell), with some but not all members of the hierarchy capable of recapitulating all components of a tumor when injected into immunocompromised mice.
CSCs have been shown to possess many characteristics that are essential to tumor initiation, invasion, and recurrence. Most notably, many CSCs are highly resistant to radiation and chemotherapy, suggesting that these cells drive recurrence [10,21]. CSCs employ a stunning arsenal of strategies for evading chemotherapy, including a slow division rate, high expression of efflux pumps, and enhanced capacity for DNA repair [21]. There is also evidence that certain CSCs can invade tissue more rapidly than other tumor cells, consistent with a special role in invasion and metastasis [22]. For this reason, significant energy has been devoted to identifying CSCs, elucidating how they contribute to tumor progression and metastasis, and developing therapies that preferentially target these cells.
Initial efforts to develop CSC-specific therapies have focused on targeting signaling pathways within CSCs that contribute to self-renewal and survival, with the goal of promoting a more differentiated phenotype susceptible to conventional cancer drugs [23,24]. However, targeting specificity remains a major challenge, given that many of these pathways (e.g. Notch, Hedgehog, Wnt) are also active within non-tumor cells.
Moreover, many of the relevant targets are intracellular proteins, creating significant challenges in drug delivery. An important opportunity to circumvent both challenges has come in the discovery that CSCs display enhanced expression of specific cell adhesion receptors, such as integrins and CD44, which cells use to attach to and transduce mechanical signals from the extracellular matrix (ECM) [25–29]. This is important, because dramatic changes to the physical environment are experienced within the tumor microenvironment, including increased mechanical stress due to interstitial pressure and increases in the density and stiffness of the ECM [26,30]. Furthermore, it is now well established that these physical changes can activate mechanotransductive signaling systems within the cell, which may collude with canonical mitogenic signaling systems to drive tumor progression [26,31]. While the lion’s share of past studies has focused on “bulk” tumor cells, several recent studies have indicated that these mechanobiological cues have special implications for CSC generation, maintenance, and metastasis [27,28]. A deeper investigation into the biophysical regulation of the CSCs represent a promising approach to unravel new CSC specific targets for pharmacological intervention.
In addition to the extrinsic biophysical signals that may regulate CSC behavior, recent reports have also shown that CSCs themselves exhibit changes in their mechanical properties when compared to the bulk tumor population [32–34]. Several reports have indicated an increase in mechanical deformability as an important identifier of CSC populations [32,33]. The identification of such biophysical “markers” has generated much excitement as it can allow for the application of advanced engineering platforms for diagnostic use, drug screening, and mechanistic discovery for the CSC population [32,33,35,36].
In this review, we will focus on how CSCs regulate and are regulated by the biophysical cues within the tumor microenvironment and how these reciprocal interactions contribute to tumorigenesis and metastasis. Further, we will describe the current state of bioengineering platforms designed to characterize CSC mechanical properties for the development of diagnostic and drug screening tools that can help accelerate the development of new therapies that target CSCs.
Regulation of CSC functions by the tumor microenvironment
The tumor microenvironment is remarkably complex and dynamic, providing a host of signaling inputs to the resident tumor cell population [30,37–39]. These cues include changes in oxygen tension, juxtacrine and paracrine signals from tumor and stromal cells, and biophysical signals such as matrix stiffness and applied loads [30,40]. The impact of these alterations is tremendous, and while much attention has been directed towards the role of hypoxia and the influence of soluble factors, this review will focus on the biophysical changes and their role in tumorigenicity and metastasis.
Over the past two decades, it has become clear that the behavior of a wide range of cell types is sensitive to mechanical inputs encoded within the tissue environment [41–45]. Mechanotransductive pathways contribute to many aspects of normal physiology, ranging from developmental morphogenesis to adult tissue repair and regeneration [46–48]. Moreover, the disruption of physiologic mechanical inputs leads to the initiation and development of a variety of diseases [49,50]. In the tumor microenvironment specifically, two mechanical changes documented in many solid tumors include: (1) an increase in compressive, tensile, and shear stress, often due to increased interstitial pressure, and (2) an increase in matrix stiffness and ECM density [26,30]. As the tumor expands, tumor cells begin to proliferate and grow in a confined volume, elevating radial and circumferential stresses within the tissue. Several studies have noted the presence of these stresses in vitro and in silico revealing applied stresses of ~0.5 kPa in colon and breast tumors [51,52]. Increased shear stresses are also observed in many cancers where increased interstitial fluid pressure leads to increased fluid flow by generating pressure gradients in the tumor microenvironment [30]. In addition, breast and pancreatic tumors exhibit an aggressive desmoplastic response where tumor cells initiate ECM remodeling by depositing increased fibronectin, tenascin, collagen, and proteoglycans while also overexpressing matrix metalloproteinases, thereby dramatically stiffening the microenvironment [26,53,54]. Within this new mechanical environment, pro-tumorigenic mechanotransductive inputs actively regulate tumor growth and spread [25,55].
CSCs and confinement
The stress experienced within the tumor microenvironment is transmitted to tumor cells, and the resulting activation of mechanotransductive signaling has been implicated in the regulation of both CSCs and bulk tumor cells. In breast cancer cells, it has been observed that increased compressive stress can induce rearrangement of actomyosin stress fibers and microtubules and enhance cell migration, reminiscent of an epithelial-mesenchymal transition [56]. Strikingly, this responsiveness to confinement was not observed in normal mammary epithelial cells but exclusively in aggressive carcinoma cells, suggesting an alteration in mechanosensing that primes tumor cells for malignant behavior. Observations such as these have spurred interest in dissecting molecular mechanisms of mechanotransduction that may be unique to or amplified within tumor cells or CSCs. In a melanoma model, confinement sensing has been shown to be cooperatively mediated by Piezo1/PKA and myosin II [57,58]. In another example, the activation of myosin II driven contractility via α4 integrin/paxillin dependent reduction of Rac1 activity promoted more efficient migration under confinement [57]. Collectively, these studies indicate that the applied mechanical stresses can influence cancer cell differentiation into CSCs as well as regulate actin dynamics to promote efficient motility [56,59].
It has been noted by many that the migration of tumor cells within confined environments is likely to require enhanced compliance or deformability. Recent evidence has shown that CSCs specifically display heightened deformability relative to either non-tumor cells or bulk tumor cells. In one study, breast CSCs displayed increased deformability, reduced nuclear stiffness, and higher invasiveness through confined spaces when compared to non-stem cancer cells [22]. Further, Harada et al. demonstrated that nuclear stiffness is regulated by lamin-A and lamin-B expression, which is often altered in cancer cells and stem cells [60]. These studies suggest that deformability and perhaps other mechanical properties enhance the ability of CSCs to invade tissue and potentially metastasize. Exciting recent work has further proposed that the high shear stresses experienced by the nucleus during this confined migration contributes to disruption of the nuclear envelope, chromatin, and nuclear DNA, thereby potentially accelerating genomic instability [60,61].
CSCs and the mechanical microenvironment
Cells sense mechanical inputs from the ECM through adhesion receptors, which trigger mechanotransductive signaling events and transmit these inputs to the cellular cytoskeleton. Engagement and clustering of integrins incudes formation of focal adhesions and related structures, which can in turn modulate signaling a variety of mechanotransductive and canonical mitogenic pathways including through MAPK, PI3K, and the Rho GTPases [25,30,62]. Interestingly, aberrant cell surface adhesion protein expression is found in many CSC populations, including overexpression of the hyaluronan/osteopontin receptor CD44, and integrin subunits α3 and α6 [3,63–68]. While there is evidence that enhanced expression of these markers is predictive of tumorigenicity, it remains unclear to what extent alterations in expression of these receptors are primary drivers of tumor progression.
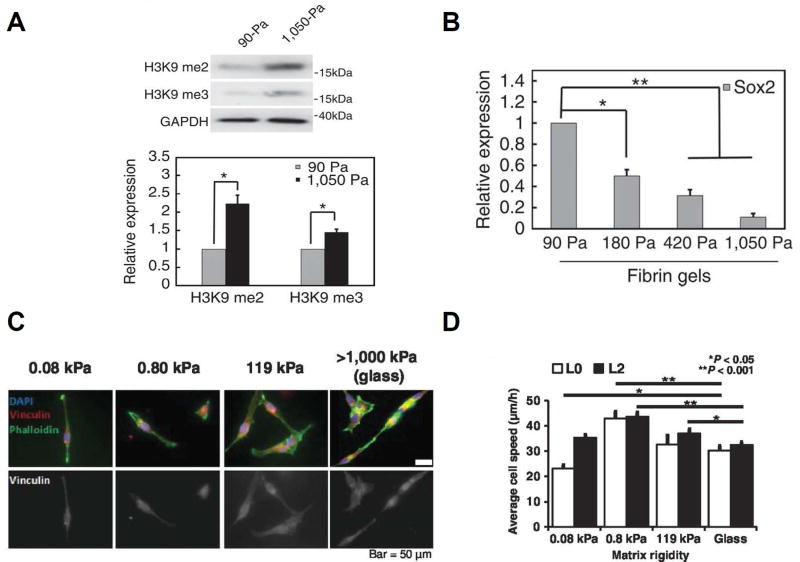
Importantly, integrin signaling is strongly dependent upon the mechanical properties of the ECM, such that stiff ECM environments promote integrin clustering, adhesion assembly, and actomyosin contractility [69,70]. This has motivated interest in in the role of ECM stiffness in regulating CSC behavior, which appears to be dependent on tumor type and stage (Figure 1). For example, tissue hypoxia and matrix stiffening synergistically cooperate to promote the generation of the breast CSC pool through the activation of integrin-linked kinase (ILK) and CD44. Further, suppression of ILK leads to a loss of the CSC phenotype even in the presence of a stiff and hypoxic microenvironment [71]. Conversely, Tan and colleagues revealed that matrix softness plays an important role in CSC maintenance through epigenetic regulation of H3K9 and Sox2 gene expression in melanoma CSCs (Figure 1A,B) [72]. The authors found that stiff environments decrease Sox2 expression and self-renewal and promote differentiation of CSCs. Our own laboratory has shown that glioblastoma CSCs display dysregulated mechanosensing with GBM CSCs exhibiting little change in proliferation, migration, or spreading as a function of ECM stiffness (Figure 1C,D) [28]. Mechanosensitivity was restored through the constitutive activation of RhoA, which decreased TIC invasive capacity both in vitro and an in vivo orthotopic xenograft model. Importantly, xenografting with mechanosensitive CSCs extended survival times and decreased tumor size relative to control CSC-based xenografts. These findings suggest that GBM CSCs may evade limitations on migration imposed by soft ECMs to aggressively and efficiently migrate through normal brain tissue, which is highly compliant. These studies highlight the important influence matrix stiffness has on CSC maintenance and behavior and reveal the need for further investigations into these mechanotransductive mechanisms.
Figure 1.
CSC mechanotransduction depends on tumor type. The melanoma CSC pool is sustained in soft physical environments; stiff environments increase H3K9 methylation (A) and reduce expression of the stem cell marker Sox2 (B) to promote differentiation and non-stem cancer cells. (C) Glioblastoma CSCs exhibit a loss of mechanosensing with no change in cell spread area and only modest changes in migration speed with matrix stiffness (D). Reproduced with permission from Tan et al (A,B) [72] from Wong et al (C,D) [28].
Overexpression of Integrins and CD44 in CSCs
In an effort to understand the mechanotransductive mechanisms that control CSC maintenance and spread, investigators have studied the consequence of overexpressed cell surface adhesion proteins in the CSC population [14,63,64,73]. As mentioned previously, overexpression of integrin α3 and α6 along with CD44 are present in many types of CSCs [63]. In GBM CSCs, integrin α3 plays a crucial role in the migration and invasion of CSCs. Invasion assays indicate that integrin α3 promotes migration of CSCs in an Erk1/2 dependent manner, and the depletion of integrin α3 via shRNA sufficiently decreased migratory capacity [64]. Moreover integrin α6 is enriched in glioblastoma CSCs and contributes to CSC tumorigenesis, with α6 knockdown reducing tumorsphere-forming capacity and promoting apoptosis [66].
CD44 has also been under active study as an adhesive regulator of CSC behavior [14,67,73]. CD44 mediates adhesion to a variety of matrix elements, including hyaluronic acid (HA) and osteopontin [74,75]. CD44-HA binding can activate promigratory pathways induced by engagement of the CD44 cytoplasmic tail with the actin cytoskeleton through mediators such as ankyrin and the ERM domain-containing proteins [76,77]. Additionally, several studies have linked CD44 to the regulation of Rho GTPases, which modulate cytoskeletal organization and migration [78]. Enrichment of CD44 has been associated with increased invasive capacity and tumorigenesis. For example, lung cancer cells highly expressing CD44 have been reported to form robust spheroid bodies, display enhanced migratory capacity, and exhibit enhanced tumorigenicity in vivo [79]. CD44-positive prostate cancer cells were reported to aggressively invade Matrigel and efficiently form tumors when injected into immunocompromised mice [80]. By contrast, CD44-negative cells were non-invasive in Matrigel and formed tumors in only in 40% of cases. Through q-PCR analysis of CD44-positive cells, the authors determined that signaling through the ERM protein Ezrin, which is downstream of CD44 binding, may be associated with invasive behavior of these cells. Collectively, the investigations of cell-matrix adhesion protein function and signaling in CSCs reveals the important role of these proteins in CSC persistence and spread. Additional work is needed to clarify the mechanotransductive components of these interactions and validate their utility as pharmacological targets.
In vitro tools for pharmacological discovery
It has long been hypothesized that deformation of cancer cells can be used as a potential biomarker or predictor of disease status. In breast, prostate, and bladder cancer models, it is established that cancer cells are consistently softer than non-cancerous cells suggesting that cell stiffness is inversely proportional to tumorigenesis and metastatic potential [32,81–83]. This idea is now being extended to CSCs, which are often more deformable than non-CSC cancer cells. For example, ovarian CSCs were found to exhibit 46%, 61%, and 72% increased deformability when compared to late stage, intermediate, and early stage ovarian cancer cells, respectively [83].
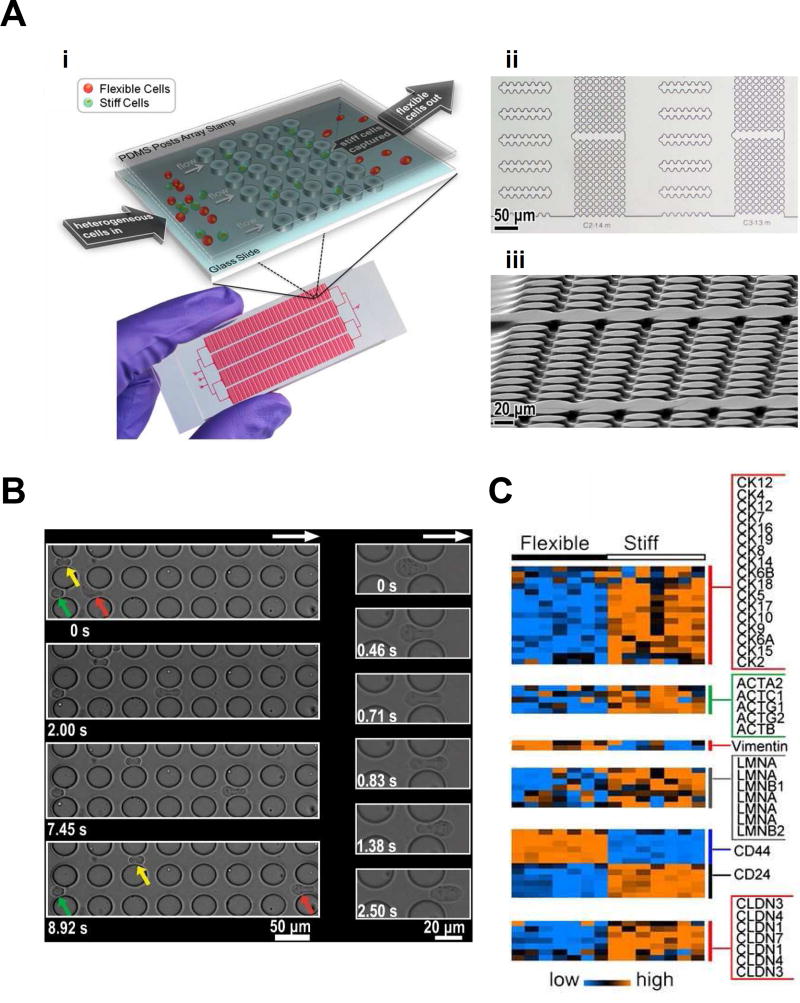
These changes in deformability have generated interest in the use of specialized engineering platforms to measure CSC mechanical properties. This in turn has created an opportunity to innovate upon traditional methods for measuring cellular mechanics, such as atomic force microscopy, micropipette aspiration, and optical tweezers, which are labor-intensive and low throughput [36,84]. Despite the accuracy of these techniques, their clinical value is limited by their relative inability to rapidly measure cell mechanical properties within large populations. Recently, microfluidic devices have helped usher in a new age of higher-throughput modalities efficient in mechanical characterization of cells [84,85]. One such microfluidic platform can probe single cell deformability at a rapid rate of approximately 2,000 cells/second using stretching extensional flow [36]. In this system, cells are fed through a syringe at a set flow rate into a cross-slot chamber that allows for inertial focusing, hydrodynamic stretching, and automated image analysis. This device revealed that undifferentiated cell populations were much more deformable than their differentiated counterparts. Another microfluidic sorting device can separate cells based upon their deformability by combining hydrodynamic force with micrometer-scale flow barriers (Figure 2A–B) [32]. Using this system, the authors were able to identify populations of highly metastatic and quiescent cells, which could then be further characterized. Analogous separation tools have been applied to harvest circulating tumor cells and subject them to downstream RNA analysis [86]. Such approaches are being coupled with single-cell sequencing technologies to gain insight into molecular mechanism (Figure 2C) [87]. These technologies are also actively being deployed in screening format to identify new lead compounds that influence tumor cell deformability [88,89].
Figure 2.
Cell sorting via cell deformability in a microfluidic device. (A) Schematic of microfluidic device, including micropost design for cell separation (i). Optical microscope (ii) and SEM (iii) images show flow channels of microfluidic device. (B) Green, yellow, and red arrows indicate three cells of different deformability flowing through the device with red being the most deformable followed by yellow and green. The most deformable cell is able to traverse a post in under 3 sec, while less deformable cells require extended times. (C) Microarray analysis of separated flexible of stiff cells reveal significant changes in mRNA levels of cytokeratins, actins, vimentin, lamins, CD44 and CD24, and claudins. Reproduced with permission from Zhang et al [32].
While cell deformability has proven to be an enormously valuable metric for describing heterogeneities within a given population, it is important to note that deformability ultimately represents a “snapshot” measurement. In reality, cellular mechanics are highly dynamic and subject to active modulation through cell cytoskeletal reorganization and contraction. The nature of this parameter is likely to introduce significant variation across measurement approaches and conditions, and so an ongoing challenge in the development of these platforms will be the adoption of more universal and generalizable metrics. Towards this end, we recently introduced an analytical framework that enables extraction of cellular viscoelastic moduli from cross-slot deformability measurements [85]. Important efforts have been underway to create high-throughput approaches to measure other properties, such as active cell contraction [90] and differential adhesion strength [91]. Finally, a new generation of devices is emerging that incorporates stromal elements with increasing sophistication. For example, tumor cell vascular extravasation has been simulated using microfluidic channels seeded with endothelial cells and subjected to fluid flow [92]. Such integrated approaches will provide exciting new avenues for the study of CSCs.
Conclusions
The concept that many solid tumors are initiated and propagated by CSCs represents a major paradigm shift in cancer biology and has motivated the creation of strategies to identify and therapeutically target CSCs. The finding that biophysical signals from the tumor microenvironment can influence CSC behavior raises the exciting possibility that mechanotransductive signaling may be targeted alongside canonical CSC mitogenic pathways. While efforts to do so are in their infancy, much work remains to identify and validate these targets as well as assess generality across multiple tumor types. These efforts will surely be aided by engineered platforms that permit high-throughput analysis of CSC mechanics and invasion in tandem with molecular-scale analyses. As these investments pay off, they should yield new diagnostic and screening tools that should contribute to novel ways of diagnosing, staging, and treating cancer.
Highlights.
Cancer stem/initiating cells (CSCs) are emerging as an important target of discovery and translational efforts
Biophysical cues in the tumor microenvironment may be key regulators of CSC maintenance and tumorigenic potential
CSCs often display unique mechanical properties relative to non-CSC tumor cells, which may enable these cells to be identified with high-throughput mechanical measurements
Mechanotransductive signaling pathways within CSCs may represent promising new therapeutic targets
Acknowledgments
The authors gratefully acknowledge the support of the NIH (1R21CA174573, 1R01NS074831, 1R21EB016359), the W. M. Keck Foundation, and the California Institute for Regenerative Medicine (RT3-07800).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016 [Internet] CA. Cancer. J. Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Wingo PA, Cardinez CJ, Landis SH, Greenlee RT, Ries LAG, Anderson RN, Thun MJ. Long-term trends in cancer mortality in the United States, 1930-1998 [Internet] Cancer. 2003;97:3133–3275. doi: 10.1002/cncr.11380. [DOI] [PubMed] [Google Scholar]
- 3.Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis [Internet] Cell Res. 2007;17:3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- 4.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. [Internet] Cancer Metastasis Rev. 2010;29:285–93. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mack GS, Marshall A. Lost in migration [Internet] Nat. Biotechnol. 2010;28:214–229. doi: 10.1038/nbt0310-214. [DOI] [PubMed] [Google Scholar]
- 6.Virchow RLK. Cellular Pathology. 1858 [Google Scholar]
- 7.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice [Internet] Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 8.Croker A, Allan A. Cancer stem cells: implications for the progression and treatment of metastatic disease [Internet] J. Cell. Mol. Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. [Internet] Nat. Rev. Cancer. 2004;4:448–56. doi: 10.1038/nrc1370. [DOI] [PubMed] [Google Scholar]
- 10.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. [Internet] Nat. Rev. Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 11.Luzzi KJ, MacDonald IC, Schmidt EE, Kerkvliet N, Morris VL, Chambers AF, Groom AC. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. [Internet] Am. J. Pathol. 1998;153:865–73. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CHM, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66 doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. [Internet] J. Clin. Invest. 2013;123:1911–8. doi: 10.1172/JCI66024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. [Internet] Oncogene. 2006;25:1696–708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 15.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. [Internet] Cancer Res. 2005;65:10946–51. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 16.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. [Internet] Cell Stem Cell. 2007;1:313–23. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. [Internet] Cancer Res. 2007;67:1030–7. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 18.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. [Internet] Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. [Internet] Nature. 2007;445:106–10. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells [Internet] Proc. Natl. Acad. Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lobo NA, Shimono Y, Qian D, Clarke MF. The Biology of Cancer Stem Cells [Internet] Annu. Rev. Cell Dev. Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- •22.Thomas D, Thiagarajan PS, Rai V, Reizes O, Lathia J, Egelhoff T, Thomas D, Thiagarajan PS, Rai V, Reizes O, et al. Increased cancer stem cell invasion is mediated by myosin IIB and nuclear translocation [Internet] Oncotarget. 2014;7:47586–47592. doi: 10.18632/oncotarget.9896. This study determined that CSC invasion is strongly facilitated by enhanced nuclear translocation, with CSCs able to squeeze through small pores much more easily than non-CSC tumor cells. The requisite nuclear deformation is driven by non-muscle myosin IIB,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. [Internet] BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 24.Ismail F, Winkler DA. Getting to the source: selective drug targeting of cancer stem cells. [Internet] ChemMedChem. 2014;9:885–98. doi: 10.1002/cmdc.201400068. [DOI] [PubMed] [Google Scholar]
- 25.Hao J, Zhang Y, Ye R, Zheng Y, Zhao Z, Li J. Mechanotransduction in cancer stem cells [Internet] Cell Biol. Int. 2013;37:888–891. doi: 10.1002/cbin.10111. [DOI] [PubMed] [Google Scholar]
- 26.Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression [Internet] Nat. Rev. Cancer. 2009;9:108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •27.Grundy TJ, De Leon E, Griffin KR, Stringer BW, Day BW, Fabry B, Cooper-White J, O’Neill GM, Giese A, Bjerkvig R, et al. Differential response of patientderived primary glioblastoma cells to environmental stiffness [Internet] Sci. Rep. 2016;6:23353. doi: 10.1038/srep23353. This study used a panel of glioblastoma CSCs to explore tumor-to-tumor variability in mechanosensing. Interestingly, glioblastoma cancer stem cells that were insensitive to substrate rigidity were more migratory and invasive in vitro. Thus, mechanosensitivity may predict tumor progression in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28.Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin J-MG, Martuscello RT, Jundi MA, Reynolds BA, Kumar S. Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. [Internet] Cancer Res. 2015;75:1113–22. doi: 10.1158/0008-5472.CAN-13-3426. This study showed that glioblastoma CSCs are surprisingly insensitive to matrix stiffness cues, but that mechanosensitivity can be restored through constitutive activation of myosin contractiity, which also has the effect of reducing invasion in vitro and in vivo while extending survival in a mouse xenograft model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jabbari E, Sarvestani SK, Daneshian L, Moeinzadeh S. Optimum 3D Matrix Stiffness for Maintenance of Cancer Stem Cells Is Dependent on Tissue Origin of Cancer Cells. [Internet] PLoS One. 2015;10:e0132377. doi: 10.1371/journal.pone.0132377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shieh AC. Biomechanical Forces Shape the Tumor Microenvironment [Internet] Ann. Biomed. Eng. 2011;39:1379–1389. doi: 10.1007/s10439-011-0252-2. [DOI] [PubMed] [Google Scholar]
- 31.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. [Internet] Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhang W, Kai K, Choi DS, Iwamoto T, Nguyen YH, Wong H, Landis MD, Ueno NT, Chang J, Qin L. Microfluidics separation reveals the stem-cell-like deformability of tumor-initiating cells. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2012;109:18707–12. doi: 10.1073/pnas.1209893109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saliba A-E, Saias L, Psychari E, Minc N, Simon D, Bidard F-C, Mathiot C, Pierga J-Y, Fraisier V, Salamero J, et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2010;107:14524–9. doi: 10.1073/pnas.1001515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, Zhou W, Jia Q, Chen J, Zhang S, Yao W, Wei F, Zhang Y, Yang F, Huang W, et al. Efficient extravasation of tumor-repopulating cells depends on cell deformability [Internet] Sci. Rep. 2016;6:19304. doi: 10.1038/srep19304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z, Lee Y, Jang J hee, Li Y, Han X, Yokoi K, Ferrari M, Zhou L, Qin L. Microfluidic cytometric analysis of cancer cell transportability and invasiveness. [Internet] Sci. Rep. 2015;5:14272. doi: 10.1038/srep14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo D. Hydrodynamic stretching of single cells for large population mechanical phenotyping. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2012;109:7630–5. doi: 10.1073/pnas.1200107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukumura D, Jain RK. Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. [Internet] J. Cell. Biochem. 2007;101:937–49. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- 38.Bissell MJ, Radisky D. Putting tumours in context. [Internet] Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radisky D, Muschler J, Bissell MJ. Order and disorder: the role of extracellular matrix in epithelial cancer. [Internet] Cancer Invest. 2002;20:139–53. doi: 10.1081/cnv-120000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: Are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingber DE. The mechanochemical basis of cell and tissue regulation. [Internet] Mech. Chem. Biosyst. 2004;1:53–68. [PubMed] [Google Scholar]
- 42.Wipff P-J, Hinz B. Myofibroblasts work best under stress. J. Bodyw. Mov. Ther. 2009;13:121–127. doi: 10.1016/j.jbmt.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 43.Chan MWC, Hinz B, McCulloch CA. Mechanical induction of gene expression in connective tissue cells. [Internet] Methods Cell Biol. 2010;98:178–205. doi: 10.1016/S0091-679X(10)98008-4. [DOI] [PubMed] [Google Scholar]
- 44.Kilarski WW, Samolov B, Petersson L, Kvanta A, Gerwins P. Biomechanical regulation of blood vessel growth during tissue vascularization. [Internet] Nat. Med. 2009;15:657–64. doi: 10.1038/nm.1985. [DOI] [PubMed] [Google Scholar]
- 45.Cho E-S, Lee K-S, Son Y-O, Jang Y-S, Lee S-Y, Kwak S-Y, Yang Y-M, Park S-M, Lee J-C. Compressive mechanical force augments osteoclastogenesis by bone marrow macrophages through activation of c-Fms-mediated signaling. [Internet] J. Cell. Biochem. 2010;111:1260–9. doi: 10.1002/jcb.22849. [DOI] [PubMed] [Google Scholar]
- 46.Farge E. Chapter eight – Mechanotransduction in Development. In. Current Topics in Developmental Biology. 2011:243–265. doi: 10.1016/B978-0-12-385065-2.00008-6. [DOI] [PubMed] [Google Scholar]
- 47.Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LCM, Wofovitz E. Fluid shear stress and the vascular endothelium: for better and for worse. [Internet] Prog. Biophys. Mol. Biol. 2003;81:177–99. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 48.Brouzés E, Farge E. Interplay of mechanical deformation and patterned gene expression in developing embryos. [Internet] Curr. Opin. Genet. Dev. 2004;14:367–74. doi: 10.1016/j.gde.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. [Internet] Cancer Cell. 2005;8:241–54. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 50.Tilghman RW, Cowan CR, Mih JD, Koryakina Y, Gioeli D, Slack-Davis JK, Blackman BR, Tschumperlin DJ, Parsons JT. Matrix Rigidity Regulates Cancer Cell Growth and Cellular Phenotype [Internet] PLoS One. 2010;5:e12905. doi: 10.1371/journal.pone.0012905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids [Internet] Nat. Biotechnol. 1997;15:778–783. doi: 10.1038/nbt0897-778. [DOI] [PubMed] [Google Scholar]
- 52.Sarntinoranont M, Rooney F, Ferrari M. Interstitial Stress and Fluid Pressure Within a Growing Tumor [Internet] Ann. Biomed. Eng. 2003;31:327–335. doi: 10.1114/1.1554923. [DOI] [PubMed] [Google Scholar]
- 53.Haage A, Schneider IC. Cellular contractility and extracellular matrix stiffness regulate matrix metalloproteinase activity in pancreatic cancer cells. [Internet] FASEB J. 2014;28:3589–99. doi: 10.1096/fj.13-245613. [DOI] [PubMed] [Google Scholar]
- 54.Gorczyca W, Holm R, Nesland JM. Laminin production and fibronectin immunoreactivity in breast carcinomas. [Internet] Anticancer Res. 1992;13:851–8. [PubMed] [Google Scholar]
- 55.Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv. Drug Deliv. Rev. 2016;99:197–205. doi: 10.1016/j.addr.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Tse JM, Cheng G, Tyrrell JA, Wilcox-Adelman SA, Boucher Y, Jain RK, Munn LL. Mechanical compression drives cancer cells toward invasive phenotype. [Internet] Proc. Natl. Acad. Sci. U. S. A. 2012;109:911–6. doi: 10.1073/pnas.1118910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hung W-C, Yang JR, Yankaskas CL, Wong BS, Wu P-H, Pardo-Pastor C, Serra SA, Chiang M-J, Gu Z, Wirtz D, et al. Confinement Sensing and Signal Optimization via Piezo1/PKA and Myosin II Pathways. [Internet] Cell Rep. 2016;15:1430–41. doi: 10.1016/j.celrep.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hung W-C, Chen S-H, Paul CD, Stroka KM, Lo Y-C, Yang JT, Konstantopoulos K. Distinct signaling mechanisms regulate migration in unconfined versus confined spaces. [Internet] J. Cell Biol. 2013;202:807–24. doi: 10.1083/jcb.201302132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •59.Lee J, Abdeen AA, Wycislo KL, Fan TM, Kilian KA. Interfacial geometry dictates cancer cell tumorigenicity [Internet] Nat. Mater. 2016;15:856–862. doi: 10.1038/nmat4610. This study reveals that the curvature of the extracellular matrix strongly regulates the ability of tumor cells to acquire stem-like properties and increased metastatic potential. [DOI] [PubMed] [Google Scholar]
- ••60.Harada T, Swift J, Irianto J, Shin J-W, Spinler KR, Athirasala A, Diegmiller R, Dingal PCDP, Ivanovska IL, Discher DE. Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival. [Internet] J. Cell Biol. 2014;204:669–82. doi: 10.1083/jcb.201308029. This report strongly implicates the relative expression of nuclear envelope proteins lamin-A and -B in governing the ability of cells to migrate through tight pores. However, while soft nuclei facilitate transmigration, they can also be damaged more easily during migration, increasing the likelihood of apoptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Denais CM, Gilbert RM, Isermann P, McGregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J. Nuclear envelope rupture and repair during cancer cell migration [Internet] Science (80-. ) 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akirakatsumi A, Wayneorr E. Integrins in Mechanotransduction *. 2004 [Google Scholar]
- 63.Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, Mclendon RE, Hjelmeland AB, et al. Cell Stem Cell Integrin Alpha 6 Regulates Glioblastoma Stem Cells. Stem Cell. 6:421–432. doi: 10.1016/j.stem.2010.02.018. [date unknown] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakada M, Nambu E, Furuyama N, Yoshida Y, Takino T, Hayashi Y, Sato H, Sai Y, Tsuji T, Miyamoto K, et al. Integrin α3 is overexpressed in glioma stem-like cells and promotes invasion [Internet] Br. J. Cancer. 2013;108:2516–2524. doi: 10.1038/bjc.2013.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seguin L, Desgrosellier JS, Weis SM, Cheresh DA, Desgrosellier JS, Cheresh DA, Mouw JK, Brooks PC, et al. Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. [Internet] Trends Cell Biol. 2015;25:234–40. doi: 10.1016/j.tcb.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cariati M, Naderi A, Brown JP, Smalley MJ, Pinder SE, Caldas C, Purushotham AD. Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. [Internet] Int. J. cancer. 2008;122:298–304. doi: 10.1002/ijc.23103. [DOI] [PubMed] [Google Scholar]
- 67.Yan Y, Zuo X, Wei D. Concise Review: Emerging Role of CD44 in Cancer Stem Cells: A Promising Biomarker and Therapeutic Target. doi: 10.5966/sctm.2015-0048. [date unknown] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Croker AK, Allan AL. Cancer stem cells: implications for the progression and treatment of metastatic disease [Internet] J. Cell. Mol. Med. 2008;12:374–390. doi: 10.1111/j.1582-4934.2007.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moore SW, Roca-Cusachs P, Sheetz MP, Ainavarapu SR, Brujic J, Huang HH, Wiita AP, Lu H, Li L, Walther KA, et al. Stretchy proteins on stretchy substrates: the important elements of integrin-mediated rigidity sensing. [Internet] Dev. Cell. 2010;19:194–206. doi: 10.1016/j.devcel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nemir S, West JL. Synthetic Materials in the Study of Cell Response to Substrate Rigidity [Internet] Ann. Biomed. Eng. 2010;38:2–20. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- ••71.Pang M-F, Siedlik MJ, Han S, Stallings-Mann M, Radisky DC, Nelson CM. Tissue Stiffness and Hypoxia Modulate the Integrin-Linked Kinase ILK to Control Breast Cancer Stem-like Cells. Cancer Res. 2016;76 doi: 10.1158/0008-5472.CAN-16-0579. This study provides evidence for the synergy of tissue stiffness and hypoxia in the generation of the CSC pool in breast cancer. The authors demonstrate that integrin-linked kinase is a critical transducer of tissue stiffness and oxygen tension in breast cancer cells and is modulated by PI3K/Akt signaling. The depletion of integrin-linked kinase abrogates the tumorigenic and metastatic potential of breast cancer cells. This report highlights the novel role of integrin-dependent pathways in the generation of breast cancer stem cell populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••72.Tan Y, Tajik A, Chen J, Jia Q, Chowdhury F, Wang L, Chen J, Zhang S, Hong Y, Yi H, et al. Matrix softness regulates plasticity of tumour-repopulating cells via H3K9 demethylation and Sox2 expression [Internet] Nat. Commun. 2014;5:717–728. doi: 10.1038/ncomms5619. This study shows that propagation of tumor-repopulating cells (TRCs) on soft matrices enhances these cells’ self-renewal capacity and tumorigenic potential. This is postulated to occur through a mechanism involving reduced histone 3 lysine residue 9 (H3K9) methylation and increased Sox2 expression This is one of the first studies to deeply explore the inteplay between matrix stiffness, epigenetic modifications, and tumor initiation capacity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? [Internet] Nat. Rev. Cancer. 2011;11:254–67. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 74.Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC. Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth [Internet] Cell Stem Cell. 2014;14:357–369. doi: 10.1016/j.stem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). [Internet] Science. 1996;271:509–12. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 76.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. [Internet] Nat. Rev. Mol. Cell Biol. 2010;11:276–87. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. [Internet] J. Cell Biol. 1994;126:1099–109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bourguignon LYW. Hyaluronan-mediated CD44 activation of RhoGTPase signaling and cytoskeleton function promotes tumor progression. [Internet] Semin. Cancer Biol. 2008;18:251–9. doi: 10.1016/j.semcancer.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu J, Xiao Z, Wong SK-M, Tin VP-C, Ho K-Y, Wang J, Sham M-H, Wong MP, Liu J, Xiao Z, et al. Lung cancer tumorigenicity and drug resistance are maintained through ALDH hi CD44 hi tumor initiating cells [Internet] Oncotarget. 2013;4:1698–1711. doi: 10.18632/oncotarget.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Klarmann GJ, Hurt EM, Mathews LA, Zhang X, Duhagon MA, Mistree T, Thomas SB, Farrar WL. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. [Internet] Clin. Exp. Metastasis. 2009;26:433–46. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Faria EC, Ma N, Gazi E, Gardner P, Brown M, Clarke NW, Snook RD. Measurement of elastic properties of prostate cancer cells using AFM [Internet] Analyst. 2008;133:1498. doi: 10.1039/b803355b. [DOI] [PubMed] [Google Scholar]
- 82.Lekka M, Laidler P, Gil D, Lekki J, Stachura Z, Hrynkiewicz AZ. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. [Internet] Eur. Biophys. J. 1999;28:312–6. doi: 10.1007/s002490050213. [DOI] [PubMed] [Google Scholar]
- •83.Babahosseini H, Ketene AN, Schmelz EM, Roberts PC, Agah M. Biomechanical profile of cancer stem-like/tumor-initiating cells derived from a progressive ovarian cancer model. [Internet] Nanomedicine. 2014;10:1013–9. doi: 10.1016/j.nano.2013.12.009. The authors analyzed the biomechanical properties of ovarian cancer stem cells compared to non-stem cancer cells at early, intermediate, and late stage cancer stages via atomic force microscopy. CSCs population were significantly softer than non-CSC tumor cells at each stage of cancer progression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Darling EM, Di Carlo D. High-Throughput Assessment of Cellular Mechanical Properties [Internet] Annu. Rev. Biomed. Eng. 2015;17:35–62. doi: 10.1146/annurev-bioeng-071114-040545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guillou L, Dahl JB, Lin J-MG, Barakat AI, Husson J, Muller SJ, Kumar S. Measuring Cell Viscoelastic Properties Using a Microfluidic Extensional Flow Device. Biophys. J. 2016;111:2039–2050. doi: 10.1016/j.bpj.2016.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •86.Hvichia GE, Parveen Z, Wagner C, Janning M, Quidde J, Stein A, Müller V, Loges S, Neves RPL, Stoecklein NH, et al. A novel microfluidic platform for size and deformability based separation and the subsequent molecular characterization of viable circulating tumor cells [Internet] Int. J. Cancer. 2016;138:2894–2904. doi: 10.1002/ijc.30007. The study describes a novel microfluidic platform that can harvest viable circulating tumor cells from peripheral blood samples through deformability based separation. Further, this platform enables downstream molecular and functional characterization including RT-PCR and array-based comparative genomic hybridization. The authors further discuss the application of single cell sequencing technologies downstream of cell harvesting as a powerful tool for mechanistic discovery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gawad C, Koh W, Quake SR. Single-cell genome sequencing: current state of the science [Internet] Nat. Rev. Genet. 2016;17:175–188. doi: 10.1038/nrg.2015.16. [DOI] [PubMed] [Google Scholar]
- 88.Chen Y, Li P, Huang P-H, Xie Y, Mai JD, Wang L, Nguyen N-T, Huang TJ. Rare cell isolation and analysis in microfluidics. [Internet] Lab Chip. 2014;14:626–45. doi: 10.1039/c3lc90136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z, Nagrath S. Microfluidics and cancer: are we there yet? [Internet] Biomed. Microdevices. 2013;15:595–609. doi: 10.1007/s10544-012-9734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aung A, Bhullar IS, Theprungsirikul J, Davey SK, Lim HL, Chiu Y-J, Ma X, Dewan S, Lo Y-H, McCulloch A, et al. 3D cardiac μtissues within a microfluidic device with real-time contractile stress readout [Internet] Lab Chip. 2016;16:153–162. doi: 10.1039/c5lc00820d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walia V, Elble RC. Enrichment for Breast Cancer Cells with Stem/Progenitor Properties by Differential Adhesion [Internet] Stem Cells Dev. 2010;19:1175–1182. doi: 10.1089/scd.2009.0430. [DOI] [PubMed] [Google Scholar]
- 92.Huang R, Zheng W, Liu W, Zhang W, Long Y, Jiang X, Joyce JA, Pollard JW, Reymond N, d’Agua BB, et al. Investigation of Tumor Cell Behaviors on a Vascular Microenvironment-Mimicking Microfluidic Chip [Internet] Sci. Rep. 2015;5:17768. doi: 10.1038/srep17768. [DOI] [PMC free article] [PubMed] [Google Scholar]