Abstract
Technology can be disruptive – forcing changes in the way we think and operate by demonstrating that “what we know, ain’t so”. Such is the case in chronic pancreatitis (CP), where imaging technologies, omics data and large human studies challenge our fundamental understanding of this life-altering syndrome. The use of new technologies highlights the fact that we define CP by end-stage features – but many of these features are also present in people who do not have CP. New evidences provide the opportunity for early diagnosis of CP, but detection of “early” end-stage features cannot be reliably used to correctly diagnose CP in people who truly have it and where treatment might provide clear benefit, and to exclude people with similar features who, after years of treatments and perhaps radical therapies, are found that they never had CP in the first place!
When it comes to new paradigms for complex systems “evidence based medicine” approaches may fail since they rely on literature built on definitions and studies using old technology, old studies and outdated theories1. For example, a major effort of 11 Professional Associations/Organizations from Germany and central Europe used the Oxford evidence based consensus process eventually defined CP as “a disease of the pancreas in which recurrent inflammatory episodes result in replacement of pancreatic parenchyma by fibrous connective tissue”, adding that “Pain presents as the main symptom of patients with chronic pancreatitis”2. This definition focuses on end-stage features and cannot be used to differentiate early CP from other diseases with abnormal imaging features, especially when pain is a major feature and the physician is under pressure to act. In the American Pancreatic Association (APA) evidence-based CP guidelines CP was not even defined. Instead, the document focused on late features, stating “Chronic pancreatitis is characterized by chronic progressive pancreatic inflammation and scarring, irreversibly damaging the pancreas and resulting in loss of exocrine and endocrine function”3. Neither of these rigorous retrospective reviews of new data, viewed through old concepts, provide compelling rational for guiding the physicians to the early diagnosis of CP, nor insights how to differentiate fibrosis, pancreatic exocrine insufficiency, diabetes mellitus and upper abdominal pain of CP from the other conditions and disorders that have overlapping features. The APA guidelines concluded with two important statement, “The diagnosis of CP still remains challenging in early stages (equivocal and mild morphologic or physiologic changes) of the disease” and “Without sufficient evidence, patients should not be mislabeled as having CP, when in fact they may have chronic abdominal pain syndrome and a remote history of ERCP-induced pancreatitis or ductal changes”3. Thus, under the old paradigm and definitions based on late disease features, only more advanced CP can be diagnosed with confidence, and early CP should not be diagnosed.
Clinical Problems
Chronic Pancreatitis is a complex disorder with features that overlap with other known and idiopathic conditions of the pancreas and abdomen. Practicing physicians face the practical problem of potentially make the diagnosis of “early CP” in a patient with some features of CP – but not the real disease, and start down a management course that includes narcotics, endoscopies, surgeries and even total pancreatectomy. On the other hand, they may miss a true diagnosis of CP in patients who may lack certain imaging features of advanced CP, denying the patient potentially effective treatments, or seeking other doctors who have a lower threshold to “make a diagnosis”, or to subject the patient to repeated diagnostic tests and therapeutic trials (especially endoscopic). Finally, it is nearly impossible to “undiagnosed” CP in a patient with a pain syndrome and abnormal imaging test who does not have CP. The challenge of diagnosing CP by one physician and undiagnosing CP by another eventually collapses to the opinion of each physician – based on personal beliefs that are often strongly held and rigorously defended.
The most common features of established and advanced CP include pancreatic atrophy, fibrosis, pain syndromes, duct distortion and strictures, calcifications, pancreatic exocrine dysfunction, pancreatic endocrine dysfunction, and dysplasia4. These clinical features arise from abnormalities in the pancreatic acinar cells, duct cells and pathways, immune responses to injury that may include fibrosis, islet of Langerhans (number and function), the peripheral and central nervous system and pain tolerance and the repair systems or injured cells that may fail, leading to pancreatic cancer. Furthermore, features of CP do not all occur suddenly, but evolve from normal to abnormal over time, with different features progressing at different rates (or not at all) in different people with true CP. However, all of these features are also present to various extents in other diseases, and many, such as fibrosis, are common variants of alcohol and smoking, diabetes and of the aging process5–7. So what is chronic pancreatitis, which features are required as essential, and can early CP be distinguished from other disorders with overlapping features?
Basic and translational researchers also struggle to develop meaningful disease models when the clinical diagnosis dependents on assessment of features that are neither necessary nor sufficient for diagnosis, and that fully overlap with other disorders that are not CP. In other words, two patients may have similar clinical signs and symptoms, and the same imaging features of moderate pancreatic fibrosis or atrophy, but one has CP and the other does not, which only becomes apparent over an indefinite period of time. Clearly, we need new approaches to make the distinction, and clarity to assist the researchers in defining the underlying mechanisms.
A New “Mechanistic” Approach
A new approach is being suggested by an international group of physician-scientists. First, they recognized that we must define the essence of CP based on the underlying mechanism of disease. If this is possible, then abnormal feature of pancreatic disease can be traced back to their origin, and similar features originating from different disease in different people can be differentiated. After nearly two years of debate, a two part “mechanistic definition” of chronic pancreatitis was proposed:
“Chronic pancreatitis is a pathologic fibro-inflammatory syndrome of the pancreas in individuals with genetic, environmental and/or other risk factors who develop persistent pathologic responses to parenchymal injury or stress.” and “Common features of established and advanced CP include pancreatic atrophy, fibrosis, pain syndromes, duct distortion and strictures, calcifications, pancreatic exocrine dysfunction, pancreatic endocrine dysfunction, and dysplasia”4.
The first part defines the origin of the problem, while the second part defines the eventual consequences of the disorder, listed as clinical features and recognized as CP by previous definitions. The “essence” of CP then, is the common underlying pathogenic mechanism that leads to the eventual clinical features. Furthermore, if focuses on the injury/stress → activation of the innate immune system → resolution of acute inflammatory response → tissue repair and regeneration sequence. The injury / inflammatory system, when out of controls, leads to all subsequent features of CP found in the systems that define the phenotype.
If we believe that the end stage features of CP are “irreversible”, and the disease proceeds from no disease to end-stage disease over time, then it may be possible to detect the underlying problem early based on detecting the pathogenic mechanism, and intervene to prevent the development of a disease that cannot be cured. So how can a mechanistic definition aid in solving the clinical dilemma of diagnosing “Early CP”?
Timing is everything
While huge gaps in knowledge remain, we do know some things for sure. In both hereditary pancreatitis (HP) and alcoholic chronic pancreatitis (for example), there is generally a progression from a normal pancreas in youth to end-stage chronic pancreatitis over time, measured in years. This process can be broken down into several stages, such that and any point in time a patient is in one, and only one state. Potentially, this concept be applied to CP in general.
First, we know that some people are perfectly healthy, have no signs or symptoms of disease, carry very dangerous susceptibility factors for their whole life without disease8. While some people with CP susceptibility factors never develop disease, they are among a larger group of people in whom the incidence and prevalence of pancreatic disease is higher then normal. Let’s call this Stage A, where risk of CP is increased for all persons in this sub-population, but there is no disease (Figure 1).
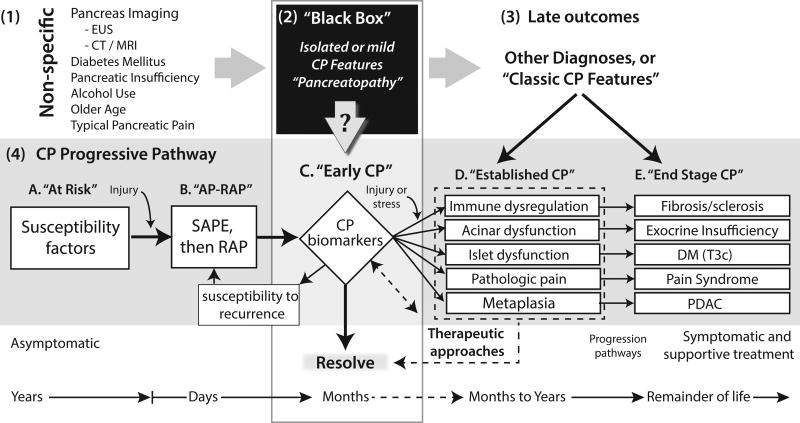
Figure.
Relationship between the mechanistic CP progressive pathways (grey box, from4) and features of CP detected in various clinical settings. (1) Non-specific features of CP. CP often presents with non-specific signs and symptoms of disease that overlap with signs and symptoms of other diseases. (2) “Black Box” is a diagnostic placeholder and represents the situation where the mechanism of diseases is unknown, and no diagnosis can be made. (3) Late outcomes. With time, most serious diseases and complex syndromes will declare themselves. IN come cases CP has progressed to more severe, irreversible features of several biological systems that allow a diagnosis of classic CP to be diagnosed. (4) The CP Progressive Pathway represents the underlying complex mechanisms responsible for a person, over their lifetime, to develop chronic pancreatitis. In Stage A and B, the CP mechanism is seldom tested for or diagnosed. Stage C represents early CP, in which the high-risk mechanism IS known, inflammatory process is active (e.g. post AP), and some features of CP are also present. In this case the early features of CP within the Black Box, combined with the clinical setting and high probability risk profile is sufficient to make the diagnosis of Early CP. The challenge is to define the strength of various combinations of susceptibility and modifying risk factors associated with relevant systems within the pancreas, and early CP features (? In arrow pointing down from the Black Box) defining diagnostic criteria for Early CP. AP-RAP, acute pancreatitis and recurrent acute pancreatitis; CP, chronic pancreatitis; DM (T3c), diabetes mellitus Type IIIc or pancreatogenic diabetes mellitus; PDAC, pancreatic ductal adenocarcinoma; SAPE sentinel acute pancreatitis event18.
Second, acute pancreatitis is a very well defined, self limited, and clinically recognized inflammatory disorder with a specific type of inflammatory response to sudden injury. It is well established that acute pancreatitis (AP), and especially recurrent acute pancreatitis (RAP) are major risk factors for eventual chronic pancreatitis – with 10%–30% of AP and RAP patients eventually developing CP9–12. Let’s call this second limited state Stage B. No one in this stage has CP, and the majority of patients recover over time.
Third, consider patients who are in a state where they are found to have one or more features of CP on sensitive imaging studies, they have pancreatitis-type pain, they have glucose intolerance that is not Type 1 diabetes mellitus (DM), nor obesity or peripheral insulin resistance (Type2 DM), and/or they have borderline pancreatic exocrine insufficiency, but they do not have evidence of end-stage chronic pancreatitis. Lets call this the “black box” (discussed below) label this as Stage C of a progressive model seen in patients with symptomatic HP or those who eventually develop alcoholic CP.
Fourth, some patients have “classic” chronic pancreatitis, but continue to have some viable pancreatic parenchyma such that endocrine and exocrine function is diminished but the gland has not failed. They may also have pain, but it can be controlled with standard measures. Let’s call this Stage D.
Fifth, some patients have nearly complete destruction of the pancreas by the inflammation-driven CP process. The gland fails, and the lost exocrine function must be replaced with oral digestive enzymes, while the endocrine function must be replace with insulin. In some cases the pain becomes uncontrolled, and surgical interventions may be the best option. We recognized that some, but not all of the features of end-stage CP may be present, since the clinical syndrome is heterogenous. Let’s call this final step in the process Stage E.
Most experts would agree that the best time to begin treating CP is early in the process. This would include some point in time after some features have developed (Stage C), and before the disease is irreversible (Stage E). This goal of early treatment requires diagnosis of Early CP – so that the correct subset of patients with “Stage C” and Early CP, can be treated to avoid progressing to Stage D, “Established CP”. This process requires the mechanistic definition of CP.
The Black Box
The term “back box” is used to refer to a complex system where the mechanism is hidden or poorly understood. Since 198413, fibrosis and morphologic changes of the pancreas have been equated with the diagnosis of CP. With high resolution imaging technologies early fibrosis can easily be detected in the pancreas, but fibrosis alone does not equal chronic pancreatitis. If fact, it is unclear what mild to extensive fibrosis in pancreatic tissue means, and where the cut off should be between “normal” and “abnormal”. Fibrosis clearly increases with age5, 14, but is this pathologic? Fibrosis is common in patients with long-standing diabetes mellitus7, 15, 16 – but is this chronic pancreatitis? Significant fibrosis is present in the pancreas of most patients with long-standing alcohol use and smoking6, but most are asymptomatic and are they to be classified as having CP. Thus, with new technologies and epidemiology studies applied to larger populations of “control patients” with diabetes, older age patients, alcohol drinkers and smokers reveal that fibrosis is common and arguably benign. Thus, the older studies that relied on fibrosis as the diagnostic criteria for chronic pancreatitis cannot be used as evidence of CP moving forward.
The problem is compounded by growing recognition of patients with “minimal change” CP, with “small duct disease”, with inflammation and atrophy rather than fibrosis, with or without pain, and with or without DM. Our best and brightest clinicians and researchers must address these conundrums.
The “Black Box” is the diagnosis of patients with early signs of symptoms of CP, whether structural, functional, or clinical. The Black Box contains patients with true, Early CP, but also contains patients with variants of normal pancreas, including the poorly defined entity of “pancreatopathy”16. Clearly, if Early CP is to be identified as a clear subset of patients with an ominous trajectory toward end-stage pancreatitis, it will need to be defined by mechanistic (and therefore predictive) criteria. The challenges is in designing models that contain all of the key elements, and then calibrating the system to be able to calculate the probability of progression to a more serious stage of disease. Otherwise, we will remain in the current dilemma of qualified and unqualified practitioners establishing their own standards and doing whatever they think is best, based on their experience, training and biases.
In summary, the “black box” thus represents the patients who have some of the clinical features of CP, but who may or may not actually have this disorder. From a pragmatic viewpoint, when a patient is presented with a report that the pancreas is abnormal, they have three questions: “Why me?”, “What will happen to me?”, and “What can I do to treat this disorder?”. Unfortunately, the homes physician usually must respond; “I do not known”, “I don’t know” and “I don’t know”.
Can the pathogenic mechanisms causing CP symptomology be defined and diagnosed?
The National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK) sponsored an international workshop in Pittsburgh, PA on July 27, 2016 immediately before Pancreas Fest 2016. Importantly, the approach was “reverse engineering”, where experts focused on the major components of the CP syndrome, including pathophysiologic risks and mechanism, exocrine complications including maldigestion, the endocrine complications (Type 3cDM and islet-acinar interactions), and mechanism of pain, including cognitive behavioral aspects. The approach was especially exciting because it recognized and embraced the new mechanistic definition, and help clinical-translational researchers in systematically defining and organizing the components of the systems, with risks and biomarkers17, currently in the Black Box. This type of exercise is critical for identifying gaps in knowledge, research opportunities and assisting translational teams developing prospective studies, including the launching a new 10 Clinical Center consortium to study chronic pancreatitis, and associated diabetes and pancreatic cancer (CPDPC) (1U01DK108306).
Conceptually, we know that a proportionally smaller and smaller subset of patients with chronic pancreatitis risk progress through sequential stages of disease prior to end-stage chronic pancreatitis4. While challenges remain in distinguishing patients with true Early CP from others in the Black Box, the risks and predictors for transition from one stage to the next, for each of the systems affected by the inflammatory response, must be determined in well-designed studies. The NIDDK-sponsored CPDPC consortium is one exciting example where it will be possible to prospectively define and improve the utility of this predictive modeling approaches. The opportunity to determine the natural history of disease, the classify and sub-classify patients on a molecular and mechanistic basis, to test and develop new biomarkers17 that are developed to monitor every aspect of the disease from detection, to diagnosis to treatment response, is exactly what is needed to obtain new data from a new paradigm.
Soon, when the patient with unexpected CP-like symptoms or features on an imaging test ask there doctor, “Why me?”, “What will happen to me?”, and “What can I do to treat this disorder?” – the answer will be, “Glad you asked, we have the answers! We can finally help prevent the development of a horrible and incurable end-stage disease”.
Acknowledgments
His research is supported by the National Institutes of Health, the Department of Defense, the National Pancreas Foundation and by the Wayne Fusaro Pancreatic Cancer Research Fund.
Footnotes
Disclosures: DCW serves as a consultant to AbbVie, Ariel Precision Medicine and Regeneron, and has equity as a cofounder of Ariel Precision Medicine.
References
- 1.Whitcomb DC. What is personalized medicine and what should it replace? Nat Rev Gastroenterol Hepatol. 2012;9:418–424. doi: 10.1038/nrgastro.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffmeister A, Mayerle J, Beglinger C, et al. S3-Consensus guidelines on definition, etiology, diagnosis and medical, endoscopic and surgical management of chronic pancreatitis German Society of Digestive and Metabolic Diseases (DGVS) Z Gastroenterol. 2012;50:1176–224. doi: 10.1055/s-0032-1325479. [English Version: http://dx.doi.org/10.1055/s-0041-107379Z Gastroenterol. 2015; 53: 1447–1495] [DOI] [PubMed] [Google Scholar]
- 3.Conwell DL, Lee LS, Yadav D, et al. American Pancreatic Association practice guidelines in chronic pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology. 2016;16:218–224. doi: 10.1016/j.pan.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen J, Papavassiliou I. Effect of aging and diffuse chronic pancreatitis on pancreas elasticity evaluated using semiquantitative EUS elastography. Ultraschall Med. 2014;35:253–258. doi: 10.1055/s-0033-1355767. [DOI] [PubMed] [Google Scholar]
- 6.Petrone MC, Arcidiacono PG, Perri F, et al. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease: do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology. 2010;10:597–602. doi: 10.1159/000314599. [DOI] [PubMed] [Google Scholar]
- 7.Ewald N, Kaufmann C, Raspe A, et al. Prevalence of diabetes mellitus secondary to pancreatic diseases (type 3c) Diabetes Metab Res Rev. 2012;28:338–342. doi: 10.1002/dmrr.2260. [DOI] [PubMed] [Google Scholar]
- 8.Khalid A, Finkelstein S, Thompson B, et al. A 93 year old man with the PRSS1 R122H mutation, low SPINK1 expression, and no pancreatitis: insights into phenotypic non-penetrance. Gut. 2006;55:728–731. doi: 10.1136/gut.2005.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yadav D, Lowenfels AB. Trends in the epidemiology of the first attack of acute pancreatitis: a systematic review. Pancreas. 2006;33:323–330. doi: 10.1097/01.mpa.0000236733.31617.52. [DOI] [PubMed] [Google Scholar]
- 10.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterol. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav D, O'Connell M, Papachristou GI. Natural history following the first attack of acute pancreatitis. Am. J. Gasterol. 2012;107:1096–1103. doi: 10.1038/ajg.2012.126. [DOI] [PubMed] [Google Scholar]
- 12.Takeyama Y. Long-term prognosis of acute pancreatitis in Japan. Clin Gastroenterol Hepatol. 2009;7:S15–S17. doi: 10.1016/j.cgh.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 13.Sarner M, Cotton PB. Classification of pancreatitis. Gut. 1984;25:756–9. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointest Endosc. 2005;61:401–406. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 15.Butler AE, Campbell-Thompson M, Gurlo T, et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohapatra S, Majumder S, Smyrk TC, et al. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas. 2016 doi: 10.1097/MPA.0000000000000609. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitcomb DC. Better Biomarkers for Pancreatic Diseases. Pancreas. 2015;44:1171–1173. doi: 10.1097/MPA.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterol. 2013;144:1292–1302. doi: 10.1053/j.gastro.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]