Abstract
The focus of critical care has evolved from saving lives to preservation of function. Morbidity rates in pediatric critical care are about double mortality rates. Morbidity includes complications of disease and medical care. In pediatric critical care, functional status morbidity is an intermediate outcome in the progression towards death, and is the result of the same factors associated with mortality including physiological profiles (PRISM), and case-mix factors. The Functional Status Scale (FSS) developed by CPCCRN, a NIH research consortium, is a validated, granular, age-independent measure of functional status that has proven valuable and practical even in large outcome studies.
Keywords: morbidity, functional status, quality, outcomes, outcomes research, critical care, pediatric critical care, pediatric intensive care
Introduction
The primary focus of critical care has evolved from saving lives by monitoring and maintaining physiological status to placing greater emphasis on the prevention of secondary injuries and preservation of function. Current pediatric intensive care unit (PICU) mortality rates approximate 2.5%–5%, decreased from 8%–18% during the early years of pediatric critical care,1 and it has been suggested that a portion of the reduced mortality rates has been an exchange for higher morbidity rates.2
Pediatric critical care does not have a consensus concept of morbidity. Despite the low mortality rates and changing primary focus of pediatric critical care to include morbidity prevention, the primary outcome for many critical care studies and assessments remains mortality. Studies which formerly could be accomplished with mortality as a legitimate and meaningful outcome are now difficult or impossible due to sample size considerations. If mortality is the primary outcome for research, quality or other studies, the sample size required may be very large, and the time required to obtain these samples may be so long as to make the results less meaningful when the study is completed.
Our aims are:
to review the conceptual framework of morbidity most relevant to pediatric critical care;
describe the uses of morbidity in research, quality, and other types of studies;
describe measures of morbidity, especially those that measure functional status;
review the foundational evidence that strongly supports the use of functional status morbidity as an equivalent or separate outcome to mortality; and
summarize the current pediatric critical care morbidity literature and the methods used to assess morbidity.
What Is Morbidity?
Morbidity is often difficult to define. While mortality is simple (alive or dead), morbidity is usually conceptualized as an important deviation from baseline and/or a deviation from the expected result of care. In the context of critical care, morbidity is frequently thought of as the ramifications of both the disease process and the care provided in the intensive care unit (ICU). It may encompass events during the inpatient stay, discharge status, or the long-term effects of the disease and the ensuing critical care interventions.
Morbidity during intensive care includes a diverse group of indicators including the development of multisystem organ dysfunction, need for vasoactive medications, days on the ventilator, length of stay, hospital-acquired infections, and other medically-focused outcomes. Morbidity, especially in the surgical literature, has increasingly been focused on inpatient complications or an unexpected hospital course associated with a procedure or its subsequent care including length of stay, adverse events, and errors. An excellent example of using inpatient complications has been developed by using the congenital heart Society of Thoracic Surgeons Congenital Heart Surgery Database.3 The selected complications of specific interest and relevance to congenital heart surgery patients include renal failure requiring dialysis, neurologic deficits at discharge, atrio-ventricular block requiring a permanent pacemaker, mechanical circulatory support, phrenic nerve injury or paralyzed diaphragm, and unplanned operation. The result of combining these complications with post-operative length of stay has been standardized for specific operations resulting in a morbidity index specifically relevant to these patients’ inpatient course, and suitable for use as a quality assessment method. Others have used more global measures of inpatient care such as cost.4 Contemporary trends such as patient- and family-centered care or cost may be may also be converted to morbidity indicators such as family and patient related changes in stress, mental health, financial status, and family functioning.5
Despite the traditional emphasis on inpatient metrics, there is growing recognition that the most important morbidities are decreases in functional status which persist or develop after the hospital stay. These may be general such as changes to activities of daily living or organ-specific changes measured by functional tests such as maximum oxygen consumption following cardiac surgery or pulmonary function tests following thoracic disease. Both types of morbidities are important. A recent review found that new functional impairment at the time of ICU discharge was reported from 10% to 36% of discharges depending on the methodology used.6 Evidence detailed below indicates that changes to functional status in critically ill children are tightly linked to physiological dysfunction (severity of illness).
The Relationship of Physiological Dysfunction to Morbidity
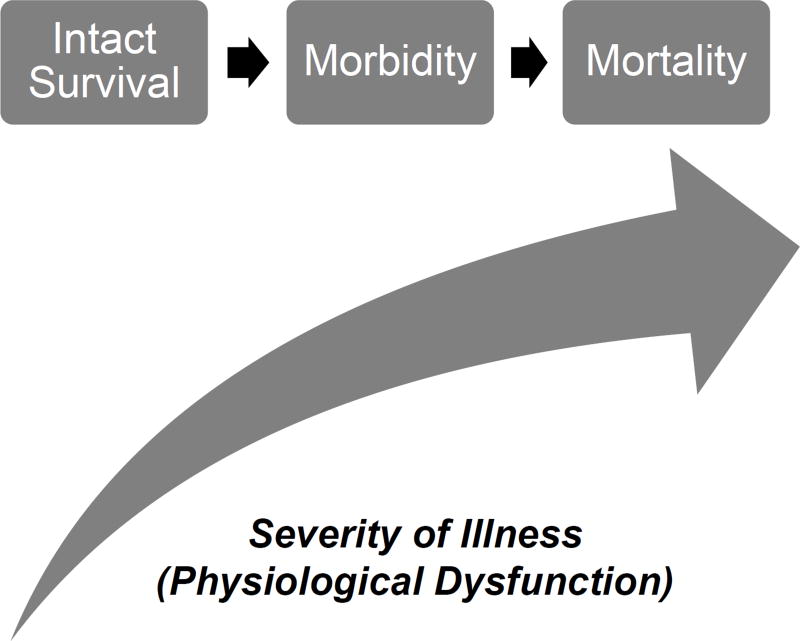
Morbidity often represents an intermediate outcome in a critically ill patient’s progression towards death, and is likely the result of the same physiologic dysfunctions that are associated with mortality (Figure 1). Therefore, the conceptual foundation of intensive care, maintaining physiological stability to prevent mortality, can be extended to morbidity, indicating that morbidity is a suitable and generalizable outcome measure for critical care quality assessments and research studies.
Figure 1.
The Conceptual Framework for Critical Care Functional Morbidity. The risk of both morbidity and mortality increase as severity of illness (physiological profiles) increases. In this conceptual framework, morbidity is an intermediate outcome on the pathway to mortality.
While it has been well known for decades that physiological dysfunction early in the PICU course is strongly associated with mortality risk, the association of physiological dysfunction with morbidity has only recently been evaluated.7 The Collaborative Pediatric Critical Care Research Network (CPCCRN) of the Eunice Kennedy Shiver National Institute of Child Health and Human Development assessed the relationship of physiological profiles measured within the first four hours of admission to the ICU to both mortality and the development of significant, new functional status morbidity at hospital discharge. This study is the Trichotomous Outcome Prediction in Critical Care (TOPICC) study. The measure of physiological profiles was the Pediatric Risk of Mortality (PRISM) score and the measure of morbidity was the Functional Status Scale (FSS, below).8,9
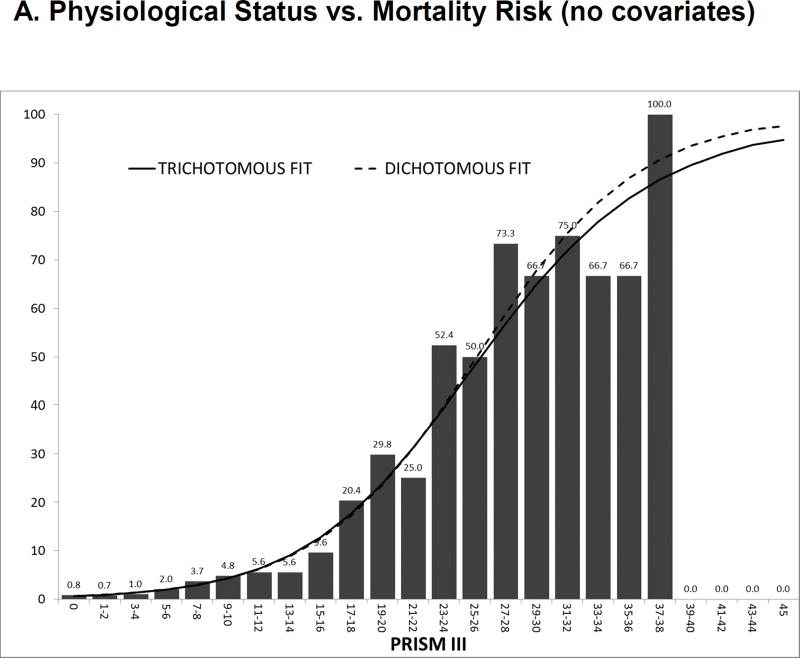
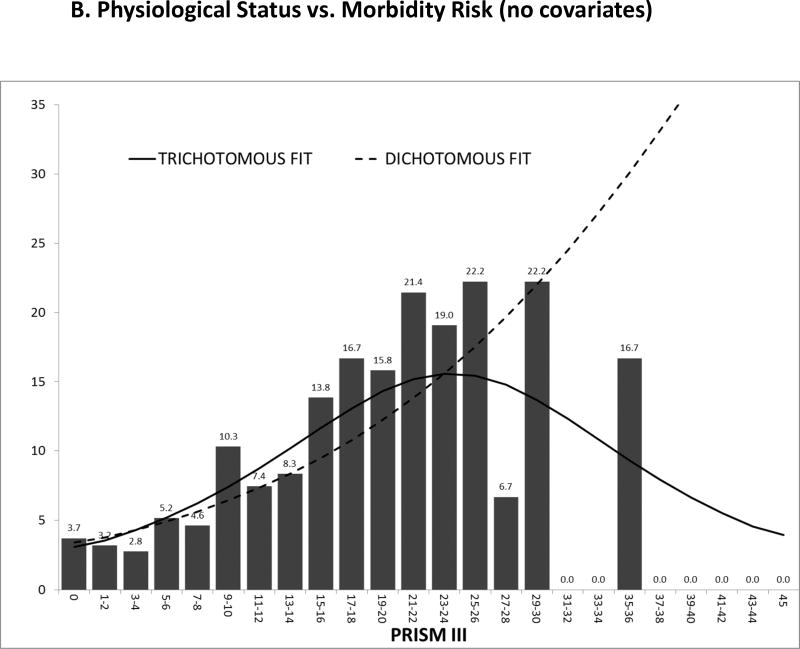
The CPCCRN study first identified a very similar relationship between physiological profiles and the development of new functional status morbidities as the relationship between physiological profiles and mortality. As the physiological instability increased, there was an increasing risk of both morbidity and mortality (Figures 2A and 2B). Next, the TOPICC study determined the factors associated with the development of morbidity and mortality for critically ill children. Table 1 compares the univariate odds ratios of developing either morbidity or mortality given the descriptive or physiological factors. In general, the risk factors for dying are also the risk factors for development of morbidity and when a variable is significant for one outcome, it is often significant for the other. Since the morbidity rate is twice as high as the mortality rate, most of the variables have higher odds ratios for morbidity than mortality. Importantly, physiological profiles measured by the four-hour PRISM score were significant for the risk of developing both morbidity and mortality, and they are even a more powerful predictor of morbidity than mortality.
Figure 2.
A and 2B The Association of Morbidity and Mortality Risk with Physiological Profiles (PRISM). Figure 2A illustrates the relationship of PRISM with mortality risk and this relationship changes little when the prediction model is dichotomous (survival/death) or trichotomous (functional status morbidity/intact survival/death). Figure 2B illustrates the association of functional status morbidity with PRISM. In the dichotomous model (functional status morbidity/other), the relationship of morbidity risk to PRISM is similar to mortality risk. However, with the trichotomous model, morbidity risk increases until morbidity risk decreases as patients with high risks die.
From Pollack MM, Holubkov R, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Critical Care Medicine 2015; 43(8):1699–1709
Table 1. Significant Risk Factors for Developing New Functional Status Morbidity and Mortality.
From Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. Aug 2015;43(8):1699–1709
Univariate odds ratios to develop Morbidity (N=351) and Mortality (N=214) based on 7560 PICU admissions. Only statistically significant factors are shown. Non-significant factors for both morbidity and mortality within each category are detailed in the footnotes.
| Variable | New morbidity (%) |
Deat h (%) |
Odds Ratios: New Morbidity Vs. No New Morbidity (Odds Ratio (95% C.I.)) |
Odds Ratios: Death Vs. No New Morbidity (Odds Ratio (95% C.I.)) |
|---|---|---|---|---|
| Age at PICU Admission (1) | ||||
| 0 day to < 7 days | 7.10 | 11.30 | 1.93 (1.12, 3.35) | 5.12 (3.09, 8.50) |
| 7 days to < 14 days | 12.00 | 9.60 | 3.40 (1.88, 6.15) | 4.53 (2.31, 8.88) |
| Primary System of Dysfunction (2) | ||||
| Respiratory | 4.40 | 2.20 | 0.88 (0.55, 1.39) | 0.24 (0.16, 0.37) |
| Cancer | 5.70 | 2.50 | 1.16 (0.60, 2.23) | 0.28 (0.12, 0.63) |
| Cardiovascular Disease - Congenital | 4.50 | 4.20 | 0.92 (0.56, 1.51) | 0.47 (0.31, 0.71) |
| Endocrine | 0 | 0.40 | <0.01 (<0.01, >999) | 0.04 (0.01, 0.30) |
| Gastrointestinal disorder | 5.70 | 3.40 | 1.18 (0.61, 2.31) | 0.39 (0.19, 0.81) |
| Musculoskeletal Condition | 3.10 | 0.30 | 0.61 (0.29, 1.30) | 0.03 (0.00, 0.24) |
| Neurologic | 7.10 | 2.40 | 1.47 (0.93, 2.34) | 0.27 (0.17, 0.43) |
| Miscellaneous | 1.80 | 1.30 | 0.34 (0.15, 0.77) | 0.14 (0.06, 0.33) |
| Intervention Category (3) | ||||
| Neurosurgery | 3.40 | 0.80 | 0.59 (0.36, 0.96) | 0.20 (0.07, 0.54) |
| Orthopedic | 2.60 | 0 | 0.45 (0.21, 0.97) | <0.01 (<0.01, >999) |
| Otolaryngology | 1.70 | 0 | 0.29 (0.14, 0.63) | <0.01 (<0.01, >999) |
| Miscellaneous | 1.60 | 1.20 | 0.27 (0.10, 0.74) | 0.31 (0.10, 0.98) |
| Acute (non-Primary) or Chronic Diagnosis of Cancer (4) | ||||
| Yes | 5.90 | 7.40 | 1.37 (0.81, 2.30) | 2.95 (1.83, 4.76) |
| Trauma (5) | ||||
| Trauma | 11.90 | 3.50 | 3.14 (2.32, 4.24) | 1.40 (0.84, 2.32) |
| Admission Source (6) | ||||
| Inpatient Unit from Same Hospital | 6.60 | 5.60 | 2.15 (1.52, 3.02) | 3.79 (2.49, 5.76) |
| Direct Admission from Referring Hospital | 7.20 | 4.90 | 2.33 (1.75, 3.09) | 3.31 (2.27, 4.83) |
| PICU Admission Status (7) | ||||
| Elective (scheduled) | 3.20 | 1.30 | 0.57 (0.44, 0.72) | 0.33 (0.23, 0.48) |
| Cardiac Arrest (8) | ||||
| Yes | 15.40 | 38.50 | 6.91 (3.88, 12.3) | 33.3 (21.3, 52.0) |
| 4-Hour PRISM Score (9) | ||||
| PRISM III (Total) | 1.11 (1.09, 1.13) | 1.23 (1.21, 1.25) | ||
| PRISM III Cardiovascular Variables | 1.12 (1.06, 1.20) | 1.44 (1.37, 1.52) | ||
| PRISM III Metabolic Variables | 1.16 (1.12, 1.21) | 1.35 (1.30, 1.41) | ||
| PRISM III Chemistry Variables | 1.14 (1.07, 1.21) | 1.46 (1.38, 1.55) | ||
| PRISM III Hematological Variables | 1.16 (1.10, 1.23) | 1.39 (1.32, 1.46) | ||
| PRISM III Neurological Variables | 1.17 (1.13, 1.21) | 1.30 (1.27, 1.34) |
Reference is Age >= 144 months. Non-significant age categories were 14 days to < 21 days, 21 days to < 1 month, 1 month to < 12 months, 12 months to < 60 months, 60 months to < 144 months.
Reference is acquired cardiovascular disease. Non-significant systems of dysfunction were hematological and renal.
Intervention Category. Reference is no intervention. Non-significant intervention categories included cardiovascular surgery, interventional catheterization, and general surgery.
Cancer. Reference is no acute or chronic cancer.
Trauma. Reference is no trauma.
Admission Source. Reference is operating room or post-anesthesia care unit. Non-significant categories are admissions from the emergency department of the same hospital.
Admission Status. Reference is emergency.
Cardiac Arrest. Reference is no cardiac arrest.
PRISM. Data shown are for each change of one PRISM point.
Next, the TOPICC study developed a model to predict the three critical care outcomes—intact survival, survival with a functional status morbidity, and death at hospital discharge—simultaneously using multivariate trichotomous logistic regression (Figure2A and 2B). When the relationship of morbidity and mortality to physiological profiles measured by the PRISM score are modeled separately, they were similar, with the risk of either mortality or morbidity increasing as physiological instability increases. But, the relationship changes when both outcomes were considered simultaneously. As physiological instability increased, both morbidity and mortality risk increased in parallel until mortality risk became dominant and the risk of morbidity decreased as those patients with a high mortality risk die. The association of morbidity risk to physiological status, when mortality risk is factored in, is an “inverted U.” Morbidity risk first increased in parallel with mortality and then decreased.
This TOPICC study demonstrated that the same relationships underlying the association of mortality to physiological status strongly influence the development of new, functional status morbidities at hospital discharge as illustrated in Figure 1. The implications are important: just as providers have the ability to influence mortality risk by appropriately identifying and treating physiological dysfunction, they have the ability to influence morbidity through the same mechanisms.
Importantly, this study was also able to develop and validate a predictor of the aforementioned three outcomes from critical care simultaneously (Table 2). The relative risks for developing morbidity or mortality are reflected in the coefficients and odds ratios. This presents the potential to use both morbidity as well as mortality as meaningful ICU outcomes for quality assessments and researching new interventions because morbidity can be adjusted for using physiological profiles and risk factors in the same way as mortality.
Table 2. Simultaneous Prediction of Morbidity and Mortality.
From Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. Aug 2015;43(8):1699–1709
| Predictors | Morbidity Coefficients (SE) |
Odds Ratios: New Morbidity vs. No New Morbidity (95% CI) |
Mortality Coefficients (SE) |
Odds Ratios: Death vs. No New Morbidity (95% CI) |
|---|---|---|---|---|
| Intercept | −3.92 (0.17) | NA | −5.51 (0.27) | NA |
| Age at PICU Admission | ||||
| 0 day to < 14 days | 0.80 (0.23) | 2.23 (1.43,3.49) | 1.64 (0.27) | 5.14 (3.00,8.79) |
| 14 days to < 1 month | 0.47 (0.44) | 1.61 (0.68,3.79) | 1.26 (0.56) | 3.53 (1.19,10.50) |
| 1 month to < 12 months | 0.39 (0.14) | 1.48 (1.13,1.93) | 0.42 (0.21) | 1.52 (1.02,2.28) |
| >12 months | Reference | Reference | Reference | Reference |
| Admission Source | ||||
| Direct admission: Referral Hospital | 0.76 (0.15) | 2.15 (1.59,2.90) | 1.09 (0.24) | 2.96 (1.87,4.70) |
| Inpatient Unit: Same Hospital | 0.87 (0.18) | 2.38 (1.67,3.39) | 1.70 (0.25) | 5.46 (3.33,8.95) |
| Emergency Department: Same Hospital | 0.11 (0.16) | 1.12 (0.81,1.53) | 0.64 (0.25) | 1.90 (1.16,3.14) |
| OR/PACU for Postoperative Care | Reference | Reference | Reference | Reference |
| Cardiac Arrest (1) | 0.97 (0.33) | 2.63 (1.38,5.00) | 1.52 (0.33) | 4.56 (2.40,8.66) |
| Acute (non-Primary) or Chronic Diagnosis of Cancer (1) | 0.25 (0.28) | 1.28 (0.74,2.21) | 0.89 (0.30) | 2.44 (1.36,4.40) |
| Trauma (1) | 1.18 (0.19) | 3.26 (2.23,4.77) | 0.81 (0.35) | 2.26 (1.13,4.51) |
| Primary System of Dysfunction | ||||
| Cardiovascular/Respiratory | Reference | Reference | Reference | Reference |
| Cancer | 0.73 (0.28) | 2.07 (1.20,3.59) | 0.90 (0.43) | 2.47 (1.06,5.74) |
| Low Risk (DKA, Hematologic, Musculoskeletal, Renal) | −0.93 (0.31) | 0.39 (0.21,0.72) | −1.69 (0.61) | 0.18 (0.06,0.61) |
| Neurologic | 0.38 (0.15) | 1.46 (1.08,1.98) | −0.07 (0.25) | 0.93 (0.57,1.54) |
| Other | −0.21 (0.23) | 0.81 (0.52,1.28) | 0.11 (0.31) | 1.11 (0.61,2.03) |
| Baseline FSS Score Categorized as Good (1,2) | −0.23 (0.13) | 0.80 (0.61,1.03) | −0.66 (0.19) | 0.52 (0.36,0.74) |
| PRISM III Neurological Score (3, 4) | 0.11 (0.02) | 1.12 (1.08,1.16) | 0.21 (0.02) | 1.24 (1.19,1.29) |
| PRISM III Non-Neurological Score (4) | 0.09 (0.01) | 1.09 (1.07,1.12) | 0.18 (0.01) | 1.19 (1.16,1.23) |
NA = not applicable
Reference is absence of the factor.
Baseline FSS score = 6 or 7.
PRISM III neurological components are pupillary reactions and mental status.
For each one point change.
Why Does Assessing Morbidity Matter For Pediatric Critical Care?
Using morbidity with or without mortality as a critical care outcome presents opportunities. First, morbidity and morbidity plus mortality represent much larger signals for quality assessment and research. New functional status morbidity at hospital discharge, significant enough that parents and health care providers would understand that life has changed at least temporarily for that child and family, is twice as common as mortality. Studies using both outcomes will take less time and be more relevant to ensuring quality of care standards or relevant research outcomes.
The most important potential use for morbidity is as a new measure of quality of care. For almost three decades, ICUs have used measures of mortality adjusted for physiological profiles and/or case-mix variables to assess the quality of care provided within an individual institution over time (internal benchmarking) or across a range of institutions using a known standard (external benchmarking). These methods compare the observed number of outcomes to the expected number of outcomes based on the physiological and case-mix profiles of the patients (standardized ratios). Low mortality rates limit the utility of mortality as an outcome for quality studies since measuring quality using mortality may require long time periods to acquire a sufficient number deaths for a reliable quality assessment. Detecting sequential changes over time will be enhanced when the outcomes are both more relevant and more frequent.
A second important and contemporary use of morbidity is for pediatric critical care research trials. Pediatric critical care trials have been stymied by the need for large patient samples or a long enrollment period to capture an adequate number of events. Using mortality as a primary outcome in intervention trials requires very large samples to avoid “fragility,” the concept which represents the number of deaths that, if changed to survivors, would have changed the statistical conclusion of the trial from significant to negative. Indeed, a recent study noted that the statistical conclusions of over half of the identified randomized clinical trials could be flipped either by using a more conservative statistical test or just changing the number of deaths to survivors by two, even in multi-centered trials in adult ICUs where mortality rates are approximately three to four times as high as pediatric units.10 Critical care in general—especially pediatric critical care, due to the relatively low mortality rate—needs a more frequent outcome than mortality for robust and reliable studies.
Measures of morbidity are already prominent in pediatric critical care research studies. For example, the Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trial investigated whether the intervention of targeted temperature management to hypothermia following cardiac arrest would improve significant changes in adaptive functioning.11 The primary outcome for THAPCA was survival with good functional status as assessed by the Vineland Adaptive Behavior Scale (VABS). The Approaches and Decisions in Acute Pediatric TBI Trial (ADAPT) is using the pediatric version of the Glasgow Outcome Scale-Extended.12 The potential assessment methods for morbidity following head injury include detailed dissection of neurocognitive function but there is not agreement on the optimal method.13 Other studies have assessed neurocognitive outcomes of tight glucose control in critically ill pediatric patients using a range of neuropsychological testing.14 However, these studies demonstrate the variability with which morbidity is defined and measured.
Measuring Morbidity
Unlike mortality, which provides a clear dichotomous outcome, morbidity is a continuum of dysfunction. For many studies, documenting morbidity at a specific time point may be sufficient, but for other studies, assessments at a time when the morbidity has stabilized is most relevant. For example, prior work has shown that morbidity following trauma usually plateaus by six months post-injury.15 For the general PICU population, however, recent evidence suggests that the incidence of significant morbidly and mortality determined at hospital discharge may double over the subsequent three years.16
A general method for measuring morbidity following critical care should include a broad range of function and be relevant to large populations in its practicality, specificity, and sensitivity. General measures of morbidity for pediatric critical care, especially if they are to be used for large population studies such as quality studies requiring a general assessment of functional status should have as many of the following attributes as possible:
Measure a clinically important outcome state
Be relevant to long-term outcomes (can be used to project to medium- and long-term outcomes)
Can be completed relatively rapidly
Can be completed without interaction with the family to allow studies to be done without informed consent and efficiently
Is reliable across the sample even if some individual patients are misclassified;
Is age independent
Is objective and sufficiently granular to limit subjective assessments
Has strong inter-rater reliability
Historically, three broad categories of methods have been used to assess morbidity in critically ill children: global measurements, health-related quality of life scales including health utilities indices, and adaptive behavior scales including neuropsychological and psychometric testing.6 Depending on the method selected, a child’s deficits in specific domain(s) (e.g. motor ability, communication skills, etc.) that are relevant to the context of the study are objectively assessed.
Global Measures of Morbidity
Global outcome scales include the Glasgow Outcome Scale (GOS)17 and its pediatric versions, the Pediatric Cerebral Performance Category (PCPC) and the Pediatric Overall Performance Category (POPC).18 These scales assign a single score to classify overall functional level without examining specific domains beyond the PCPC’s focus on cognitive performance. The GOS and POPC/PCPC are relatively straightforward to administer but do not have uniformly objective and granular classifications for the assessment, leading to the potential for poor inter-rater reliability and poor precision. The second generation GOS are the GOS–Extended (GOS-E)19 and its pediatric version, the GOS-E Pediatrics (GOS-E Peds).12 The GOS-E scores use a short structured interview with the patient or family to determine functional status.
The POPC and PCPC are intended to estimate short-term functioning based on a projection by health care providers at hospital discharge. They are widely used following critical illness because of their ease of assessment, face validity due to their similarity to the GOS, and statistically significant (but weak) relationship to long-term neuro-psychological tests,20 and have been used in large pediatric critical care studies.21 The scores include: 1 for good, 2 for mild disability, 3 for moderate disability, 4 for severe disability, 5 for vegetative state or coma, and 6 for death. Completion of the POPC/PCPC generally takes only a few minutes and does not require parent/guardian or patient participation for completion. Unfortunately, the PCPC/POPC method lacks both precision and reproducibility. Inter-rater agreement of this system was only 76% – 80% when there was inclusion of a neighboring class.21 That is, the agreements were only satisfactory if patients were classified as good to moderate disability, mild to severe disability, or moderate to very severe disability. Lack of precision was evident when the POPC/PCPC was compared to the more objective and granular Functional Status Scale (FSS, see below).22
The GOS-E Peds is primarily used as an outcome for brain injury studies, although it has the potential for outcome assessment for other conditions. It has domains adapted to children (consciousness, independence in the home, independence outside the home, school/work, social and leisure activities, family and friendships, and return to normal life) and separates two age groups (“younger” and “older” patients) in an attempt to account for developmental stages. The GOS-E Peds was validated in 159 children (average age 81 ± 57 months) at a single site.12 Overall, the GOS-E Peds was well-correlated with the GOS (correlation >0.8), and reasonably correlated with the composite Vineland Adaptive Behavior Score (VABS) (correlation > 0.6), VABS domains (all correlations >0.45), Bayley (correlation >0.6), other intelligence scores (correlations >0.55) but less well-correlated with parent ratings scales. The best results were obtained in older children with the worst head injuries. This suggests that it be further validated prior to use in any non-TBI group and in younger children with less severe TBI. The GOS-E Peds has not been assessed for inter-rater reliability (although the adult version has very good inter-rater reliability and has even been done with a mail questionnaire). Importantly, the validation assessments were done three months following the injury as a structured interview with professionals who were not blinded to other results. The GOS-E Peds was used as a secondary outcome in the “Cool Kids” trial which was terminated after only 77 patients23 and is a primary outcome in the Approaches and Decisions in Acute Pediatric TBI Trial (ADAPT).24
Health-Related Quality of Life (HRQoL)
HRQoL measures attempt to gain an understanding of a child’s overall functional status by using the concepts of quality of life. The most popular methods are the PedsQL and the Child Health Questionnaire.25–28 Most health-related quality of life methods assess various combinations of different domains (e.g., social, emotional, school functioning, physical, etc.). The responses of children as young as five years of age may be used to complete the questionnaires. It is important to recognize that assessments are fundamentally subjective and patient, parent, and health care provider assessments often diverge.29 HRQoL methods have multiple versions which may include interviews, in person questionnaires/surveys, telephone surveys, long forms, short forms, and disease- or condition-specific versions. A recent review found that, overall, there is a significant decrease in HRQoL for pediatric ICU patients, although this has been measured by different methods and follow-up periods. This review also examined over 20 different measures of HRQoL, concluding there are neither HRQoL tools specific to the post-PICU population nor a consensus as to the best method applicable to general post-ICU studies.30
Adaptive Behavior Scales
Adaptive behavior scales are commonly used and currently popular for research and for individual patient assessments. They focus on skill domains considered important to normal functioning and are adjusted for developmental age through evaluation of domains such as social and cognitive skills. The most commonly used are the Vineland Adaptive Behavior Scales (VABS) and the Adaptive Behavior Assessment Scale (ABAS).31,32 Both methods are available in different formats.
The ABAS is a questionnaire, and is commonly used by schools and in environments where questionnaires are more practical. The VABS is a psychometric instrument that is especially useful in the evaluation of children with intellectual disability, chronic disease, TBI, mental health conditions, and many types of developmental delays, as well as part of a battery of neuropsychological tests.
The VABS must be administered by a trained interviewer or psychologist. The VABS assesses personal independence and social responsibility using information relevant to day-to-day activities necessary to take care for oneself and to get along with others. There are multiple versions of the VABS that include short versions that can be administered by telephone. Unfortunately, despite its popularity, the VABS has not been assessed for validity in the peer-reviewed literature in several decades.33,34 In general, the VABS is believed to function very well, especially in those with mild and moderate levels of functioning, but it may perform less well in those with severe and profound levels of dysfunction, and it can be logistically difficult to administer to large numbers of patients.
The Functional Status Scale (FSS)
In an effort to develop a method using the principles of both activities of daily living and adaptive behavior that could be easily and accurately applied to larger patient populations, researchers with the CPCCRN developed the Functional Status Scale (FSS). The FSS was developed by the CPCCRN based on consensus input from pediatricians, pediatric neurologists, pediatric developmental psychologists, pediatric nurses, pediatric intensivists, and pediatric respiratory therapists from 11 institutions. The FSS (Table 3) assesses functioning in the domains of mental status, sensory functioning, communication, motor functioning, feeding, and respiratory status.
Table 3. Functional Status Scale.
Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics 2009 Jul;124(1):e18–28. doi: 10.1542/peds.2008-1987.
| Normal (Score = 1) |
Mild Dysfunction (Score = 2) |
Moderate Dysfunction (Score = 3) |
Severe Dysfunction (Score = 4) |
Very Severe Dysfunction (Score = 5) |
|
|---|---|---|---|---|---|
| Mental status | Normal sleep/wake periods; appropriate responsiveness | Sleepy but arousable to noise/touch/movement and/or periods of social non-responsiveness | Lethargic and/or irritable | Minimal arousal to stimuli (stupor) | Unresponsive, coma, and/or vegetative state |
| Sensory functioning | Intact hearing and vision and responsive to touch | Suspected hearing or vision loss | Not reactive to auditory stimuli or to visual stimuli | Not reactive to auditory stimuli and to visual stimuli | Abnormal responses to pain or touch |
| Communication | Appropriate noncrying vocalizations, interactive facial expressiveness, or gestures | Diminished vocalization, facial expression, and/or social responsiveness | Absence of attention-getting behavior | No demonstration of discomfort | Absence of communication |
| Motor functioning | Coordinated body movements, normal muscle control, and awareness of action and reason | 1 limb functionally impaired | ≥2 limbs functionally impaired | Poor head control | Diffuse spasticity, paralysis, or decerebrate/decorticate posturing |
| Feeding | All food taken by mouth with age-appropriate help | Nothing by mouth or need for age-inappropriate help with feeding | Oral and tube feedings | Parenteral nutrition with oral or tube feedings | All parenteral nutrition |
| Respiratory status | Room air and no artificial support or aids | Oxygen treatment and/or suctioning | Tracheostomy | Continuous positive airway pressure treatment for all or part of the day and/or mechanical ventilatory support for part of the day | Mechanical ventilatory support for all of the day and night |
Functional status for each domain is categorized from “normal” (1) to “very severe dysfunction” (6), with aggregate scores ranging from 6 (best) to 30 (worst). Construct validity was established by correlating the performance of the FSS with adaptive behavior as measured by the ABAS-II. Adaptive behavior was selected as a similar but not identical measure of function, recognizing that correlation between adaptive behavior scores purporting to measure the same functions is only moderate. Discriminant validity was established by receiver operating characteristic (ROC) curve analysis. Other patient factors including age, elective/emergency admission status, operative status, patient type), and study site, were investigated to determine if they were independently associated with FSS after adjusting for the ABAS-II using multivariable linear regression. FSS showed a consistent, moderate to strong association with ABAS-II across these other patient factors. The intraclass correlation of the total FSS was 0.95, indicating overall high reproducibility.
Since the FSS can be determined on admission based on parent recall or medical records, it enables a comparison of pre-illness with post-illness functioning. The FSS was recently used in a CPCCRN study of >10,000 pediatric ICU patients and was an excellent metric with sufficient precision and reliability for prediction based on physiological profiles.7 In this study, a new significant functional status change was defined as a change of three points or more for two major reasons. First, investigators felt that this magnitude of change would be very evident to both parents and providers. Second, 95% of the patients with a change of three points or more had a change of at least two points in a single FSS domain, indicating that the change had clearly occurred and was unlikely the result of a data collection error. This study established the practical advantages of the FSS for assessing hospitalized children by demonstrating that it can be assessed in less than five minutes from the medical record or conversations with health professions caring for the patient. Recently, the FSS was used to assess discharge status in a large pediatric trauma cohort.35
Known Morbidity Following Pediatric Intensive Care
Morbidity, including physical, psychosocial, and neuro-cognitive deficits is common in intensive care conditions. A recent article found in a general, unselected population of PICU patients that new functional status morbidity assessed with the FSS was 33% higher than mortality at hospital discharge and both the morbidity and mortality rates doubled in the three years after initial hospital discharge.16 Almost as many children demonstrated worsening of their functional status or died (38%) as survived without a change in functional status (44%). Less than 10% of children exhibited functional gains over time. Long-term function was associated with indicators of severity of illness including the need for invasive therapies such as use of mechanical ventilation and use of vasoactive medications.
Although it is not possible to precisely compare morbidity and mortality rates over time because of the different research methods, data from several decades ago demonstrated a PICU mortality rate of 4.6% and a PICU morbidity rate of 3.1% (based on a two point or greater POPC change), whereas recent data from the TOPICC study (based on a FSS change of three or more points from baseline to hospital discharge) had a reversal of these percentages, with a hospital mortality rate of 2.4% and morbidity rate of 4.8%.2,21 Thus, the “morbidity plus mortality rate” has decreased only from 7.7% to 7.2%, which has been mirrored in other studies.2,21,36 Importantly, these rates are not severity- or risk-adjusted, but the potential shift from mortality to morbidity is consistent with the clinical observations of many clinicians.
In the TOPICC study, morbidities affected essentially all types of patients and age groups in relatively equal measure. New morbidities occurred with relatively equal risk in those with all diagnostic groups and all degrees of baseline functional compromise. They also occurred in almost all operative groups, with the highest rates in cardiac surgery and general surgery and in only 3.1% of neurosurgical patients. Importantly, functional morbidity as well as mortality rates differed by more than 300% among the sites, indicating functional morbidity may be used as a robust and relevant measure of quality, effectiveness, and efficacy.
Prior research has used PCPC/POPC to determine risk factors for developing a new morbidity during critical illness. Using the VPS database, Bone et al. identified children who survived their ICU admission but had worsening of PCPC/POPC scores.37 New functional or cognitive morbidities were noted in 10.3% and 3.4% of survivors, respectively. Multivariate analysis identified trauma, oncologic, and neurologic diagnoses as particularly high-risk. As seen in other studies, patients who required significant invasive support (mechanical ventilation, renal replacement therapy, CPR, or ECMO) were also prone to development of new morbidities during admission, consistent with other studies using neuropsychological testing, VABS and HRQoL scales.38–40
In addition to the general knowledge about ICU-generated morbidities, some research has looked at development of new morbidities (or specific types of morbidities) in specific disease states.
Psychological Morbidity and Family Stress
In addition to the physical and cognitive outcomes associated with ICU care, researchers have also examined the psychiatric burden faced by children and families following critical illness. Given the physical and emotional stresses faced by patients and their families in critical care settings it is unsurprising that there are psychological ramifications of these illnesses. A review by Davydow et al. showed a patient incidence of PTSD symptoms ranging from 10–28% and depression symptoms ranging from 7–13%.41 These rates are higher than have been seen in other patient populations, including the pediatric oncology population as well as children sustaining traumatic injury. Factors such as ICU length of stay and severity of illness at the time of ICU admission were associated with a higher prevalence of symptoms. This contrasts to studies in the adult population, where evidence is mixed as to whether these factors are associated with worse post-ICU psychiatric morbidity. This may be an instructive reminder as to the limitations of adapting research conducted in adults to the pediatric population.
Congenital Heart Surgery
Significant functional status morbidity at hospital discharge is approximately 50% greater than mortality for children following pediatric cardiac surgery. Discharge morbidity is associated with the same factors as mortality from congenital heart surgery including the severity of the anatomic anomaly, the difficulty of the surgical palliation or repair, and the physiological dysfunction in the immediately post-operative period. The trichotomous outcome predictor (above) performs well predicting morbidity as well as mortality in congenital heart surgery patients as well the general ICU population.42
Trauma
The 2006 Institute of Medicine report, “Emergency Care for Children: Growing Pains,” acknowledged the need for better outcomes including functional status at hospital discharge.43 The National Trauma Data Bank, the leading US trauma registry and most commonly used database for injured children from over 700 facilities, uses only mortality as a hospital discharge outcome for both ICU and non-ICU children even though mortality is substantially less than 3%.44 In trauma research, most functional outcome studies have been used only in specific age groups, have been performed only among children with traumatic brain injury, or have been assessed in a research setting, e.g., the Pediatric Evaluation of Disability Inventory (PEDI),45 the WEE Functional Injury Measure (WeeFIM),46 and the GOS-E Peds. Few studies have evaluated functional measures of injury outcomes across a range of injury types and severity or have been validated across a wide age range.47
Conclusions
Pediatric critical care has improved mortality rates over time but may have exchanged mortality for the development of new morbidities. Morbidity is linked to the same physiological factors as mortality in critical illness. A variety of methods have been used to characterize morbidity in the pediatric critical care literature. Consensus on the most appropriate method to assess patient morbidities and integration of such a method into pediatric critical care research (including large database use) will offer the next step forward in caring for critically ill children. The FSS developed by the CPCCRN is a granular, age-independent, and validated method that has been valuable in large-sample critical care studies.
Morbidity assessments should be available from the medical record to ensure they are available for routine studies of quality and available for other large-scale studies. Similarly, databases must incorporate appropriate morbidity measures in their quality and research studies. Currently, large databases such as those of the Society of Thoracic Surgery (STS), American College of Surgeons (ACS) Trauma Registry, and the Pediatric Health Information System (PHIS) do not include a patient-level functional status morbidity assessment.
Key Points.
Morbidity is an important outcome that can be measured even for large studies. There are many measures of morbidity that can be selected based on the context of the study.
In Pediatric Critical Care, functional status is an “intermediate” outcome on the pathway to death that is significantly associated with physiological instability (measured by the Pediatric Risk of Mortality (PRISM) score).
Morbidity risk in pediatric critical care can be measured using physiological profiles (PRISM) and other case-mix factors and used for quality assessment in a manner similar to death.
New functional status morbidity rates are about double mortality rates.
The Functional Status Scale (FSS) developed by the Collaborative Pediatric Critical Care Research Network is a granular method of measuring functional status and new functional status morbidity that is applicable to large-sample studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Julia Heneghan, Critical Care Medicine, Children’s National Medical Center, 111 Michigan Ave NW, Washington, DC 20010.
Murray M Pollack, Department of Pediatrics, George Washington University School of Medicine and Health Sciences, Critical Care Medicine, Children’s National Medical Center, 111 Michigan Ave NW, Washington, DC 20010.
References
- 1.Pollack MM, Ruttimann UE, Getson PR. Accurate prediction of the outcome of pediatric intensive care. A new quantitative method. N Engl J Med. 1987 Jan 15;316(3):134–139. doi: 10.1056/NEJM198701153160304. [DOI] [PubMed] [Google Scholar]
- 2.Pollack MM, Holubkov R, Funai T, et al. Pediatric Intensive Care Outcomes: Development of New Morbidities During Pediatric Critical Care. Pediatr Crit Care Med. 2014;15(9):821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobs ML, O'Brien SM, Jacobs JP, et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013 Apr;145(4):1046–1057. e1041. doi: 10.1016/j.jtcvs.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronman MP, Hall M, Slonim AD, et al. Charges and lengths of stay attributable to adverse patient-care events using pediatric-specific quality indicators: a multicenter study of freestanding children's hospitals. Pediatrics. 2008 Jun;121(6):e1653–1659. doi: 10.1542/peds.2007-2831. [DOI] [PubMed] [Google Scholar]
- 5.Boyd JM, Burton R, Butler BL, et al. Development and Validation of Quality Criteria for Providing Patient- and Family-centered Injury Care. Ann Surg. 2016 Sep 08; doi: 10.1097/SLA.0000000000002006. [DOI] [PubMed] [Google Scholar]
- 6.Ong C, Lee JH, Leow MK, et al. Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review. Pediatr Crit Care Med. 2016 May;17(5):e247–259. doi: 10.1097/PCC.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 7.Pollack MM, Holubkov R, Funai T, et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit Care Med. 2015 Aug;43(8):1699–1709. doi: 10.1097/CCM.0000000000001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack MM, Holubkov R, Funai T, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med. 2016 Jan;17(1):2–9. doi: 10.1097/PCC.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack MM, Holubkov R, Glass P, et al. Functional Status Scale: new pediatric outcome measure. Pediatrics. 2009 Jul;124(1):e18–28. doi: 10.1542/peds.2008-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridgeon EE, Young PJ, Bellomo R, et al. The Fragility Index in Multicenter Randomized Controlled Critical Care Trials. Crit Care Med. 2016 Jul;44(7):1278–1284. doi: 10.1097/CCM.0000000000001670. [DOI] [PubMed] [Google Scholar]
- 11.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015 May 14;372(20):1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma. 2012 Apr 10;29(6):1126–1139. doi: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCauley SR, Wilde EA, Anderson VA, et al. Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J Neurotrauma. 2012 Mar 01;29(4):678–705. doi: 10.1089/neu.2011.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesotten D, Gielen M, Sterken C, et al. Neurocognitive development of children 4 years after critical illness and treatment with tight glucose control: a randomized controlled trial. Jama. 2012 Oct 24;308(16):1641–1650. doi: 10.1001/jama.2012.12424. [DOI] [PubMed] [Google Scholar]
- 15.Polinder S, Meerding WJ, Toet H, et al. Prevalence and prognostic factors of disability after childhood injury. Pediatrics. 2005 Dec;116(6):e810–817. doi: 10.1542/peds.2005-1035. [DOI] [PubMed] [Google Scholar]
- 16.Pinto NPPE, Kim TY, Ladner P, et al. Long-term Function After Pediatric Critical Illness: Results From The Survivor Outcomes Study. Ped Crit Care Med. 2017 doi: 10.1097/PCC.0000000000001070. in press. [DOI] [PubMed] [Google Scholar]
- 17.Jennett BBM. Assessment of outcome after severe brain damage: a practical scale. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 18.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatric. 1992;121:69–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 19.Jennett B, Snoek J, Bond MR, et al. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981 Apr;44(4):285–293. doi: 10.1136/jnnp.44.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiser DH, Long N, Roberson PK, et al. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000 Jul;28(7):2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 21.Fiser DH, Tilford JM, Roberson PK. Relationship of illness severity and length of stay to functional outcomes in the pediatric intensive care unit: a multi-institutional study. Critical Care Medicine. 2000 Apr;28(4):1173–1179. doi: 10.1097/00003246-200004000-00043. [DOI] [PubMed] [Google Scholar]
- 22.Pollack MM, Holubkov R, Funai T, et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014 Jul;168(7):671–676. doi: 10.1001/jamapediatrics.2013.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adelson PD, Wisniewski SR, Beca J, et al. Comparison of hypothermia and normothermia after severe traumatic brain injury in children (Cool Kids): a phase 3, randomised controlled trial. The Lancet. Neurology. 2013 Jun;12(6):546–553. doi: 10.1016/S1474-4422(13)70077-2. [DOI] [PubMed] [Google Scholar]
- 24.Larsen GY, Schober M, Fabio A, et al. Structure, Process, and Culture Differences of Pediatric Trauma Centers Participating in an International Comparative Effectiveness Study of Children with Severe Traumatic Brain Injury. Neurocrit Care. 2016 Jun;24(3):353–360. doi: 10.1007/s12028-015-0218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varni JW, Seid M, Knight TS, et al. The PedsQL 4.0 Generic Core Scales: sensitivity, responsiveness, and impact on clinical decision-making. J Behav Med. 2002 Apr;25(2):175–193. doi: 10.1023/a:1014836921812. [DOI] [PubMed] [Google Scholar]
- 26.Varni JW, Limbers CA, Neighbors K, et al. The PedsQLTM Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011 Feb;20(1):45–55. doi: 10.1007/s11136-010-9730-5. [DOI] [PubMed] [Google Scholar]
- 27.Drotar D, Schwartz L, Palermo TM, et al. Factor structure of the child health questionnaire-parent form in pediatric populations. Journal of pediatric psychology. 2006 Mar;31(2):127–138. doi: 10.1093/jpepsy/jsi078. [DOI] [PubMed] [Google Scholar]
- 28.Raat H, Bonsel GJ, Essink-Bot ML, et al. Reliability and validity of comprehensive health status measures in children: The Child Health Questionnaire in relation to the Health Utilities Index. J Clin Epidemiol. 2002 Jan;55(1):67–76. doi: 10.1016/s0895-4356(01)00411-5. [DOI] [PubMed] [Google Scholar]
- 29.Jardine J, Glinianaia SV, McConachie H, et al. Self-reported quality of life of young children with conditions from early infancy: a systematic review. Pediatrics. 2014 Oct;134(4):e1129–1148. doi: 10.1542/peds.2014-0352. [DOI] [PubMed] [Google Scholar]
- 30.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015 Jul;41(7):1235–1246. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 31.Sparrow SCD, Balla D. Vineland-II. Vineland Adaptive Behaviror Scales - Survey Forms Manual. Second. AGS Publishing; 2005. [Google Scholar]
- 32.Harrison PLOT. ABAS II. Adaptive Behavior Assessment System. second. PsychCorp; 2003. [Google Scholar]
- 33.Raggio DJ, Massingale TW. Comparison of the Vineland Social Maturity Scale, the Vineland Adaptive Behavior Scales--survey form, and the Bayley Scales of Infant Development with infants evaluated for developmental delay. Perceptual and motor skills. 1993 Dec;77(3 Pt 1):931–937. doi: 10.2466/pms.1993.77.3.931. [DOI] [PubMed] [Google Scholar]
- 34.Raggio DJ, Massingale TW, Bass JD. Comparison of Vineland Adaptive Behavior Scales-Survey Form age equivalent and standard score with the Bayley Mental Development Index. Perceptual and motor skills. 1994 Aug;79(1 Pt 1):203–206. doi: 10.2466/pms.1994.79.1.203. [DOI] [PubMed] [Google Scholar]
- 35.Bennett TD, Dixon RR, Kartchner C, et al. Functional Status Scale in Children With Traumatic Brain Injury: A Prospective Cohort Study. Pediatr Crit Care Med. 2016 Dec;17(12):1147–1156. doi: 10.1097/PCC.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010 Sep;11(5):549–555. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 37.Bone MF, Feinglass JM, Goodman DM. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr Crit Care Med. 2014 Sep;15(7):640–648. doi: 10.1097/PCC.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 38.Ebrahim S, Singh S, Hutchison JS, et al. Adaptive behavior, functional outcomes, and quality of life outcomes of children requiring urgent ICU admission. Pediatr Crit Care Med. 2013 Jan;14(1):10–18. doi: 10.1097/PCC.0b013e31825b64b3. [DOI] [PubMed] [Google Scholar]
- 39.Als LC, Nadel S, Cooper M, et al. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: a prospective study of school-aged children. Crit Care Med. 2013 Apr;41(4):1094–1103. doi: 10.1097/CCM.0b013e318275d032. [DOI] [PubMed] [Google Scholar]
- 40.Als LC, Tennant A, Nadel S, et al. Persistence of Neuropsychological Deficits Following Pediatric Critical Illness. Crit Care Med. 2015 Aug;43(8):e312–315. doi: 10.1097/CCM.0000000000001075. [DOI] [PubMed] [Google Scholar]
- 41.Davydow DS, Richardson LP, Zatzick DF, Katon WJ. Psychiatric morbidity in pediatric critical illness survivors: a comprehensive review of the literature. Arch Pediatr Adolesc Med. 2010 Apr;164(4):377–385. doi: 10.1001/archpediatrics.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berger JTHR, Reeder R, Wessel DL, et al. Morbidity and Mortality Prediction in Pediatric Heart Surgery: Physiological Profiles and Surgical Complexity. J Thorac Cardiovasc Surg. 2017 doi: 10.1016/j.jtcvs.2017.01.050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Committee on the Future of Emergency Care. Future of Emergency Care: Emergency Care for Children: Growing Pains. 2006 [Google Scholar]
- 44.Amer Coll of Surgeon. ACS NTDB National Trauma Data Standard: Data Dictionary. 2015 [Google Scholar]
- 45.Dumas HM, Haley SM, Carey TM, et al. The relationship between functional mobility and the intensity of physical therapy intervention in children with traumatic brain injury. Pediatric physical therapy : the official publication of the Section on Pediatrics of the American Physical Therapy Association. 2004 Fall;16(3):157–164. doi: 10.1097/01.PEP.0000136004.69289.01. [DOI] [PubMed] [Google Scholar]
- 46.Shaklai S, Peretz R, Spasser R, et al. Long-term functional outcome after moderate-to-severe paediatric traumatic brain injury. Brain injury. 2014;28(7):915–921. doi: 10.3109/02699052.2013.862739. [DOI] [PubMed] [Google Scholar]
- 47.Gabbe BJ, Simpson PM, Sutherland AM, et al. Functional and health-related quality of life outcomes after pediatric trauma. J Trauma. 2011 Jun;70(6):1532–1538. doi: 10.1097/TA.0b013e31820e8546. [DOI] [PubMed] [Google Scholar]