Abstract
Influenza viruses are a major public health problem worldwide. Although influenza has been extensively researched, there are still many aspects that are not fully understood such as the effects of within and between-hosts dynamics and their impact on behavior change. Here, we develop mathematical models with multiple infection stages and estimate parameters based on within-host data to investigate the impact of behavior change on influenza dynamics. We divide the infected population into three and four groups based on the age of the infection, which corresponds to viral load shedding. We consider within-host data on viral shedding to estimate the length and force of infection of the different infectivity stages. Our results show that behavior changes, due to exogenous events (e.g., media coverage) and disease symptoms, are effective in delaying and lowering an epidemic peak. We show that the dynamics of viral shedding and symptoms, during the infection, are key features when considering epidemic prevention strategies. This study improves our understanding of the spread of influenza virus infection in the population and provides information about the impact of emergent behavior and its connection to the within and between-hosts dynamics.
Keywords: Mathematical Model, Epidemiology, Influenza, Media, Behavior Change, Symptoms
1. INTRODUCTION
Even with the presence of vaccines and antiviral medication, seasonal and influenza pandemics lead to approximately 3 to 5 million cases of severe illness and 250,000 to 500,000 deaths worldwide.(1) There are various protective measures that can be used to reduce the spread of the influenza including treatment of infectives with antiviral drugs, vaccination, and social-distancing measures such as quarantine, school-closures, and isolation.(2, 3) However, these methods, in particular, antiviral drugs and early diagnosis of infectives, are associated with a high cost(4, 5) and vaccines may not be readily available.(6)
Other alternatives to reduce the likelihood of infection include limiting close contact with other individuals, staying home when sick, covering mouth and nose when coughing or sneezing, washing hands, and other protective measures.(7) A review(8) on hands hygiene in preventing transmission of severe acute respiratory syndrome (SARS) suggests that hand washing is an effective measure against disease transmission. During the 2003 SARS epidemic, residents of Hong Kong rapidly employed individual preventive strategies, which might have contributed to controlling the public health crisis.(9) Several studies have analyzed the use of facemasks in preventing the spread of disease and have showed that they can be effective in reducing transmission.(10, 11)
Furthermore, a survey conducted in the spring of 2009 about the novel influenza A (H1N1), demonstrated that 16–20% of Americans refrained from crowded places and 20% avoided contact with individuals not in their household(12) (for additional information see Table I in Ref. [12]). Changes in human behavior are thought to be one of the major contributors to reducing the spread of disease, especially in the absence of pharmaceutical interventions.(13–17)
There are many factors that can influence an individual to alter his/her behavior including demographic and psychological factors.(18) Infected individuals may reduce the number of contacts with others due to onset of disease symptoms.(3) Individuals who are not infected or are in asymptomatic stages of an infection may take protective measures due to media risk communication,(19) for example, they may avoid crowded places and wash their hands more often, to lower the risk of becoming infected. Reasons affecting behavior change of individuals in asymptomatic and symptomatic infection stages differ, which might significantly impact the disease dynamics and this is the reason why we distinguish between them in our study.
Influenza infection is typically accompanied by severe symptoms such as fever, cough, sore throat, and muscle or body aches.(20) In addition, the infection stimulates the immune system leading to additional symptoms such as mucosal inflammation.(21–23) In particular, in Ref. [23] it was shown that viral shedding correlates with the rise of local and systemic symptoms. Similarly, the levels in nasal lavage fluids were found to be directly associated with viral titers, temperature, mucus production, and symptoms scores in volunteers experimentally infected with influenza A/Texas/36/91 (H1N1).(21) In another study, levels of cytokines and chemokines were characterized and were found to increase during influenza infection and correlate with symptoms.(22) In a review article on influenza infection, it was concluded that the total symptoms scores and viral shedding curves exhibited analogous dynamics (see Figure 5 in Ref. [24]). Moreover, the infectivity of an individual changes with the age of infection (i.e., the time since the individual was infected), as it is proportional to the degree of viral shedding.(25) Based on these studies, here we divided the infected population into groups according to the age of the infection, which is proportional to viral load shedding.
Changes in human behavior, due to disease symptoms, may impact the transmission rate, modeling assumptions for forecasting disease dynamics, and public health recommendations. Omitting spontaneous changes in human behavior when modeling the spread of infectious diseases might lead to an overestimation of the number of cases and the secondary rates of infection.(26) Here, we consider potential behavior changes possibly due to the impact of information in the media about the severity of the disease (e.g., a deadly epidemic might lead to drastic changes in human behavior, whereas a mild epidemic might lead to minimal changes in behavior) and due to the severity of symptoms.
Public health strategies during an influenza epidemic are influenced by the previous occurrences of epidemics. In addition to those experiences, mathematical models are needed to test prevention strategies based on changes in mobility and contact patterns, viral evolution, and technological and medical advances. Mathematical models are influential for preparing for disease outbreaks(2, 27–34) and modeling of real-time epidemics.(35–39) Moreover, a number of within-host mathematical models have given insight about the dynamics of influenza virus infection and immune responses.(40–48) There are number of models considering the impact of changes in human behavior(15, 16, 49–51) (also see review Ref. [52]). However, there are few models that link information obtained from within-host modeling to the epidemiological model parameters.(43, 53–56) None of the models mentioned have investigated the impact of changes in behavior and infectivity based on within-host data.
In this study, we design mathematical models with multiple infection stages and estimated parameters based on within-host data to investigate the impact of the behavior change on influenza dynamics. We show that the viral shedding dynamics and symptoms during the infection are key features when considering the adoption of precautionary measures during an epidemic.
2. MATERIALS AND METHODS
2.1. Mathematical Model and Parameters
We develop a model to study the dynamics of influenza infection with parameters dependent on the within-host data profiles. The model consists of susceptible (S), infected (I), and recovered (R) individuals. We assume that the infected population is divided into n groups, i.e., where the subscript j = 1, …, n is based on time since infection, that is the age of the infection, which is directly related to viral load shedding. An infected individual progresses from to after 1/pi days, i.e., the average rate of progression is pi, where i = 1, …, n − 1. Individuals recover at the rate pn and gain permanent immunity to that circulating strain of influenza. The infection rate for each stage is denoted by βi, where i = 1, …, n.
In the model, susceptible individuals can change their behavior based on the perceived risk of infection, which might be due to amount of information available through several media sources on emerging influenza epidemics. The fraction of the population that changes its behavior immediately after becoming infected is represented by ρ. Also, infected individuals are likely to change their behavior due to the severity of the disease symptoms. The fraction of the population that alters its behavior at the end of the initial stage, at the onset of symptoms, and at the end of the most symptomatic stage are denoted by μ and ω, respectively. In our model, superscripts B and N represent the individuals that change their behavior and individuals that do not alter their behavior, respectively. Individuals that are taking preventive measures are assumed to have a reduced force of infection by a factor of η.
For simplicity and in agreement with the available within-host data, we consider models with three and four stages, i.e., n = 3 or 4 (further discussed below). The mathematical model for the three-stage infection (n = 3) with behavior changes is given by the following system of equations:
| (1) |
A mathematical model for the four-stage infection with behavior changes can be easily extended from the three-stage model given by Eq. (1), by adding additional groups of infectives, and the fraction of the population that alters its behavior when moving to the last stage of the disease, γ. Schematic diagrams of the three-stage and four-stage model are shown in Figures 1 and 2, respectively.
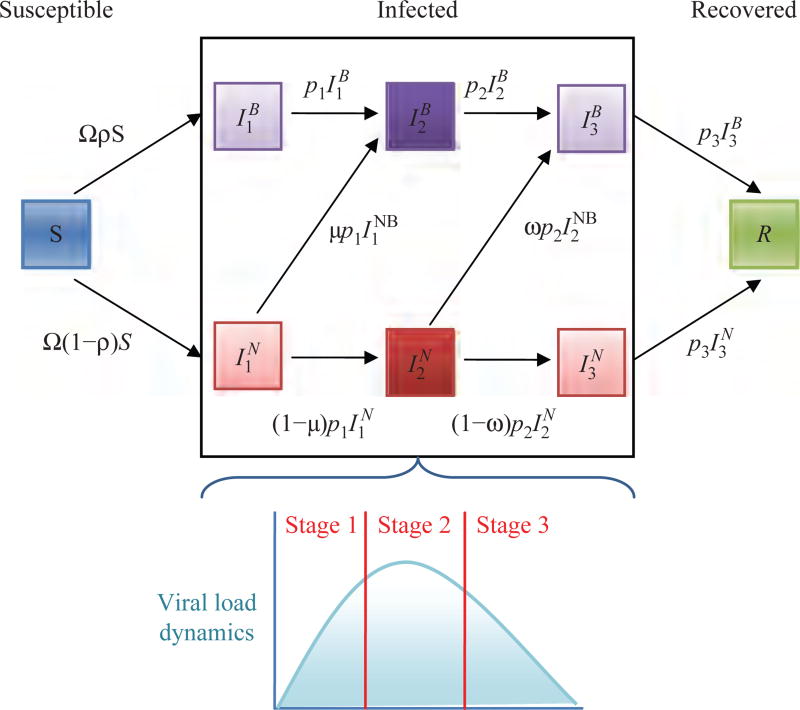
Fig. 1.
Schematic illustration of the three-stage model given by Eq. (1), where and the superscripts B and N represent individuals that changed their behavior and individuals that did not change their behavior, respectively.
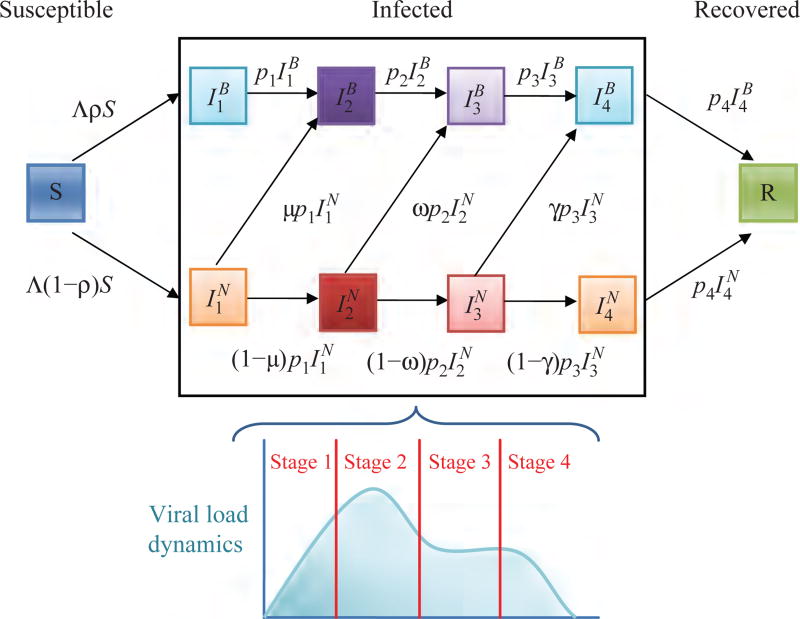
Fig. 2.
Schematic illustration of the four-stage model given by extending Eq. (1), where and the superscripts B and N represent individuals that changed their behavior and individuals that did not change their behavior, respectively.
Following Ref. [25], we assumed that the population is fully susceptible since we are interested in analyzing an outbreak; however, this assumption may be changed by lowering the initial susceptible population by a desired factor. We assume that the fraction of the population that adjusts its behavior (ρ, μ, ω, and γ) is between 0 and 1; where 0 means no one changed their behavior and 1 means that the whole population changed their behavior. Similarly, the reduction in infection rate, due to the behavior change (η), is assumed to be between 0 and 1; where 0 represents no reduction in the number of contacts and 1 stands for complete isolation. Variables and parameters of three-stage and four-stage models are summarized in Tables I and II.
Table I.
Variables of the three and four-stage models.
| Variable | Definition | |
|---|---|---|
| S | Number of susceptible individuals | |
|
|
Number of infected individuals not changing the behavior at the infection-stage j for j = 1, 2, and 3 for the three-stage model and j = 1, 2, 3, and 4 for the four-stage model | |
|
|
Number of infected individuals changing the behavior at the infection-stage j for j = 1, 2, and 3 for the three-stage model and j = 1, 2, 3, and 4 for the four-stage model | |
| R | Number of recovered individuals |
Table II.
Parameter definitions and values.
| Symbol | Definition | Unit | Baseline | Range | Reference |
|---|---|---|---|---|---|
| N | Total population | People | 1 million | 0–300 million | [11] |
| 1/N | Initially infected fraction of the population | 1 | 0.00001 | 0–1 | [11] |
| initial value: | |||||
| βj | Infection rate | People−1 Day−1 | For three-stage: | 0–1 | [11, 58, 63, 64] |
| β2 = 0.23 × 10−6 | See text | ||||
| β1 = β3 = 0.4 × β2 | |||||
| For four-stage: | |||||
| 1/pj | Duration of the stage j | Days | 1/p1 = 1 | 0–7 | See text |
| For three-stage: | |||||
| 1/p2 = 4, 1/p3 = 2 | |||||
| For four-stage: | |||||
| 1/p2 = 1/p3 = 1/p4 = 2 | |||||
| ρ, μ, ω, γ | Fraction of the population that changed the behavior immediately after the infection, or during stages 1, 2, or 3, respectively | 1 | 0.2 | 0–1 | See text |
| η | Reduction in infection rate due to the behavior change | 1 | 0.2 | 0–1 | See text |
2.2. Estimation of Within-Host Parameters
In order to obtain a biologically realistic relation of viral load to symptoms and infectivity, we considered the data published in Ref. [41]. Further details about the data and the design of the experiment can be found in the original manuscript;(57) in short, the study consists of daily nasal washes of six volunteers injected with 104.2TCID50 of cloned wild-type influenza A/Hong Kong/123/77 (H1N1). We assumed that the infectivity and symptoms vary with the age of infection, as they are proportional to the amount of viral shedding.
We consider a profile of the viral load based on Ref. [41] to estimate the length of the different infectivity stages. For the three-stage model, we divide the viral load profile into three stages with lengths 1, 4, and 2 days, respectively (Fig. 1). The first phase is the stage before the viral peak, the second stage contains the viral peak, and the third stage is the continuation of the viral load decline. To obtain the infectivity at each stage, we take the second phase of the three-stage model as a baseline infection rate (β = β2) and express the infection rates in the remaining stages in terms of β, which results in the first and third phase to have the infection rate of β1 = β3 = 0.4 × β (see Fig. 1 and Table II). The resulting infectivity profiles are similar to the baseline values used in Ref. [25] taken from Ref. [58].
Following the bimodal behavior of viral load of the data, the mathematical model presented in Ref. [48], and studies of influenza virus infection,(59, 60) we partitioned the profile of viral load into four stages with lengths 1, 2, 2, and 2 days, respectively for the four-stage model (Fig. 2). The first phase is the stage before the viral peak, the second stage contains the viral peak, the third stage is the viral plateau or a second minor peak, and the fourth is the viral load decline. Similar to the three-stage model, we estimate the infection rates as follows: β2 = 1.25 × β, β1 = β4 = 0.4 × β, and β3 = 0.75 × β (see Fig. 2 and Table II).
2.3. Basic Reproductive Ratio
The basic reproductive ratio (ℜ0) is the average number of new infections caused by a single case in a fully susceptible population. Typically, if ℜ0 > 1 then an epidemic will arise and if ℜ 0 < 1 then an epidemic will not occur.
We used the Next Generation Method to find ℜ0.(61) We let F be the matrix of new infections and V the matrix of transfers between groups evaluated at the infection-free steady state (S0 = N and ). ℜ0 is given by the dominant eigenvalue of FV−1 evaluated at the infection-free steady state.(61)
For the three-stage model, given by Eq. (1), the basic reproductive ratio (ℜ0) is given by the expression:
| (2) |
The basic reproductive ratio for the four-stage model can be similarly calculated using the Next Generation Method(61) and it is given by
| (3) |
Notice that when there is no behavior change, the basic reproductive number for the three-staged model can be expressed as .
In the Results section, we discuss the dependence of the basic reproductive ratio on the model parameters.
3. RESULTS
3.1. Impact of Behavior Change on Basic Reproductive Ratio
The basic reproductive ratios for three and four-stage models, ℜ0 and , given by Eqs. (2) and (3), respectively, depend on the fraction of the population that changed their behavior immediately after the infection, during stages 1, 2, and 3 (ρ, μ, ω, and γ, respectively); the reduction in infection rate due to the behavior change (η), and other parameters. It also follows that the basic reproductive ratio also depends on the infection rate, which changes based on the number of contacts and transmissibility of the influenza strain. In Table III we tested the impact of ρ, μ, ω, and γ on the basic reproductive ratios for three and four-stage models.
Table III.
Impact of the fraction of the population that changed their behavior immediately after the infection, or during stages 1 or 2, (ρ, μ, and ω), respectively, on the basic reproductive ratio of three and four-stage models, ℜ0 and , respectively.
| Parameter variations | ℜ0 for the three-stage model |
for the four-stage model |
||
|---|---|---|---|---|
|
|
|
|
||
| η = 20% | Baseline* | Special case** |
Baseline* | Special case** |
| ρ = μ = ω = γ =0% | 1.196 | 1.426 | 1.196 | 1.426 |
| ρ = μ = ω = γ =5% | 1.172 | 1.397 | 1.167 | 1.391 |
| ρ = μ = ω = γ =10% | 1.149 | 1.370 | 1.141 | 1.358 |
| ρ = μ = ω = γ =20% | 1.108 | 1.322 | 1.094 | 1.302 |
| ρ = μ = γ =0%, ω =20% | 1.189 | 1.419 | 1.175 | 1.396 |
| μ = ω = γ =0%, ω=20% | 1.148 | 1.369 | 1.148 | 1.369 |
| ρ = ω = γ =0%, ρ =20% | 1.152 | 1.373 | 1.152 | 1.373 |
| ρ = μ = ω =0%, γ =20% | – | – | 1.189 | 1.419 |
|
| ||||
| η = 50% | Baseline* | Special case** | Baseline* | Special case** |
|
| ||||
| ρ = μ = ω = γ =5% | 1.136 | 1.355 | 1.124 | 1.228 |
| ρ = μ = ω = γ =10% | 1.079 | 1.287 | 1.058 | 1.257 |
| ρ = μ = ω = γ =20% | 0.976 | 1.165 | 0.945 | 1.119 |
| ρ = μ = γ =0%, ω =20% | 1.178 | 1.408 | 1.143 | 1.350 |
| μ = ω = γ =0%, ρ=20% | 1.076 | 1.283 | 1.076 | 1.283 |
| ρ = ω = γ =0%, μ =20% | 1.086 | 1.293 | 1.086 | 1.293 |
| ρ = μ = ω =0%, γ =20% | – | – | 1.178 | 1.408 |
Notes:
Parameters given in Table II.
In the special case: for the Three-Stage Model we take the following infection rates: 0.4 × β2, 1.25 × β2, 0.4 × β2 for stages 1, 2, and 3, respectively, and for the Four-Stage Model we take: 0.4 × β2, 1.25 × β2, 1.25 × β2, 0.4 × β2 for stages 1, 2, 3, and 4, respectively. The rest of the parameters are baseline stated in Table II.
We compute the basic reproductive ratio for the baseline (parameter values listed in Table II) and for a special case (parameter values listed under Table III) for each of the models. In the baseline case, ℜ0 and range between 1.0–1.2 (Table III) for various scenarios when 0–20% of individuals altered their behavior. In the special case, infection rates are higher, resulting in a greater basic reproductive number; ℜ0 and range from 1.2 to 1.4. In both of these cases, the basic reproductive ratios for both models are within the ranges presented in Ref. [62].
We conclude that has the highest value when there is no behavior change (Table III), which means that the transmission of the disease is the fastest. In contrast, as more people alter their behavior by reducing number of contacts and following CDC recommendations, the lower the basic reproductive ratio (Table III). For instance, if 20% of individuals in all stages change their behavior, this results in significantly reduced values of ℜ0 and . An important observation from Table III is that when 20% of individuals alter their behavior for the duration of the disease, immediately after the infection or at the end of the first stage of an infection, the results show a lower ℜ0 and , than when the same percentage of individuals adjust their daily routine at the later infection stages.
Moreover, the basic reproductive ratio for the three and four-stage models, ℜ0 and , are given by Eqs. (2) and (3), respectively, depend on p2 (1/p2 is the length of the most symptomatic and infectious phase). In the three-stage model, the most symptomatic phase is assumed to be four days and for the four-stage model it is taken to be two days. Hence, for the range of model parameters (Table II), we obtain (Table III).
3.2. Sustained Behavior Change Due to Media and Upon the Onset of Symptoms Greatly Influences the Spread of Disease
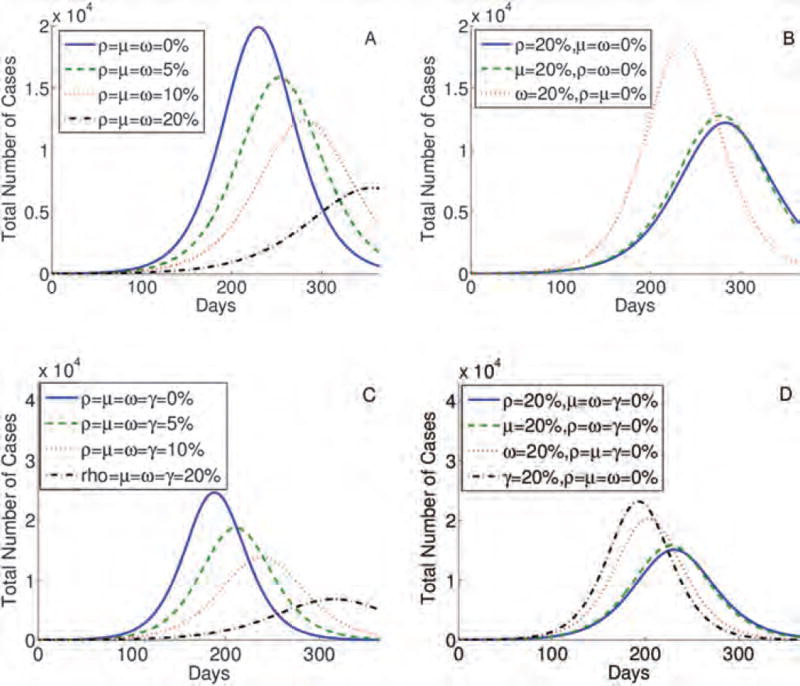
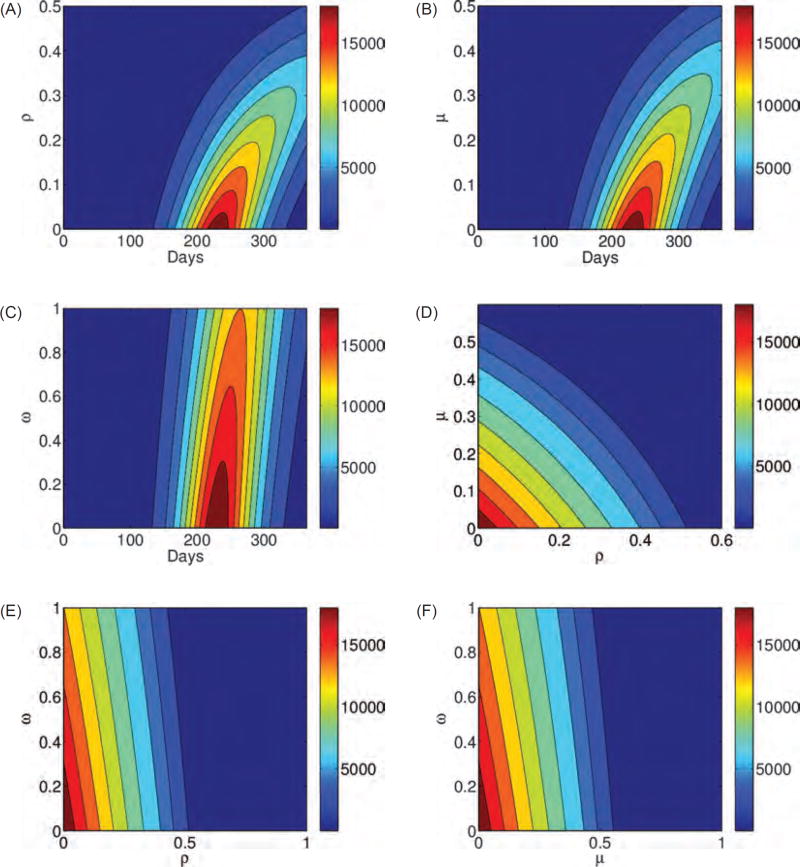
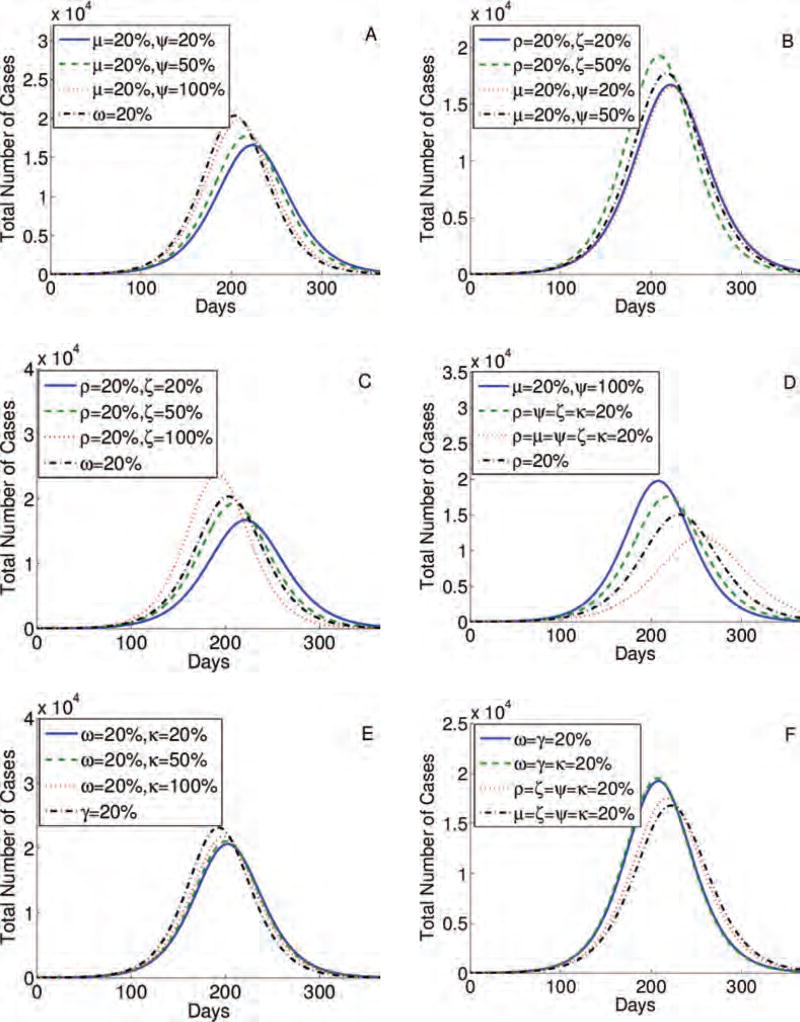
The highest epidemic peak (approximately 2 × 104 cases) is obtained when individuals do not change their behavior (case ρ = μ = ω = 0% in Fig. 3(A)). When 20% of individuals alter their behavior in all stages (ρ = μ = ω = 20% in Fig. 3(A)), the peak is the lowest and the epidemic is delayed the longest, which can give time to develop a vaccine and other preventive strategies. Contour plots presented in Figures 4(A)–(F) show that the percentage of individuals altering their behavior immediately after the infection (ρ), at the end of the first stage, and at the onset of symptoms, (μ). The simulations show that these changes result in a lower epidemic peak than the one obtained by the same percentage of individuals changing their routines if they are implemented in the last stage of infection (ω). In particular, 20% of individuals altering their behavior immediately upon infection or at the onset of symptoms reduce the peak by approximately half (Fig. 3(B)). To conclude, in Figures 3 and 4, we observe that prolonged changes in behavior in these early stages of the infection are more effective than adjustments in daily routine during the last phase of an infection. Results are similar for the four-stage model (Figs. 3(C) and (D)).
Fig. 3.
Simulations for the three-stage (A) and (B) and four-stage (C) and (D) models, testing the impact of the sustained behavior change immediately after the infection, during stages 1, 2, and 3 (ρ, μ, ω, and γ) respectively. The higher the percentage of individuals following CDC recommendations the lower and more delayed epidemic peak (A) and (C). Also, the earlier the behavior is changed and sustained the lower and more delayed epidemic peak (B) and (D).
Fig. 4.
Contour plots A, B, and C of the three-stage model, given by Eq. (1), illustrate the total number of cases as a function of the fraction of the population that changed the behavior immediately after the infection, or during stages 1 or 2, (ρ, μ, and ω), respectively, and sustained these alteration in their daily routine. Contour plots D, E, and F show the peak of the total number of cases as a function of ρ and μ, ρ and ω, ω and μ, respectively. The higher the percentage of individuals following CDC recommendations and the earlier this preventive behavior is employed and sustained the lower and more delayed epidemic peak. Results are similar for the four-stage model.
3.3. Interrupted Behavior Change
We also varied the three and four-stage models by including a reverse change of behavior of infected individuals, who decide to no longer follow CDC recommendations on disease prevention methods (Figs. 5 and 6). The fraction of the population that goes back to the pre-infection daily routine while being in the first stage of the infection, at the onset of symptoms, is denoted by ξ. Similarly, individuals reversing their behavior at the end of the second and third stage of the infection (for the four-stage model) are given by ψ and κ, respectively. These scenarios are important because individuals either working or attending school are only able to take a few days off, which may be insufficiently long to fully recover from influenza infection and they may have to choose which days to miss.
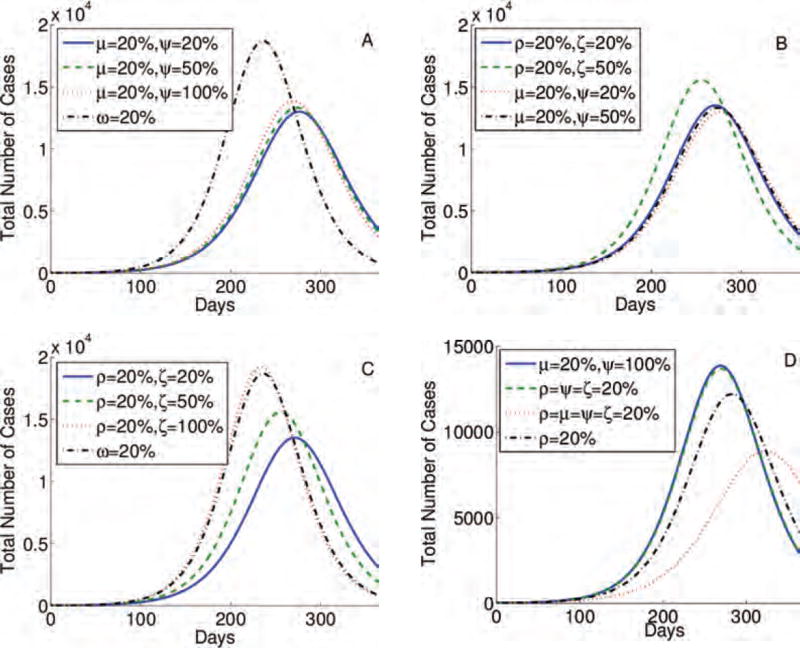
Fig. 5.
Simulations for the three-stage model, given by Eq. (1), testing the impact of the behavior change immediately after the infection, or during stages 1 or 2, (ρ, μ, and ω), respectively, which is interrupted during stages 1 or 2 (ξ and ψ), respectively. We compared variations of behavior changes in different infection stages and their reversions.
Fig. 6.
Simulations for the four-stage model, given by an extension of Eq. (1), testing the impact of the behavior change immediately after the infection, or during stages 1, or 2, or 3 (ρ, μ, ω, and γ), respectively, which is interrupted during stages 1, or 2, or 3 (ξ, ψ, and κ), respectively. We compared variations of behavior changes in different stages and their reversions.
The epidemic peak is lower and more delayed when the same percentage of individuals changes its behavior at the onset of symptoms, i.e., they are moving from stage I1 to I2, even if all of them stop following CDC recommendations at the end of the most asymptomatic stage (I2). In addition, this result holds even when the same percentage of those changing their behavior during the last stages of the infection (I3) (Figs. 5(A) and 6(A)). Moreover, the epidemic peak is similar even when 20% of individuals change their behavior immediately upon infection, most likely due to the media risk communication, and when the same percentage of population alter their schedules at the onset of symptoms, even if 20% of them will resume their daily routines when entering the infection stage that follows (Figs. 5(B) and 6(B)). Epidemic severity is lower when 20% of individuals begin following CDC recommendations immediately upon infection, even if 20% or 50% of them resume their daily schedules at the onset of symptoms (this case may be unlikely), than 20% of individuals changing their daily routines at the end of the most asymptomatic stage (Figs. 5(C) and 6(C)). In Figures 5(D) and 6(D)–(F), we show further combinations of behavior changes and their impact on the time and amplitude of an epidemic peak. We conclude that it is most effective when individuals change their behavior immediately upon infection and when the onset of symptoms begins than during the last stage (s) of an infection.
4. CONCLUSIONS AND DISCUSSION
We showed that behavioral changes greatly influence the dynamics of the spread of influenza. It was shown in Ref. [26] that the epidemic peak is achieved in four weeks when using an SIR model. However, when behavioral changes are introduced, the epidemic peak may be delayed and significantly lowered (see Fig. 3 in Ref. [26] and Figs. 3–6).
Predictions from both models, three and four-stage, show that that prolonged change of behavior in the early stages of the infection is more effective than adjustments in daily routine during the last phase of an infection (Figs. 3 and 4). We also found that the epidemic peak is lowered and further delayed when individuals change their behavior immediately upon infection or at the onset of symptoms, than during the last stage of an infection, even if a certain percentage of the population stops following disease preventive recommendations (Figs. 5 and 6).
Our results regarding basic reproductive numbers for both models suggests that the most optimal strategy is to follow CDC recommendations even before the disease symptoms begin or immediately at the onset of symptoms and sustain these protective activities for the duration of the disease (Table III). Hence, media risk communication plays an important role in warning individuals in asymptomatic stages of an infection to take preventive measures during an outbreak.
Considering spontaneous behavior change in a model is beneficial, especially if the model results are being used to guide public health policies. In addition, behavioral changes can influence disease control strategies and estimation of the epidemiological parameters, thus, epidemiological predictions may be inaccurate if behavior is not considered.
Our results suggest that it is crucial to educate the public about non-pharmaceutical interventions that can reduce their probability of becoming infected. More research is needed about effective communication strategies and compliance by various demographic characteristics.
Acknowledgments
This work is partially supported by an ASPIRE grant from the Office of the Vice President for Research at the University of South Carolina (Kasia A. Pawelek) and a National Science Foundation S-STEM under Grant Number 1259283 (Cristian Salmeron). Research reported in this publication was also partially supported by the Modeling of Infectious Disease Agent Study within the National Institute of General Medical Sciences of the National Institutes of Health under Award Number U01GM097658 (Sara Del Valle). We thank Ruy M. Ribiero and Libin Rong for useful discussions. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References and Notes
- 1.WHO. Influenza (Seasonal) Fact Sheet Nr. 2009;211 [Google Scholar]
- 2.Longini IM, Jr, Halloran ME, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am. J. Epidemiol. 2004;159:623. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- 3.Haber MJ, Shay DK, Davis XM, Patel R, Jin X, Weintraub E, Orenstein E, Thompson WW. Effectiveness of interventions to reduce contact rates during a simulated influenza pandemic. Emerg Infect. Dis. 2007;13:581. doi: 10.3201/eid1304.060828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muennig PA, Khan K. Cost-effectiveness of vaccination versus treatment of influenza in healthy adolescents and adults. Clin. Infect. Dis. 2001;33:1879. doi: 10.1086/324491. [DOI] [PubMed] [Google Scholar]
- 5.Mauskopf JA, Cates SC, Griffin AD, Neighbors DM, Lamb SC, Rutherford C. Cost effectiveness of zanamivir for the treatment of influenza in a high risk population in Australia. Pharmacoeconomics. 2000;17:611. doi: 10.2165/00019053-200017060-00007. [DOI] [PubMed] [Google Scholar]
- 6.Robertson JS, Inglis SC. Prospects for controlling future pandemics of influenza. Virus Res. 2011;162:39. doi: 10.1016/j.virusres.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Preventing the Flu: Good Health Habits Can Help Stop Germs. CDC; Atlanta, Georgia: 2010. [Google Scholar]
- 8.Fung IC, Cairncross S. Effectiveness of handwashing in preventing SARS: A review. Trop Med. Int. Health. 2006;11:1749. doi: 10.1111/j.1365-3156.2006.01734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lau JT, Yang X, Tsui H, Kim JH. Monitoring community responses to the SARS epidemic in Hong Kong: From day 10 to day 62. J. Epidemiol Community Health. 2003;57:864. doi: 10.1136/jech.57.11.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang CS, Wong CY. Factors influencing the wearing of facemasks to prevent the severe acute respiratory syndrome among adult Chinese in Hong Kong. Prev. Med. 2004;39:1187. doi: 10.1016/j.ypmed.2004.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tracht SM, Del Valle SY, Hyman JM. Mathematical modeling of the effectiveness of facemasks in reducing the spread of novel influenza A (H1N1) PLoS One. 2011;5:e9018. doi: 10.1371/journal.pone.0009018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.SteelFisher GK, Blendon RJ, Bekheit MM, Lubell K. The public’s response to the 2009 H1N1 influenza pandemic. New England Journal of Medicine. 2010;362:e65. doi: 10.1056/NEJMp1005102. [DOI] [PubMed] [Google Scholar]
- 13.Del Valle S, Hethcote H, Hyman JM, Castillo-Chavez C. Effects of behavioral changes in a smallpox attack model. Math Biosci. 2005;195:228. doi: 10.1016/j.mbs.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 14.Epstein JM, Parker J, Cummings D, Hammond RA. Coupled contagion dynamics of fear and disease: Mathematical and computational explorations. PLoS One. 2008;3:e3955. doi: 10.1371/journal.pone.0003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poletti P, Caprile B, Ajelli M, Pugliese A, Merler S. Spontaneous behavioural changes in response to epidemics. J. Theor. Biol. 2009;260:31. doi: 10.1016/j.jtbi.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Funk S, Gilad E, Watkins C, Jansen VA. The spread of awareness and its impact on epidemic outbreaks. Proc. Natl. Acad Sci. USA. 2009;106:6872. doi: 10.1073/pnas.0810762106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bootsma MC, Ferguson NM. The effect of public health measures on the 1918 influenza pandemic in U.S. cities. Proc. Natl. Acad. Sci. USA. 2007;104:7588. doi: 10.1073/pnas.0611071104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bish A, Michie S. Demographic and attitudinal determinants of protective behaviours during a pandemic: A review. Br. J. Health Psychol. 2010;15:797. doi: 10.1348/135910710X485826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slater MD, Rasinski KA. Media exposure and attention as mediating variables influencing social risk judgments. Journal of Communication. 2005;55:810. [Google Scholar]
- 20.CDC. Flu Symptoms and Severity. CDC; Atlanta, Georgia: 2011. [Google Scholar]
- 21.Hayden FG, Fritz R, Lobo MC, Alvord W, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza a virus infection. Relation to symptom formation and host defense. J. Clin. Invest. 1998;101:643. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fritz RS, Hayden FG, Calfee DP, Cass LM, Peng AW, Alvord WG, Strober W, Straus SE. Nasal cytokine and chemokine responses in experimental influenza a virus infection: Results of a placebo-controlled trial of intravenous zanamivir treatment. J. Infect. Dis. 1999;180:586. doi: 10.1086/314938. [DOI] [PubMed] [Google Scholar]
- 23.Skoner DP, Gentile DA, Patel A, Doyle WJ. Evidence for cytokine mediation of disease expression in adults experimentally infected with influenza a virus. J. Infect. Dis. 1999;180:10. doi: 10.1086/314823. [DOI] [PubMed] [Google Scholar]
- 24.Carrat F, Vergu E, Ferguson NM, Lemaitre M, Cauchemez S, Leach S, Valleron AJ. Time lines of infection and disease in human influenza: A review of volunteer challenge studies. Am. J. Epidemiol. 2008;167:775. doi: 10.1093/aje/kwm375. [DOI] [PubMed] [Google Scholar]
- 25.Carrat F, Luong J, Lao H, Salle AV, Lajaunie C, Wackernagel H. A ‘small-world-like’ model for comparing interventions aimed at preventing and controlling influenza pandemics. BMC Med. 2006;4:26. doi: 10.1186/1741-7015-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poletti P, Ajelli M, Merler S. The effect of risk perception on the 2009 H1N1 pandemic influenza dynamics. PLoS One. 2011;6:e16460. doi: 10.1371/journal.pone.0016460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke DS. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germann TC, Kadau K, Longini IM, Jr, Macken CA. Mitigation strategies for pandemic influenza in the United States. Proc. Natl. Acad. Sci. USA. 2006;103:5935. doi: 10.1073/pnas.0601266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu JT, Riley S, Fraser C, Leung GM. Reducing the impact of the next influenza pandemic using household-based public health interventions. PLoS Med. 2006;3:e361. doi: 10.1371/journal.pmed.0030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciofi degli Atti ML, Merler S, Rizzo C, Ajelli M, Massari M, Manfredi P, Furlanello C, Scalia Tomba G, Iannelli M. Mitigation measures for pandemic influenza in Italy: An individual based model considering different scenarios. PLoS One. 2008;3:e1790. doi: 10.1371/journal.pone.0001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davey VJ, Glass RJ, Min HJ, Beyeler WE, Glass LM. Effective, robust design of community mitigation for pandemic influenza: A systematic examination of proposed US guidance. PLoS One. 2008;3:e2606. doi: 10.1371/journal.pone.0002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halloran ME, Ferguson NM, Eubank S, Longini IM, Jr, Cummings DA, Lewis B, Xu S, Fraser C, Vullikanti A, Germann TC, Wagener D, Beckman R, Kadau K, Barrett C, Macken CA, Burke DS, Cooley P. Modeling targeted layered containment of an influenza pandemic in the United States. Proc. Natl. Acad. Sci. USA. 2008;105:4639. doi: 10.1073/pnas.0706849105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merler S, Ajelli M, Rizzo C. Age-prioritized use of antivirals during an influenza pandemic. BMC Infect Dis. 2009;9:117. doi: 10.1186/1471-2334-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balcan D, Hu H, Goncalves B, Bajardi P, Poletto C, Ramasco JJ, Paolotti D, Perra N, Tizzoni M, Van den Broeck W, Colizza V, Vespignani A. Seasonal transmission potential and activity peaks of the new influenza A(H1N1): A Monte Carlo likelihood analysis based on human mobility. BMC Med. 2009;7:45. doi: 10.1186/1741-7015-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gojovic MZ, Sander B, Fisman D, Krahn MD, Bauch CT. Modelling mitigation strategies for pandemic (H1N1) CMAJ. 2009;181:673. doi: 10.1503/cmaj.091641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baguelin M, Hoek AJ, Jit M, Flasche S, White PJ, Edmunds WJ. Vaccination against pandemic influenza A/H1N1v in England: A real-time economic evaluation. Vaccine. 2010;28:2370. doi: 10.1016/j.vaccine.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Lee BY, Brown ST, Korch GW, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, Grefenstette JJ, Bailey RR, Assi TM, Burke DS. A computer simulation of vaccine prioritization, allocation, and rationing during the 2009 H1N1 influenza pandemic. Vaccine. 2010;28:4875. doi: 10.1016/j.vaccine.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ajelli M, Merler S, Pugliese A, Rizzo C. Model predictions and evaluation of possible control strategies for the 2009 A/H1N1v influenza pandemic in Italy. Epidemiol. Infect. 2011;139:68. doi: 10.1017/S0950268810001317. [DOI] [PubMed] [Google Scholar]
- 40.Saenz RA, Quinlivan M, Elton D, Macrae S, Blunden AS, Mumford JA, Daly JM, Digard P, Cullinane A, Grenfell BT, McCauley JW, Wood JL, Gog JR. Dynamics of influenza virus infection and pathology. J. Virol. 2010;84:3974. doi: 10.1128/JVI.02078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baccam P, Beauchemin C, Macken CA, Hayden FG, Perelson AS. Kinetics of influenza a virus infection in humans. J. Virol. 2006;80:7590. doi: 10.1128/JVI.01623-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Handel A, Longini IM, Jr, Antia R. Towards a quantitative understanding of the within-host dynamics of influenza a infections. J. R. Soc. Interface. 2010;7:35. doi: 10.1098/rsif.2009.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handel A, Longini IM, Jr, Antia R. Neuraminidase inhibitor resistance in influenza: Assessing the danger of its generation and spread. PLoS Comput. Biol. 2007;3:e240. doi: 10.1371/journal.pcbi.0030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao H, Hollenbaugh JA, Zand MS, Holden-Wiltse J, Mosmann TR, Perelson AS, Wu H, Topham DJ. Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza a virus. J. Virol. 2007;84:6687. doi: 10.1128/JVI.00266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith AM, Adler FR, Perelson AS. An accurate two-phase approximate solution to an acute viral infection model. J. Math. Biol. 2010;60:711. doi: 10.1007/s00285-009-0281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J. Virol. Methods. 1998;74:57. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 47.Smith AM, Ribeiro RM. Modeling the viral dynamics of influenza a virus infection. Crit. Rev. Immunol. 2010;30:291. doi: 10.1615/critrevimmunol.v30.i3.60. [DOI] [PubMed] [Google Scholar]
- 48.Pawelek KA, Huynh GT, Quinlivan M, Cullinane A, Rong L, Perelson AS. Modeling within-host dynamics of influenza virus infection including immune responses. PLoS Comput. Biol. 2012;8:e1002588. doi: 10.1371/journal.pcbi.1002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poletti P, Ajelli M, Merler S. Risk perception and effectiveness of uncoordinated behavioral responses in an emerging epidemic. Math. Biosci. 2012;238:80. doi: 10.1016/j.mbs.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Del Valle SY, Mniszewski SM, Hyman JM. Modeling the impact of behavior changes on the spread of pandemic influenza, Modeling the Interplay Between Human Behavior and Spread of Infectious Diseases. Springer-Verlang; New York: 2013. pp. 59–77. [Google Scholar]
- 51.Pawelek KA, Oeldorf-Hirsch A, Rong L. Modeling the impact of twitter on influenza epidemics. Mathematical Biosciences and Engineering. 2014;11:1337. doi: 10.3934/mbe.2014.11.1337. [DOI] [PubMed] [Google Scholar]
- 52.Funk S, Salathe M, Jansen VA. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface. 2010;7:1247. doi: 10.1098/rsif.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andre JB, Ferdy JB, Godelle B. Within-host parasite dynamics, emerging trade-off, and evolution of virulence with immune system. Evolution. 2003;57:1489. doi: 10.1111/j.0014-3820.2003.tb00357.x. [DOI] [PubMed] [Google Scholar]
- 54.Antia R, Levin BR, May RM. Within-host population dynamics and the evolution and maintenance of microparasite virulence. The American Naturalist. 1994;144:457. [Google Scholar]
- 55.Ganusov VV, Bergstrom CT, Antia R. Within-host population dynamics and the evolution of microparasites in a heterogeneous host population. Evolution. 2002;56:213. doi: 10.1111/j.0014-3820.2002.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 56.Gilchrist MA, Sasaki A. Modeling host-parasite coevolution: A nested approach based on mechanistic models. J. Theor. Biol. 2002;218:289. doi: 10.1006/jtbi.2002.3076. [DOI] [PubMed] [Google Scholar]
- 57.Murphy BR, Rennels MB, Douglas RG, Jr, Betts RF, Couch RB, Cate TR, Jr, Chanock RM, Kendal AP, Maassab HF, Suwanagool S, Sotman SB, Cisneros LA, Anthony WC, Nalin DR, Levine MM. Evaluation of influenza A/Hong Kong/123/77 (H1N1) ts-1A2 and cold-adapted recombinant viruses in seronegative adult volunteers. Infect. Immun. 1980;29:348. doi: 10.1128/iai.29.2.348-355.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cauchemez S, Carrat F, Viboud C, Valleron AJ, Boelle PY. A bayesian MCMC approach to study transmission of influenza: application to household longitudinal data. Stat. Med. 2004;23:3469. doi: 10.1002/sim.1912. [DOI] [PubMed] [Google Scholar]
- 59.Murphy BR, Chanock RM, Clements ML, Anthony WC, Sear AJ, Cisneros LA, Rennels MB, Miller EH, Black RE, Levine MM, Betts RF, Douglas RG, Jr, Maassab HF, Cox NJ, Kendal AP. Evaluation of A/Alaska/6/77 (H3N2) cold-adapted recombinant viruses derived from A/Ann Arbor/6/60 cold-adapted donor virus in adult seronegative volunteers. Infect. Immun. 1981;32:693. doi: 10.1128/iai.32.2.693-697.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jao RL, Wheelock EF, Jackson GG. Production of interferon in volunteers infected with Asian influenza. J. Infect. Dis. 1970;121:419. doi: 10.1093/infdis/121.4.419. [DOI] [PubMed] [Google Scholar]
- 61.van den Driessche P, Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29. doi: 10.1016/s0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 62.Longini IM, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings DA, Halloran ME. Containing pandemic influenza at the source. Science. 2005;309:1083. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- 63.Chowell G, Ammon CE, Hengartner NW, Hyman JM. Transmission dynamics of the great influenza pandemic of 1918 in Geneva, Switzerland: Assessing the effects of hypothetical interventions. J. Theor. Biol. 2006;241:193. doi: 10.1016/j.jtbi.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Del Valle SY, Hyman JM, Hethcote HW, Eubank SG. Mixing patterns between age groups in social networks. Social Networks. 2007;29:539. [Google Scholar]