Abstract
Gene therapies have great potential in regenerative medicine; however, clinical translation has been inhibited by low stability and limited transfection efficiencies. Herein, we incorporate collagen-mimetic peptide (CMP)-linked polyplexes in collagen scaffolds to increase DNA stability by up to 400% and enable tailorable in vivo transgene expression at 100-fold higher levels and 10-fold longer time periods. These improvements were directly linked to a sustained interaction between collagen and polyplexes that persisted during cellular remodeling, polyplex uptake, and intracellular trafficking. Specifically, incorporation of CMPs into polyethylenimine (PEI) polyplexes preserved serum-exposed polyplex-collagen activity over a period of 14 days, with 4 orders-of-magnitude more intact DNA present in CMP-modified polyplex-collagen relative to unmodified polyplex-collagen after a 10 day incubation under cell culture conditions. CMP-modification also altered endocytic uptake, as indicated by gene silencing studies showing a nearly 50% decrease in transgene expression in response to caveolin-1 silencing in modified samples versus only 30% in unmodified samples. Furthermore, cellular internalization studies demonstrated that polyplex-collagen association persisted within cells in CMP polyplexes, but not in unmodified polyplexes, suggesting that CMP linkage to collagen regulates intracellular transport. Moreover, experiments in an in vivo repair model showed that CMP modification enabled tailoring of transgene expression from 4 to 25 days over a range of concentrations. Overall, these findings demonstrate that CMP decoration provides substantial improvements in gene retention, altered release kinetics, improved serum-stability, and improved gene activity in vivo. This versatile technique has great potential for multiple applications in regenerative medicine.
Keywords: Non-viral gene delivery, collagen, collagen-like peptides, collagen-mimetic peptides
Graphical Abstract
Introduction
Regenerative medicine has the potential to restore function to damaged tissues or organs, and its promise has prompted the development of a wide range of biomaterials and drug delivery approaches [1–8]. To guide complicated remodeling and repair processes, biomaterial-based scaffolds are commonly employed to provide a framework for cellular growth and tissue deposition [9–12]. While traditional scaffolds have been engineered with emphasis on providing biocompatible, structural support, focus in recent years has shifted towards materials designs with the capacity to guide more complex aspects of reparative processes [11–15]. To serve as good analogues of the extracellular matrix (ECM), scaffolds should act as cell-responsive structures capable of dynamically interacting with cells during cellular ingrowth, proliferation, and phenotypic commitment. For this purpose, the stable incorporation and controllable delivery of bioactive cues, such as therapeutic proteins or DNA, are essential [10, 12, 16, 17].
The ECM serves as a natural reservoir for bioactive cues such as growth factors (GFs), whose release and activity are controlled by both ECM affinity and ECM turnover. ECM turnover is a tightly-regulated process that consists of two primary steps: protease-mediated ECM degradation and endocytic uptake of the resulting ECM fragments. The ECM undergoes continuous remodeling; however turnover rates are elevated during development and regeneration, and can also occur in response to signals transmitted from ECM receptors or ECM-modifying proteins such as matrix metalloproteinases (MMPs) [18–20]. In turn, cell-triggered, localized delivery of ECM-bound cues (e.g. GFs), with tightly regulated and distinct release kinetics, is achieved in direct coordination with other reparative processes [21–24]. Similar mechanisms that exploit ECM remodeling have evolved to enable entry of other moieties and signals into cells with high efficiency. Viruses bind to ECM to enhance their cellular availability, preserve their stability, and increase the chance of internalization via interaction with specific cellular receptors [21, 25, 26], and ECM endocytic pathways are also commonly used to facilitate cellular uptake of viruses via integrin-dependent pathways [21, 25, 26], especially via collagen-binding integrins such as α2β1 [26, 27]. ECM components have even been found to act as bridges that directly facilitate binding and initiate viral internalization [21].
The ability to harness ECM turnover would also have key benefits for synthetic materials engineered to control therapeutic delivery. Enzymatically degradable polymer matrices of both synthetic [13, 14] and natural origin [10, 16, 17] have been designed to confer proteolytic sensitivity such that tissue remodeling and scaffold degradation are synchronized, and these approaches have been shown to dramatically improve therapeutic stability and activity. For instance, matrix metalloproteinase (MMP) degradable polymers [14, 28] and tethers [16, 17] have been used to coordinate the delivery of stabilized GFs such as vascular endothelial growth factor (VEGF) and bone morphogenetic protein 2 (BMP-2) with cellular remodeling during diabetic ulcer healing [1, 16, 29–32] and bone regeneration [10, 33], respectively. Additionally, scaffolds have also been designed to achieve improved, localized gene delivery and thereby provide a compelling alternative for creating GF-rich environments; gene manipulations are less expensive, more stable, and have a proven capacity to elicit improved therapeutic effects with orders of magnitude (~2000-fold) dose reductions compared to those necessary for topical administration of GFs [34]. Furthermore, gene delivery mimics endogenous repair, using host cells to coordinate localized, sustained GF production and in situ, on-demand delivery of nascent proteins with authentic post-translational modifications [35]. These characteristics are of particular value in tissues such as chronic wounds due to their extended healing over months and spatiotemporal heterogeneity [35]. For instance, in vivo studies have demonstrated that incorporation of GF-encoding DNA into both polymer-based [36] and protein-based [37] scaffolds can promote transgene expression over periods ranging from days to weeks, leading to essential reparative processes where no healing or minimal healing was previously observed.
Despite the potential benefits of scaffold-mediated gene transfer, existing materials have been unable to provide sufficient healing activity due to their lack of sufficient in vivo stability and prolonged delivery. Improved delivery strategies thus are vital for clinical translation. Currently, DNA is most commonly incorporated into polymer- and protein-based scaffolds through either simple encapsulation approaches or adsorption methods employing non-covalent interactions between the substrate and the DNA or DNA carrier (e.g. electrostatic, hydrophobic, or van der Waals interactions). Such methods offer simplicity and have the capacity, as noted above, to achieve cell-triggered release through use of protease-degradable materials [11, 13, 28, 38]. However, existing methods for incorporating DNA within biomaterials often fail to retain gene carriers in the delivery site for prolonged periods in vivo and off-target delivery is problematic. For instance, collagen scaffolds containing electrostatically entrapped, PDGF-B-encoding adenovirus accelerated healing in experimental rat wound models, yet vector escape and immunogenicity were apparent and hindered clinical translation [39]. While the use of non-viral gene delivery can potentially minimize immunogenicity concerns, non-viral approaches often fail to induce complete repair in preclinical models [40], and insufficient gene transfer efficacy has been identified as the major limiting factor. Furthermore, most existing technologies for sustained therapeutic delivery are not suitable for many tissue repair applications because of the complexity of the healing process, which can include extended healing periods over months and multiple out-of-phase healing cascades within repair sites. A reliable strategy to retain active gene carriers until cells initiate repair and facilitate efficient gene transfer is essential.
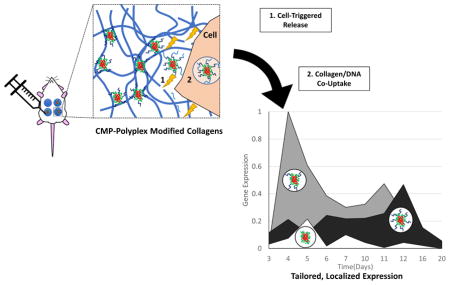
In this work, our goal was to demonstrate that the anchoring of polyplexes via specific interactions with collagen could be used to achieve stable gene expression in vivo by harnessing collagen turnover as a driver for not only gene release, but also enhanced activity. Our studies employ collagen-mimetic peptides (CMPs), which comprise primarily a collagen-like (GXY)n motif and have the unique capacity to stably integrate within native collagen through a reversible strand-exchange process [41–49]. Our prior work was the first to demonstrate that CMP-based integration of polyplexes could be used to retain stabilized DNA on collagen with improved control over the timing and extent of gene delivery for periods of a week to over a month (2-fold longer than that of unmodified polyplexes), and the direct correlation of transgene expression with MMP concentrations. In this work, we evaluated the persistence of the collagen-polyplex interaction and the capacity to drive gene delivery via endocytic collagen turnover. CMP-modified PEI polyplexes were incorporated into collagen gels [50–52] and long-term transfection studies and DNA integrity assays were employed to monitor polyplex efficacy and stability. Collagen-polyplex co-internalization studies and gene silencing experiments were used to assess mechanisms of cellular uptake, particularly via caveolar mechanisms [53, 54]. Additionally, extended studies in murine models were used to determine the feasibility of CMP-based mechanisms for improving gene transfer in vivo, and its potential application in regenerative medicine.
Materials and Methods
Materials
Fmoc-protected amino acids were purchased from Anaspec (Fremont, CA). H-Rink amide ChemMatrix® resin was purchased from PCAS Biomatrix (Quebec, Canada). O-Benzotriazole- N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU) was purchased from Novabiochem (San Diego, CA). High performance liquid chromatography (HPLC)-grade N,N-dimethyl formamide (DMF), acetonitrile, trifluoroacetic acid (TFA), Lipofectamine® RNAiMAX Reagent, and cell culture reagents, including Dulbecco's modified Eagle's medium (DMEM), Opti-MEM® I Reduced Serum Media (Opti-MEM), Dulbecco's phosphate buffered saline (PBS), and trypsin were purchased from Fisher Scientific (Fairlawn, NJ). Fetal bovine serum (FBS) was purchased from Corning (Manassas, MA). Piperidine, 4-methylmorpholine, all cleavage cocktail components, branched PEI (25 kDa), collagen type I-fluorescein isothiocyanate (FITC), and syringes were purchased from Sigma-Aldrich (St. Louis, MO). Type I bovine collagen was purchased from Advanced BioMatrix (San Diego, CA). DNA gel loading dye and Alexa Fluor 350 NHS ester were purchased from Thermo Fisher Scientific (Waltham, MA). pCMV-GLuc plasmid was purchased from New England Biolabs (Ipswich, MA) and pCMV-MetLuc-mem plasmid was purchased from Clontech (Mountain View, CA), and both plasmids were amplified in NEB 5-α electrocompetent E. coli purchased from New England Biolabs. The plasmids were purified from bacterial culture using a Qiagen Megaprep Kit (Valencia, CA), following the manufacturer's protocols. Caveolin-1 siRNA and caveolin-1 Antibody (N-20) was purchased from Santa Cruz Biotechnology (Dallas, Texas) and Goat Anti-Rabbit IgG (Horseradish Peroxidase (HRP)) was purchased from Abcam (Cambridge, MA). Collagenase I was purchased from Worthington Biochemical Corp (Lakewoord, NJ). Growth factor reduced (GFR) phenol red free Matrigel® was purchased from Corning (Corning, NY), BMP-2 was purchased from GenScript (Piscataway, NJ), and coelenterazine was purchased from Clontech and GoldBio (Yorba Linda, CA).
Preparation of Modified Collagen Gels
The GPP-based CMP [GPP: (GPP)3GPRGEKGERGPR(GPP)3GPCCG] GPP was synthesized via Fmoc solid phase peptide synthesis, conjugated to PEI using Michael-type addition chemistry, and subsequently, the GPP-PEI was used to prepare GPP-modified polyplexes as described in [52]. Using a variation of well-established polyplex formation protocols [53, 54], equivolumetric solutions of PEI and DNA suspended in 20 mM HEPES buffer (pH 6.0) were combined to yield a final solution with an amine to phosphate ratio (N:P) ratio of 10. To incorporate the GPP, a specified percent of PEI was replaced with the GPP-PEI conjugate after preincubation at 50°C for 30 minutes to prevent triple-helical hybridization of the GPP. Collagen gels with GPP-immobilized or encapsulated unmodified polyplexes were constructed by re-suspending dehydrated polyplex in neutralized type I bovine collagen-solution (4 mg/mL, pH 7.4) as described [52]. 250 μL of this solution was added to each well of an 8-well plate (0.8 cm2 surface area/well) and incubated for 3 h at 4°C to enable CMP-collagen hybridization then overnight at 37°C to allow for gelation. After visually confirming gelation, the gels were thoroughly washed with PBS and water.
Polyplex-Serum Activity Studies
To determine whether GPP-mediated immobilization enhanced the serum stability of the polyplexes, GPP-PEI polyplex-modified collagen gels and GPP-free PEI polyplex-encapsulating collagen gels were prepared with pCMV-Gluc plasmid. GPP-PEI polyplex-modified gels were created with polyplex containing different amounts of GPP-PEI (10, 20, or 50% GPP-PEI/Total PEI) whereas GPP-free polyplex-encapsulating gels were created similarly but with polyplex containing 0% GPP-PEI/Total PEI. These gels were preincubated under physiologically relevant conditions (37°C, 5% CO2) in media containing a range of serum concentrations (0.25, 10, 20, or 50% (v/v) FBS and 1% penicillinstreptomycin (P/S) for up to 2 weeks. After the specified gel preincubation period, the gels were washed 3 times with PBS and 3 times with DMEM supplemented with 1% P/S and 10% FBS. Subsequently, NIH/3T3 cells were plated at a density of 50,000 cells/cm2 in complete media (DMEM supplemented with 1% P/S and 10% FBS). The cells were allowed to adhere over a period of 6 h before treatment with tumor necrosis factor-alpha (TNF-α) (10 ng/mL), a well-known stimulator of MMP production [55]. Gene expression subsequently was monitored over several days after which cells were recovered by subjecting the gels to a 30 minute collagenase digestion (0.2 mg/mL in serum-free DMEM supplemented with 0.1% BSA) at 37°C and centrifugation for 4 min at 400 x g. As previously reported, elevated MMP activity was confirmed using a SensoLyte® 520 Generic MMP Assay Kit Fluorimetric, following the manufacturer's protocol, and gene expression was monitored via detection of the luminescence of GLuc secreted into the conditioned media, via the Bio-Lux® Gaussia Luciferase Assay.
DNA Stability Experiments
To assess the stability of the DNA incorporated into the collagen scaffolds, an ethidium bromide exclusion assay was performed. Polyplex-containing collagen gels were produced as previously described with 20% GPP-modified polyplex. 20% GPP-modified polyplex were further examined because 20% falls within the middle of the range of the degrees of GPP modification found to significantly enhance transfection in prior work, and could therefore provide insight on the increases in transfection efficiency obersved in polyplex modified with lower (10%) and higher amounts (50%) of GPP. The gels were incubated in media (DMEM supplemented with 1% P/S and 10% FBS) under physiologically relevant conditions (37°C, 5% CO2) for 2 weeks. During the incubation, media was collected from each sample 10, 12, and 14 day after starting the incubation. To examine the stability of the collected DNA, heparin was added to dissociate the polyplexes, as previously described [20]. Equal amounts of recovered DNA (0.1 μg, based on quantification using a spectrophotometric analysis) were analyzed with 1% agarose gels stained with 0.5 μg ethidium bromide per mL of TBE [tris/borate/ethylenediaminetetra-acetic acid (EDTA)] buffer. Forty μL of recovered DNA solution was added to 8 μL of 6x gel loading dye, and 40 μL of the mixture was added to each well. The gels were run at 100 V for 1 h and imaged with a BioRad Gel Doc XR (Brookhaven, CT). To analyze the gel, the ImageJ (National Institutes of Health) measurement tool was used.
Colocalization Studies
To determine if polyplexes co-internalized with collagen fragments during collagen remodeling and polyplex uptake, fluorescence-based colocalization studies were conducted. FITC-labeled collagen was incorporated into the collagen gels (25% FITC-collagen (m/m)), and 25% of the PEI used to form the polyplexes was prelabeled with Alexa Fluor 350 via Michael-type addition chemistry, according to the manufacturer’s protocols. Unreacted dye was removed via dialysis (MWCO: 1000 Da). Otherwise, polyplex-modified collagen gels were prepared as previously described and subsequently preincubated in media supplemented with 10% FBS for a specified period of time ranging up to 2 weeks. NIH/3T3 cells were seeded on the surface of the gels, and after a 4 day incubation under the same conditions in the polyplex-serum activity studies, the gels were subjected to a 30 minute collagenase digestion (0.2 mg/mL in serum-free DMEM supplemented with 0.1% BSA) at 37°C. Cells were recovered by centrifugation for 4 min at 400 x g. Recovered cells were replated into 24-well plates (5000 cells/cm2). Six hours post-plating, the cells were fixed using 4% paraformaldehyde (PFA) for 15 min at room temperature. Additionally, the cells were treated with Trypan blue (10% v/v in PBS) to quench extracellular fluorescence[12]. The cells were imaged using a Leica DMI6000 B inverted microscope (Wetzlar, Germany). Image analysis tools in ImageJ were used to quantify intracellular colocalization of the polyplex and collagen via Mander’s Coefficient (M1) analysis. The results for the 0% and 20% GPP-modified polyplex samples are presented in Fig. 3, while the M1 values calculated for the 10% and 50% samples are within the supplemetal information (Figure S3).
Figure 3.
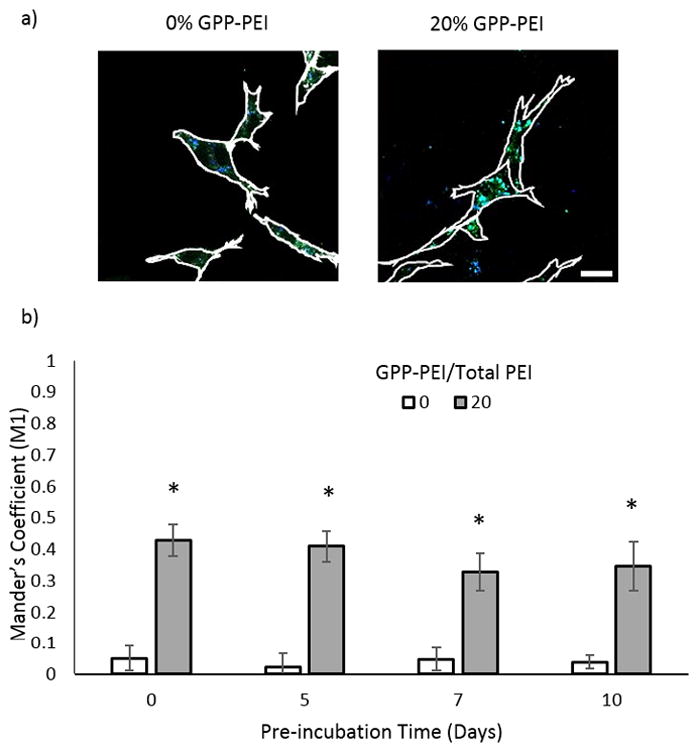
Collagen-polyplex colocalization study. a) Representative fluorescent microcopy images of cells collected from gels preincubated for 5 days. The scale bar is 25 μm. b) Quantitative analysis of intracellular collagen-polyplex association by calculation of Mander’s coefficients for colocalization of FITC-collagen with Alexa Fluor 350-polyplexes in NIH/3T3 cells. The data for the 0, 5, and 7 day preincubations represent the mean +/−standard deviation of 10 separately analyzed cells. For the 10 day preincubation, 5 separately analyzed cells were analyzed.
Caveolin-1 Silencing Studies
To determine whether CMP-modification encouraged cellular uptake via caveolin-1-mediated endocytosis, caveolin-1 silencing studies were conducted. Initially, NIH/3T3 cells were seeded at a density of 80,000 cells/well in 6-well tissue culture treated plates (Corning). After a 24 h recovery, cells were transfected with caveolin-1 siRNA complexes (1 μg siRNA/well) made using a commercial transfection agent (Lipofectamine® RNAiMAX Reagent), according to the manufacturer’s protocols. The complexes were prepared by mixing equal volumes (100 μL each) of the transfection agent solution (6 μL of Lipofectamine RNAiMAX Reagent diluted in 94 μL Opti-MEM) and siRNA solution (1 μg suspended in Opti-MEM) and allowing complexation to occur for 15 minutes. During complexation, the preplated cells were washed with Opti-MEM and covered with a fresh layer of Opti-MEM (800 μL/well). 200 μL of the siRNA complex solution was subsequently added to each well and these solutions were incubated with the cells at 37°C for 6 h. After the incubation period, the cells were washed 3 times with PBS, and fresh DMEM (containing 1% P/S and 10% FBS) was subsequently added. After 24, 48, or 72 h, the extent of gene silencing was assessed by Western blotting using caveolin-1 antibody (N-20) (rabbit polyclonal IgG) and Goat Anti-Rabbit IgG (HRP). As a control, the same procedures were carried out using a non-coding siRNA sequence in place of the caveolin-1 siRNA.
After confirming the extent to which caveolin-1 was silenced, gene transfer studies employing caveolin-1 silencing were conducted. 24 h after transfection with the siRNA complexes, the cells were collected using standard cell recovery protocols involving treatment with trypsin (0.25% v/v). The cells were subsequently seeded onto collagen gels containing pCMV-GLuc and varying amounts of GPP-PEI, and the expression of Gaussia Luciferase was monitored after a 4 day incubation under the same conditions in the polyplex-serum acivity studies via detection of luminescence using a Glomax Multimodal Plate reader (Sigma), as described in [52].
In Vivo Gene Delivery Experiments
An Institutional Animal Care and Use Committee-approved protocol was used for all animal studies. In all studies, male, 8 week-old (CD-1) white mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) were used. Lyophilized polyplex solutions were prepared as previously described using pCMV-MetLuc-mem. pCMV-MetLuc-mem encodes for a membrane-bound luciferase (MetLuc-mem) that displays on the cell surface, making it readily accessible to substrate and available for repeated in vivo imaging. To prepare the polyplexes for injection, the lyophilized polyplexes were re-suspended in Matrigel® solution supplemented with BMP-2 (100 μg DNA/mL and 5 μg BMP-2/mL). The mixtures were vortexed and allowed to incubate on ice for approximately 1.5 h to allow bubbles formed during vortexing to disperse and for strand invasion to occur. A 1 mL sterile syringe was then used to draw up the solutions and the filled syringes were kept on ice to prevent gelation. Mice were then anesthetized using isoflurane and once under anesthesia, the abdomen of the mice were shaven and disinfected with isopropanol. Immediately before injection, the syringes containing the Matrigel® solution were briefly warmed at room temperature and mice were injected subcutaneously with 300 μL of solution into each of four different locations on the abdomen using an 18-gauge needle. A visible pellet immediately formed at each site of injection. Each polyplex-containing pellet contained equal concentrations of Matrigel®, BMP-2, and polyplex, while control solutions included only Matrigel® and BMP-2. To visualize MetLuc-mem expression, images of the mice were obtained using a Caliper In vivo Imaging System Lumina (IVIS®) (Perkin Elmer, Waltham, Massachusetts), after the mice were injected subcutaneously in the vicinity of each pellet with 50 μL of luciferase substrate (coelenterazine). To prepare the substrate solutions, lyophilized coelenterazine was suspended in ethanol (5 mg/mL), and the substrate stock solution was diluted into PBS to a concentration of 0.5 μg/μL immediately before injection. Images of the mice were obtained every minute following substrate injection over a period of 45 minutes until MetLuc-mem expression was no longer detectable. The Living Image® software region-of-interest (ROI) tool was subsequently used to analyze the images and determine when total luminescence within uniform areas surrounding the injection sites plateaued. Total luminescence within the areas surrounding the control pellet with no polyplex was substracted as background within each mouse. Alternatively, the area of expression was defined as the sum of pixels within the unform areas or ROI. The study was replicated in a total of 4 mice.
Data Processing and Statistical Analysis
All studies were performed atleast in triplicate. Statistical analysis was carried out using ANOVA with a pairwise comparisons post hoc test (Tukey Test) with statistical significance accepted at p< 0.05. Significant differences were shown by asterisks in the figures.
Results
Polyplex Activity under Physiological Relevant Conditions
A series of transfection experiments was conducted to determine whether using GPPs to immobilize polyplexes in collagen would impart stability under physiologically relevant conditions over extended periods of time. To simulate the in vivo wound environment, GPP-PEI polyplex-modified collagen gels and GPP-free PEI polyplex-encapsulating collagen gels were prepared and preincubated in media containing increasing amounts of serum for 1, 7, or 14 d. Subsequent to this preincubation, NIH/3T3 cells were plated on the modified collagen gels, treated with TNF-α to induce MMP expression, and allowed to grow for 4 day before reporter protein expression (GLuc) was monitored by analyzing luminescence in conditioned media. The luminescence detected 4 days post-plating is reported in Fig. 1. Maximum expression was detected in samples containing unmodified polyplex (0% GPP-PEI/Total PEI) after a 1 day preincubation period with 0.25% (v/v) FBS. However, the level of expression in the 0% GPP-PEI samples rapidly decreased when the samples were preincubated with higher concentrations of FBS, with a 44% decrease in luminescence when the FBS concentration increased from 0.25% to 10%, an additional 58% decrease when the FBS was increased from 10% to 20%, and a further 75% decrease when the FBS was increased from 20% to 50%. Levels of expression in samples containing unmodified polyplex became undetectable at all preincubation times longer than 1 d.
Figure 1.
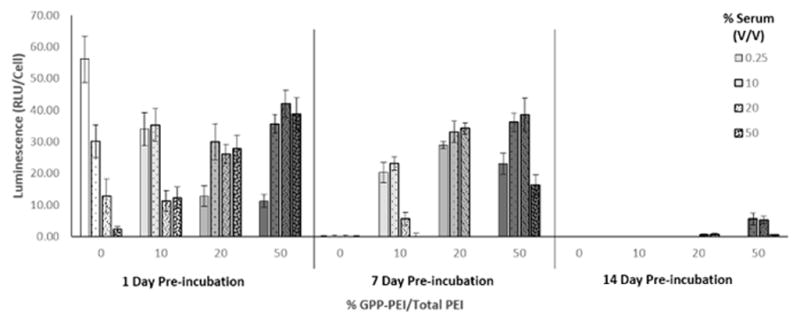
Polyplex activity studies. Polyplex-containing collagen gels were preincubated at 37°C in media containing a range of serum concentrations (0.25%–50% (v/v)) for up to 2 weeks. NIH/3T3 cells were plated onto the preincubated gels and treated with TNF-α to stimulate MMP expression. The data represent the luminescence in the media due to luciferase expression by the cells after 4 d on the gels. Each data point represents the mean +/− standard deviation for a total of eight separately prepared and analyzed samples.
Levels of expression in the GPP-PEI polyplex-modified gels were maintained more consistently in the presence of serum (FBS). In the samples preincubated for 1 d, all of the GPP-modified samples had higher expression levels than the corresponding 0% GPP-PEI/Total PEI samples, with the exception of the samples preincubated in 0.25% FBS; under these conditions, expression in the 0% GPP-PEI samples was approximately 43% greater than the expression levels in any of the GPP-modified samples. After 1 day of preincubation, the 10% GPP-PEI/Total PEI polyplexes had the highest levels of expression when preincubated with either 0.25 or 10% FBS, and expression levels decreased by approximately 33% when these samples were preincubated in media containing either 20% or 50% FBS. The opposite trend was noted in the more extensively modified polyplexes, with the 20% and 50% GPP-PEI/Total PEI polyplexes exhibiting the lowest levels of expression after preincubation with 0.25% (v/v) FBS. The luminescence detected in the 20% and 50% GPP-PEI samples increased approximately 120% and 320%, respectively, when these samples were preincubated with any of the higher concentrations of serum (10%, 20%, or 50%) versus preincubation with 0.25% (v/v) FBS.
After longer preincubation periods, the differences in the levels of expression in samples containing unmodified polyplex (0% GPP-PEI) vs. GPP-modified polyplex became more pronounced. When samples were preincubated for 1 week, expression levels in the unmodified samples were undetectable, whereas in the 10% GPP-PEI/Total PEI polyplex samples, the levels of luminescence remained detectable but decreased approximately 34%, 35%, 50%, and 85% for the 0.25%, 10%, 20%, and 50% FBS (v/v) samples, respectively, as compared with the luminescence in 1 day preincubated samples. In the 0.25% FBS samples modified with 20% and 50% GPP-PEI polyplexes, the levels of expression increased by approximately 51% and 65%, respectively, compared to the corresponding 1 day preincubated samples. Levels of expression were higher when the 20% and 50% GPP-PEI samples were preincubated for 1 week with 10% and 20% FBS, as compared with 0.25% or 50% FBS, and the overall level of expression significantly increased in the 20% GPP-PEI/Total PEI samples when these samples were incubated for 7 day vs. 1 day at these FBS concentrations. After a 14 day preincubation, significant expression was only detected in the 50% GPP-PEI/Total PEI modified gels that were preincubated with 10% and 20% FBS.
DNA Integrity under Physiological Relevant Conditions
To determine if the integrity of the DNA was preserved under physiologically relevant conditions that would be encountered by the scaffold in vivo pre-cellular invasion, DNA was recovered from the media collected from polyplex-containing collagen gels after various serum preincubation periods. The integrity of the DNA was assessed by agarose gel electrophoresis (Fig. 2). The three bands characteristic of plasmid DNA (open circular, linearized, and supercoiled) were visible in each sample collected from the GPP-PEI polyplex-modified gels. These three bands notably faded in intensity when samples were collected from gels that were preincubated for longer time periods. For example, there was a 37% decrease in the plasmid band intensity observed in the 20% GPP-PEI polyplex-modified sample collected after a 12 day incubation, and a 53% decrease in the plasmid band intensity in the sample collected after 14 d, as compared to those in the 10 day sample. Fluorescence was also observed in the wells for each preincubated 20% GPP-PEI sample. In comparison, fluorescence from the three plasmid bands was difficult to detect with the naked eye in the samples collected from collagen gels containing 0% GPP-PEI polyplex, even though the same quantity of DNA was employed in the polyplexes as indicated by spectrophotometric analysis. Based upon image analysis of the bands, the 0% GPP-PEI sample band intensities exhibited decreases of 22% and 53%, respectively, for samples collected after 12 day and 14 day vs. 10 d. Fluorescence/DNA was also observed in the wells of samples collected from the GPP-PEI polyplex-modified gels. DNA immobilization in the wells suggested that complexation of DNA with PEI remained intact despite the addition of heparin, a method commonly used to completely dissociate DNA polyplexes [52].
Figure 2.
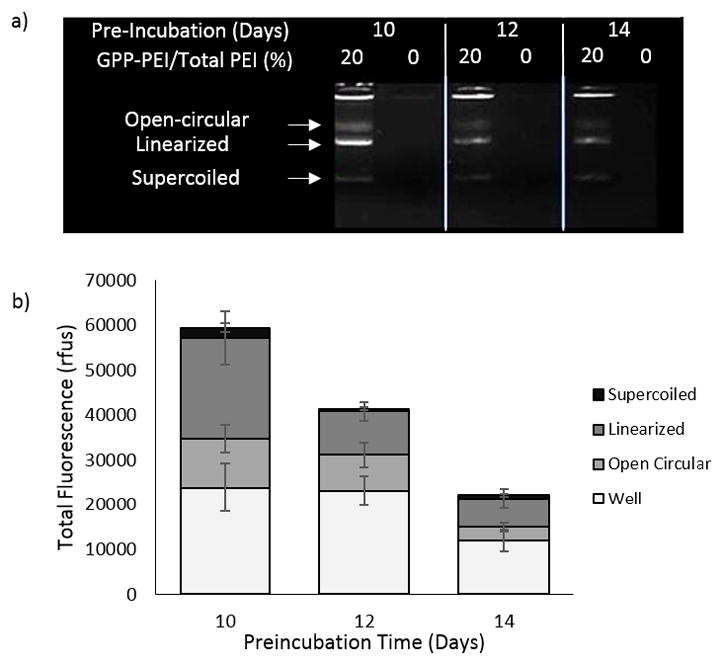
DNA stability analysis. a) Representative agarose gel showing the integrity of DNA recovered from 20% and 0% GPP-PEI/Total PEI polyplex-containing collagen gels after various preincubation time periods in media (DMEM supplemented with 10% FBS b) Quantification of gel fluorescence observed in GPP-modified samples where the mean +/− standard deviation is for a total of three separately prepared and analyzed samples run within the same gel.
Collagen-Polyplex Co-internalization Study
Cell-triggered release and efficient cell-uptake are essential for high efficiency expression in vivo. To determine the extent to which endocytic collagen remodeling was able to drive the gene delivery process in the GPP-PEI polyplex-modified collagen versus the GPP-free polyplex-encapsulated collagen, confocal microscopy was used to examine the cellular co-internalization of polyplex and collagen for 20% GPP-PEI/collagen samples. 20% GPP-PEI/collagen samples were analyzed because the highest levels of transfection were observed within these samples post incubation in 10% FBS supplemented media for up to a 10 day preincubation period. In order to study co-localization of the two materials, FITC-labeled collagen was incorporated into the collagen gels (25% (m/m) FITC-collagen) and 25% of the PEI (m/m) used in the polyplexes was labeled with Alexa Fluor 350. Gels were preincubated in media supplemented with 10% FBS for a specified period of time ranging from 5 days up to 2 weeks; subsequently, NIH/3T3 cells were plated on the preincubated gels, and after a 4 day incubation, the cells were recovered and replated for imaging. Intracellular colocalization of the polyplex and collagen was quantified via Mander’s Coefficient (M1) analysis where M1 ranges from 0 to 1 and is defined as the ratio of the summed intensities of pixels from the blue image (polyplex) for which the intensity in the green channel (collagen) is over zero to the total intensity in the blue channel (polyplex). Therefore, M1 is a good indicator of the share of the blue signal (polyplex) corresponding with a signal in the green channel (collagen) over its total intensity.
As shown in Fig. 3, the Mander’s Coefficient was an order of magnitude greater in samples modified with GPP as compared to the unmodified samples. In the samples containing unmodified polyplex, the Mander’s coefficient indicated insignificant colocalization between the polyplex and collagen after each incubation period, whereas in the samples modified with 20% GPP-PEI polyplex, the Mander’s Coefficient was approximately 0.4 after each incubation period. The Mander’s Coefficient for each sample remained constant, independent of preincubation time. In Fig. 3, representative images of the cells recovered from the collagen gels are presented. Notably, less polyplex uptake was detected in the unmodified samples versus the GPP-modified versus particularly after longer preincubation periods. These findings are consistent with previous data confirming that the unmodified polyplexes were retained for shorter periods, and therefore less polyplex was available for uptake after longer preincubations.
Intracellular Trafficking Study
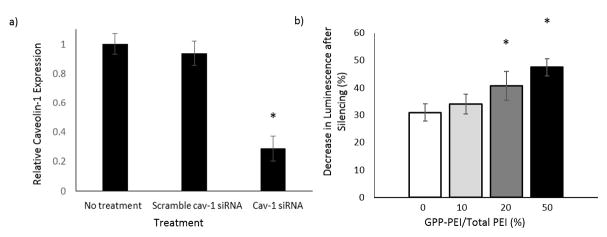
Collagen endocytosis occurs naturally during tissue remodeling through a mechanism involving MMP-mediated release of collagen fragments followed by α2β1-integrin and caveolin-1-regulated uptake [56]. To examine whether GPP-modification and collagen association affected the endocytic uptake of polyplexes, transfection experiments were conducted as previously described, but using NIH/3T3 cells with silenced caveolin-1 expression cultured in media supplemented with collagenase (Fig. 4). Significant reductions in gene expression were observed following caveolin-1 suppression in all samples, suggesting that both the unmodified and GPP-modified polyplexes were internalized via caveolar endocytosis. Notably, the decrease in expression with caveolin-1 silencing was directly dependent upon the amount of GPP-PEI/total PEI incorporated into the polyplex. In both the 20% and 50% GPP-PEI/total PEI polyplex samples, the changes in luminescence after silencing were significantly greater (30% and 53% greater, respectively) than that observed in the unmodified sample.
Figure 4.
Cellular uptake of GPP-modified polyplex post caveolin-1 silencing. a) Caveolin-1 (cav-1) silencing efficiency was determined 2 days post-treatment via western blots. Expression levels relative to a non-treated control were also examined 1 and 3 days post treatment (Fig. S1). b) Changes in polyplex endocytic pathways mediated by GPP-modification and continued association with collagen fragments were elucidated in caveolin-1 silencing transfection experiments. Each data point represents the mean +/− standard deviation for a total of six separately prepared and analyzed samples.
Expression in an in vivo Model
A simple in vivo model was used to test the efficacy of the CMP-based gene transfer approach during tissue repair, using subcutaneous ECM depots to mimic the wound environment. 10% and 50% GPP modified polyplex were employed to determine the impact of varying the degree of CMP modification on expression within an in vivo model over the range of GPP modifications determined to impact transgene expression in the previous in vitro studies. The lack of an excision simplified the model while allowing for the examination of gene expression in a similar environment, containing the same cell types/tissues, and conceivably a minor inflammatory response triggered by the foreign body injection (unmeasured). Matrigel® was employed as the delivery substrate due to its prior successful implementation in both wound and bone healing studies and its potential to hybridize with CMPs due to its high collagen content [37] As observed in Fig. 5b–c, insignificant levels of luminescence were detected, at each of the time points, in the pellets devoid of polyplex within the representative mouse. Meanwhile, in the samples containing unmodified polyplex, only short periods (2–3 d) of low-level luminescence were detected at 2 to 4 days post-injection. Prolonged, localized expression was observed only in pellets containing GPP-modified polyplex. Expression was observed within the pellets for periods of 16 days and 20 days in the 10% and 50% GPP-modified polyplex samples, respectively. Furthermore, the initial area over which expression was observed at 4 days post-injection was approximately 70% smaller in the 50% versus the 10% GPP-PEI polyplex sample. In addition to being smaller, the expression in the 50% GPP-PEI sample remained consistently localized over the entire time period over which expression was detectable. While the observed expression appeared markedly more localized and of longer duration in the 50% GPP-PEI polyplex samples as compared to the 10% samples, the overall expression levels were significantly higher in the 10% GPP-PEI polyplex. For instance, in the represenative mouse, expression was first detected in the 10% GPP-PEI sample on the same day the maximum level of expression was detected, e.g. 4 days post-injection. A week after injection, the levels of expression in the 10% GPP-PEI samples had decreased by more than 75% and expression levels became insignificant by day 16. In the 50% GPP-PEI samples, expression was overall more consistent from day to day. Expression was first detected on day 3 and remained relatively consistent until day 10 before reaching a maximum level of expression on day 11 and then decreasing. The maximum luminescence detected in the 50% GPP-PEI sample was approximately half that detected in the 10% GPP-PEI sample. In Fig. 5d, the replicates are reported as a median value in order to clearly demonstrate the spread of replicates, while highlighting the consistant capacity of CMP-modification to achieve tailorable, sustained expression within a collagen-based scaffold. Maxiumum transgene expression was simulary detected within the 10% GPP-PEI polyplex samples within the first week post injection and determined to be approximately 80% that detected in the 50% GPP-PEI polyplex sample. Expression in the rapidly decreased within the 10% sample after a week period, decreasing by nearly 70% by day 14, while maximum expression was not achieved in the 50% sample until day 12. Additionally, the area in which expression was observed (Fig. 5c and d), was determined to be overall smaller and more consistent in the 50% GPP modified polyplex samples versus the 10% modified polyplex over the duration of the work. For instance, within the representative mouse, the maximum area of expression in the 50% sample was over 3.1-fold greater than that overserved in the 10% sample. The 0% areas of expression were not included due to their much lower values relative to the GPP modified samples.
Figure 5.
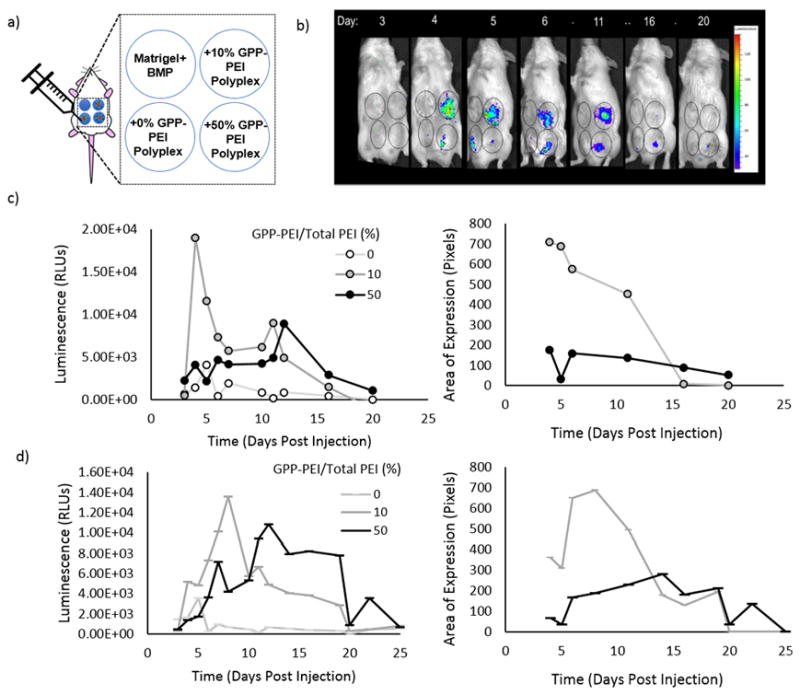
In vivo application of CMP-modified polyplex. a) Schematic indicating the location and contents of each subcutaneous pellet on the abdomens of CD-1 mice. Each solution contained MatrigelTM, BMP-2, and polyplex where indicated, and the solutions formed visible pellets immediately after injection. DNA encoded for a membrane bound form of Metridia Luciferase to permit in vivo imaging. b) In vivo images of a representative mouse at various time points post injection, taken using an IVIS (Exposure: 5 s, Binning: Large, f/stop: 1) indicating luminescence over a maximum period of 20 d. c) Quantification of transgene expression and the area over which expression was identified. This study was replicated in four mice (SI).
Discussion
Tissue repair is a complicated process largely driven by dynamic ECM remodeling. In localized delivery applications, particularly those aimed at tissue repair, substrate-mediated DNA delivery offers many advantages through its improved capacity to control retention/release of gene cargoes both spatially and temporally, as well as to increase serum-stability through steric inhibition [13, 38]. Furthermore, delivery systems can be purposefully engineered to synchronize cell-mediated production of healing factors with natural tissue remodeling/healing via design/application of cell responsive-materials. Accordingly, the properties of many native, dynamic ECM proteins, such as collagen, make them attractive delivery substrates; however, substrate-mediated gene delivery approaches continue to face major obstacles when applied in healing tissues due to the exceptionally harsh, nuclease-rich environment and prolonged repair periods, which can extend over weeks to months. To address these issues, we sought to use triple-helix strand invasion to integrate gene cargoes into collagen matrices to both abrogate DNA losses due to nuclease degradation, and directly harness native endocytic repair processes for improved gene localization, optimal release kinetics, and efficient intracellular trafficking. Our previous experiments demonstrated the ability to tailor the release/retention of PEI polyplex from collagen, one of the most well-established, natural biomaterials, through the incorporation of different amounts of adjustable CMP-linkers into the polyplex [52], and also showed enhancements in transfection efficiency of GPP-PEI polyplexes delivered from collagen. In this work, we demonstrate the utility of the CMP materials in vivo to harness the tissue remodeling process to trigger gene stabilization and delivery. Our results indicate the benefits of the CMP approach for producing enhanced, prolonged, and better localized gene expression in an in vivo model.
During tissue repair, collagen remodeling proceeds through processes regulated by MMP-1, α2β1 integrin, and caveolin-1 [56]. Our prior studies showed that significant transfection was contingent on the stimulation of MMP expression [52], providing the first evidence of cell-responsive delivery via collagen remodeling mechanisms. Furthermore, the improvement in transfection efficiency in the GPP-PEI polyplex-modified gels compared to the unmodified polyplex gels was vastly enhanced when gels were preincubated under physiologically relevant conditions in the presence of serum over long periods of time, indicating fundamental differences in the surface properties and stability of the polyplexes by virtue of their interactions with collagen. In particular, transfection studies with gels preincubated in serum concentrations of 0.25–50% (v/v) FBS over time periods ranging from 1 day up to 2 weeks demonstrated that GPP-PEI incorporation had a significant effect on gene expression as a function of both time and serum concentration (Fig. 1). For example, expression levels rapidly decreased when the unmodified structures were incubated with higher serum concentrations and for longer periods, and for preincubation periods over a week, no significant expression was detected. In contrast, significant levels of expression were observed in the GPP-PEI polyplex samples even after a week-long preincubation period when the polyplex contained 10%–50% GPP/Total PEI, and the 50% GPP-PEI/Total PEI samples exhibited marked expression even after a 2-week long preincubation with serum. Other matrix-mediated gene delivery approaches have demonstrated the capacity to facilitate expression for similar periods[38, 57, 58], however the capacity to preserve polyplex activity under physiologically relevant conditions (high serum concentrations at 37°C) before cell invasion as demonstrated by our preincubation studies, has largely been unstudied. Similar studies may serve as a useful predictor of gene-activated matrix stability in applications in which cells are not initially encapsulated within the matrix and cellular invasion is delayed such as wound repair or tissue engineering.
Differences in gene expression levels are likely due to differences in both polyplex retention/release kinetics as well as differences in gene stability/processing associated with collagen linkage. As previously observed, approximately 20% less DNA was initially retained in the unmodified polyplex samples as compared with the GPP-PEI polyplex samples, and the DNA that was retained was released approximately 1.63x, 2.10x, and 3.25x faster than the DNA in the 10, 20, and 50% GPP-PEI polyplexes, respectively, over 15 day in PBS [52]. While the release kinetics were likely not identical in the media containing serum, our current study suggests that GPP modification and multivalency still improve polyplex retention relative to unmodified polyplex retention on collagen even during preincubation with a range of serum concentrations for up to 2-weeks. In the unmodified polyplex samples, upon release into the serum-supplemented media, the unmodified polyplex’s high density of positive surface charges likely led to many interactions with negatively charged serum proteins such as albumin, leading to rapid aggregation and loss of viability within an hour [13, 59]. Due to the faster release kinetics of the gel containing the unmodified polyplex, compounded by the fact it initially held less polyplex, the collagen’s concentration of viable polyplex decreased at a significantly faster rate leading to lower levels of gene expression, which would also be exacerbated by the higher concentrations of serum expediting the aggregation of released polyplex. Differences in expression levels as a function of time were also observed for polyplex containing different amounts of GPP-PEI/Total PEI, with samples containing higher amounts of GPP-PEI/Total PEI (10%, 20%, and 50%) exhibiting higher expression levels particularly after longer time periods of preincubation in serum.
The increase in expression observed in the more extensively modified polyplexes at higher serum concentrations was also likely due to a combination of effects stemming from scaffold and/or polyplex interactions with serum proteins. While collagen encourages cellular adhesion, serum gradients have been shown to encourage cellular in-growth, enhanced mobility, and increased proliferation [60, 61]. In substrate-mediated delivery, cellular access to DNA, which is determined by cell mobility and DNA scaffold concentration, is one of the largest determinants of gene expression. Preincubation of the gels with serum likely leads to the absorption of serum proteins onto the scaffold, making the environment more hospitable to the cells. In unpublished observations, cells plated on these samples appeared more likely enter the scaffold and remain in the scaffold, unlike in the scaffolds preincubated in lower levels of serum, where it was observed cells were predominantly located in the top layers of the gels or adhered to the bottom of the polystyrene plate. We also postulate that increased association with collagen may have encouraged favorable interaction between the polyplexes and serum proteins, leading to alterations in the cellular uptake and subsequent intracellular trafficking of the released gene-containing structures. FBS contains a myriad of proteins, including adhesion mediators as well as signaling and transport proteins. One possibility is pre-exposure to serum allowed the positively charged polyplexes to interact with negatively charged serum proteins while still remaining stably bound to collagen. This interaction would decrease the charge of the polyplex, diminishing the tendency for polyplexes to aggregate or be degraded post release. Pre-exposure of PEI polyplex and other positively charged nanoparticles to serum has previously been documented to have this effect [62, 63].
The integration of polyplex in collagen, along with serum protein adsorption, has also been shown to affect cellular uptake mechanisms, with these interactions suggested to lead to greater endocytic uptake and/or more efficient intracellular trafficking to the nucleus [63, 64]. In fact, our studies supported the existence of these effects in the CMP-polyplex materials, showing that the higher amounts of GPP peptide affected not only polyplex release kinetics, but the polyplex’s final composition. In addition to mediating affinity with the collagen scaffold, the GPP peptide encouraged the polyplex to associate with the collagen fragments post-release, as shown in the colocalization study (Fig. 3). The consistent and significant differences between the Mander’s overlap coefficient (M1) for collagen association with the GPP polyplex, versus the unmodified polyplex, even after extended preincubation periods, indicated a continued GPP-mediated association of GPP-PEI polyplex with collagen following liberation and cellular uptake. Moreover, M1 values range between 0 to 1, therefore the mean M1 value of 0.38 for the 20% GPP modified samples at the different time points is indicative of significant overlap between the two components comparable to those recorded for labeled components initially incorporated into polyplex during formulation [65]. The continued CMP-collagen interaction is consistent with previous studies that have shown that the serum-stability of the almost-neutral CMPs allows them to associate with remodeling collagen in highly complex environments including in vivo murine tumor, bone abnormality, and wound models. [45, 47–49]. Continued association of the polyplex with collagen fragments has many potential benefits. It may further reduce the surface charge of the polyplex, reducing interaction with serum proteins and aggregation. In other works, neutral, hydrophilic polymers, such as poly(ethylene glycol) (PEG), or anionic polymers, such as alginate or hyaluronic acid, are used to help shield the high density of positive charges of the PEI [13, 59, 66]. In our system, collagen fragments may play a similar role and afford the released polyplex with both increased serum stability and resistance to aggregation and dissociation. Increased plasmid stability in GPP-PEI polyplex-modified gels was confirmed using gel electrophoresis. As shown in Fig. 2, intact plasmid DNA was present in the media collected from all GPP-PEI polyplex-modified samples, even after a 14 day preincubation, indicating that the DNA was at least partially protected. After longer preincubation, the plasmid bands were reduced in intensity, indicating a degree of degradation, but the fluorescence intensity still remained significantly higher than the levels observed in the unmodified polyplex samples. Because equal amounts of DNA were loaded into each well, the result suggests that CMP-mediated DNA incorporation into the collagen gel protected the DNA more effectively than DNA retained through non-specific interactions. We hypothesize that GPP-PEI polyplexes were able to better preserve the integrity of the DNA because of their slower release kinetics, causing DNA to remain bound for longer periods, and because of the continued association of polyplex with collagen fragments post-release from the scaffold. The fluorescence observed in the wells of the samples collected from the GPP-PEI polyplex-modified gels suggests that complexes of the DNA with either PEI, a serum protein, and/or collagen, are present, and these structures were not dissembled with heparin as readily as those collected in our previous, serum-free study [52].
The collagen fragments may also impart other collagen-specific benefits. Collagen has been used to mediate gene delivery in various forms, ranging from macroscopic porous sponges to nanoparticles (100–300 nm in diameter) [9, 67–69]. Atelocollagen-mediated delivery has been shown to increase cellular uptake and nuclease resistance when compared to typical polycation transfer methods and is capable of transferring genes into both dividing and non-dividing cells [67, 68]. While the majority of current studies utilize atelocollagen for localized delivery, previous works have shown atelocollagen-nucleic acid nanoparticles could be successfully implemented for systemic delivery, lengthening circulation time and increasing treatment efficiency [57, 67, 68]. The collagen fragments also have a series of integrin binding sites, which may aid in encouraging cellular internalization. To better understand the impact of the GPP peptide on polyplex uptake mechanisms, transfection experiments were conducted in which the caveolar endocytosis pathway was blocked. The caveolar endocytic pathway was chosen because previous studies with PEI polyplex have shown that this pathway initiates high efficiency intracellular trafficking to the nucleus and expression, and therefore targeting this pathway is desirable [53, 54]. The GPP peptide, as well as endogenous collagen, contains an α2β1-integrin binding site, which known to be initiate to caveolar endocytosis by collagen fragments. We discovered a direct correlation between the percent of GPP-PEI/Total PEI incorporated into the polyplex and the impact caveolin-1 silencing had on transfection. The decrease in transfection between the unmodified polyplex vs. the 20% and 50% GPP-PEI/Total PEI polyplexes (30% and 53%, respectively), was significant (p-value < 0.05). The results confirm that the GPP peptide acts not only as a tether to control release, but also as a ligand, providing a better-defined, efficient pathway way into the cells. The change in cellular uptake was likely due to the synergistic effects of the proline-rich CMP and collagen fragments. Further experimentation would be necessary to elucidate which effect was prevalent.
In vitro studies proved to be accurate predictors of the system’s efficacy in vivo. Animal models have demonstrated that CMPs can be used to detect areas of excessive remodeling, such as tumors and joints [47], and CMPs can anchor cytoactive factors and collagen in wound beds to improve wound closure and granulation [48]. Utilizing a mouse model, in vivo gene expression in polyplex encapsulating or GPP-PEI polyplex-modified subcutaneous Matrigel® pellets was monitored. Matrigel® is a widely used, commercially available substrate that consists of basement membrane ECM components such as type IV collagen [70]. While type IV collagen has a lower triple helical content than type I collagen [71] significant CMP-type IV collagen interactions have been documented, albeit with lower efficiency than CMP-type I collagen interactions [45]. Type IV collagen has also been successfully employed in wound and bone in past studies [37, 70, 72]. Through GPP modification of the polyplex, we demonstrated the ability to enhance, extend, and localize expression, even from the type IV collagen-rich pellets. Levels of expression in the pellets with GPP-PEI polyplex were higher and the duration of expression was directly dependent on the amount of GPP-PEI/Total PEI in the polyplex. For instance, maximum expression in the 10% GPP-PEI/Total PEI pellet is 100-fold greater than in the unmodified polyplex pellet and significant expression is observed for approximately 20 days versus less than a week in the unmodified samples. Alternatively, expression in the 50% GPP-PEI/Total PEI pellet was observed for up to 25 days. This relationship was likely due to a difference in release kinetics, stability, and cellular internalization and is supported by the observation that level of expression in the 50% GPP-PEI/Total PEI pellet was generally lower than that observed in the 10% GPP-PEI/Total PEI pellet, but was more localized and occurred over a longer duration. Our work, as well as other works with CMPs, have demonstrated that the incorporation of multiple CMPs onto a nanostructure/polymers dramatically affects mobility in collagen-based scaffolds due to the affinity CMPs have, particularly for remodeled collagen [73]. The reversible, serum-stable reaction prevents CMP-modified materials from leaving the delivery site[48], and as demonstrated here, can be used to both control and localize release. Other matrix-mediated gene delivery approaches have achieved similar expression periods as achieved in this work ranging from days[74, 75] to a month[76] within similar subcutaneous animal implants, while some have achieved multi-month expression periods [58]. However, our in vivo results demonstrate the ease in which expression can be tailored via variation of DNA carrier GPP composition and the potential benefits of promoting localized delivery post carrier release through GPP targeting of remodeled collagen. These unique characteristics make CMP-mediated delivery both innovative and of great value in complicated applications like wound repair which benefit from controlled multi-therapeutic delivery[8] and avoidance off-target delivery[77].
Additionally, while other matrix-mediated gene delivery approaches have demonstrated similar duration of expression to that we report, our work differs substantially in that most approaches rely solely on scaffold degradation to mediate DNA delivery, limiting localized responsiveness and enhancing the danger of off-target delivery. The application of protease-sensitive scaffolds has provided a means of engineering localized, cell-triggered gene transfer activity [11, 12], but has not eliminated the danger of vector escape [39] or addressed potential issues associated with achieving gene delivery tailored to the irregular environments common in wound repair. CMP-modification of non-viral vectors has provided a method for tailoring release/retention from collagen, and additional studies strongly suggest the reversible nature of CMP/collagen hybridization synergistically prevent vector escape from the delivery site through re-association with the collagen scaffold and surrounding remodeled collagen [44, 47]. Furthermore, unlike most matrix-mediated approaches, CMP-modification directly addresses obstacles associated with both the extra- and intracellular environments. CMP-modification has been demonstrated to not only enhance vector availability/stability in the delivery site, but also to provide a ligand promoting cellular uptake through endocytic pathways linked to high efficiency expression. Additional studies have demonstrated that the CMPs’ unique triple helical structure is integral in achieving enhanced cellular binding and cell penetration [78]. For instance, CMPs were successfully used to enhance liposome targeting of melanoma cells through specific ligand/receptor interactions, whereas no binding to non- targeted cells was recorded [79]. Given the many obstacles that inhibit gene delivery, the introduction of a novel multifunctional peptide has enormous potential for accelerating vector optimization required for pragmatic/clinical application. While multifunctional peptide vectors have been developed, typically through the fusion of peptide moieties known to overcome a particular barriers (ex: DNA condensation, cellular internalization/targeting, nuclear localization), only a small fraction have demonstrated utility in vivo, suggesting that the practice of studying the effects of these moieties individually is not sufficient [80]. The continued development of multifunctional CMPs and continued studies examining the impact of CMP-modification on different aspects of vector efficiency has enormous potential in advancing gene delivery applications.
Conclusion
Protease-sensitive biomaterials, including collagen, have been employed to synchronize the delivery of cues with tissue remodeling and the reparative process. The availability and biocompatibility of collagen have secured its place as a versatile biomaterial, both as a bioactive scaffold and as a cell-responsive reservoir for additional therapeutics. While many techniques for modifying collagen with therapeutics have been developed, the biomimetic approach utilizing CMPs has many advantages including its highly specific yet physical nature and its versatility in regards to tuning release/retention. In this work, CMP-modification was shown to modulate and enhance delivery of PEI polyplex from collagen-based scaffolds both in vitro and in vivo. Variation of the amount of GPP-PEI/Total PEI in PEI polyplexes demonstrated the capacity to control release and stabilize polyplex, even in the presence of high concentrations of serum under physiologically relevant conditions for up to 2 weeks. The cell-responsive nature of the GPP-polyplex-modified collagen substrates was clearly indicated by the fact that significant transfection was only observed in the presence of MMPs/collagen remodeling. Furthermore, the colocalization of the GPP-PEI polyplex with collagen following polyplex release from the scaffold improved DNA structural integrity and enhanced the targeting of the polyplexes into a high efficiency endocytic pathway leading to gene expression. The unique properties of the GPP peptide allowed for its use as both an adjustable tether, via CMP-collagen hybridization, and as an endocytic ligand. This method allowed for the “hijacking” of the natural process of collagen remodeling to overcome major obstacles in gene delivery, such as improving vector activity in the presence of serum and providing a well-defined intracellular pathway, in addition to serving as a cell-responsive trigger for release. As suggested by our in vivo work, these properties can translate to even more complex systems and have great potential in the treatment of sites in which excessive collagen remodeling occurs.
Supplementary Material
Statement of Significance.
In this work, we demonstrate a novel approach for stably integrating DNA into collagen scaffolds to exploit the natural process of collagen remodelling for high efficiency non-viral gene delivery. The incorporation of CMPs into DNA polyplexes, coupled with the innate affinity between CMPs and collagen, not only permitted improved control over polyplex retention and release, but also provided a series of substantial and highly unique benefits via the stable and persistent linkage between CMP-polyplexes and collagen fragments. Specifically, CMP-modification of polyplexes was demonstrated to (i) control release over nearly a month, (ii) improve vector stability under physiological-like conditions, and (iii) provide ligands able to efficiently transfer genes via endocytic collagen pathways. These unique properties overcome key barriers inhibiting non-viral gene therapy.
Acknowledgments
This work was partially supported by the National Science Foundation (NSF) under Grant nos. 1605130 and 1159466. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily represent the views of the NSF. This work was also partially supported by the National Institutes of Health under Grant no. R01 AR067247, and under a Centers of Biomedical Research Excellence (COBRE) Grant no. 5P20GM104316.
Footnotes
Conflict of interest statement: The authors have declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tan Q, Chen B, Yan X, Lin Y, Xiao ZF, Hou XL, Dai JW. Promotion of diabetic wound healing by collagen scaffold with collagen-binding vascular endothelial growth factor in a diabetic rat model. Journal of Tissue Engineering and Regenerative Medicine. 2014;8(3):195–201. doi: 10.1002/term.1513. [DOI] [PubMed] [Google Scholar]
- 2.Furth ME, Atala A, Van Dyke ME. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials. 2007;28(34):5068–5073. doi: 10.1016/j.biomaterials.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 3.Tibbitt MW, Anseth KS. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnology and Bioengineering. 2009;103(4):655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malafaya PB, Silva GA, Reis RL. Natural-origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews. 2007;59(4–5):207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: State of the art and future trends. Macromolecular Bioscience. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 6.Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science. 2011;36(8):981–1014. [Google Scholar]
- 7.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. Journal of the Royal Society Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen F-M, Zhang M, Wu Z-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31(24):6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 9.Stenzel KH, Miyata T, Rubin AL. COLLAGEN AS A BIOMATERIAL. Annual Review of Biophysics and Bioengineering. 1974;3:231–253. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 10.Vo TN, Kasper FK, Mikos AG. Strategies for controlled delivery of growth factors and cells for bone regeneration. Advanced Drug Delivery Reviews. 2012;64(12):1292–1309. doi: 10.1016/j.addr.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei Y, Segura T. DNA delivery from matrix metalloproteinase degradable poly (ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30(2):254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tokatlian T, Shrum CT, Kadoya WM, Segura T. Protease degradable tethers for controlled and cell-mediated release of nanoparticles in 2- and 3-dimensions. 2010;31(31):8072–8080. doi: 10.1016/j.biomaterials.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeney M, Onyiah S, Zhang Z, Tong XM, Han LH, Yang F. Modulating polymer chemistry to enhance non-viral gene delivery inside hydrogels with tunable matrix stiffness. Biomaterials. 2013;34(37):9657–9665. doi: 10.1016/j.biomaterials.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 14.Lau TT, Wang DA. Bioresponsive hydrogel scaffolding systems for 3D constructions in tissue engineering and regenerative medicine. Nanomedicine. 2013;8(4):655–668. doi: 10.2217/nnm.13.32. [DOI] [PubMed] [Google Scholar]
- 15.Yao L, Daly W, Newland B, Yao S, Wang W, Chen BKK, Madigan N, Windebank A, Pandit A. Improved axonal regeneration of transected spinal cord mediated by multichannel collagen conduits functionalized with neurotrophin-3 gene. Gene Therapy. 2013;20(12):1149–1157. doi: 10.1038/gt.2013.42. [DOI] [PubMed] [Google Scholar]
- 16.Ehrbar M, Djonov VG, Schnell C, Tschanz SA, Martiny-Baron G, Schenk U, Wood J, Burri PH, Hubbell JA, Zisch AH. Cell-demanded liberation of VEGF(121) from fibrin implants induces local and controlled blood vessel growth. Circulation Research. 2004;94(8):1124–1132. doi: 10.1161/01.RES.0000126411.29641.08. [DOI] [PubMed] [Google Scholar]
- 17.Geer DJ, Swartz DD, Andreadis ST. Biomimetic delivery of keratinocyte growth factor upon cellular demand for accelerated wound healing in vitro and in vivo. American Journal of Pathology. 2005;167(6):1575–1586. doi: 10.1016/S0002-9440(10)61242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu PF, Takai K, Weaver VM, Werb Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harbor Perspectives in Biology. 2011;3(12) doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill SE, Parks WC. Metalloproteinases and their inhibitors: Regulators of wound healing. International Journal of Biochemistry & Cell Biology. 2008;40(6–7):1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biology. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Beer C, Pedersen L. Matrix fibronectin binds gammaretrovirus and assists in entry: New light on viral infections. Journal of Virology. 2007;81(15):8247–8257. doi: 10.1128/JVI.00312-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Briquez PS, Hubbell JA, Martino MM. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Advances in Wound Care. 2015;4(8):479–489. doi: 10.1089/wound.2014.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKleroy W, Lee TH, Atabai K. Always cleave up your mess: targeting collagen degradation to treat tissue fibrosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2013;304(11):L709–L721. doi: 10.1152/ajplung.00418.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy WL, Mooney DJ. Controlled delivery of inductive proteins, plasmid DNA and cells from tissue engineering matrices. Journal of Periodontal Research. 1999;34(7):413–419. doi: 10.1111/j.1600-0765.1999.tb02275.x. [DOI] [PubMed] [Google Scholar]
- 25.Boulant S, Stanifer M, Lozach PY. Dynamics of Virus-Receptor Interactions in Virus Binding, Signaling, and Endocytosis. Viruses-Basel. 2015;7(6):2794–2815. doi: 10.3390/v7062747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siljamaki E, Rintanen N, Kirsi M, Upla P, Wang W, Karjalainen M, Ikonen E, Marjomaki V. Cholesterol Dependence of Collagen and Echovirus 1 Trafficking along the Novel alpha 2 beta 1 Integrin Internalization Pathway. Plos One. 2013;8(2) doi: 10.1371/journal.pone.0055465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marjomaki V, Pietiainen V, Matilainen H, Upla P, Ivaska J, Nissinen L, Reunanen H, Huttunen P, Hyypia T, Heino J. Internalization of Echovirus 1 in caveolae. Journal of Virology. 2002;76(4):1856–1865. doi: 10.1128/JVI.76.4.1856-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elangovan S, D'Mello SR, Hong L, Ross RD, Allamargot C, Dawson DV, Stanford CM, Johnson GK, Sumner DR, Salem AK. The enhancement of bone regeneration by gene activated matrix encoding for platelet derived growth factor. Biomaterials. 2014;35(2):737–747. doi: 10.1016/j.biomaterials.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai HJ, Kuan CH, Wu HC, Tsai JC, Chen TM, Hsieh DJ, Wang TW. Tailored design of electrospun composite nanofibers with staged release of multiple angiogenic growth factors for chronic wound healing. Acta Biomaterialia. 2014;10(10):4156–4166. doi: 10.1016/j.actbio.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Losi P, Briganti E, Errico C, Lisella A, Sanguinetti E, Chiellini F, Soldani G. Fibrin-based scaffold incorporating VEGF- and bFGF-loaded nanoparticles stimulates wound healing in diabetic mice. Acta Biomaterialia. 2013;9(8):7814–7821. doi: 10.1016/j.actbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 31.Ti DD, Hao HJ, Xia L, Tong C, Liu JJ, Dong L, Xu SJ, Zhao YL, Liu HL, Fu XB, Han WD. Controlled Release of Thymosin Beta 4 Using a Collagen-Chitosan Sponge Scaffold Augments Cutaneous Wound Healing and Increases Angiogenesis in Diabetic Rats with Hindlimb Ischemia. Tissue Engineering Part A. 2015;21(3–4):541–549. doi: 10.1089/ten.TEA.2013.0750. [DOI] [PubMed] [Google Scholar]
- 32.Xie ZW, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, Tang LP, Yang J, Nguyen KT. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomaterialia. 2013;9(12):9351–9359. doi: 10.1016/j.actbio.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sawyer AA, Song SJ, Susanto E, Chuan P, Lam CXF, Woodruff MA, Hutmacher DW, Cool SM. The stimulation of healing within a rat calvarial defect by mPCL-TCP/collagen scaffolds loaded with rhBMP-2. Biomaterials. 2009;30(13):2479–2488. doi: 10.1016/j.biomaterials.2008.12.055. [DOI] [PubMed] [Google Scholar]
- 34.Doukas J, Chandler LA, Gonzalez AM, Gu DL, Hoganson DK, Ma CL, Nguyen T, Printz MA, Nesbit M, Herlyn M, Crombleholme TM, Aukerman SL, Sosnowski BA, Pierce GF. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Human Gene Therapy. 2001;12(7):783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- 35.Falanga V. Wound healing and its impairment in the diabetic foot. The Lancer. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 36.Lei YG, Huang SX, Sharif-Kashani P, Chen Y, Kavehpour P, Segura T. Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds. Biomaterials. 2010;31(34):9106–9116. doi: 10.1016/j.biomaterials.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
-
37.Choi SH, Chunsik Bae, HanHojae KS, Eon KangSeongsoo. Effect of Matrigel for Bone Graft using Hydroxyapatite/Poly ε-caprolactone Scaffold in a Rat Calvarial Defect Model. Journal of Veterinary Clinics. 2010;27(4):325–329.
 [Google Scholar]
[Google Scholar] - 38.Bengali Z, Pannier AK, Segura T, Anderson BC, Jang JH, Mustoe TA, Shea LD. Gene delivery through cell culture substrate adsorbed DNA complexes. Biotechnology and Bioengineering. 2005;90(3):290–302. doi: 10.1002/bit.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu DL, Nguyen T, Gonzalez AM, Printz MA, Pierce GF, Sosnowski BA, Phillips ML, Chandler LA. Adenovirus encoding human platelet-derived growth factor-B delivered in collagen exhibits safety, biodistribution, and immunogenicity profiles favorable for clinical use. Molecular Therapy. 2004;9(5):699–711. doi: 10.1016/j.ymthe.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns. 2009;35(2):171–180. doi: 10.1016/j.burns.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan TR, Stahl PJ, Yu SM. Matrix-Bound VEGF Mimetic Peptides: Design and Endothelial-Cell Activation in Collagen Scaffolds. Advanced Functional Materials. 2011;21(22):4252–4262. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan TR, Stahl PJ, Li Y, Yu SM. Collagen-gelatin mixtures as wound model, and substrates for VEGF-mimetic peptide binding and endothelial cell activation. Acta Biomaterialia. 2015;15:164–172. doi: 10.1016/j.actbio.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y, Mo XA, Kim D, Yu SM. Template-Tethered Collagen Mimetic Peptides for Studying Heterotrimeric Triple-Helical Interactions. Biopolymers. 2011;95(2):94–104. doi: 10.1002/bip.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Targeting collagen strands by photo-triggered triple-helix hybridization. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(37):14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Ho D, Meng H, Chan TR, An B, Yu H, Brodsky B, Jun AS, Yu SM. Direct Detection of Collagenous Proteins by Fluorescently Labeled Collagen Mimetic Peptides. Bioconjugate Chemistry. 2013;24(1):9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y, Yu SM. Targeting and mimicking collagens via triple helical peptide assembly. Current Opinion in Chemical Biology. 2013;17(6):968–975. doi: 10.1016/j.cbpa.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Foss CA, Pomper MG, Yu SM. Imaging Denatured Collagen Strands In vivo and Ex vivo via Photo-triggered Hybridization of Caged Collagen Mimetic Peptides. Jove-Journal of Visualized Experiments. 2014;(83):e51052. doi: 10.3791/51052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chattopadhyay S, Guthrie KM, Teixeira L, Murphy CJ, Dubielzig RR, McAnulty JF, Raines RT. Anchoring a cytoactive factor in a wound bedpromotes healing. Journal of Tissue Engineering and Regenerative Medicine. 2016;10(12):1012–1020. doi: 10.1002/term.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chattopadhyay S, Raines RT. Collagen-Based Biomaterials for Wound Healing. Biopolymers. 2014;101(8):821–833. doi: 10.1002/bip.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krishna OD, Kiick KL. Supramolecular Assembly of Electrostatically Stabilized, Hydroxyproline-Lacking Collagen-Mimetic Peptides. Biomacromolecules. 2009;10(9):2626–2631. doi: 10.1021/bm900551c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Krishna OD, Jha AK, Jia XQ, Kiick KL. Integrin-mediated adhesion and proliferation of human MSCs elicited by a hydroxyproline-lacking, collagen-like peptide. Biomaterials. 2011;32(27):6412–6424. doi: 10.1016/j.biomaterials.2011.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urello MA, Kiick KL, Sullivan MO. A CMP-based method for tunable, cell-mediated gene delivery from collagen scaffolds. Journal of Materials Chemistry B. 2014;2(46):8174–8185. doi: 10.1039/c4tb01435a. [DOI] [PubMed] [Google Scholar]
- 53.Larsen JD, Reilly MJ, Sullivan MO. Using the Epigenetic Code To Promote the Unpackaging and Transcriptional Activation of DNA Polyplexes for Gene Delivery. Molecular Pharmaceutics. 2012;9(5):1041–1051. doi: 10.1021/mp200373p. [DOI] [PubMed] [Google Scholar]
- 54.Reilly MJ, Larsen JD, Sullivan MO. Intracellular Trafficking of a Histone-Mimetic Polyplex to Promote Nucleus-Specific Self-Unpackaging. Molecular Therapy. 2011;19(7):1388–1389. [Google Scholar]
- 55.Zhu XX, Liu Q, Wang MM, Liang MR, Yang X, Xu X, Zou HJ, Qiu JH. Activation of Sirt1 by Resveratrol Inhibits TNF-alpha Induced Inflammation in Fibroblasts. Plos One. 2011;6(11) doi: 10.1371/journal.pone.0027081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi F, Harman J, Fujiwara K, Sottile J. Collagen I matrix turnover is regulated by fibronectin polymerization. American Journal of Physiology-Cell Physiology. 2010;298(5):C1265–C1275. doi: 10.1152/ajpcell.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pannier AK, Shea LD. Controlled release systems for DNA delivery. Molecular Therapy. 2004;10(1):19–26. doi: 10.1016/j.ymthe.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 58.Salvay DM, Zelivyanskaya M, Shea LD. Gene delivery by surface immobilization of plasmid to tissue-engineering scaffolds. Gene Therapy. 2010;17(9):1134–1141. doi: 10.1038/gt.2010.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song HM, Wang G, He B, Li L, Li CX, Lai YS, Xu XH, Gu ZW. Cationic lipid-coated PEI/DNA polyplexes with improved efficiency and reduced cytotoxicity for gene delivery into mesenchymal stem cells. International Journal of Nanomedicine. 2012;7:4637–4648. doi: 10.2147/IJN.S33923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orsi S, De Capua A, Guarnieri D, Marasco D, Netti PA. Cell recruitment and transfection in gene activated collagen matrix. Biomaterials. 2010;31(3):570–576. doi: 10.1016/j.biomaterials.2009.09.054. [DOI] [PubMed] [Google Scholar]
- 61.Chen HH, Zhao S, Song JG. TGF-beta 1 suppresses apoptosis via differential regulation of MAP kinases and ceramide production. Cell Death and Differentiation. 2003;10(5):516–527. doi: 10.1038/sj.cdd.4401171. [DOI] [PubMed] [Google Scholar]
- 62.Rhaese S, von Briesen H, Rubsamen-Waigmann H, Kreuter J, Langer K. Human serum albumin-polyethylenimine nanoparticles for gene delivery. Journal of Controlled Release. 2003;92(1–2):199–208. doi: 10.1016/s0168-3659(03)00302-x. [DOI] [PubMed] [Google Scholar]
- 63.Nicoli E, Syga MI, Bosetti M, Shastri VP. Enhanced Gene Silencing through Human Serum Albumin-Mediated Delivery of Polyethylenimine-siRNA Polyplexes. Plos One. 2015;10(4) doi: 10.1371/journal.pone.0122581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle Size and Surface Chemistry Determine Serum Protein Adsorption and Macrophage Uptake. Journal of the American Chemical Society. 2012;134(4):2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 65.Reitan NK, Sporsheim B, Bjorkoy A, Strand S, Davies CD. Quantitative 3-D colocalization analysis as a tool to study the intracellular trafficking and dissociation of pDNA-chitosan polyplexes. Journal of Biomedical Optics. 2012;17(2) doi: 10.1117/1.JBO.17.2.026015. [DOI] [PubMed] [Google Scholar]
- 66.Bahadur KCR, Thapa B, Xu PS. Design of Serum Compatible Tetrary Complexes for Gene Delivery. Macromolecular Bioscience. 2012;12(5):637–646. doi: 10.1002/mabi.201100464. [DOI] [PubMed] [Google Scholar]
- 67.Minakuchi Y, Takeshita F, Kosaka N, Sasaki H, Yamamoto Y, Kouno M, Honma K, Nagahara S, Hanai K, Sano A, Kato T, Terada M, Ochiya T. Atelocollagen-mediated synthetic small interfering RNA delivery for effective gene silencing in vitro and in vivo. Nucleic Acids Research. 2004;32(13) doi: 10.1093/nar/gnh093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeshita F, Minakuchi Y, Nagahara S, Honma K, Sasaki H, Hirai K, Teratani T, Namatame N, Yamamoto Y, Hanai K, Kato T, Sano A, Ochiya T. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12177–12182. doi: 10.1073/pnas.0501753102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J, Lee IL, Lim WS, Chia SM, Yu H, Leong KW, Mao HQ. Evaluation of collagen and methylated collagen as gene carriers. International Journal of Pharmaceutics. 2004;279(1–2):115–126. doi: 10.1016/j.ijpharm.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 70.Gorelik JV, Paramonov BA, Blinova MI, Diakonov IA, Kukhareva LV, Pinaev GP. Matrigel increases the rate of split wound healing and promotes keratinocyte 'take' in deep wounds in rats. Cytotechnology. 2000;32(2):79–86. doi: 10.1023/A:1008192111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microscopy Research and Technique. 2008;71(5):357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tigges U, Hyer EG, Scharf J, Stallcup WB. FGF2-dependent neovascularization of subcutaneous Matrigel plugs is initiated by bone marrow-derived pericytes and macrophages. Development. 2008;135(3):523–532. doi: 10.1242/dev.002071. [DOI] [PubMed] [Google Scholar]
- 73.Li Y, San BH, Kessler JL, Kim JH, Xu QG, Hanes J, Yu SM. Non-Covalent Photo-Patterning of Gelatin Matrices Using Caged Collagen Mimetic Peptides. Macromolecular Bioscience. 2015;15(1):52–62. doi: 10.1002/mabi.201400436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scherer F, Schillinger U, Putz U, Stemberger A, Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. Journal of Gene Medicine. 2002;4(6):634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]
- 75.Zhang XQ, Tang HH, Hoshi R, De Laporte L, Qiu HJ, Xu XY, Shea LD, Ameer GA. Sustained transgene expression via citric acid-based polyester elastomers. Biomaterials. 2009;30(13):2632–2641. doi: 10.1016/j.biomaterials.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meilander-Lin NJ, Cheung PJ, Wilson DL, Bellamkonda RV. Sustained in vivo gene delivery from agarose hydrogel prolongs nonviral gene expression in skin. Tissue Engineering. 2005;11(3–4):546–555. doi: 10.1089/ten.2005.11.546. [DOI] [PubMed] [Google Scholar]
- 77.Boateng JS, Matthews KH, Stevens HNE, Eccleston GM. Wound healing dressings and drug delivery systems: A review. Journal of Pharmaceutical Sciences. 2008;97(8):2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 78.Shinde A, Feher KM, Hu C, Slowinska K. Peptide internalization enabled by folding: triple helical cell-penetrating peptides. Journal of Peptide Science. 2015;21(2):77–84. doi: 10.1002/psc.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rezler EM, Khan DR, Lauer-Fields J, Cudic M, Baronas-Lowell D, Fields GB. Targeted drug delivery utilizing protein-like molecular architecture. Journal of the American Chemical Society. 2007;129(16):4961–4972. doi: 10.1021/ja066929m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Raad M, Teunissen EA, Mastrobattista E. Peptide vectors for gene delivery: from single peptides to multifunctional peptide nanocarriers. Nanomedicine. 2014;9(14):2217–2232. doi: 10.2217/nnm.14.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.