Abstract
Background
The mechanism of kidney injury in hematopoietic stem cell transplantation (HSCT)–associated thrombotic microangiopathy (TA-TMA) is not completely understood. Renal C4d staining is a marker of classic complement activation and endothelial injury and has been described in preliminary reports of HSCT recipients with TA-TMA. Our objective was to evaluate complement in the pathogenesis of small vessel injury in children receiving HSCT. We hypothesized that kidney tissue from children with TA-TMA would more frequently show C4d deposition compared with HSCT recipients without histologic TA-TMA.
Methods
We reviewed kidney specimens (biopsy or autopsy) from children who had undergone HSCT at a single center. Using histologic criteria alone, subjects were divided into TA-TMA (n=8) and non–TA-TMA (control) groups (n=12). C4d staining was performed by immunohistochemistry and evaluated on arterioles, peritubular capillaries, glomeruli, and tubular basement membranes.
Results
Diffuse or focal renal arteriolar C4d staining was more common in subjects with histologic TA-TMA (75%) compared with controls (8%). Rare peritubular capillary C4d staining was present in 50% of TA-TMA samples and was absent in controls. Glomerular C4d staining was seen at a similar frequency in cases and controls, whereas tubular basement membrane staining was less frequently observed and only in subjects with TA-TMA.
Conclusions
Arteriolar C4d deposition may be a pathologic marker of TA-TMA, implicating localized complement fixation in HSCT recipients with kidney disease secondary to small vessel injury. Further studies to better characterize the preferential arteriolar C4d staining may identify a renal compartment of injury, possibly explaining the dramatic hypertension seen in TA-TMA.
Keywords: Thrombotic microangiopathy, Hematopoietic cell transplantation, Complement, Pediatrics
The mechanisms of endothelial injury in hematopoietic stem cell transplantation (HSCT)–associated thrombotic microangiopathy (TA-TMA) are not completely understood (1, 2). HSCT recipients are exposed to multiple potential endothelial insults, including chemotherapy, radiation, infections, and calcineurin inhibitors for graft-versus-host disease (GVHD) prophylaxis (1, 3, 4).
In TA-TMA, microangiopathic hemolytic anemia is associated with endothelial thrombosis and small vessel obstruction (4–7). Damage to the renal vasculature can lead to hypertension, proteinuria, acute kidney injury, and chronic kidney disease (8–10). This is significant because HSCT recipients requiring acute dialysis have a mortality rate approaching 90%, chronic kidney disease is a complication in more than 15% of bone marrow transplant patients, and survivors of HSCT have a risk of end-stage kidney disease 16 times higher than the general population (11–14).
The histologic findings of TA-TMA are virtually identical to those seen in other kidney diseases associated with microangiopathy, including diarrhea positive hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and, occasionally, antibody-mediated kidney transplant rejection (1, 5, 15). Atypical hemolytic uremic syndrome, which is also indistinguishable from TA-TMA by pathology, is associated with disruptions in the alternative pathway of complement (16).
C4d staining is a marker of classic complement activation by tissue-specific antibodies or direct endothelial injury (17, 18). Recent uncontrolled reports noted peritubular capillary (PTC) and glomerular C4d deposits in kidney samples from HSCT recipients with TA-TMA (19, 20). Our objective was to expand on these findings by examining if kidney tissue from patients with TA-TMA more frequently demonstrated C4d deposition compared with HSCT recipients that did not have histologic evidence of microangiopathy in the kidney.
RESULTS
Study Population
We identified a total of 21 specimens from 20 pediatric HSCT recipients with available kidney tissue for C4d testing. Of these 21 specimens, 9 (from 8 subjects) had histologic evidence of TA-TMA, 6 from biopsy tissue, and the remaining 3 from autopsy tissue. One subject with TA-TMA had a biopsy and autopsy with similar renal findings; therefore, only the autopsy findings were included in the analysis. The 12 HSCT recipients without histology-proven renal TA-TMA served as controls. All 12 of these control samples were from kidney tissue obtained at the time of autopsy. The time from HSCT until the kidney specimen was obtained was a median (interquartile range) of 233 days (91–511 days) for the subjects with TA-TMA and 172 days (78–453 days) for the control subjects (P=0.59).
Clinical Characteristics
The clinical characteristics of the study population are shown in Table 1. There was no significant difference in age, gender, the occurrence of GVHD (in at-risk allogeneic recipients), or the need for dialysis between subjects with TA-TMA and HSCT controls. The subjects with TA-TMA were more likely to have an underlying diagnosis of malignancy, whereas the controls were more likely to have an immunodeficiency. The subjects with TA-TMA more commonly had received an autologous transplant and myeloablative conditioning. There was no difference in the prevalence of viral infections between the two groups.
TABLE 1.
Clinical characteristics of the study population
| Variable | Subjects with histology proven renal TA-TMA (n=8) | HSCT recipients without renal TA-TMA on histology (n=12) |
|---|---|---|
| Age (years) | 3.7 (2.0–4.7) [0.6–5.0] | 3.2 (1.4–17.1) [0.9–28.1] |
| Gender (male) | 3 (37.5) | 8 (66.7) |
| Underlying diagnosisa | ||
| Malignancy | 6 (75.0) | 2 (16.7) |
| Immunodeficiency | 1 (12.5) | 9 (75.0) |
| Bone marrow failure | 1 (12.5) | 1 (8.3) |
| Type of transplanta | ||
| Allogeneic | 2 (25.0) | 11 (91.7) |
| Autologous | 6 (75.0) | 1 (8.3) |
| Type of conditioninga | ||
| Myeloablative | 7 (87.5) | 4 (33.3) |
| Reduced Intensity | 1 (12.5) | 8 (66.6) |
| GVHD in those at risk (allogeneic) | 1/2 (50) | 3/11 (27.3) |
| Viral infection | 4 (50.0) | 4 (33.3) |
| Dialysis | 6 (75) | 5 (41.7) |
| Day after HSCT of dialysis initiation (day+) | 163 (36–271) [1–2186] | 37 (16–79) [9–871] |
| Able to stop dialysis | 3/6 (50) | 0/5 (0) |
| Day after HSCT biopsy or autopsy | 233 (91–511) [19–2281] | 172 (78–453) [9–887] |
| specimen was obtained (day+) | ||
| Days after dialysis initiation biopsy or autopsy specimen was obtained | 51.5 (3–391) [j36 to 539] | 5 (3–17) [0–135] |
P<0.05 between cases and controls.
Data expressed as n (%) or median (25th–75th percentile) [range].
HSCT, hematopoietic stem cell transplant; GVHD, graft-versus-host disease; TA-TMA, transplant-associated thrombotic microangiopathy.
Table 2 shows the transplant characteristics and outcome for the study population. In the TA-TMA group, five subjects were alive at last follow-up. One patient died from relapsed neuroblastoma while in kidney failure from TA-TMA and the remaining two subjects died from TA-TMA–related complications. Three control subjects had no histologic abnormalities in renal tissue, whereas the other nine subjects had glomerular, tubular, or interstitial compartment injury without evidence of TA-TMA.
TABLE 2.
HSCT characteristics and C4d staining of kidney tissue
| ID | Diagnosis | HSCT type | Conditioning regimen | Cause of death | Renal pathology findings | PTC C4d stain | Arteriolar C4d stain | Glomerular C4d stain | Tubular BM C4d stain |
|---|---|---|---|---|---|---|---|---|---|
| TA-TMA group | |||||||||
| 1 | NB | Auto | MA | Alive at last follow-up | TMA | Rare | Diffuse | — | Focal |
| 2 | NB | Auto | MA | Alive at last follow-up | TMA | — | Diffuse | Diffuse | — |
| 3 | MB | Auto | MA | Alive at last follow-up | TMA | Rare | Focal | Rare | Rare |
| 4 | AMT | Allo | MA | Alive at last follow-up | TMA | — | Focal | — | — |
| 5a | NB | Auto | MA | Relapsed NB | TMA | Rare | Diffuse | — | — |
| 6 | NB | Auto | MA | TA-TMA | TMA | — | — | Rare | — |
| 7 | HLH | Allo | RIC | TA-TMA | TMA | — | — | Focal | — |
| 8 | NB | Auto | MA | Alive at last follow-up | TMA | Rare | Diffuse | Focal | Focal |
| Control group | |||||||||
| 1 | XLP | Allo | RIC | Necrotizing encephalopathy | GS/TA | — | — | Focal | — |
| 2 | FA | Allo | MA | ARDS/adenoviral infection | TN | — | — | Rare | — |
| 3 | HLH | Allo | RIC | Pulmonary hypertension | Normal | — | — | Focal | — |
| 4 | CID | Allo | RIC | Pseudomembranous colitis | ↑ Vascular CT | — | — | Diffuse | — |
| 5 | WAS | Allo | RIC | ARDS | TA/IF | — | Focal | Diffuse | — |
| 6 | XLP | Allo | RIC | Adenoviral infection | GS/TA | — | — | Focal | — |
| 7 | HLH | Allo | RIC | Chronic GVHD | Abscesses | — | — | Focal | — |
| 8 | IPEX | Allo | RIC | Septic shock | Normal | — | — | Rare | — |
| 9 | HLH | Allo | RIC | Disseminated fungal infection | TN | — | — | Focal | — |
| 10 | HLH | Allo | MA | Adenoviral infection | TN | — | — | — | — |
| 11 | ALL | Allo | MA | Sepsis/relapsed leukemia | Normal | — | — | Rare | — |
| 12 | EWS | Auto | MA | ARDS/relapsed EWS | Infarcts | — | — | — | — |
Both biopsy and autopsy with similar findings.
Diffuse if >50% tissue staining, focal if 10% to 50% of tissue, rare if 1% to 10% of tissue, or negative (—) if 0%.
ALL, acute lymphoblastic leukemia; Allo, allogeneic HSCT; AMT, amegakaryocytic thrombocytopenia; ARDS, acute respiratory distress syndrome; Auto, autologous HSCT; BM, basement membrane; CID, combined immunodeficiency; CT, connective tissue; EWS, Ewing sarcoma; FA, Fanconi anemia; GS, glomerular sclerosis; GVHD, graft-versus-host disease; HLH, hemophagocytic lymphohistiocytosis; IF, interstitial fibrosis; IPEX, immunodysregulation polyendocrinopathy enteropathy X-linked syndrome; MA, myeloablative; MB, medulloblastoma; NB, neuroblastoma; PTC, peritubular capillary; RIC, reduced intensity conditioning; TA-TMA, transplant-associated thrombotic microangiopathy; TA, tubular atrophy; TN, tubular necrosis; WAS, Wiskott-Aldrich syndrome; XLP, X-linked lymphoproliferative disease.
Regarding medication exposure, routine fungal prophylaxis included fluconazole (autologous HSCT) or voriconazole (allogeneic HSCT). Fevers were empirically treated with meropenem and ciprofloxacin. Two allogeneic HSCT recipients were receiving cyclosporine that was stopped after TA-TMA was diagnosed. Two patients received foscarnet, ganciclovir, and cidofovir for refractory cytomegalovirus.
Association of C4d Staining with Histologic TA-TMA
Tables 2 shows C4d staining results in the kidney tissue analyzed from our study population. Diffuse (>50% of tissue specimen) or focal (10%–50%) arteriolar C4d staining was shown to be more common in TA-TMA specimens (n=6 of 8 [75%]) compared with controls (n=1 of 12 [8%]; P=0.004).
Among the six subjects with TA-TMA and arteriolar C4d staining, four subjects had diffuse and two had focal staining. These four patients with diffuse arteriolar staining had a longer period of time (median, 69.5 days; interquartile range, 33.5–101 days) from a clinical diagnosis of TA-TMA until tissue diagnosis than the four TA-TMA patients with either focal or negative arteriolar staining (time from clinical to tissue diagnosis of 22.5 days; interquartile range, 15–26.5 days; P=0.06). In the two subjects with TA-TMA and negative C4d arteriolar staining, potential triggers were conditioning chemotherapy in one (who also had severe multiorgan toxicity from chemotherapy) and cytomegalovirus viremia in the other. The one control subject with arteriolar C4d staining (focal) was diagnosed with tubular atrophy/interstitial fibrosis on histology (Table 2).
Rare (1%–5% of tissue specimen) PTC C4d staining was present in 50% of the subjects with TA-TMA and was absent in controls. Any glomerular C4d staining was common and similar in both groups and was seen in 5 of 8 (63%) subjects with TA-TMA and 10 of 12 (83%) of control subjects (P=0.35). Focal or rare tubular basement membrane staining was found in 3 of 8 (38%) of the subjects with TA-TMA and was absent in controls.
DISCUSSION
Renal arteriolar C4d deposition after HSCT was significantly more common in children with histologically confirmed renal TA-TMA compared with controls (75% vs. 8%; P=0.004). This staining correlated with pathologic evidence of TMA observed in the arterioles on hematoxylin–eosin preparations (Fig. 1). Rare PTC and tubular basement membrane C4d staining was found only in subjects with TA-TMA but in 50% or less of cases. Glomerular staining appeared similar in both groups and was presumably nonspecific.
FIGURE 1.
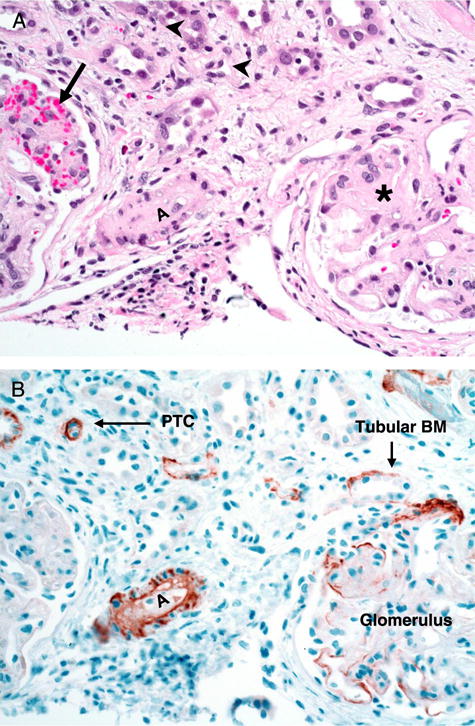
Representative renal biopsy specimen from a subject with histologic evidence of TA-TMA after hematopoietic stem cell transplantation. A, hematoxylin–eosin staining (magnification, ×20) of renal cortex with glomeruli. Glomeruli show variable degrees of obliteration of the capillary lumens and thickened capillary walls (asterisk) with mild to moderate mesangial matrix expansion. There is also evidence of red blood cell fragmentation and extravasation (arrow). There are no inflammatory infiltrates in glomeruli. Small arterioles (A) also show obliteration of the vessel lumen due to sloughed endothelial cells, intimal proliferation, and extracellular matrix deposition. There is mild tubular atrophy as well as mild interstitial inflammation (arrowheads). B, C4d staining (magnification, ×20) of corresponding tissue section shows diffuse positive staining in the degenerating small arteriole (A) with microangiopathic changes. Focal or rare C4d stain is noticeable in some tubular basal membranes (Tubular BM), PTC, and glomeruli. PTC, peritubular capillary; TA-TMA, transplant-associated thrombotic microangiopathy.
To the best of our knowledge, this is the first report of arteriolar C4d deposition in TA-TMA after HSCT. Arteriolar C4d staining has been observed in tissue from patients receiving kidney, liver, small bowel, and heart transplants but, in contrast to PTC C4d deposition, has not been associated with antibody-mediated transplant rejection or shorter allograft survival (21, 22). Additionally, arteriolar C4d deposition has been noted in the kidneys of nontransplant patients with “vascular disease” (23). The significance of arteriolar staining in solid organ transplant recipients is not known but may be related to complement activation from endothelial injury secondary to either hypertension or calcineurin inhibitor therapy (21, 23). This may explain, or be the result of, the dramatic hypertension often seen in TA-TMA (8, 9).
Our results expand on previous reports (19, 20) by examining C4d in all four renal tissue compartments (PTC, arterioles, glomeruli, and basement membrane) in pediatric HSCT recipients, by identifying arteriolar C4d deposition, and including control patients who also received a HSCT. Mii et al. (19, 20) examined kidney tissue from seven adult subjects with acute and/or chronic GVHD with histologic TA-TMA. C4d staining was performed on kidney biopsy and autopsy specimens using immunofluorescence (IF) or immunohistochemistry (IHC) a median of 7 months after HSCT. No HSCT controls without TA-TMA were included in their analysis. Similar to our findings where glomerular C4d staining appeared to be nonspecific (as it was positive in 75% of our entire study population), Mii et al. noted diffuse C4d deposition in the glomerular capillaries in 57% of their subjects with TA-TMA. The authors also identified less frequent staining of PTCs and the tubular basement membrane. Several subjects had evidence of arteriolar hyalinosis and arteriolar TA-TMA, but arteriolar C4d was not reported. They concluded that renal TA-TMA might be induced by antibody deposition and complement-mediated endothelial cell injury in chronic GVHD, although no antiendothelial or anti–human leukocyte antigen (HLA) antibodies could be detected in their study subjects.
Additionally, Troxell et al. (24) reviewed pathology records over an 8-year period at two centers and identified 15 kidney biopsy specimens in adult HSCT recipients. C4d staining performed by IHC was negative in the PTCs of the two subjects with histologic TA-TMA. One biopsy with acute tubular necrosis and interstitial nephritis after HSCT showed focal (10%–20%) PTC C4d staining. Glomerular subepithelial C4d deposition was found in the biopsies from seven patients with nephrotic syndrome from membranous nephropathy. Again, arteriolar staining was not reported.
Our observation that isolated glomerular staining for C4d may be a nonspecific finding after HSCT is supported by other studies in transplant recipients. Troxell et al. (25) noted that mesangial C4d staining in the absence of other histologic abnormalities could be identified on frozen, but not paraffin-fixed, tissue after kidney transplantation. Others have also reported that glomerular C4d deposition is a nonspecific finding in HSCT recipients and patients with kidney transplant glomerulopathy (19–21, 23). After kidney transplantation, unlike PTC staining, glomerular C4d may not be associated with worse allograft survival (22).
PTC C4d is strongly correlated with worse kidney allograft survival and likely reflects the presence of complement fixing donor-specific anti-HLA antibodies (23, 26–28). C4d has been correlated with tissue injury and worse clinical outcomes in recipients of other solid organ transplants, including heart (29), pancreas (30), and liver (31) allografts. The specificity of C4d for graft rejection is less clear after lung (32) or composite tissue (face or hands) transplantation (33, 34).
The combination of histologic evidence of TMA and PTC C4d deposition has been reported by some after kidney transplantation (15, 35), whereas others were unable to demonstrate this association (36). In a large study of kidney allograft biopsies, concomitant PTC C4d staining and TMA portended more severe glomerular injury, whereas arteriolar damage was more severe in those with TMA and negative PTC C4d staining (37).
In nontransplant patients with native kidney disease, C4d-positive staining of PTCs is rare (38), whereas glomerular staining is commonly reported in known complement-mediated diseases and other conditions with immune complex deposition, such as lupus nephritis (23, 39). In fact, glomerular C4d deposition has been associated with histologic findings of TMA in patients with lupus (39, 40). During pregnancy, C4d deposition in the umbilical vein has been associated with premature birth (41) and C4d staining of the placenta with fetal loss in patients with lupus (42).
This evidence from both transplant and nontransplant patients supports that extraglomerular C4d is a specific marker of tissue injury and is unlikely to be a benign pathologic finding in most cases. However, C4d staining is not always associated with disease. In blood group-mismatched kidney transplant recipients, PTC C4d staining has not been associated with inflammation or worse graft outcomes possibly due to accommodation by endothelial tissue that becomes resistant to the effects of complement (38). It is worth noting that one of our control subjects had focal arteriolar C4d staining without histologic evidence of TA-TMA (possible false-positive). This subject was diagnosed with tubular atrophy/interstitial fibrosis. Although we are unaware of any prior reports of false-positive C4d staining in patients with interstitial fibrosis, Banff criteria excludes scoring areas of kidney allograft specimens with interstitial fibrosis. Recent data in renal transplantation support that inflammation in areas of interstitial fibrosis/tubular atrophy may be associated with worse allograft survival independent of C4d staining (43).
In the present study, we graded pathology specimens on the degree of C4d staining (diffuse, focal, rare, or negative) in each of four tissue compartments (arterioles, PTCs, glomeruli, and tubular basement membranes). We defined C4d positivity by IHC as diffuse or focal according to kidney transplant guidelines (17, 44). It is unclear why subjects with TA-TMA had different arteriolar C4d staining patterns (diffuse, focal, negative). We speculate that subjects with TA-TMA and diffuse arteriolar staining had a relatively longer period of time between their clinical and tissue diagnosis, allowing time for more C4d to be deposited. Similarly, in kidney transplant recipients, it has been shown that the association between different C4d staining patterns and clinical disease may depend on the time since transplantation (22, 45).
C4d may not be sensitive for TA-TMA in all subjects, as shown by two of our patients with histologic TMA and negative arteriolar, PTC, and tubular basement membrane staining. False-negative C4d staining can occur if the analyzed tissue is necrotic (23). Because there are several potential triggers for TA-TMA (viral infections, conditioning chemotherapy, calcineurin inhibitor use, and GVHD), the pathologic mechanism of injury may differ (2). Similarly, after kidney transplantation, the concept of C4d-negative antibody-mediated rejection is increasingly being investigated as a mechanism of complement-independent endothelial damage by antibodies or other inflammatory mediators (natural killer cells and macrophages) (38, 46).
The strengths of our analysis include a comparison of findings in patients with TA-TMA and HSCT controls without microangiopathy, the quantification of C4d staining in multiple renal tissue compartments, and the blinded grading of tissue specimens. Despite a relatively low number of study subjects, our study represents C4d analysis in the largest cohort of HSCT recipients to date, a population where a renal biopsy has a particularly high risk of bleeding complications from thrombocytopenia and hypertension (24). One limitation of our analysis was the lack of frozen tissue for IF testing. Some authors consider IF to be more sensitive than IHC for the detection of C4d (21, 23, 38). However, a study in 138 kidney transplant biopsies compared both techniques and found that IHC, when referenced to IF, had a specificity of 98% and sensitivity of 87% to identify C4d (25). The authors noted that, despite decreased sensitivity, IHC may provide several benefits over IF including ease of tissue preparation and the capacity to compare light microscopy and C4d staining results in the same plane (25).
Our sample was limited to an assessment of only kidney tissue. TA-TMA most commonly damages the kidney but has also been reported to affect the lungs and gastrointestinal tract (47, 48). It would important to learn if complement fixation is a ubiquitous process or if the kidney is preferentially affected due to its fenestrated glomerular endothelium and unique expression of complement regulatory proteins (16, 49).
Given the high proportion of autologous recipients in our study, we are unable to draw conclusions regarding the role of GVHD in TA-TMA (19, 20). Nevertheless, our findings may bring new insights to the pathogenesis of TA-TMA in HSCT patients by possibly implicating localized complement fixation in renal arterioles. Complement activation may occur secondary to as yet unidentified antibodies or via antibody-independent mechanisms of endothelial damage in response to endothelium damaging triggers such as chemotherapy, radiation, or viruses (2). Further research is needed to better understand the role of complement in small vessel endothelial injury in allogeneic and autologous patients with TA-TMA.
Current treatment options for TA-TMA have not been evaluated in controlled trials. Reported therapies include therapeutic plasma exchange, defibrotide, rituximab, and/or discontinuation of calcineurin inhibitors in allogeneic recipients. Novel therapies include agents to decrease endothelial damage (statins and bosentan), block complement activation (eculizumab), or prevent tissue fibrosis (angiotensin-converting enzyme inhibitors) (2, 50–53).
In conclusion, we report a strong association of arteriolar C4d deposition with TA-TMA, possibly representing complement-mediated injury targeting this specific renal tissue compartment in children receiving HSCT. We support the recommendation by others to consider C4d staining in kidney tissue from patients with clinical signs of TMA (38). We acknowledge that kidney biopsy remains a challenging procedure after HSCT. However, kidney injury after HSCT can be multifactorial and the exact cause is often unknown (10). If arteriolar C4d staining aids in the diagnosis of TA-TMA, this may encourage a kidney biopsy in appropriately selected patients. These patients include those early in the course of their disease (before tissue injury becomes irreversible), allogeneic recipients with concomitant GVHD in whom biopsy results can guide the use of calcineurin inhibitor therapy, and in other patients with clinical concern for TA-TMA but no obvious triggering event (i.e., viral infection). More in-depth study using standard techniques (10) such as C4d staining may increase our understanding of the mechanisms of tissue injury in this population and help to develop rational therapeutic strategies.
MATERIALS AND METHODS
We reviewed all kidney biopsy and autopsy specimens from children undergoing HSCT at Cincinnati Children’s Hospital Medical Center over 5 years until October 2012. Specimens were included in the analysis if stored kidney tissue was available for C4d testing. The Cincinnati Children’s Hospital Institutional Review Board approved the protocol and waived the requirement for informed consent.
We used histologic criteria to divide specimens into those with evidence of TA-TMA in the kidney and controls without TA-TMA. TA-TMA was defined as small vessel fibrin thrombosis, mesangiolysis, subendothelial injury, intima separation, and vascular obstruction (Fig. 1) (1, 4). Clinical data abstracted from the medical record included histologic diagnosis, subject age, gender, underlying diagnosis, diagnosis group (malignancy, immunodeficiency, or bone marrow failure), type of transplant (allogeneic versus autologous), type of conditioning (myeloablative vs. reduced intensity), specific conditioning chemotherapy, GVHD prophylaxis, presence of GVHD after transplantation, infectious complications, and the need for dialysis. The time the specimen was obtained in relation to transplant was also calculated.
C4d staining was performed by IHC on formalin-fixed, paraffin-embedded 4 Km tissue sections. No frozen tissue was available for IF. Rabbit anti-human C4d polyclonal antibody (1:20 titer) was applied to sections at 42-C for 32 min. The presence of antigen was visualized with an I-View DAB detection kit. C4d staining was graded by two pathologists (H.J.Y. and G.L.) blinded to each subject’s original pathologic diagnosis. C4d staining was evaluated on arterioles, PTCs, glomeruli, and tubular basement membranes (Fig. 1) and graded as diffuse (if staining involved >50% of the evaluated tissue), focal (10%–50% of tissue specimen), rare (1%–10% of tissue specimen), or negative (0%). Because IF has been reported as more sensitive for C4d staining than IHC (21, 38), we defined positive staining with our IHC assay as diffuse or focal. This lower cutoff defining positive staining is consistent with Banff criteria for the diagnosis of antibody-mediated kidney transplant rejection (17).
Descriptive statistics are presented as n (%) for categorical variables and median (25th–75th percentile and range) for continuous variables. To examine for differences between patients with a pathologic diagnosis of TA-TMA and controls, continuous variables were analyzed using Wilcoxon rank-sum test and categorical variables using Fisher’s exact test. A two-sided P<0.05 was considered statistically significant. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC).
Footnotes
The authors declare no funding or conflicts of interest.
References
- 1.Batts ED, Lazarus HM. Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant. 2007;40:709. doi: 10.1038/sj.bmt.1705758. [DOI] [PubMed] [Google Scholar]
- 2.Laskin BL, Goebel J, Davies SM, et al. Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation-associated thrombotic microangiopathy. Blood. 2011;118:1452. doi: 10.1182/blood-2011-02-321315. [DOI] [PubMed] [Google Scholar]
- 3.Nakamae H, Yamane T, Hasegawa T, et al. Risk factor analysis for thrombotic microangiopathy after reduced-intensity or myeloablative allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2006;81:525. doi: 10.1002/ajh.20648. [DOI] [PubMed] [Google Scholar]
- 4.Changsirikulchai S, Myerson D, Guthrie KA, et al. Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol. 2009;4:345. doi: 10.2215/CJN.02070508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stavrou E, Lazarus HM. Thrombotic microangiopathy in haematopoietic cell transplantation: an update. Mediterr J Hematol Infect Dis. 2010;2:e2010033. doi: 10.4084/MJHID.2010.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siami K, Kojouri K, Swisher KK, et al. Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation. 2008;85:22. doi: 10.1097/01.tp.0000297998.33418.7e. [DOI] [PubMed] [Google Scholar]
- 7.Chan GS, Lam MF, Au WY, et al. Clinicopathologic analysis of renal biopsies after haematopoietic stem cell transplantation. Nephrology (Carlton) 2008;13:322. doi: 10.1111/j.1440-1797.2007.00915.x. [DOI] [PubMed] [Google Scholar]
- 8.Glezerman IG, Jhaveri KD, Watson TH, et al. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:976. doi: 10.1016/j.bbmt.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laskin BL, Goebel J, Davies SM, et al. Early clinical indicators of transplant-associated thrombotic microangiopathy in pediatric neuroblastoma patients undergoing auto-SCT. Bone Marrow Transplant. 2011;46:682. doi: 10.1038/bmt.2010.182. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani S. Chronic kidney disease in long-term survivors of hematopoietic cell transplantation: epidemiology, pathogenesis, and treatment. J Am Soc Nephrol. 2006;17:1995. doi: 10.1681/ASN.2006020118. [DOI] [PubMed] [Google Scholar]
- 11.Parikh CR, Coca SG. Acute renal failure in hematopoietic cell transplantation. Kidney Int. 2006;69:430. doi: 10.1038/sj.ki.5000055. [DOI] [PubMed] [Google Scholar]
- 12.Cohen EP, Drobyski WR, Moulder JE. Significant increase in end-stage renal disease after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;39:571. doi: 10.1038/sj.bmt.1705643. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MJ, Parikh CR, Inrig JK, et al. Chronic kidney disease after hematopoietic cell transplantation: a systematic review. Am J Transplant. 2008;8:2378. doi: 10.1111/j.1600-6143.2008.02408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kersting S, Koomans HA, Hene RJ, et al. Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant. 2007;39:359. doi: 10.1038/sj.bmt.1705599. [DOI] [PubMed] [Google Scholar]
- 15.Noris M, Remuzzi G. Thrombotic microangiopathy after kidney transplantation. Am J Transplant. 2010;10:1517. doi: 10.1111/j.1600-6143.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 16.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 17.Mengel M, Sis B, Haas M, et al. Banff 2011 Meeting report: new concepts in antibody-mediated rejection. Am J Transplant. 2012;12:563. doi: 10.1111/j.1600-6143.2011.03926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 19.Mii A, Shimizu A, Kaneko T, et al. Renal thrombotic microangiopathy associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Pathol Int. 2011;61:518. doi: 10.1111/j.1440-1827.2011.02704.x. [DOI] [PubMed] [Google Scholar]
- 20.Mii A, Shimizu A, Masuda Y, et al. Renal thrombotic microangiopathy associated with chronic humoral graft versus host disease after hematopoietic stem cell transplantation. Pathol Int. 2011;61:34. doi: 10.1111/j.1440-1827.2010.02608.x. [DOI] [PubMed] [Google Scholar]
- 21.Batal I, Girnita A, Zeevi A, et al. Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol. 2008;21:1490. doi: 10.1038/modpathol.2008.152. [DOI] [PubMed] [Google Scholar]
- 22.Kikic Z, Regele H, Nordmeyer V, et al. Significance of peritubular capillary, glomerular, and arteriolar C4d staining patterns in paraffin sections of early kidney transplant biopsies. Transplantation. 2011;91:440. doi: 10.1097/TP.0b013e3182052be8. [DOI] [PubMed] [Google Scholar]
- 23.Colvin RB. Antibody-mediated renal allograft rejection: diagnosis and pathogenesis. J Am Soc Nephrol. 2007;18:1046. doi: 10.1681/ASN.2007010073. [DOI] [PubMed] [Google Scholar]
- 24.Troxell ML, Pilapil M, Miklos DB, et al. Renal pathology in hematopoietic cell transplantation recipients. Mod Pathol. 2008;21:396. doi: 10.1038/modpathol.3801011. [DOI] [PubMed] [Google Scholar]
- 25.Troxell ML, Weintraub LA, Higgins JP, et al. Comparison of C4d immunostaining methods in renal allograft biopsies. Clin J Am Soc Nephrol. 2006;1:583. doi: 10.2215/CJN.00900805. [DOI] [PubMed] [Google Scholar]
- 26.Mauiyyedi S, Crespo M, Collins AB, et al. Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol. 2002;13:779. doi: 10.1681/ASN.V133779. [DOI] [PubMed] [Google Scholar]
- 27.Bohmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 28.Haas M, Rahman MH, Racusen LC, et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 29.Fedrigo M, Gambino A, Tona F, et al. Can C4d immunostaining on endomyocardial biopsies be considered a prognostic biomarker in heart transplant recipients? Transplantation. 2010;90:791. doi: 10.1097/TP.0b013e3181efd059. [DOI] [PubMed] [Google Scholar]
- 30.Drachenberg CB, Torrealba JR, Nankivell BJ, et al. Guidelines for the diagnosis of antibody-mediated rejection in pancreas allografts-updated Banff grading schema. Am J Transplant. 2011;11:1792. doi: 10.1111/j.1600-6143.2011.03670.x. [DOI] [PubMed] [Google Scholar]
- 31.Kozlowski T, Andreoni K, Schmitz J, et al. Sinusoidal C4d deposits in liver allografts indicate an antibody-mediated response: diagnostic considerations in the evaluation of liver allografts. Liver Transpl. 2012;18:641. doi: 10.1002/lt.23403. [DOI] [PubMed] [Google Scholar]
- 32.Yousem SA, Zeevi A. The histopathology of lung allograft dysfunction associated with the development of donor-specific HLA alloantibodies. Am J Surg Pathol. 2012;36:987. doi: 10.1097/PAS.0b013e31825197ae. [DOI] [PubMed] [Google Scholar]
- 33.Landin L, Cavadas PC, Ibanez J, et al. CD3+-mediated rejection and C4d deposition in two composite tissue (bilateral hand) allograft recipients after induction with alemtuzumab. Transplantation. 2009;87:776. doi: 10.1097/TP.0b013e318198dbc7. [DOI] [PubMed] [Google Scholar]
- 34.Kanitakis J, McGregor B, Badet L, et al. Absence of C4d deposition in human composite tissue (hands and face) allograft biopsies: an immunoperoxidase study. Transplantation. 2007;84:265. doi: 10.1097/01.tp.0000266899.93315.52. [DOI] [PubMed] [Google Scholar]
- 35.Chan GS, Tse KC, Lam MF, et al. Thrombotic microangiopathy in an allograft kidney: a diagnostic challenge. Histopathology. 2006;48:775. doi: 10.1111/j.1365-2559.2006.02338.x. [DOI] [PubMed] [Google Scholar]
- 36.Troxell ML, Norman D, Mittalhenkle A. Glomerular fibrin thrombi in ABO and crossmatch compatible renal allograft biopsies. Pathol Res Pract. 2011;207:15. doi: 10.1016/j.prp.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 37.Meehan SM, Kremer J, Ali FN, et al. Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin J Am Soc Nephrol. 2011;6:395. doi: 10.2215/CJN.05870710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen D, Colvin RB, Daha MR, et al. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen D, Koopmans M, Kremer Hovinga IC, et al. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460. doi: 10.1002/art.23662. [DOI] [PubMed] [Google Scholar]
- 40.Shen Y, Chen XW, Sun CY, et al. Association between anti-beta2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: a prospective study of 155 cases. Lupus. 2010;19:1195. doi: 10.1177/0961203310368409. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen D, Buurma A, Goemaere NN, et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225:502. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 43.Mannon RB, Matas AJ, Grande J, et al. Inflammation in areas of tubular atrophy in kidney allograft biopsies: a potent predictor of allograft failure. Am J Transplant. 2010;10:2066. doi: 10.1111/j.1600-6143.2010.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mengel M, Bogers J, Bosmans JL, et al. Incidence of C4d stain in protocol biopsies from renal allografts: results from a multicenter trial. Am J Transplant. 2005;5:1050. doi: 10.1111/j.1600-6143.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 45.Papadimitriou JC, Drachenberg CB, Ramos E, et al. Antibody-mediated allograft rejection: morphologic spectrum and serologic correlations in surveillance and for cause biopsies. Transplantation. 2013;95:128. doi: 10.1097/TP.0b013e3182777f28. [DOI] [PubMed] [Google Scholar]
- 46.Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 47.Perkowska-Ptasinska A, Sulikowska-Rowinska A, Pazik J, et al. Thrombotic nephropathy and pulmonary hypertension following autologous bone marrow transplantation in a patient with acute lymphoblastic leukemia: case report. Transplant Proc. 2006;38:295. doi: 10.1016/j.transproceed.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 48.Hewamana S, Austen B, Murray J, et al. Intestinal perforation secondary to haematopoietic stem cell transplant associated thrombotic microangiopathy. Eur J Haematol. 2009;83:277. doi: 10.1111/j.1600-0609.2009.01267.x. [DOI] [PubMed] [Google Scholar]
- 49.Fang CJ, Richards A, Liszewski MK, et al. Advances in understanding of pathogenesis of aHUS and HELLP. Br J Haematol. 2008;143:336. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- 50.Worel N, Greinix HT, Leitner G, et al. ABO-incompatible allogeneic hematopoietic stem cell transplantation following reduced-intensity conditioning: close association with transplant-associated microangiopathy. Transfus Apher Sci. 2007;36:297. doi: 10.1016/j.transci.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:571. doi: 10.1016/j.bbmt.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 52.Choi CM, Schmaier AH, Snell MR, et al. Thrombotic microangiopathy in haematopoietic stem cell transplantation: diagnosis and treatment. Drugs. 2009;69:183. doi: 10.2165/00003495-200969020-00004. [DOI] [PubMed] [Google Scholar]
- 53.de Latour RP, Xhaard A, Fremeaux-Bacchi V, et al. Successful use of eculizumab in a patient with post-transplant thrombotic microangiopathy. Br J Haematol. 2013;161:279. doi: 10.1111/bjh.12202. [DOI] [PubMed] [Google Scholar]


