Abstract
Background
HIV-infection is associated with dramatic changes in the intestinal mucosa. The impact of other viral pathogens is unclear.
Methods
Eighty biopsies from left and right colon (n=63) and terminal ileum (n=17) were collected from 19 HIV-infected and 22 HIV-uninfected subjects. Levels of cytomegalovirus (CMV) and Epstein Barr Virus (EBV) DNA were measured by droplet-digital (dd)PCR. Mucosal gene expression was measured via multiplex-assay. Microbiome analysis was performed using bacterial 16S-rDNA-pyrosequencing. The effect of CMV and EBV replication on the microbiome composition and mRNA-expression of selected cytokines (IL-6, IFN-γ, IL-1β, CCL2, IL-8 IFN-β1) was evaluated.
RESULTS
Overall, CMV and EBV were detected in at least one intestinal site in 60.5% and 78.9% of subjects, respectively. HIV-infected individuals demonstrated less detectable CMV (p=0.04); CMV was more frequently detected in terminal ileum than colon (p=0.04). Detectable EBV was more frequent among HIV-infected (p=0.05) without differences by intestinal site. The number of operational taxonomic units did not differ by CMV or EBV detection status. Among HIV-infected subjects, higher CMV was only associated with lower relative abundance of Actinobacteria in the ileum (p=0.03). Presence of CMV was associated with up-regulated expression of all selected cytokines in the ileum (p<0.02) and higher expression of IL-8 and IFN-β1 in the colon (P<0.05) of HIV-uninfected subjects, but not among HIV-infected. EBV had no effect on cytokine expression or microbiome composition whatsoever.
CONCLUSIONS
These results illustrate a complex interplay between HIV-infection, intestinal CMV replication and mucosal gut environment, and highlight a possible modulatory effect of CMV on the microbial and immune homeostasis.
INTRODUCTION
Infection with Human Immunodeficiency Virus (HIV) is associated with alterations in the gut-associated lymphoid tissue (GALT), which occur early in the course of infection and contributes to persistent immune dysfunction and HIV disease progression [1, 2]. Mucosal HIV replication and consequent depletion of GALT-associated CD4+ T-cells is associated with epithelial barrier damage and increased translocation of bacterial products from the gut into systemic blood circulation (microbial translocation) [1–4]. In turn, microbial translocation is associated with systemic immune activation and predicts disease progression and mortality in untreated and treated HIV-infected individuals [1, 2, 5–7].
Recent studies described the effect of HIV-infection on the composition of the gut microbiome [8–10], and on the mucosal gene expression profile [11]. HIV-infection is associated with decreased diversity of the host intestinal microbiome, and “dysbiosis” which is characterized by a loss of bacterial taxa that are typically considered commensal (i.e., Lactobacilli and Bifidobacteria) with an increase in pathogenic bacteria (i.e., Pseudomonas and Proteobacteria) [8, 9]. HIV-infection is associated with up-regulation in the expression of various genes involved in cellular and humoral immune response, cytokine signaling and other relevant cellular pathways [11].
HIV is not the only chronic viral infection, which can replicate and persist in the gut mucosa. Cytomegalovirus (CMV) and Epstein Bar Virus (EBV), are prevalent among HIV-infected individuals, and can replicate within the intestinal mucosa causing severe diseases in the setting of immune suppression [12, 13]. The introduction of antiretroviral therapy (ART) reduced the incidence of AIDS-related viral colitis [14]. However, co-infection with CMV (and EBV to lesser extent) continues to contribute to morbidity and mortality in HIV-infected individuals and is associated with increased inflammation, immune dysfunction, senescence and HIV-disease progression [15, 16]. One study found that CMV replication in the gut was associated with intestinal damage in asymptomatic ART-suppressed HIV-infected individuals [17]. The authors demonstrated that CMV can productively infect intestinal epithelial cells and disrupts tight junctions, compromising epithelial barrier function. The CMV-induced pro-inflammatory cytokine IL-6 was highlighted as a key factor in this process, and attenuation of CMV replication by letermovir prevented CMV-induced loss of epithelial integrity. These data highlight the role of CMV as a possible co-factor in intestinal epithelial barrier dysfunction and suggest a possible novel target to prevent inflammation during HIV-infection.
METHODS
Clinical Study Subjects and Study Design
Colonic mucosal biopsy samples were obtained from the Rush University Medical Center (RUMC) Institutional Review Board (IRB)-approved gastrointestinal (GI) repository, from which 19 HIV-infected subjects and 22 controls were matched by demographic variables (age, sex and race). All subjects gave written informed consent for the use of tissue samples and data becoming part of an RUMC IRB GI-repository. Subjects were recruited from the same geographic area with the endoscopy being performed at the RUMC Endoscopy Lab. Colonoscopies were performed for colon cancer screening, constipation, minor rectal bleeding, and intermittent loose stools. Mucosal biopsies were obtained from the terminal ileum, right and left colon with a standard 2.2 mm biopsy forceps. Biopsies were then placed in a cryovial, flash frozen in liquid nitrogen and stored at −80°C, until the time of analysis. The characteristics of the study subjects were previously described [8, 11], and are summarized in Table 1. Overall, all HIV-infected subjects were on ART and all but three had HIV RNA below the limit of detection, at the time of sample collection.
Bacterial 16S ribosomal RNA (rRNA) Gene Sequencing, Sequence Processing and Quality Assessment
Sequencing
Sequencing procedures and analysis of generated data were previously described [8]. Briefly, DNA was extracted using a commercially available FastDNA Spin Kit for Soil, (MP Biomedicals, Solon, OH USA). The 16S rDNA gene was pyrosequenced on a 454 GS FLX platform, using the 28F forward primer, 5′-GAGTTTGATCNTGGCTCAG-3′ and the 519R reverse primer 5′-GNTTTACNGCGGCKGCTG-3′ were used for amplification of the variable regions V1-3 with barcoding. Two sequence runs were performed with about equal distribution of HIV and control cases in both runs.
Sequence Processing
The sequencing files were processed using custom C# and python scripts as well as python scripts in the Quantitative Insights Into Microbial Ecology (QIIME) software pipeline (VirtualBox Versions 1.5 and 1.6) [18–21]. Sequence outputs were filtered for low quality sequences (i.e., any sequences that are <200 or >1000 base pairs (bps), presenting any nucleotide mismatches to either the barcode or the primer, sequences including >6 homopolymer runs or ambiguous bases, sequences with an average quality score of >25) and were truncated at the reverse primer. Sequences were denoised using USEARCH [22], and chimera checked with UCHIME[23] and Chimera Slayer [24] as previously described [8]. Operational taxonomic units (OTUs) were picked using uclust [22] at 97% similarity, and representative sequences were generated. Sequences were aligned with PyNAST [25] and taxonomy assignment was performed in QIIME 1.6VB against the QIMME 1.6 version of Greengenes database [26, 27] using the RDP classifier [28, 29] with an 80% bootstrap threshold.
CMV and EBV DNA Measurements
Levels of CMV and EBV DNA were quantified by droplet digital PCR (ddPCR) [30, 31]. Copy numbers were calculated as the mean of replicate PCR measurements and normalized to one million cells, determined by RPP30 [32].
Assessment of gene expression
Gene expression analysis was performed via an Affymetrix (Santa Clara, California, USA) custom QuantiGene multi-plex assay [11]. We included 40 genes of interest (Supplementary Table 1) and two housekeeping genes: β-Actin and glyceraldehyde 3- phosphate dehydrogenase (GAPDH). Samples were prepared as tissue homogenates using Affymetrix lysis buffer and processed according to the manufacturer’s instructions and mRNA levels were determined using a Luminex-based custom multiplex bead array (Affymetrix).
Background values were subtracted from the raw intensity readings for each sample. Values that were less than or equal to 0 were set to 1 (floor effect), and all values were log2 transformed [11]. These values were normalized to the average of the two housekeeping genes.
Statistical analysis
For the following analyses, left and right colons were combined to create the site variable with 2 levels: colon vs. ileum. All analyses were performed in the R statistical language (ver. 3.3) [33], unless noted otherwise. All tests were performed 2-tailed at the 5% significance level without α adjustment for the following reasons: 1) we reduced the number of outcomes to be tested by applying a dimension-reduction technique (i.e., cytokine analysis), 2) we applied multilevel models where group comparisons tend to be more conservative because the groups’ point estimates as well as their intervals were “shrunk” and shifted closer to each other [34], and 3) our primary mode of analysis is Bayesian regression models, which calibrates evidence for a particular group difference using a single corresponding prior distribution without considering any other differences or any future assessments of the same group comparisons.
Analysis of CMV and EBV
To model subjects’ propensities to have detectable CMV and EBV, we used Bayesian hierarchical regression models [35]. Specifically, hierarchical models first estimate an intercept for the reference group (i.e., HIV-uninfected controls) and then an intercept for the HIV-subject group as a difference from the reference group. Second, within each group, the varying intercepts for the subjects are estimated as deviations from the group intercepts. We used a binomial family with a logit link, since 61.9% (65/105) and 45.7% (48/105) of the non-missing CMV and EBV DNA counts were undetectable (i.e., 0), respectively. For both outcomes, models included site (i.e., terminal ileum or colon) and HIV as fixed effects and individual identifier (ID) as a random intercept. A weakly informative prior distribution of N(0, 4) was used. Hierarchical models were evaluated using with R-hat values for model convergence (<1.1 = convergence) and the difference in the Leave-One-Out Information Criterion (ΔLOOIC) for model fit. We also provided the so-called Bayesian p-value (PB=[1-p(θ|X>0)]*2) for reference. The model-estimated individual intercepts were used as predictors to estimate how individual’s propensities to shed CMV and EBV affected cytokine activity levels and relative abundances of microbiome bacteria in the subsequent analyses. Raw CMV and EBV data divided by HIV status and intestinal site are included as supplementary material (Supplementary Figure 1).
Analysis of Cytokine Activity
Of the 41 subjects, 2 healthy control and 3 HIV-infected individuals were excluded because no cytokine data were available. Of the remaining 36 subjects, 31 had complete data (18 HIV-uninfected controls and 13 HIV-infected). The major challenge in analyzing the cytokine data was to summarize the high dimensional data of 40 cytokines sampled in 2 sites while handling missing values. To meet these challenges, we applied factor analysis for mixed data (FAMD) [36]. In particular, we used a version of FAMD that can iteratively estimates missing values, implemented in the missMDA R package [37]. We would retain dimensions that together explained at least 60% of the variances of the 40 cytokines to empirically identify sets of 3 cytokines that mostly contributed to forming each dimension. Afterwards, we used linear mixed-effects regression models to examine how CMV or EBV intercept levels, HIV status, and intestinal site interacted to influence activities of our set of 3 pre-selected cytokines. The CMV and EBV intercepts were dichotomized for easier interpretation. In addition to the empirically selected 3 cytokines, CMV can induce IL-6, IFN-γ and IL-1β, therefore those cytokines were selected a priori to be part of the analysis [38–40]. Raw cytokine data (divided by HIV status, intestinal site and CMV/EBV detectability) are included as supplementary material (Supplementary Figures 2 and 3).
Analysis of Microbiome Data
Of the 41 subjects, 2 healthy-control individuals and one HIV-infected individual were excluded because microbiome data were available. To model microbiome bacterial abundance data, we applied a Bayesian hierarchical regression model with the HIV status, site, and CMV/EBV intercept as fixed effects and subject ID as a random intercept to each phylum separately. Similarly to the cytokine analysis described above, the CMV and EBV intercepts were dichotomized for easier interpretation. Each model included 2- and 3-way interactions among the fixed effects. If the maximum count with a particular phylum equaled to 1, a binomial family was used; otherwise, a negative binomial family was used to model the count data. We evaluated the probability of an estimated parameter contained a zero or not with a 95% posterior credible interval. For a reference purpose, “p-values” are provided by computing the proportion of posterior samples that were greater or less than 0. We performed a principal coordinates analysis of all the samples using the Unifrac metric at the OTU level according to the HIV and CMV/EBV detection status. Raw microbiome data (divided by HIV status, intestinal site and CMV/EBV detectability) are included as supplementary material (Supplementary Figures 4 and 5).
Sensitivity Analysis
To control for potential confounding effects of relevant covariates, we performed sensitivity analysis for each outcome with sexual orientation, sex, age, body Mass index (BMI), HIV RNA viral load (dichotomized to detected versus non detected), and race.
RESULTS
Study cohort
There were no statistically significant differences in terms of age, gender, and race among the HIV-infected and the control subjects (Table 1). Among HIV-infected subjects, the mean CD4+ T cell count was 425 cells/μl. The HIV RNA levels were below the limit of detection (<75 copies/ml) for 16 out of 19 patients; the remaining 3 subject had viral loads of 179, 1094 and 4604 copies /ml.
Table 1.
| HIV-Uninfected Controls (n=22) | HIV-Infected Subjects (n=19) | |
|---|---|---|
| Age in years, Median (IQR) | 54.5 (51–59) | 52 (50–55) |
| Sex, Male/Female | 17/5 | 16/3 |
| Sexual Orientation | ||
| Homosexual | 16 | 12 |
| Heterosexual | 4 | 3 |
| Not known | 2 | 4 |
| BMI in kg, Median (IQR) | 29.7 (25.7–31) | 24.8 (22.7–32.3) |
| Race | ||
| African American | 12 | 8 |
| Asian | 1 | 1 |
| Hispanic | 6 | 6 |
| White | 3 | 4 |
| CD4, Median (IQR) | N/A | 334 (220–581) |
| HIV RNA cp/mL, Median (min-max) | - | nd (nd-4604) |
| Ileum Biopsy Samples | 14 | 15 |
| Right Colon Biopsy Samples | 22 | 19 |
| Left Colon Biopsy Samples | 22 | 16 |
Among the 19 HIV-positive individuals, 12 self-identified as men who have sex with men (MSM), 4 as heterosexuals and for 5 participants sexual orientation was unknown. Among the 22 HIV-negative individuals, 16 were heterosexual, 4 MSM, and 2 had unknown orientation.
Summary of Sequencing Data
We obtained 1,079,589 raw sequences, and 322,061,108 raw bases with an average of 8,849 sequences/sample at an average length of 298 bps/sequence. After quality–filtering, 455,452 total sequences and an average of 3,733 sequences per sample were available, that were processed for the rest of the analysis as described in the methods. The sequences were rarified to the minimum number of high-quality sequences in all samples and normalized by total count for the diversity analyses [8].
Model results for intestinal CMV and EBV replication
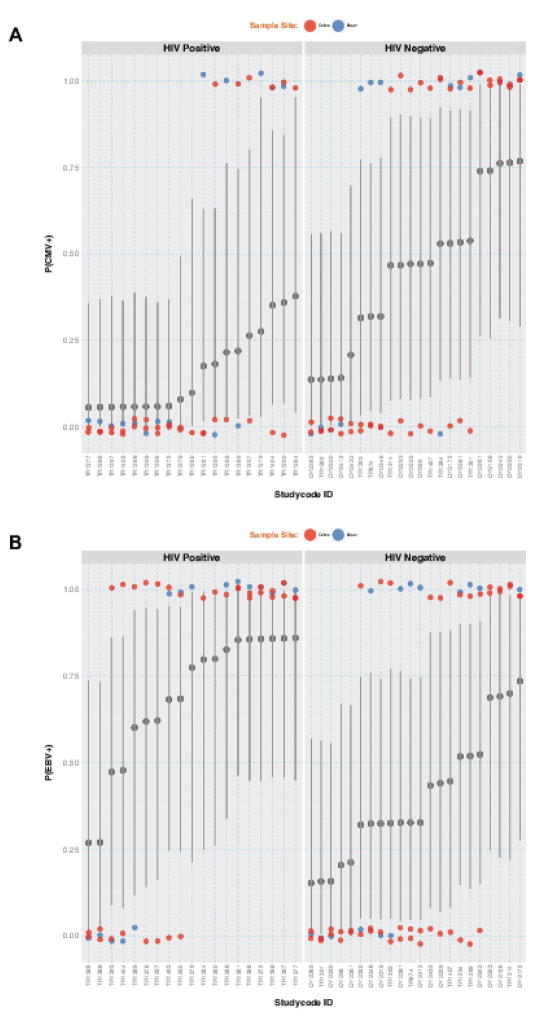
Irrespective of HIV status, CMV and EBV were detected in at least one intestinal site in 60.5% and 78.9% of the study participants, respectively. Both variables were analyzed with Bayesian hierarchical regression models with a binomial family with random intercepts. HIV-infected individuals were less likely to present detectable CMV than HIV-uninfected controls (median OR = 0.19 [95% Credible Interval = 0.03 ~ 0.78], PB=0.02, Figure 1A), and CMV was more likely to be detected in the terminal ileum than in the colon (OR = 3.20 [1.002 ~ 11.21], PB=0.05). HIV-infected subjects were more likely to have detectable EBV than HIV-uninfected controls (OR = 3.92 [1.08 ~ 21.41], PB=0.04, Figure 1B), while the odds of detecting EBV in the ilium was similar to that in the colon (OR=1.80 [0.64~5.76], PB=0.3).
Figure 1. Posterior medians and 95% intervals for probabilities of detecting CMV (pCMV, Panel A) and EBV (pEBV, Panel B) DNA, derived from Bayesian hierarchical regression models.
Y-axis represents individual study participants. Small dots represent observed data with colors indicating sample sites (red = colon, blue = ileum). Large grey dots, vertical grey lines, and the horizontal grey line indicate estimated random intercepts for subjects, 95% intervals, and the group intercepts, respectively. Observed values are jittered to avoid overlap.
Model Results for Cytokine Activity Levels
Since CMV can induce IL-6, IFN-γ and IL-1β, those cytokines were selected a priori to be part of the analysis [38–40] and were not included in the following factor analysis. In the 36 subjects (20 control and 16 HIV-infected) with cytokine data, the FAMD method was applied to 37 cytokine activities, as continuous variables and site as a categorical variable, first imputing missing data and then extracting dimensions that together explained at least 60% of the variance: Dimension 1 explained 62.9% of the variance. Therefore, 3 cytokines that contributed most to forming Dimension 1 were included in the final analysis: CCL2, IL-8, IFN-β1. In the subsequent analysis, the activity levels for six selected cytokines (i.e., three empirically selected CCL2, IL-8, IFN-β1 and three selected a priori IL-6, IFN-γ and IL-1β) are modeled against intestinal site, HIV status, and CMV/EBV intercept levels.
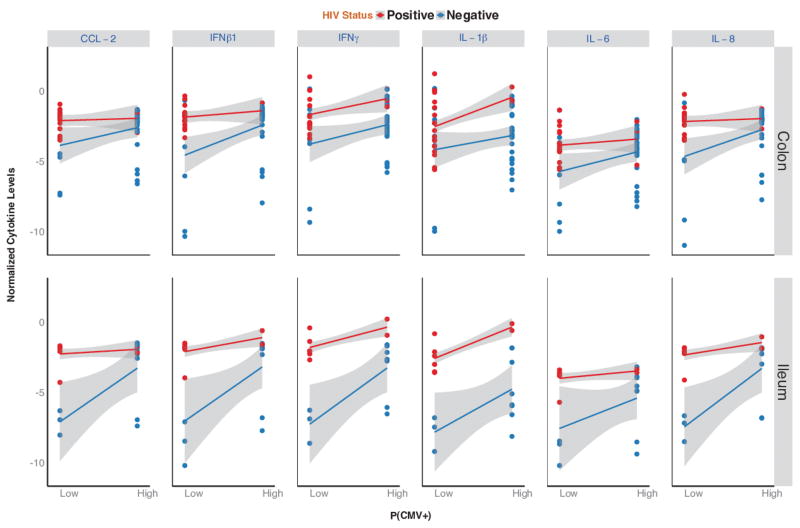
The subsequent model revealed a significant interaction between HIV status, intestinal site, cytokine type and probability of detecting CMV (P<0.001). The CMV intercept was dichotomized at the median (p(CMV+)= 0.27) to facilitate the interpretation. Specifically among the HIV-uninfected controls, higher probabilities of detecting CMV was associated with significantly up-regulated expression of all 6 pre-selected cytokines in the ileum (all P<0.02) and higher expression of IL-8 and IFN-β1 in the colon (P<0.05, Figure 2). Presence of CMV was not associated with any difference in mucosal gene expression among HIV-infected subjects (all P>0.14). When CMV was detected at lower probabilities, gene expression levels were significantly higher in HIV-infected participants than in HIV-uninfected controls both in the ileum (all P<0.001) and in the colon (all P<0.05, except for IL-1β: p=0.09). Conversely, when CMV was detectable with higher probability, gene expression was not significantly different between the two groups in the colon (all P>0.17, except for IL-1β: p=0.04) or in the ileum (all P>0.07, except for IL-1β: p<0.01). There was no significant effect of detectable EBV on any colonic mucosal biopsy site in either group (all P>0.17).
Figure 2. Cytokine levels for six selected cytokines (CCL-2, INFβ1, IFNγ, IL1β, IL-6 and IL-8) for HIV positive (in red) and HIV uninfected controls (in blue) divided by intestinal site (colon versus ileum).
X-axis shows probability of detecting CMV DNA (low versus high); Y-axis shows cytokine levels (normalized to the average of the two housekeeping genes). The lines and grey areas represent model-based estimates and their 95% confidence intervals
Model results for Intestinal Microbiome Composition
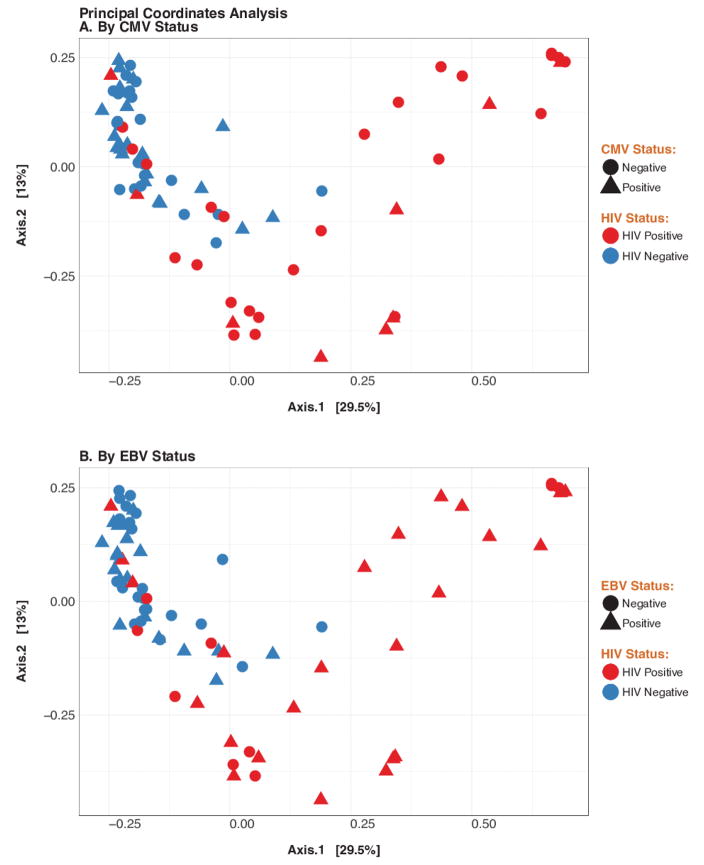
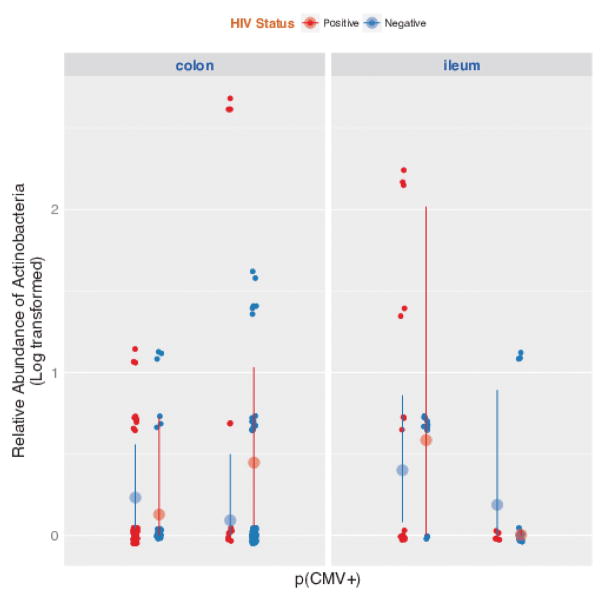
The relative abundance of the main phyla in the colon and ileum was assessed in relation to HIV-status and presence of CMV or EBV. While HIV-infection was associated with significantly lower beta-diversity [8], the number of OTU did not differ according to CMV or EBV detection status. Principal coordinates analysis of all of the samples at the OTU level confirmed clustering of samples by HIV-status, but not by CMV or EBV (Figure 3). Among HIV-infected subjects, a higher probability of detecting CMV was associated with lower relative abundance of Actinobacteria (p=0.03) in the ileum. CMV was not associated with any significant shift in the microbiome composition of HIV-uninfected controls. There was no significant association between detectable EBV and the microbiome composition for either group or site.
Figure 3. Principal coordinates analyses (PCA) of gut microbiome beta diversity among HIV-infected and uninfected control subjects, by CMV and EBV replication status.
Panel A shows PCA applied to the Unifrac metric colored by HIV status and shaped by CMV status. Similarly, Panel B shows PCA of all samples colored by HIV status and shaped by EBV status; x and y-axes represent first and second principal coordinates, respectively.
Sensitivity Analysis
All described analyses were repeated with relevant covariates to control for potential confounding effects. First, to assess the potential effect of sexual orientation among the HIV-positive group, we divided HIV-positives into MSM (n=10~12), heterosexuals (n=0~4), and unknown sexual orientation (n=3~4). Note that sample sizes are variables because of the missing values for some outcomes. We did not observe any significant differences between MSM and heterosexual people on CMV/EBV shedding, cytokine expression, or microbiome composition, regardless of how patients with unknown sexual orientation were imputed (e.g., as MSM, heterosexual, or not imputed, ps>0.2). Second, we assessed the potential confounding effects of other relevant covariates – sex, age, BMI, viral load (dichotomized into detected (n=3) vs non-detected (n=15)), and race on each of the outcome. The only significant covariate effect was the effect of sex on cytokines: males had overall higher cytokine levels averaged across the 6 included in the model (1.66±0.80, p=0.03). More importantly, all significant effects remained significant (ps<0.05) regardless of which covariate was included in the model.
DISCUSSION
Microbial translocation is linked to mucosal and systemic inflammation and is predictive of disease progression and mortality in HIV-infected individuals [1, 5–7, 41]. It is important to understand what factors, other than HIV itself, might influence the gut microbiome composition and the mucosal inflammatory profile. After primary HIV-infection, T-cell reconstitution in the gastrointestinal tract never reaches the levels observed in uninfected patients [42, 43] and intestinal mucosa could serve as an important sanctuary of viral reactivation. For example, CMV and EBV can replicate in the gut and might contribute to local and systemic inflammation during HIV-infection [13].
In our study, we investigated the effect of subclinical CMV and EBV replication in the intestinal mucosa on cytokine expression and microbiome composition of HIV-infected subjects receiving ART and -uninfected controls.
We observed that CMV and EBV are frequently detected in the intestinal mucosa of virally suppressed HIV-infected and -uninfected subjects. HIV-infected subjects had an increased probability of detecting EBV compared to HIV-negative subjects. Surprisingly, the HIV-infected subjects had significantly less CMV compared to HIV-uninfected subjects. This is inconsistent with previous studies showing increased CMV shedding rates in blood and other mucosal sites [13], especially genital and oral mucosa, and might be a consequence of depletion of target cells in the gut of HIV-infected subjects.
In a previous study, we observed that HIV-infection was associated with up-regulation in the expression of various genes involved in cellular and humoral immune response, cytokine signaling and other relevant cellular pathways, compared to HIV-uninfected controls [11]. Using the same dataset, we investigated how the presence of detectable intestinal CMV and EBV might affect mucosal cytokine expression.
To summarize the high-dimensional dataset, we used factor analysis for mixed data to compute dimensions that together explained >60% of the total variance and a set of three cytokines were identified (i.e., CCL2, IL-8, IFN-β1). Based on prior knowledge, IL-6, IFN-γ and IL-1β were also included in the analysis. We found that the presence of detectable CMV was associated with significantly up-regulated expression of all selected cytokines in the ileum, as well as increased IL-8 and IFN-β1 gene expression in the colon of HIV-uninfected controls. Conversely, the presence of CMV was not associated with any difference in mucosal gene expression among HIV-infected subjects. In fact, when CMV was detectable, gene expression levels in the terminal ileum of HIV-uninfected controls was similar to those of HIV-infected subjects. There was no significant effect of EBV replication on any gut mucosal biopsy site in both groups.
In summary, similar to what was described for other mucosal sites [15, 44], our data highlight a possible immune-modulatory role of CMV replication in the gut of HIV-uninfected subjects. Both HIV and CMV are associated with similar pro-inflammatory changes in the intestinal mucosa, but no additive effect was observed. It is unknown if the observed increase in cytokine expression in the gut of HIV-uninfected subjects with detectable CMV, might be associated with a compromised mucosal epithelial function initiating microbial translocation or any other clinical outcome.
Subsequently, we evaluated if presence of CMV or EBV was associated with any changes in the intestinal microbiome composition. While HIV-infection was associated with significantly lower beta-diversity [8], the number of OTU did not differ according to CMV or EBV detection status. When looking at the relative abundance of the main phyla in the colon and ileum, we observed that higher levels of CMV were significantly associated with lower relative abundance of Actinobacteria (P=0.03) in the ileum of HIV-infected individuals. Conversely, CMV was not associated with any significant shift in the microbiome of HIV-uninfected controls.
There are several limitations to the current study. This exploratory microbiome analysis was not powered to specifically address changes in the microbiome and cytokine composition and their relationship with virological outcomes. In addition, sexual preference was not matched between groups and this might have affected our results [45]. We only detected CMV and EBV DNA and we did not look for the presence of structural proteins, so we cannot definitively establish presence of active viral replication. Lastly, since CMV and EBV replication likely presents a patched pattern of infected cells in mucosal tissues, our study will be subject to sampling error. As a result, the observed links between CMV and EBV replication and gut mucosal changes need to be confirmed in a larger cohort of study subjects. Despite the mentioned limitations and even if its effect is much less dramatic than HIV, CMV could play a modulatory role on the microbiome composition during HIV-infection and affect the immune homeostasis in the gut.
Supplementary Material
Figure 4. Relative abundance for Actinobacteria for HIV positive (in red) and HIV uninfected controls (in blue) divided by intestinal site (colon versus ileum) and by CMV detection status.
X-axis shows probability of detecting CMV DNA (low versus high); Y-axis shows relative abundance (log transformed) of Actinobacteria phylum. The large dots and vertical lines represent means and their 95% confidence intervals
Acknowledgments
We are grateful to all the participant, the CFAR Genomic and Translational Virology Cores.
Footnotes
Author Contributions
SG and AC participated in the study design and data analysis, and wrote the primary version of the manuscript; MN performed the primary data analysis and wrote the primary version of the manuscript; EAM, PAE, RMV, AK, JL, PC participated in the study design, enrolled study participants an performed endoscopic procedures and revised the manuscript; SL and DMS contributed to the study design, enrolled all participants, and revised the manuscript; SL performed laboratory experiments and revised the manuscript. ALL participated in the study design, participated in the data analyses, and wrote the primary version of the manuscript. All authors read and approved the final manuscript.
Competing Interests
No conflicts of Interests to declare.
Financial Disclosure
This work was supported primarily by a grant from the National Institutes of Health, University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research, P30-AI027763 (CNIHR), California HIV Reserch Program Ideal award to Sara Gianella, by the department of Veterans Affairs, the James B. Pendleton Charitable Trust and additional grants from the National Institutes of Health: AI100665, MH100974, MH097520, DA034978, AI007384, AI027763, AI106039, AI43638, AI074621, AI036214, MH101012, UL1TR000100, CARE U19 AI096113 and AI068636-09. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–71. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 2.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blodget E, Shen C, Aldrovandi G, et al. Relationship between microbial translocation and endothelial function in HIV infected patients. PLoS One. 2012;7:e42624. doi: 10.1371/journal.pone.0042624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klatt NR, Harris LD, Vinton CL, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchetti G, Cozzi-Lepri A, Merlini E, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 2011;25:1385–94. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 7.Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nature reviews Microbiology. 2012;10:655–66. doi: 10.1038/nrmicro2848. [DOI] [PubMed] [Google Scholar]
- 8.Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–94. doi: 10.1038/mi.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV-1 pathogenesis: a two-way street. AIDS. 2016;30:2737–51. doi: 10.1097/QAD.0000000000001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voigt RM, Keshavarzian A, Losurdo J, et al. HIV-associated mucosal gene expression: region-specific alterations. AIDS. 2015;29:537–46. doi: 10.1097/QAD.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto H, Kimura Y, Murao T, et al. Severe Colitis Associated with both Epstein-Barr Virus and Cytomegalovirus Reactivation in a Patient with Severe Aplastic Anemia. Case Rep Gastroenterol. 2014;8:240–4. doi: 10.1159/000365546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gianella S, Massanella M, Wertheim JO, Smith DM. The Sordid Affair between Human Herpesvirus and Human Immunodeficiency Virus. J Infect Dis. 2015;212:845–52. doi: 10.1093/infdis/jiv148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer KL, Weinberg A. Cytomegalovirus infection in the era of HAART: fewer reactivations and more immunity. J Antimicrob Chemother. 2004;54:582–6. doi: 10.1093/jac/dkh396. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ML, Lederman MM, Gianella S. Partners in Crime: The Role of CMV in Immune Dysregulation and Clinical Outcome During HIV Infection. Curr HIV/AIDS Rep. 2016;13:10–9. doi: 10.1007/s11904-016-0297-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianella S, Letendre S. CMV and HIV: A Dangerous “Pas de Deux”. J Infect Dis. 2016 doi: 10.1093/infdis/jiw217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maidji E, Somsouk M, Rivera JM, Hunt PW, Stoddart CA. Replication of CMV in the gut of HIV-infected individuals and epithelial barrier dysfunction. PLoS Pathog. 2017;13:e1006202. doi: 10.1371/journal.ppat.1006202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolcott RD, Gontcharova V, Sun Y, Dowd SE. Evaluation of the bacterial diversity among and within individual venous leg ulcers using bacterial tag-encoded FLX and titanium amplicon pyrosequencing and metagenomic approaches. BMC microbiology. 2009;9:226. doi: 10.1186/1471-2180-9-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sen R, Ishak HD, Estrada D, Dowd SE, Hong E, Mueller UG. Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci U S A. 2009;106:17805–10. doi: 10.1073/pnas.0904827106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishak HD, Plowes R, Sen R, et al. Bacterial diversity in Solenopsis invicta and Solenopsis geminata ant colonies characterized by 16S amplicon 454 pyrosequencing. Microbial ecology. 2011;61:821–31. doi: 10.1007/s00248-010-9793-4. [DOI] [PubMed] [Google Scholar]
- 21.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 23.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas BJ, Gevers D, Earl AM, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011;21:494–504. doi: 10.1101/gr.112730.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics. 2010;26:266–7. doi: 10.1093/bioinformatics/btp636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME journal. 2012;6:610–8. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner JJ, Koren O, Hugenholtz P, et al. Impact of training sets on classification of high-throughput bacterial 16s rRNA gene surveys. The ISME journal. 2012;6:94–103. doi: 10.1038/ismej.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saemann MD, Bohmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2000;14:2380–2. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 30.Strain MC, Lada SM, Luong T, et al. Highly Precise Measurement of HIV DNA by Droplet Digital PCR. PLoS One. 2013;8:e55943. doi: 10.1371/journal.pone.0055943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gianella S, Anderson CM, Var SR, et al. Replication of Human Herpesviruses Is Associated with Higher HIV DNA Levels during Antiretroviral Therapy Started at Early Phases of HIV Infection. J Virol. 2016;90:3944–52. doi: 10.1128/JVI.02638-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massanella M, gianella S, Lada SM, richman DD, strain MC. Quantification of Total and 2-LTR (Long terminal repeat) HIV DNA, HIV RNA and Herpesvirus DNA in PBMCs. bio-protocol. 2015 doi: 10.21769/bioprotoc.1492. http://www.bio-protocol.org/e1492. [DOI] [PMC free article] [PubMed]
- 33.Team RC. The R Project for Statistical Computing. 2016 https://wwwr-projectorg/
- 34.Gelman A, Hill H, Yajima M. Why We (Usually) Don’t Have to Worry About Multiple Comparisons. J Res Educ Eff. 2012;5:189–211. [Google Scholar]
- 35.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis. 3 2013. [Google Scholar]
- 36.Le LS, Josse J, Husson F. FactoMineR: An R Package for Multivariate Analysi. Journal of Statistical Software. 2008;25:1–18. [Google Scholar]
- 37.Josse J, Husson F. missMDA: A Package for Handling Missing Values in Multivariate Data Analysis. Journal of Statistical Software. 2016;70:1–31. [Google Scholar]
- 38.Carlquist JF, Edelman L, Bennion DW, Anderson JL. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-kappaB. J Infect Dis. 1999;179:1094–100. doi: 10.1086/314734. [DOI] [PubMed] [Google Scholar]
- 39.Almeida GD, Porada CD, St Jeor S, Ascensao JL. Human cytomegalovirus alters interleukin-6 production by endothelial cells. Blood. 1994;83:370–6. [PubMed] [Google Scholar]
- 40.Bruewer M, Luegering A, Kucharzik T, et al. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 41.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–8. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 42.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–59. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–17. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gianella S, Strain MC, Rought SE, et al. Associations between virologic and immunologic dynamics in blood and in the male genital tract. J virol. 2012;86:1307–15. doi: 10.1128/JVI.06077-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noguera-Julian M, Rocafort M, Guillen Y, et al. Gut Microbiota Linked to Sexual Preference and HIV Infection. EBioMedicine. 2016;5:135–46. doi: 10.1016/j.ebiom.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.