Background
Hemorrhagic shock (HS) is an important cause of mortality worldwide. HS is commonly associated with civilian and combat trauma, upper and lower gastrointestinal bleeding, obstetric and gynecologic bleeding, ruptured aneurysms, and iatrogenic vascular injury [1-5]. In the United States, HS accounts for 40% of the deaths after severe traumatic injury, which is the main cause of death in individuals younger than 44 years of age [1, 2].
HS rapid lethality is a consequence of severely reduced tissue perfusion resulting in inadequate delivery of oxygen. Indeed, about one out of every two patients with HS due to severe trauma die before arrival at the hospital [2], and one out of every two patients who make it to the hospital die within 3 hours of admission [6]. Patients surviving the initial shock and resuscitation have an elevated incidence of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (up to 20% of intensive care unit patients), further aggravating the patient's condition and contributing to HS overall morbidity and mortality [6-8]. The current standard of care for HS consists of timely intervention to control bleeding, volume replacement, and whole blood or blood component therapy [9]. Recent advances in HS shock treatment, however, have led to disappointingly small improvements in patient survival and in the incidence of ALI [3, 10, 11].
HS-associated lung injury is generally thought to develop in part due to reperfusion, which can cause cellular stress and the release of damage-associated molecular pattern (DAMP) molecules and inflammatory mediators, leading to leukocyte and vascular endothelial cell (EC) activation, increased capillary permeability, neutrophil infiltration, and tissue injury [12-15]. Cold-inducible RNA-binding protein (CIRP) is a highly conserved nuclear protein upregulated by hypoxia and mild hypothermia [16, 17]. We have discovered that, during HS, CIRP translocates from the nucleus to the cytoplasm, and is subsequently released in the circulation [18]. Once released, CIRP acts as a damage-associated molecular pattern molecule (DAMP) to increase HS severity and mortality rate [18, 19]. We have recently shown that CIRP injection causes healthy mice to develop lung injury, as evidenced by lung histology, neutrophil infiltration, and local production of TNF-α and IL-1β [20]. CIRP also induced lung vascular EC activation leading to increased cell-surface expression of the adhesion molecules E-selectin and ICAM-1, activation of the NRLP3 inflammasome, and increased vascular permeability [20]. These observations suggest that CIRP may play a critical role in the development of lung injury associated with HS.
We have recently identified C23 as an oligopeptide derived from the human CIRP protein (Ser110-Glu125) that binds with very high affinity to the CIRP receptor [18]. Furthermore, C23 inhibits CIRP-induced phagocyte secretion of TNF-α [18]. As such, we hypothesized that, by blocking CIRP, adjuvant treatment with C23 should decrease activation of leukocytes and EC and, thus, attenuate lung injury caused by HS. Therefore, in this study we sought to determine the effects of C23 on systemic and pulmonary injury in a mouse model of HS.
Methods
Animals
Male C57BL/6 mice (25-30 g) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in temperature controlled rooms with 12-h light cycles and fed a standard mouse diet. Mice were acclimated for one week before experimentation. All experiments involving live animals were carried out in accordance with the National Institutes of Health guidelines for the use of experimental animals and were reviewed and approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research.
Animal model of HS
At the beginning of the experiment, mice were anesthetized with isoflurane. After identification of the femoral nerves, the right and left femoral arteries were cannulated with PE-10 polyethylene tubing (BD, Sparks, MD) containing a small amount of heparin (2 IU/ml) in normal saline solution. The left femoral artery cannula was connected to a digital blood pressure analyzer (BPA; Digi-Med, Louisville, Ky) for continuous monitoring of the mean arterial pressure (MAP) and heart rate (HR), and the right femoral artery cannula was used for controlled hemorrhage and fluid replacement. After cannulation, mice were observed until stabilization of the MAP at 90 mmHg. HS was induced by slow withdrawal of blood through the right femoral arterial cannula over 10-15 min, until the MAP reached 25 mmHg. The MAP was then maintained at 25 ± 3 mmHg for 90 min by withdrawal or replenishment of shed blood (Fig. 1A). Sham-operated animals underwent the same surgical procedures but were neither hemorrhaged nor resuscitated.
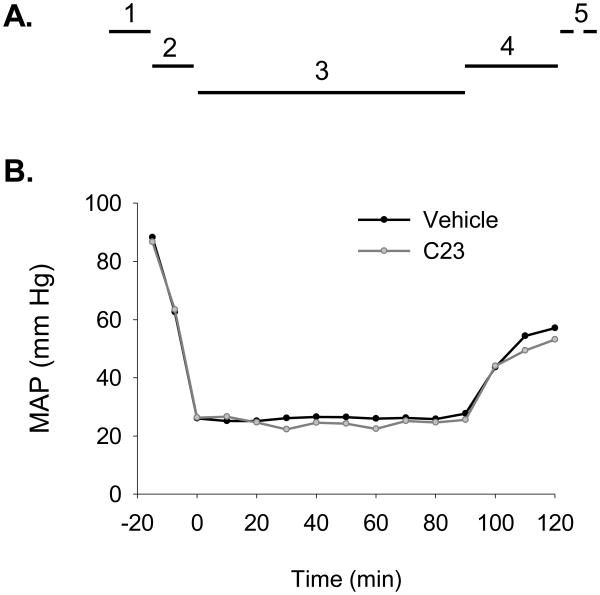
Figure 1. Hemorrhagic shock model and mean arterial pressure (MAP).
(A) The model of hemorrhagic shock used consisted of (1) femoral artery cannulation and MAP stabilization time, 10-15 min; (2) controlled hemorrhage, 15 min; (3) hemorrhagic shock, 90 min; (4) resuscitation with normal saline solution and adjuvant treatment with C23 or vehicle, 30 min; and (5) observation period until blood and lung tissue collection, 210 min. (B) Mice adjuvantly treated with vehicle or C23 (n=6 per group) had similar MAP measurements from the femoral artery cannulation stage up to the end of the resuscitation period.
Resuscitation and adjuvant treatment
At 90 min of HS, mice received low-volume (two times the volume of shed blood) resuscitation with room-temperature normal saline solution, infused over 30 min via the right femoral artery. Shed blood was not used for resuscitation, and animals did not receive heparin at any moment. Synthetic C23 (GRGFSRGGGDRGYGG) was obtained from GenScript USA Inc. (Piscataway, NJ; >95% purity). In addition to the resuscitation fluid, each mouse was randomly allocated to receive only normal saline solution (vehicle) or adjuvant treatment with C23 (8 μg/kg BW). After resuscitation, isoflurane anesthesia was discontinued and mice were returned to their cages.
Collection of blood and lungs
At 4.5 hours after the end of resuscitation, mice were anesthetized with isoflurane for collection of blood and lungs. After clotting, blood samples were centrifuged at 1000g for 10 min at 4°C, and the resulting serum samples were stored at ‐80°C until assayed. A section of lung tissue was preserved in 10% formalin for histopathological analysis. The remaining lung tissue was flash-frozen in liquid nitrogen and stored at -80°C until assayed.
Determination of organ injury markers
Serum levels of aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were determined using specific colorimetric enzymatic assays (Pointe Scientific, Canton, MI) according to the manufacturer's instructions.
Assessment of lung IL-1β, TNF-α and IL-6 mRNA
The mRNA expression of lung IL-1β, TNF-α, and IL-6 proinflammatory cytokines was assessed by real-time quantitative PCR (qPCR). RNA was extracted from lung tissues with TRIzol reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed into cDNA using murine leukemia virus reverse transcriptase (ThermoFisher Scientific, Waltham, MA). qPCR reactions were carried out in 25 μl containing 0.08 μmol of each forward and reverse primer (Table I), 5 μl cDNA, 6.5 μl H2O, and 12.5 μl SYBR Green PCR Master Mix (ThermoFisher Scientific). Amplification was conducted in duplicates in a 7300 real-time thermocycler (Applied Biosystems, Foster City, CA) with a thermal profile of 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. Relative expression of mRNA normalized to mouse β-actin was determined using the 2(-ΔΔCt) method [21].
Table I. Primer sequences used for cDNA amplification of mouse genes.
| Protein | Gene | RefSeq | Forward primer | Reverse primer |
|---|---|---|---|---|
| β-actin | Actb | NM_007393 | CGTGAAAAGATGACCCAGATCA | TGGTACGACCAGAGGCATACAG |
| IL-1β | Il1b | NM_008361 | CAGGATGAGGACATGAGCACC | CTCTGCAGACTCAAACTCCAC |
| TNF-α | Tnfa | X02611 | AGACCCTCACACTCAGATCATCTTC | TTGCTACGACGTGGGCTACA |
| IL-6 | Il6 | NM_031168 | CCGGAGAGGAGACTTCACAG | CAGAATTGCCATTGCACAAC |
Measurement of myeloperoxidase (MPO) activity
MPO activity in the lungs was determined in duplicates using the peroxidase catalyzed reaction. Lung samples were sonicated in 50 mM potassium phosphate buffer containing 0.5% hexadecyltrimethylammonium bromide. After centrifugation, the sonicate supernatant was diluted in reaction solution containing o-dianisidine hydrochloride and H2O2. The MPO activity was defined as the rate of change in the 460-nm optical density (OD) over 1 min, as previously described [18].
HE staining
Formalin-fixed lung samples were embedded in paraffin, microsectioned at 4 μm, and stained with hematoxylin and eosin (HE). Histological examination was performed by an examiner blinded to treatment allocation. Lung injury score was assessed using a semi-quantitative light microscopy evaluation, as previously described [22].
Lung extravasation assay
Lung vascular leakage was quantified using the Evans blue dye (EBD)-labeled albumin extravasation assay [23]. At 3.5 hours after the end of resuscitation,mice were injected EBD (30 mg/kg in 100 μl; Sigma-Aldrich, St. Louis, MO) via the jugular vein. At 1 hour after EBD administration (4.5 hours after the end of resuscitation) the lungs were perfused with heparinized (1 U/ml) normal saline via right ventricle injection to remove intravascular dye. The left lung was removed for photographic documentation. The right lung was dehydrated for 48 hours at 60°C, immersed in formamide (4 ml/g wet weight, Sigma-Aldrich) at 37°C for 24 h, and centrifuged at 5,000g for 30 min. The amount of extracted EBD in the supernatant was quantified by spectrophotometry. The concentration of extravasated EBD-albumin in lung homogenates was expressed as ng EBD per mg of dry lung tissue.
Determination of ICAM1 protein levels
Lung tissues were homogenized in radioimmunoprecipitation assay (RIPA) cell lysis buffer (10 mM Tris-HCl, pH 7.5, 120 mM NaCl, 1% NP-40, 1% sodium deoxycholate and 0.1% SDS) containing a protease inhibitor cocktail (Roche, Indianapolis, IN). The total protein concentration was determined using a colorimetric assay (Bio-Rad, Hercules, CA). The extracted protein (25 μg) was fractionated on a Bis-Tris gel and transferred to 0.2 μm nitrocellulose membrane. The membranes were blocked and incubated overnight with rabbit polyclonal anti-ICAM-1 antibodies (1:500; M-19; Santa Cruz Biotech, Dallas, TX) at 4°C. The membranes were then incubated with horseradish peroxidase-labeled secondary antibodies, reacted with chemiluminescence peroxidase substrate (ECL, Amersham Biosciences, Piscataway, NJ), and exposed to radiograph film. Band densities were determined using NIH ImageJ software. Protein loading for each lane was normalized using β-actin protein levels.
Statistical analysis
All normality distributed data was expressed as mean ± standard error of the mean (SE) and compared using Student's t-test or analysis of variance (ANOVA) using Student-Newman-Keul's post-hoc analysis. Non-normally distributed data were compared using ANOVA on Ranks. Differences in values were considered significant if p < 0.05. All statistical analyses were performed using SigmaStat (Systat Software Inc, Chicago, IL). Due to limited statistical power, lack of statistically significant differences between groups was considered non-informative.
Results
Adjuvant treatment with C23 does not alter the arterial blood pressure after resuscitation for HS
Mice were subjected to controlled hemorrhage for 90 min followed by resuscitation with normal saline solution plus adjuvant treatment with vehicle or C23 (Fig. 1A). MAP measurements prior to the resuscitation period were identical between mice adjuvantly treated with vehicle or C23 (Fig. 1B) indicating that both groups were subjected to shock of similar severity. During and at the end of the resuscitation period, MAP measurements between mice adjuvantly treated with vehicle or C23 were also similar (Fig. 1B), suggesting that C23 has no clinically significant intrinsic vasoactive activity.
Adjuvant treatment with C23 attenuates organ injury after HS
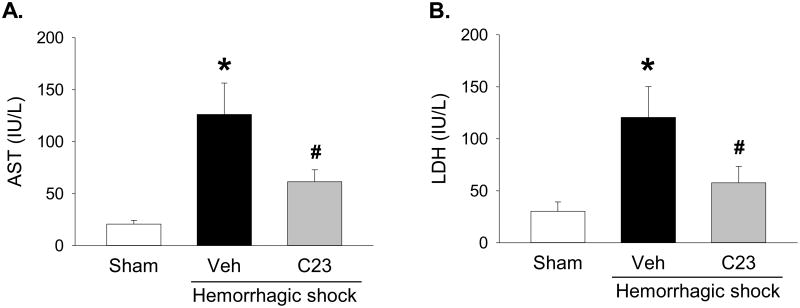
AST and LDH are ischemic organ injury markers released mainly by the liver, but also by myocardial and skeletal muscle, kidneys, brain, and red blood cells [24]. Thus, to determine whether adjuvant treatment with C23 attenuates organ injury after HS, we measured the serum levels of AST and LDH. Compared with sham animals, the serum levels of AST and LDH were significantly elevated 30.2- and 29.5-fold, respectively, in hemorrhaged-animals adjuvantly treated with vehicle (Fig. 2). Compared with vehicle, adjuvant treatment with C23 significantly reduced serum levels of AST and LDH by 51.3% and 52.2%, respectively (Fig. 2). These results indicate that adjuvant treatment of HS with C23 attenuates overall ischemic organ injury.
Figure 2. Adjuvant treatment with C23 attenuates organ injury after hemorrhagic shock.
At 4.5 hours (210 min) after resuscitation, (A) AST and (B) LDH serum levels were significantly elevated in mice adjuvantly treated with vehicle (Veh), and significantly decreased in mice adjuvantly treated with C23. Data are presented as means ± SE (n=6 per group). *p < 0.05 vs. sham, #p < 0.05 vs. vehicle; one-way ANOVA followed by Student-Newman-Keuls test.
Adjuvant treatment with C23 decreases lung proinflammatory cytokines and neutrophil infiltration after HS
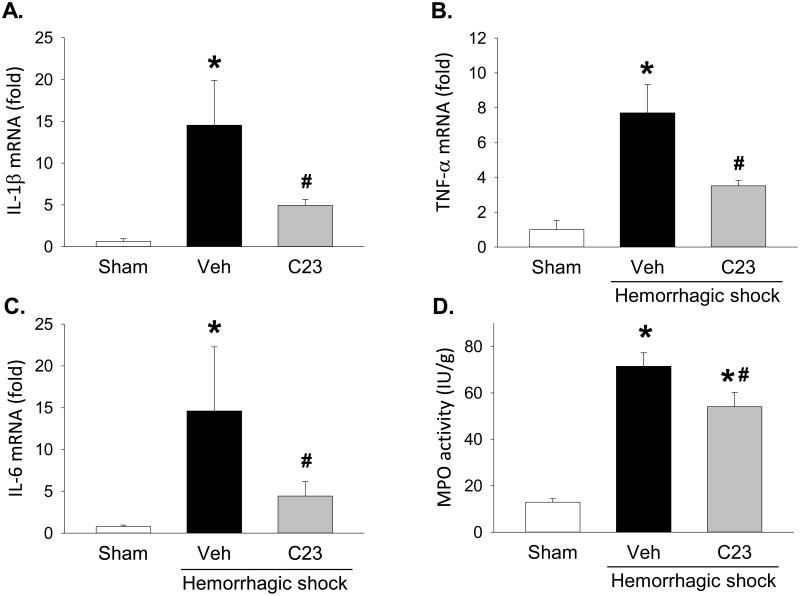
CIRP, released during sepsis and shock, contributes to lung injury with local production of proinflammatory mediators and neutrophil infiltration [20]. To evaluate whether adjuvant treatment with C23 reduces the expression of proinflammatory cytokines in the lung, we measured lung mRNA levels of IL-1β, TNF-α, and IL-6. Compared with sham animals, the expression levels of IL-1β, TNF-α, and IL-6 were 22.6-, 7.7-, and 19-fold higher in hemorrhaged-animals adjuvantly treated with vehicle (Fig. 3A-C). Compared with vehicle, adjuvant treatment with C23 significantly lowered the expression levels of IL-1β, TNF-α, and IL-6 by 66.1%, 54.4%, and 69.7%, respectively (Fig. 3A-C). Infiltrating neutrophils are an important source of proinflammatory cytokines in indirect lung injury. To determine whether adjuvant treatment with C23 reduces lung neutrophil infiltration, we measured lung myeloperoxidase (MPO) activity, which is proportional to the number of activated neutrophils in the tissue. Compared with sham animals, lung MPO activity was 5.5-fold higher in hemorrhaged-animals adjuvantly treated with vehicle (Fig. 3D). Compared with vehicle, adjuvant treatment with C23 significantly reduced MPO activity by 24.3% (Fig. 3D). Together, these results indicate that adjuvant treatment of HS with C23 attenuates lung inflammation.
Figure 3. C23 attenuates lung inflammation after HS.
At 4.5 hours after resuscitation, lung gene expression levels of (A) IL-1β, (B) TNF-α, and (C) IL-6 were significantly elevated in mice adjuvantly treated with vehicle (Veh) and significantly decreased in mice adjuvantly treated with C23. (D) Myeloperoxidase (MPO) activity was also elevated in the lungs of hemorrhaged mice and decreased in the lungs of mice adjuvantly treated with C23. Data are presented as means ± SE (n=6 per group). *p < 0.05 vs. sham, #p < 0.05 vs. vehicle; one-way ANOVA followed by Student-Newman-Keuls test.
Adjuvant treatment with C23 attenuated lung histological injury after HS
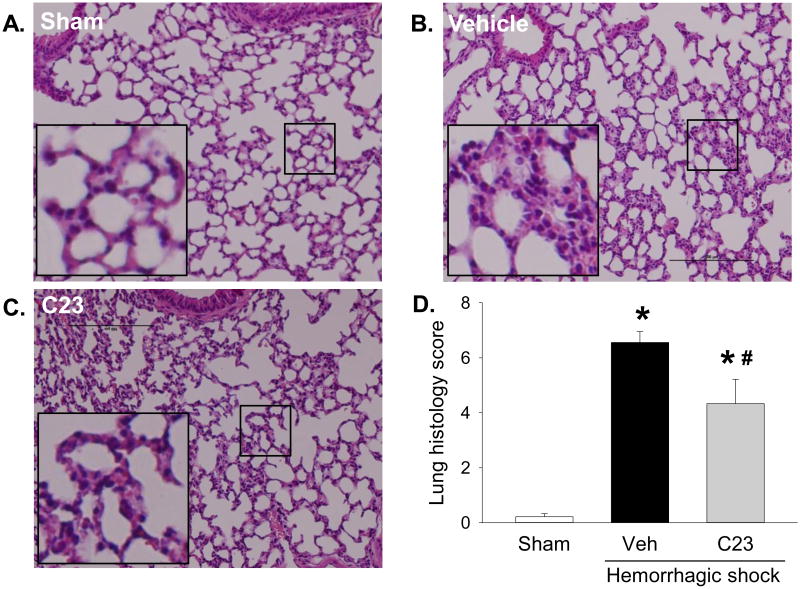
To assess C23's effect on changes in lung morphology caused by HS, we examined HE-stained lung sections with light microscopy. At 4.5 hours after HS, the lungs of hemorrhaged mice had alveolar wall thickening and interstitial accumulation of neutrophils (Fig. 4A-C). However, the lung injury score of mice adjuvantly treated with C23 was significantly reduced by 33.9% compared with vehicle (Fig. 4D). The improvement in lung alveolar edema and inflammatory infiltrates suggest that adjuvant treatment of HS with C23 attenuates lung injury.
Figure 4. C23 improves lung histological architecture after HS.
At 4.5 hours after resuscitation, (A) the lungs of sham mice showed normal histological architecture, while the lungs of hemorrhaged mice treated with (B) vehicle or (C) C23 displayed alveolar wall thickening with predominantly interstitial neutrophil accumulation. Representative photomicrographs, 200×; bar, 100 μm; inset, magnified region indicated by square. (D) The histological injury score, graded by an investigator blinded to the interventions, was significantly higher in mice adjuvantly treated with vehicle (Veh) than in mice treated with C23. Data are presented as means ± SE (n=3 per group). *p < 0.05 vs. sham, #p < 0.05 vs. vehicle; one-way ANOVA followed by Student-Newman-Keuls test.
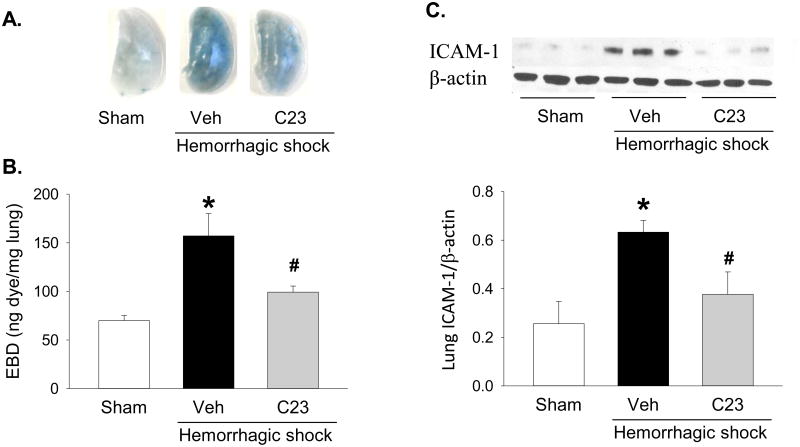
Adjuvant treatment with C23 reduced lung permeability and attenuates the activation of endothelial cells in the lungs after HS
CIRP mediates lung vascular endothelial cell (EC) barrier dysfunction and EC activation [20]. We investigated whether adjuvant treatment with C23 attenuates vascular permeability using the EBD extravasation assay. Compared with sham mice, the lungs of HS mice treated with vehicle had a blue tint (Fig 5A), and accumulated a significantly elevated (2.2-fold) amount of amount of EBD (Fig 5A,B). Adjuvant treatment with C23, on the other hand, significantly decreased EBD extravasation by 36.8% (Fig 5A,B). The decrease in EBD extravasation indicates that adjuvant treatment with C23 attenuates vascular EC barrier dysfunction after HS. To determine whether C23 was also able to reduce EC activation, we quantified ICAM-1 protein expression in the lungs of sham and HS mice (Fig. 5C). Compared with sham mice, ICAM-1 levels were increased 2.5-fold in the lungs of HS mice treated with vehicle (Fig. 5C). In contrast, adjuvant treatment with C23 significantly decreased lung expression of ICAM-1 by 40.3% (Fig. 5C). These results suggest that adjuvant treatment with C23 ameliorates alveolar wall edema and neutrophil infiltration by reducing EC activation after HS.
Figure 5. C23 attenuates lung endothelial cell barrier dysfunction and activation after HS.
At 4.5 hours after resuscitation, Evans blue dye (EBD)-albumin extravasation was significantly higher in hemorrhaged mice adjuvantly treated with vehicle (Veh) than in sham mice, and significantly lower in mice adjuvantly treated with C23, as indicated by (A) visual detection of EBD-albumin on the surface of the lungs (representative images) and (B) spectrophotometric quantification of EBD after extraction with formamide. (C) Compared with sham mice, ICAM-1 protein expression was significantly elevated in the lungs of mice adjuvantly treated with vehicle and significantly decreased in mice adjuvantly treated with C23. Representative Western blot showing three samples per group. Data are presented as means ± SE (n=6 per group). *p < 0.05 vs. sham, #p < 0.05 vs. vehicle; one-way ANOVA followed by Student-Newman-Keuls test.
Discussion
Most HS therapeutic studies have focused on the oncotic pressure and timing of fluid administration, transfusion of blood and blood components, and minimization of hypothermia and acidosis [25]. Lung injury after trauma, however, develops rapidly irrespective of resuscitation strategy [26]. We hypothesized that, in the context of isolated hemorrhage, adjuvant treatment with C23 would decrease lung injury severity in mice subjected to HS. Indeed we observed that mice receiving C23 had lower circulating levels of organ injury markers and attenuated lung injury after HS. As such, administration of fluid replacement together with a CIRP-targeting peptide is novel translational approach with potential to be developed for future therapeutic use in patients with HS.
CIRP is a 172-amino acid nuclear protein an amino-terminal RNA-recognition domain and a carboxy-terminal arginine-glycine rich domain which stabilize RNA translation [27-29]. CIRP is constitutively expressed in most tissues at low levels [27, 30, 31]. Cellular stressors such as radiation [28, 32-34], hypothermia [35], and hypoxia [16] upregulate CIRP expression, induce its translocation from the nucleus to the cytosol, promote its uptake by stress granules, and cause its release to the extracellular space [28]. Accordingly, we have shown that CIRP is released during hemorrhagic and distributive shock [18]. Extracellular CIRP can cause phagocyte release of proinflammatory mediators and vascular EC activation [18, 20, 36, 37]. Indeed, both wild-type mice administered anti-CIRP neutralizing antibodies and CIRP knockout mice have significantly increased survival after HS [18]. We have also discovered that extracellular CIRP plays a critical role in the pathogenesis of lung injury. The lungs of healthy mice injected with recombinant mouse (rm) CIRP have increased expression of proinflammatory cytokines, neutrophil infiltration, alveolar wall edema and infiltration, vascular permeability barrier dysfunction, EC activation, and apoptotic cells [20].
Using surface plasmon resonance (SPR) analysis to estimate the apparent dissociation constant (Kd) between CIRP and potential receptors [38], we have shown that CIRP binds with high affinity to TLR4 (Kd = 6.17 ×10-7 M), MD2 (Kd = 3.02 ×10-7 M), and the TLR4/MD2 complex (Kd = 2.39 ×10-7 M) [18]. With the intention of indentifying potential antagonists for CIRP, we screened 32 oligopeptides (15-mers) covering the entire sequence of human CIRP and identified C23 as the oligopeptide with the highest affinity for MD2 (Kd = 2.97 × 10-8 M) [18]. C23's high affinity binding to the TLR4/MD2 complex is clearly evident when compared with LPS (LPS-TLR4, Kd = 1.41 ×10-5 M; LPS-MD2, Kd = 2.33 ×10-6 M) or HMGB1 (HMGB1-TLR4/MD2, Kd = 1.5 ×10-6 M) [39, 40]. Indeed, a computational modeling indicated that C23 fits into MD2's pocket (unpublished observations; W.-L. Yang and P. Wang), further supporting C23's potential to displace CIRP's binding to the TLR4/MD2 complex. Additionally, pre-incubation with C23 prevented rmCIRP-induced release of TNF-α from the human monocyte THP-1 cell line [18], suggesting that C23 blocks CIRP activity. As hypothesized, adjuvant treatment of HS with C23 resulted in decreased lung proinflammatory cytokine expression, reduced lung neutrophil infiltration, improved lung histology score, improved lung vascular permeability barrier, and decreased lung EC activation. All of these outcomes have pathogenic or prognostic value in lung injury, and have been elicited by CIRP injection. As such, our findings suggest that targeting CIRP is a feasible and rational approach to improve HS-associated morbidity.
In this study, we administered C23 along with fluid resuscitation at 90 minutes of shock. This is time point is delayed but relevant, and applicable to patients with HS due to trauma as well as other causes, such as patients with gynecologic and digestive tract hemorrhage. The resuscitation scheme used consisted of rapid administration of isotonic crystalloid solution. Although colloid solutions are often preferred, saline solutions seem to be just as effective [41-43]. We intentionally avoided resuscitation with blood or blood-derived products to avoid transfusion-related lung injury [44], which might confound the interpretation of our results. Even though our resuscitation's dose and timing may be suboptimal, they were sufficient to adequately recover the MAP to around 60 mmHg, and were equal in the vehicle and C23 groups, allowing for fair comparison. C23 was administered by intravenous infusion alongside volume resuscitation because, as a peptide, C23's half-live is expected to be short and a bolus injection might not produce the same lung injury attenuation observed with the 30-minute infusion. We then waited for 4.5 hours to measure the effects of adjuvant treatment with C23 on outcomes selected based on their relevance for lung injury pathophysiology and clinical severity, as well as their induction by extracellular CIRP [20]. Therefore, the model, the resuscitation scheme, and the selection of outcomes were all clinically relevant.
Finally, we should consider this study's potential limitations. The dose of C23 was decided based on pilot studies in septic shock (manuscript in preparation), but it is not yet clear whether better results would be obtained with higher doses or continuous infusion. C23's pharmacotoxicology has not yet been studied, but significant side-effects are unlikely considering the benign phenotype of CIRP knock-out mice [45, 46]. Since the PaO2/FiO2 ratio was not measured, we were unable to diagnose ARDS or to correlate the degree of respiratory shunt with other lung injury findings. Furthermore, since our model consisted of hemorrhage alone, it may not be possible to extrapolate our findings to more complex scenarios such as hemorrhage associated with trauma and hemorrhage associated with coagulopathies. Finally, while the investigators were blinded only for the histology analysis, lack of blinding for other endpoints is an unlikely source of bias, since all other measurements were determined directly and independently.
Conclusions
We have discovered that adjuvant treatment with C23 ameliorates HS-associated lung injury. These findings highlight the importance of a recently described novel pathway linking CIRP to the development of lung injury. Since CIRP is also released during septic shock [18], stroke [37], and hepatic ischemia and reperfusion [36], future studies should consider targeting CIRP with C23 as a new potential therapeutic strategy to treat patients with these and other related conditions.
Acknowledgments
Not applicable.
Funding. This study was supported by NIH grant R01 HL076179 (PW).
Footnotes
Declarations: Ethics approval. All experiments involving live animals were carried out in accordance with the National Institutes of Health guidelines for the use of experimental animals and were reviewed and approved by the Institutional Animal Care and Use Committee at the Feinstein Institute for Medical Research.
Availability of data and materials. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests. One of the authors (P.W.) is an inventor of the PCT applications WO/2010/120726: “Treatment of inflammatory diseases by the inhibition of cold shock proteins” and US 61/881,798: “Peptides inhibiting cold-inducible RNA binding protein activity.” These patent applications cover the fundamental concept of inhibiting CIRP for the treatment of inflammatory diseases. The other authors have no conflict of interest.
Authors' Contributions. W.-L.Y. and P.W. conceived and designed the experiments; F.Z. performed the experiments; F.Z. and M.B. analyzed the data; F.Z. and M.B. wrote the manuscript and prepared the figures; and W.-L.Y. and P.W. reviewed the manuscript and supervised the research. All authors read and approved the final manuscript.
References
- 1.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World J Surg. 2007;31(7):1507–11. doi: 10.1007/s00268-007-9087-2. [DOI] [PubMed] [Google Scholar]
- 2.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 3.Pidcoke HF, Aden JK, Mora AG, Borgman MA, Spinella PC, Dubick MA, Blackbourne LH, Cap AP. Ten-year analysis of transfusion in Operation Iraqi Freedom and Operation Enduring Freedom: increased plasma and platelet use correlates with improved survival. J Trauma Acute Care Surg. 2012;73(6 Suppl 5):S445–52. doi: 10.1097/TA.0b013e3182754796. [DOI] [PubMed] [Google Scholar]
- 4.Rudstrom H, Bergqvist D, Bjorck M. Iatrogenic vascular injuries with lethal outcome. World J Surg. 2013;37(8):1981–7. doi: 10.1007/s00268-013-2061-2. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004;8(5):373–81. doi: 10.1186/cc2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holcomb JB, del Junco DJ, Fox EE, Wade CE, Cohen MJ, Schreiber MA, Alarcon LH, Bai Y, Brasel KJ, Bulger EM, et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–36. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369(9572):1553–64. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 8.Reilly JP, Bellamy S, Shashaty MG, Gallop R, Meyer NJ, Lanken PN, Kaplan S, Holena DN, May AK, Ware LB, et al. Heterogeneous phenotypes of acute respiratory distress syndrome after major trauma. Ann Am Thorac Soc. 2014;11(5):728–36. doi: 10.1513/AnnalsATS.201308-280OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossaint R, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernandez-Mondejar E, Hunt BJ, Komadina R, Nardi G, Neugebauer E, et al. Management of bleeding following major trauma: an updated European guideline. Crit Care. 2010;14(2):R52. doi: 10.1186/cc8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CH, Hsieh WH, Chou HC, Huang YS, Shen JH, Yeo YH, Chang HE, Chen SC, Lee CC. Liberal versus restricted fluid resuscitation strategies in trauma patients: a systematic review and meta-analysis of randomized controlled trials and observational studies*. Crit Care Med. 2014;42(4):954–61. doi: 10.1097/CCM.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 11.Roberts I, Shakur H, Ker K, Coats T. Antifibrinolytic drugs for acute traumatic injury. Cochrane Database Syst Rev. 2011;(1) doi: 10.1002/14651858.CD004896.pub3. CD004896. [DOI] [PubMed] [Google Scholar]
- 12.Korff S, Loughran P, Cai C, Fan J, Elson G, Shang L, Pires SS, Lee YS, Guardado J, Scott M, et al. TLR2 on Bone Marrow and Non-Bone Marrow Derived Cells Regulates Inflammation and Organ Injury in Cooperation with TLR4 During Resuscitated Hemorrhagic Shock. Shock. 2016 doi: 10.1097/SHK.0000000000000650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodhi CP, Jia H, Yamaguchi Y, Lu P, Good M, Egan C, Ozolek J, Zhu X, Billiar TR, Hackam DJ. Intestinal Epithelial TLR-4 Activation Is Required for the Development of Acute Lung Injury after Trauma/Hemorrhagic Shock via the Release of HMGB1 from the Gut. J Immunol. 2015;194(10):4931–9. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diebel LN, Liberati DM, Ledgerwood AM, Lucas CE. Changes in lymph proteome induced by hemorrhagic shock: the appearance of damage-associated molecular patterns. J Trauma Acute Care Surg. 2012;73(1):41–50. doi: 10.1097/TA.0b013e31825e8b32. discussion 1. [DOI] [PubMed] [Google Scholar]
- 15.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wellmann S, Buhrer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, Fujita J, Seeger K. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117(Pt 9):1785–94. doi: 10.1242/jcs.01026. [DOI] [PubMed] [Google Scholar]
- 17.Al-Fageeh MB, Smales CM. Alternative promoters regulate cold inducible RNA-binding (CIRP) gene expression and enhance transgene expression in mammalian cells. Mol Biotechnol. 2013;54(2):238–49. doi: 10.1007/s12033-013-9649-5. [DOI] [PubMed] [Google Scholar]
- 18.Qiang X, Yang WL, Wu R, Zhou M, Jacob A, Dong W, Kuncewitch M, Ji Y, Yang H, Wang H, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19(11):1489–95. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Y, Dong H, Zhong Y, Huang J, Lv J, Li J. The cold-inducible RNA-binding protein (CIRP) level in peripheral blood predicts sepsis outcome. PLoS One. 2015;10(9):e0137721. doi: 10.1371/journal.pone.0137721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang WL, Sharma A, Wang Z, Li Z, Fan J, Wang P. Cold-inducible RNA-binding protein causes endothelial dysfunction via activation of Nlrp3 inflammasome. Sci Rep. 2016;6:26571. doi: 10.1038/srep26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Hirano Y, Aziz M, Yang WL, Wang Z, Zhou M, Ochani M, Khader A, Wang P. Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury. Crit Care. 2015;19:53. doi: 10.1186/s13054-015-0782-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Standiford TJ, Kunkel SL, Lukacs NW, Greenberger MJ, Danforth JM, Kunkel RG, Strieter RM. Macrophage inflammatory protein-1 alpha mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155(3):1515–24. [PubMed] [Google Scholar]
- 24.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Sayad M, Noureddine H. Recent advances of hemorrhage management in severe trauma. Emerg Med Int. 2014;2014:638956. doi: 10.1155/2014/638956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Schwacha MG, Dubick MA, Cap AP, Darlington DN. Trauma-related acute lung injury develops rapidly irrespective of resuscitation strategy in the rat. Shock. 2016;46(3 Suppl 1):108–14. doi: 10.1097/SHK.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama H, Higashitsuji H, Yokoi H, Itoh K, Danno S, Matsuda T, Fujita J. Cloning and characterization of human CIRP (cold-inducible RNA-binding protein) cDNA and chromosomal assignment of the gene. Gene. 1997;204(1-2):115–20. doi: 10.1016/s0378-1119(97)00530-1. [DOI] [PubMed] [Google Scholar]
- 28.Yang C, Carrier F. The UV-inducible RNA-binding protein A18 (A18 hnRNP) plays a protective role in the genotoxic stress response. J Biol Chem. 2001;276(50):47277–84. doi: 10.1074/jbc.M105396200. [DOI] [PubMed] [Google Scholar]
- 29.Yang R, Zhan M, Nalabothula NR, Yang Q, Indig FE, Carrier F. Functional significance for a heterogenous ribonucleoprotein A18 signature RNA motif in the 3′-untranslated region of ataxia telangiectasia mutated and Rad3-related (ATR) transcript. J Biol Chem. 2010;285(12):8887–93. doi: 10.1074/jbc.M109.013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, Okuno H, Millan JL, Matsuda T, Yoshida O, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152(1):289–96. [PMC free article] [PubMed] [Google Scholar]
- 31.Nishiyama H, Xue JH, Sato T, Fukuyama H, Mizuno N, Houtani T, Sugimoto T, Fujita J. Diurnal change of the cold-inducible RNA-binding protein (Cirp) expression in mouse brain. Biochem Biophys Res Commun. 1998;245(2):534–8. doi: 10.1006/bbrc.1998.8482. [DOI] [PubMed] [Google Scholar]
- 32.Fornace AJ, Jr, Alamo I, Jr, Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988;85(23):8800–4. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheikh MS, Carrier F, Papathanasiou MA, Hollander MC, Zhan Q, Yu K, Fornace AJ., Jr Identification of several human homologs of hamster DNA damage-inducible transcripts. Cloning and characterization of a novel UV-inducible cDNA that codes for a putative RNA-binding protein. J Biol Chem. 1997;272(42):26720–6. doi: 10.1074/jbc.272.42.26720. [DOI] [PubMed] [Google Scholar]
- 34.Barenco M, Tomescu D, Brewer D, Callard R, Stark J, Hubank M. Ranked prediction of p53 targets using hidden variable dynamic modeling. Genome biology. 2006;7(3):R25. doi: 10.1186/gb-2006-7-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xue JH, Nonoguchi K, Fukumoto M, Sato T, Nishiyama H, Higashitsuji H, Itoh K, Fujita J. Effects of ischemia and H2O2 on the cold stress protein CIRP expression in rat neuronal cells. Free Radic Biol Med. 1999;27(11-12):1238–44. doi: 10.1016/s0891-5849(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 36.Godwin A, Yang WL, Sharma A, Khader A, Wang Z, Zhang F, Nicastro J, Coppa GF, Wang P. Blocking cold-inducible RNA-binding protein protects liver from ischemia-reperfusion injury. Shock. 2015;43(1):24–30. doi: 10.1097/SHK.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou M, Yang WL, Ji Y, Qiang X, Wang P. Cold-inducible RNA-binding protein mediates neuroinflammation in cerebral ischemia. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbagen.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahnefeld C, Drewianka S, Herberg FW. Determination of kinetic data using surface plasmon resonance biosensors. Methods Mol Med. 2004;94:299–320. doi: 10.1385/1-59259-679-7:299. [DOI] [PubMed] [Google Scholar]
- 39.Shin HJ, Lee H, Park JD, Hyun HC, Sohn HO, Lee DW, Kim YS. Kinetics of binding of LPS to recombinant CD14, TLR4, and MD-2 proteins. Mol Cells. 2007;24(1):119–24. [PubMed] [Google Scholar]
- 40.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107(26):11942–7. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R, Investigators SS. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Eng J Med. 2004;350(22):2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 42.Alderson P, Schierhout G, Roberts I, Bunn F. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2000;(2) doi: 10.1002/14651858.CD000567. CD000567. [DOI] [PubMed] [Google Scholar]
- 43.Annane D, Siami S, Jaber S, Martin C, Elatrous S, Declere AD, Preiser JC, Outin H, Troche G, Charpentier C, et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013;310(17):1809–17. doi: 10.1001/jama.2013.280502. [DOI] [PubMed] [Google Scholar]
- 44.Kelher MR, Banerjee A, Gamboni F, Anderson C, Silliman CC. Antibodies to major histocompatibility complex class II antigens directly prime neutrophils and cause acute lung injury in a two-event in vivo rat model. Transfusion. 2016;56(12):3004–11. doi: 10.1111/trf.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakurai T, Itoh K, Higashitsuji H, Nonoguchi K, Liu Y, Watanabe H, Nakano T, Fukumoto M, Chiba T, Fujita J. Cirp protects against tumor necrosis factor-alpha-induced apoptosis via activation of extracellular signal-regulated kinase. Biochim Biophys Acta. 2006;1763(3):290–5. doi: 10.1016/j.bbamcr.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Masuda T, Itoh K, Higashitsuji H, Nakazawa N, Sakurai T, Liu Y, Tokuchi H, Fujita T, Zhao Y, Nishiyama H, et al. Cold-inducible RNA-binding protein (Cirp) interacts with Dyrk1b/Mirk and promotes proliferation of immature male germ cells in mice. Proc Natl Acad Sci U S A. 2012;109(27):10885–90. doi: 10.1073/pnas.1121524109. [DOI] [PMC free article] [PubMed] [Google Scholar]