Abstract
Objective
Both frailty and falls occur at earlier than expected ages among HIV-infected individuals, but the contribution of frailty to fall risk in this population is not well understood. We examined this association among participants enrolled in AIDS Clinical Trials Group (ACTG) A5322.
Design
A prospective, multi-center cohort study of HIV-infected men and women ≥40 years.
Methods
Frailty assessment included a 4-meter walk, grip strength, and self-reported weight loss, exhaustion, and low physical activity. Multinomial logistic regression assessed the association between baseline frailty, grip, and 4-meter walk and single and recurrent (2+) falls over the next 12 months; logistic regression assessed effect modification by several factors on association between frailty and any (1+) falls.
Results
Of 967 individuals, 6% were frail, 39% pre-frail, and 55% non-frail. Eighteen percent had ≥1 fall, and 7% had recurrent falls. In multivariable models, recurrent falls were more likely among frail (OR=17.3; 95% CI=7.03-42.6) and pre-frail (OR=3.80; 95% CI=1.87-7.72) than non-frail individuals. Significant associations were also seen with recurrent falls and slow walk and weak grip. The association between frailty and any falls was substantially stronger among individuals with peripheral neuropathy.
Conclusions
Aging HIV-infected pre-frail and frail individuals are at significantly increased risk of falls. Incorporation of frailty assessments or simple evaluations of walk speed or grip strength in clinical care may help identify individuals at greatest risk for falls. Peripheral neuropathy further increases fall risk among frail persons, defining a potential target population for closer fall surveillance, prevention, and treatment.
Keywords: frailty, falls, HIV-infected, aging, peripheral neuropathy
Introduction
Falls are a significant risk factor for health-related complications and mortality in older adults, and are associated with a substantial health care burden and cost [1]. The likelihood of injuries resulting from falls increases with age; these injuries can exacerbate already-existing problems of physical inactivity and weakness [2]. The prevalence of both frailty and falls increase with age, and the association between these two common age-associated morbidities has been extensively documented in community-dwelling and institutionalized populations [3, 4]. Among middle-aged and older HIV-infected individuals, including those with well-suppressed infection, frailty and impairments in physical function are common [5-8], and one study found that the prevalence of falls among middle-aged HIV-infected adults with a mean age of 52 years paralleled that of HIV-uninfected adults 65 years or older [9]. While the number of older HIV-infected adults in the United States continues to rise due to enhanced survival and life expectancy, research into risk factors for falls in HIV-infected individuals remains limited [10-12]. Only one study to-date has considered the association between frailty and falls; frailty was found to be strongly associated with recurrent (2 or more) falls in the prior 12 months in a cross-sectional analysis of 359 HIV-infected men and women 45-65 years of age, with exhaustion being the frailty component with the strongest association [9].
The AIDS Clinical Trials Group (ACTG) A5322 study is a prospective observational study of 1035 HIV-infected adults 40 years of age and older. In this analysis, our specific aims were to determine the proportion of A5322 participants who experienced falls over a 12-month period, overall and by frailty status; to summarize the number and proportion of falls resulting in medical treatment or a bone fracture; to examine the association between frailty status and incidence of single and recurrent falls; and to assess several factors (sex, age, comorbidities, peripheral neuropathy and neurocognitive impairment) as potential effect modifiers of the association between frailty and any falls.
Methods
Participants
The ACTG Study A5322 examines long-term health outcomes of older HIV-infected men and women. All participants received their initial antiretroviral treatment (ART) regimen through an ACTG clinical trial and were previously followed long-term after their trial participation in another ACTG observational study, A5001 [13]. A5322 participants were enrolled at one of 32 clinical research sites in the U.S. including Puerto Rico between November 2013 and July 2014. Ongoing visits every 6 months include medical chart abstraction, targeted physical examinations, fasting laboratory tests, repository biological specimen collection, interviews and self-administered questionnaires for collection of behavioral information, and evaluations of frailty, neurocognitive function, and instrumental activities of daily living. All participants signed a written informed consent before enrollment, and A5322 has been approved by local institutional review boards at each site.
The current analysis is a prospective evaluation of the association between baseline (A5322 entry) frailty and self-reported falls occurring over the subsequent two study visits (6 and 12 months). All A5322 participants who underwent a complete frailty evaluation at baseline and had information available regarding falls over one or both of the two subsequent study visits were included in this analysis.
Outcome
Falls are assessed at every visit, beginning with the 6 month visit, using self-reported responses obtained through interview-administered questionnaires. During the interview, participants are instructed that a fall is defined as an unexpected event in which the individual loses their balance and lands on the floor, ground or a lower level or hits an object. Events resulting from a major medical event such as a stroke or an overwhelming external hazard such as being pushed are not considered falls. Participants are asked about occurrence of falls (1, 2, 3-5, >5) in the past 6 months. For participants who report a fall, the need for medical attention and the occurrence of bone fracture are also reported. In this analysis the number of falls was calculated across both time points for participants who completed both the 6 and 12 month falls interview. For persons who completed only one interview the total number of falls reported at that visit was included. Participants with missing information regarding falls at either 6 or 12 months were assumed to not have had any falls during the relevant time period.
Exposure
In A5322, frailty is assessed using a modified version of the Fried's frailty assessment [14] as previously described [15], which consists of 5 components: grip strength (the average of three grip assessments using a dynamometer), gait speed (4-meter timed walk), and self-reported unintentional weight loss (≥10 lbs over the prior year), exhaustion, and limitations in vigorous physical activities. In this analysis the baseline frailty assessment was used. Participants were categorized as frail (3-5 components), pre-frail (1 or 2 components), or non-frail (no components). The individual frailty components of gait speed (≤ 1 meter/second vs > 1 meter/second) and grip strength (cutoffs for weak grip strength based on sex and BMI) were also examined as exposures [14].
Confounders
Potential confounders of the association between frailty and falls were identified based on previous literature [16-18]. We evaluated these variables using a directed acyclic graph (DAG) [19] to identify a minimally sufficient list of variables to include in the multivariable models. Each potential confounder was added one at a time to the univariable model, and any variable that changed the coefficient (log odds) for any category of the effect of the frailty measure (3-category frailty, grip strength, or gait speed) on falls by ≥ 15 percent was kept in the multivariable model. The baseline variables considered as potential confounders were: age (continuous; 40-49, 50-59, 60+ years); sex; race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic/other); education (<high school, high school, >high school); medical insurance (no coverage, Medicare/Medicaid, private insurance/other); BMI (underweight (<18.5 kg/m2), normal weight (18.5-<25 kg/m2), overweight (25-30 kg/m2), obese (>30 kg/m2); waist circumference cutoff (men >102 cm, women >88 cm); use of antihypertensive medication; use of antidepressant/anxiolytic medication; comorbidities established via chart abstraction (diabetes mellitus, hypertension, kidney disease, liver disease, cardiovascular disease, stroke, chronic hepatitis C virus infection [positive antibody], cancer [within past 5 years]); recreational substance use (marijuana, cocaine, heroin, amphetamines, other non-prescribed drugs); alcohol use (heavy/binge, moderate, light or none); cigarette smoking (current/prior/never); peripheral neuropathy (absent or hypoactive ankle reflexes bilaterally or mild, moderate, or severe loss in vibration perception bilaterally) [20]; any history of didanosine, stavudine or zalcitabine use; physical activity (≥3 or more days in past 7 of moderate/vigorous activity vs. < 3 days); history of AIDS-defining conditions (any/none); and neurocognitive function (continuous; impaired). Neurocognitive function is assessed with the A5001 Neuroscreen [21], which uses normalized, age-, sex- and race/ethnicity-adjusted z-scores of the Trail-Making A, Trail-Making B, and Digit Symbol tests. One overall continuous score is obtained from the average of the three z-scores. Impairment is defined as having at least one z-score two standard deviations below the mean or at least two z-scores one standard deviation below the mean on two separate tests.
Effect Modifiers
A subset of the above variables (sex, age, comorbidities, peripheral neuropathy, neurocognitive impairment) were examined to explore whether the association between the frailty measures and any falls differed between categories of any of these variables.
Statistical Analyses
For descriptive analyses and to examine the association between frailty measures and falls, participants were categorized into one of three fall categories: no falls reported over 12 months, single fall, and recurrent falls (≥2). In the effect modification analyses, the outcome was defined as any falls (≥1) versus no falls to avoid the problem of sparse data.
The number and proportion of individuals with falls over the 12-month period after baseline was calculated, overall and by frailty status, as well as the proportion of participants who sought medical attention for a fall and the proportion of participants who experienced a fracture as a result of a fall. Participant characteristics by fall group were compared using chi-square tests for categorical variables and Kruskal-wallis tests for continuous variables.
Associations between the 3 frailty measures and single and recurrent falls over the 12-month period were assessed using separate multinomial logistic regression models. Multinomial logistic regression was used rather than ordinal logistic regression because the score test statistics were significant for all three ordinal logistic regression models (frailty and falls: χ2=18.26, p-value<0.0001, gait speed and falls: χ2= 28.40, p-value<0.0001, and grip strength and falls: χ2=9.8464, p-value=0.0017), indicating that the proportional odds assumption of ordinal logistic regression is not met.
To examine potential effect modification by sex, age, comorbidities, neuropathy, and neurocognitive function on the association between frailty and falls, we first reviewed cross-tabulations of frailty and falls stratified by categories of each potential effect modifier. Where there was qualitative evidence of effect modification based on the stratified tables, models with an interaction term between the potential effect modifier and the frailty variable (3-category frailty, gait speed, or grip strength) were evaluated [22].
Using sensitivity analyses, we explored the reasonableness of our assumption that individuals with missing falls information at either the 6- or 12-month visit had no falls. In these sensitivity analyses, we repeated model-building for the association between frailty and falls, and for effect modification on frailty and falls, restricted to individuals with falls information at both visits. All analyses were conducted using SAS version 9.4.
Results
Nine hundred-sixty seven of the 1035 A5322 participants (93%) had a frailty assessment at baseline and a falls interview at either the 6-month or 12-month visit and were therefore included in these analyses. Eighty-four percent of individuals completed falls interviews at both visits; 9.1% at the 6-month visit only, and 6.5% at 12 months only.
Of the 967 individuals, 174 (18%) had at least one fall over the 12-month period, with 106 (11%) reporting one fall and 68 (7%) recurrent falls. Fifty-nine percent of the recurrent fallers fell 3 or more times. Twenty-one percent of participants who reported any fall sought medical attention for a fall, and 5% sustained a fracture as a result of the fall. Selected demographic, clinical and behavioral characteristics, stratified by number of falls (0, 1 and 2+) are summarized in Table 1.
Table 1. Select Demographic, Clinical and Behavioral Characteristics of A5322 Participants (N=967).
| Fall Category | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | No Falls (N=793) |
One Fall (N=106) |
Recurrent Falls (N=68) |
Total (N=967) |
P-valuea | |
| Age at baseline (Years) | Median (Q1-Q3) | 50 (45-56) | 53 (48-60) | 53 (50-58) | 51 (46-56) | <.001 |
| 40-49 | 366 (46.2%) | 37 (34.9%) | 16 (23.5%) | 419 (43.3%) | <.001 | |
| 50-59 | 315 (39.7%) | 42 (39.6%) | 40 (58.8%) | 397 (41.1%) | ||
| ≥60 | 112 (14.1%) | 27 (25.5%) | 12 (17.6%) | 151 (15.6%) | ||
| Sex | M | 650 (82.0%) | 85 (80.2%) | 49 (72.1%) | 784 (81.1%) | 0.13 |
| F | 143 (18.0%) | 21 (19.8%) | 19 (27.9%) | 183 (18.9%) | ||
| Race/Ethnicity | White, non-Hispanic | 372 (46.9%) | 59 (55.7%) | 34 (50.0%) | 465 (48.1%) | 0.20 |
| Black, non-Hispanic | 234 (29.5%) | 29 (27.4%) | 24 (35.3%) | 287 (29.7%) | ||
| Hispanic + other | 185 (23.3%) | 18 (17.0%) | 10 (14.7%) | 213 (22.0%) | ||
| Missing | 2 (0.3%) | 0 (0.0%) | 0 (0.0%) | 2 (0.2%) | ||
| Years of Education | < High school | 115 (14.5%) | 17 (16.0%) | 10 (14.7%) | 142 (14.7%) | 0.06 |
| High school | 160 (20.2%) | 23 (21.7%) | 24 (35.3%) | 207 (21.4%) | ||
| > High school | 518 (65.3%) | 66 (62.3%) | 34 (50.0%) | 618 (63.9%) | ||
| Health Insurance | No coverage | 159 (20.1%) | 8 (7.5%) | 9 (13.2%) | 176 (18.2%) | <.001 |
| Medicare/Medicaid | 231 (29.1%) | 42 (39.6%) | 37 (54.4%) | 310 (32.1%) | ||
| Private/other | 393 (49.6%) | 55 (51.9%) | 22 (32.4%) | 470 (48.6%) | ||
| Missing | 10 (1.3%) | 1 (0.9%) | 0 (0.0%) | 11 (1.1%) | ||
| Smoking Status | Never | 330 (41.6%) | 41 (38.7%) | 27 (39.7%) | 398 (41.2%) | 0.58 |
| Prior | 263 (33.2%) | 40 (37.7%) | 19 (27.9%) | 322 (33.3%) | ||
| Current | 200 (25.2%) | 25 (23.6%) | 22 (32.4%) | 247 (25.5%) | ||
| Current Alcohol Use | Abstainer | 302 (38.1%) | 31 (29.2%) | 31 (45.6%) | 364 (37.6%) | 0.34 |
| Light Drinker | 283 (35.7%) | 39 (36.8%) | 24 (35.3%) | 346 (35.8%) | ||
| Moderate Drinker | 49 (6.2%) | 8 (7.5%) | 5 (7.4%) | 62 (6.4%) | ||
| Heavy Drinker | 129 (16.3%) | 24 (22.6%) | 8 (11.8%) | 161 (16.6%) | ||
| Missing | 30 (3.8%) | 4 (3.8%) | 0 (0.0%) | 34 (3.5%) | ||
| Current Substance Use | No | 597 (75.3%) | 71 (67.0%) | 52 (76.5%) | 720 (74.5%) | 0.08 |
| Yes | 155 (19.5%) | 31 (29.2%) | 15 (22.1%) | 201 (20.8%) | ||
| Missing | 41 (5.2%) | 4 (3.8%) | 1 (1.5%) | 46 (4.8%) | ||
| Vigorous or Moderate Activities/Week | <3 days | 341 (43.0%) | 54 (50.9%) | 38 (55.9%) | 433 (44.8%) | 0.15 |
| ≥3 days | 404 (50.9%) | 51 (48.1%) | 29 (42.6%) | 484 (50.1%) | ||
| Missing | 48 (6.1%) | 1 (0.9%) | 1 (1.5%) | 50 (5.2%) | ||
| BMIb (kg/m2) | Normal weight | 261 (32.9%) | 36 (34.0%) | 16 (23.5%) | 313 (32.4%) | 0.51 |
| Obese | 215 (27.1%) | 32 (30.2%) | 24 (35.3%) | 271 (28.0%) | ||
| Overweight | 312 (39.3%) | 38 (35.8%) | 27 (39.7%) | 377 (39.0%) | ||
| Underweight | 5 (0.6%) | 0 (0.0%) | 1 (1.5%) | 6 (0.6%) | ||
| Waist Circumference above Cutoffc | No | 525 (66.2%) | 71 (67.0%) | 40 (58.8%) | 636 (65.8%) | 0.45 |
| Yes | 268 (33.8%) | 35 (33.0%) | 28 (41.2%) | 331 (34.2%) | ||
| Comorbidities | No | 163 (20.6%) | 19 (17.9%) | 17 (25.0%) | 199 (20.6%) | 0.53 |
| Yes | 630 (79.4%) | 87 (82.1%) | 51 (75.0%) | 768 (79.4%) | ||
| History of AIDS-defining Condition | No | 638 (80.5%) | 86 (81.1%) | 51 (75.0%) | 775 (80.1%) | 0.54 |
| Yes | 155 (19.5%) | 20 (18.9%) | 17 (25.0%) | 192 (19.9%) | ||
| Peripheral Neuropathy | No | 492 (62.0%) | 58 (54.7%) | 39 (57.4%) | 589 (60.9%) | 0.25 |
| Yes | 296 (37.3%) | 48 (45.3%) | 29 (42.6%) | 373 (38.6%) | ||
| Missing | 5 (0.6%) | 0 (0.0%) | 0 (0.0%) | 5 (0.5%) | ||
| Didanosine/Stavudine Used | No | 676 (85.2%) | 91 (85.8%) | 60 (88.2%) | 827 (85.5%) | |
| Yes | 117 (14.8%) | 15 (14.2%) | 8 (11.8%) | 140 (14.5%) | 0.794 | |
| Anxiety/Depression Medication | No | 552 (69.6%) | 67 (63.2%) | 26 (38.2%) | 645 (66.7%) | <.001 |
| Yes | 241 (30.4%) | 39 (36.8%) | 42 (61.8%) | 322 (33.3%) | ||
| Hypertension Medication | No | 512 (64.6%) | 63 (59.4%) | 38 (55.9%) | 613 (63.4%) | 0.24 |
| Yes | 281 (35.4%) | 43 (40.6%) | 30 (44.1%) | 354 (36.6%) | ||
| Neurocognitive Function | Median (Q1-Q3) | 0.43 (-0.33-1.07) | 0.33 (-0.20-1.10) | -0.28 (-0.97-0.58) | 0.37 (-0.38-1.03) | <.001 |
| Neurocognitive Impairment | No | 633 (79.8%) | 87 (82.1%) | 44 (64.7%) | 764 (79.0%) | 0.002 |
| Yes | 112 (14.1%) | 12 (11.3%) | 20 (29.4%) | 144 (14.9%) | ||
| Missing | 48 (6.1%) | 7 (6.6%) | 4 (5.9%) | 59 (6.1%) | ||
From Kruskall-Wallis test for continuous age; from chi-square test for all other comparisons.
BMI = body mass index.
Cutoff = 102 cm for men, 88 cm for women.
There was no use of zalcitabine.
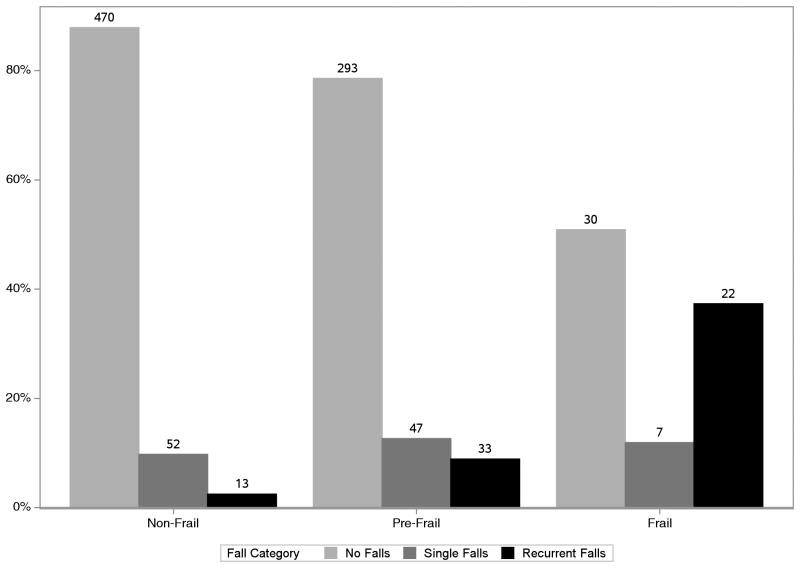
Overall, 59 participants (6%) were classified as frail, and 373 (39%) as pre-frail. Fig 1 shows the proportion of individuals with falls by frailty status. Twelve percent of non-frail, 22% of pre-frail and 49% of frail individuals had one or more falls.
Figure 1. Proportion of Participants with Single and Recurrent Falls by Baseline Frailty Status.
Chi-square test p-value <0.001, comparing the proportion of individuals with single and recurrent falls by frailty group (non-frail, pre-frail and frail).
Association between frailty and falls
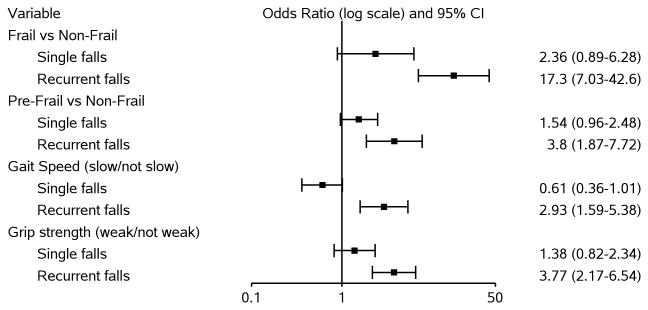
As shown in Fig 2, compared to non-frail participants, recurrent falls were more likely among pre-frail (adjusted odds ratio [aOR]=3.80, 95% confidence interval [CI]=1.87, 7.72) and frail (aOR=17.3, 95% CI=7.03, 42.6) participants. There was increased odds of single falls among both pre-frail (aOR=1.54; 95% CI=0.96, 2.48) and frail participants (aOR=2.36; 95% CI=0.89, 6.28) although these associations did not reach statistical significance. Regarding individual components of the frailty assessment, participants with weak grip strength had a higher odds of recurrent falls than those without weak grip strength (aOR=3.77, 95% CI=2.17, 6.54). Similarly, participants with slow gait speed were more likely than those without slow gait to experience recurrent falls (aOR=2.93, 95% CI=1.59, 5.38). Neither gait speed nor grip strength were associated with single falls.
Figure 2. Multivariable Associations between Frailty Characteristics and Falls.
Frailty-falls model adjusted for: age (categorical), race/ethnicity, alcohol use, current substance use, neurocognitive function. Gait speed-falls model adjusted for: age (continuous), race/ethnicity, education level, current alcohol use, neurocognitive function. Grip strength-falls model adjusted for: age (categorical), race/ethnicity, current substance use, physical activity, diabetes, health insurance, current alcohol use.
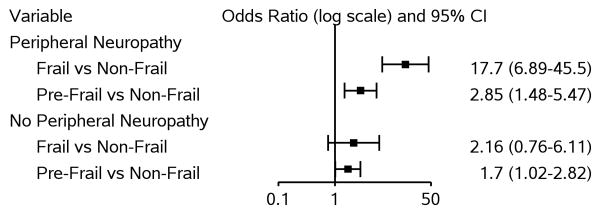
Three hundred seventy-three participants (38.6%) had peripheral neuropathy. Fifty-five (14.7%) had moderate to severe loss of vibration bilaterally and 278 (74.5%) had absent or hypoactive reflexes bilaterally (35 had both moderate/severe vibration loss and absent/hypoactive reflexes bilaterally). Most of the others had mild loss of vibration bilaterally with normal reflexes. Among individuals with peripheral neuropathy, frail participants were significantly more likely to experience falls (aOR=17.7, 95% CI=6.89, 45.5) than non-frail individuals, while among those without peripheral neuropathy, the association between frailty and falls was weaker and not significant (aOR=2.16, 95% CI=0.76, 6.11; p-value for interaction term=0.003) (Fig 3). The effect modification of peripheral neuropathy was observed only for frailty and not for pre-frailty, grip strength or gait speed. There was some qualitative evidence of effect modification by sex on the association between frailty and falls but this interaction was not statistically significant (frail vs. non-frail: aOR for males=7.82 [95% CI=3.69, 16.6], aOR for females=3.66 [95% CI=0.95, 14.1]; p-value for interaction=0.21). There was no evidence of effect modification by age, comorbidities or neurocognitive impairment on the associations between any of the frailty measures and falls.
Figure 3. Effect Modification by Peripheral Neuropathy on Association between Frailty Status and Any (1+) Falls.
Model adjusted for: age, race/ethnicity, current alcohol use, current substance use, and neurocognitive function; interaction term between peripheral neuropathy and frailty status also included.
In the sensitivity analyses restricted to persons with falls information at both the 6- and 12-month visits, our results were essentially the same, with the following exceptions: the odds ratio for frailty and single falls was larger and statistically significant (aOR=3.03, 95% CI=1.09, 8.39) than in the primary analyses, and the odds ratio for grip strength and single falls, while similar to that in the primary analysis, did not reach statistical significance (aOR=1.45, 95% CI=0.81, 2.62).
Discussion
We identified a strong association between frailty and recurrent falls in this cohort of older HIV-infected men and women. Frail individuals had substantially higher odds of falling two or more times over the subsequent 12 month period. Also, ours is the first study to report upon an association between pre-frailty and falls in HIV-infected adults, although several previous studies of HIV-uninfected individuals have identified this association [4, 5, 23]. In particular, the two objectively measured components of frailty – weak grip strength and slow 4-meter walk – were associated with significantly higher odds of recurrent falls.
The association between frailty and fall occurrence was substantially stronger for recurrent than for single falls. Recurrent fallers and persons who fall less frequently likely comprise very different groups in terms of overall health status. Occasional fallers may be more physically active and their falls more likely due to accidents, while recurrent fallers are more likely to have impaired balance and strength, reduced reaction time, and diminished functional reserve capacity [18, 24]. The majority of recurrent fallers (59%) in our study reported falling 3 or more times over the 12-month follow-up period, indicating a relatively high frequency of falls.
The magnitude of the overall association we identified between frailty and falls is substantially greater than previously observed in studies of older HIV-uninfected individuals. In a review and meta-analysis of prospective studies that assessed risk of future falls among community-dwelling individuals 60 years of age and older, the odds ratios for frailty and recurrent falls ranged from 1.38 to 3.56, and for pre-frailty and falls from 0.90 to 1.62 [4]. Our findings are more consistent with those observed in a previous study of HIV-infected individuals [9]. In this study and in ours, the effect sizes were substantially larger, despite the fact that the individuals being evaluated were on average almost 10 years younger. The reason for this difference in effect size is likely multi-factorial. One explanation could be that a large proportion of our study population – over 80% - was male. We found that the association between frailty and falls was more pronounced among men than women, although this difference was not statistically significant. However, the aforementioned meta-analysis of HIV-uninfected older individuals attributed the high heterogeneity of effect sizes between frailty status and falls largely to differences in the proportion of men in these studies, and it was also noted that the association between frailty and falls seemed stronger among males than females [4].
Another potential explanation for the strong association we observed between frailty and falls is that HIV-infected individuals in our cohort have multiple comorbid conditions that may affect their risk of falls through different mechanisms (such as weakness, impaired balance, neuropathy, and polypharmacy). In particular, over a third of our cohort reported peripheral neuropathy. In the previously published analysis of frailty and falls among HIV-infected individuals, a similar proportion (38%) had peripheral neuropathy [9]. Indeed, we found strong evidence for effect modification by peripheral neuropathy on the association between frailty and falls; among individuals with peripheral neuropathy, the association was very strong, while among those without peripheral neuropathy it was more modest and also more in line with effect sizes observed in HIV-uninfected populations. Peripheral neuropathy and frailty can affect fall risk through overlapping yet distinct mechanisms of action, such that their combined effect is larger than the sum of their individual effects. Peripheral neuropathy, manifested in part through sensory loss, has deleterious effects on gait, balance and postural stability [25]. Frail individuals, who are defined by their limited reserves, lower limb weakness and diminished ability to respond to stressors, are likely unable to recover quickly after losing their balance. Future studies comparing HIV-infected and HIV-uninfected individuals are needed to confirm the stronger association among HIV-infected individuals between frailty and falls, and to examine reasons for this effect modification.
The protective association of slow walk speed on the incidence of single falls was unexpected. However, in a population-based cohort study of community-dwelling elderly individuals, those who were classified as normal to slow walkers had a lower overall risk of falling than did individuals classified as fast walkers [26], and may suggest that the former category of individuals have already employed compensatory mechanisms to minimize fall risk.
Among our study's strengths is its large sample size, which allowed us to examine both pre-frailty and frailty, individual components of frailty, and effect modification while adjusting for important confounding variables. Ours is the first prospective study to evaluate frailty and falls among HIV-infected individuals. There was a relatively short recall period required for falls, which may have reduced the likelihood of underestimating falls [2]. There are also several limitations to mention. This A5322 cohort of middle-aged and older men and women currently has a relatively small number of frail individuals as well as recurrent fallers, resulting in some imprecise effect estimates. The small number of frail persons studied precluded examination of effect modification on frailty and recurrent falls. We assumed those with missing falls information at a visit experienced no falls, which could have underestimated the incidence of falls in the cohort. Additionally, this cohort is comprised of a group of participants who have a history of long-term voluntary participation in both clinical trials and observational studies, and the results of this study may therefore not be generalizable to all HIV-infected older adults in the U.S.
Conclusion
Aging HIV-infected pre-frail and frail individuals are at significantly increased risk of falls compared to non-frail HIV-infected individuals, and this association appears to be stronger than previously observed in HIV-uninfected individuals. Incorporation of frailty assessments or simple evaluations of 4 meter walk speed or grip strength in routine clinical care may help identify individuals at greatest risk for falls and, hence, those who would benefit most from falls prevention interventions. Peripheral neuropathy further increases fall risk among frail persons and this may define a potential target population for closer fall surveillance, prevention, and treatment.
Acknowledgments
KT and KME designed the study. MA conducted the statistical analysis under the supervision of KT. KT drafted the article. All authors contributed to interpretation of results, critically reviewed the article, and approved the final version.
We thank the study volunteers who participated in A5322, all the ACTG clinical units who enroll and follow participants, and the ACTG.
Funding: This work was supported by the following institutes of the National Institutes of Health: the National Institute of Allergy and Infectious Diseases (NIAID) [AI068636, AI069432, AI068634, AI069423, AI069452, AI067039, AI106701 (and AI069494 to SLK)], the National Institute of Mental Health (NIMH), and the National Institute of Dental and Craniofacial Research (NIDCR), and also by the National Institute of Aging (NIA) [K23AG050260; R01AG054366] to KME. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research was also supported by the Veterans Administration: VISN10 Geriatric Research Educational and Clinical Centers, Louis Stokes Cleveland Veterans Administration Medical Center and the CWRU Center for AIDS Research P30 AI036219 to RCK. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
RCK receives grant support from Gilead Sciences, FJP is a consultant and/or on the speakers bureau for Gilead Sciences, Janssen Pharmaceuticals, Merck and Co. and Bristol Myers Squibb. BT has received honoraria and/or grant support to Northwestern University from ViiV Healthcare, Gilead Sciences, Glaxo Smith Kline, and Janssen. KME has received grant support from Gilead Sciences and has served as a consultant for Theratechnologies.
Footnotes
Data presented previously at the 2017 Conference on Retroviruses and Opportunistic Infections (CROI).
References
- 1.Centers for Disease Control and Prevention (US) Important facts about falls. [Accessed on April 12, 2017]; Available from: https://www.cdc.gov/homeandrecreationalsafety/falls/adultfalls.html.
- 2.Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988;36(7):613–616. doi: 10.1111/j.1532-5415.1988.tb06155.x. [DOI] [PubMed] [Google Scholar]
- 3.Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752–762. doi: 10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(12):1027–1033. doi: 10.1016/j.jamda.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Gustafson DR, Shi Q, Thurn M, Holman S, Minkoff H, Cohen M, et al. Frailty and constellations of factors in aging HIV-infected and uninfectedwWomen--the Women's Interagency HIV Study. J Frailty Aging. 2016;5(1):43–48. doi: 10.14283/jfa.2016.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levett TJ, Cresswell FV, Malik MA, Fisher M, Wright J. Systematic review of prevalence and predictors of frailty in individuals with human immunodeficiency virus. J Am Geriatr. 2016;64(5):1006–1014. doi: 10.1111/jgs.14101. [DOI] [PubMed] [Google Scholar]
- 7.Erlandson KM, Schrack JA, Jankowski CM, Brown TT, Campbell TB. Functional impairment, disability, and frailty in adults aging with HIV infection. Curr HIV/AIDS Rep. 2014;11(3):279–290. doi: 10.1007/s11904-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brothers TD, Kirkland S, Guaraldi G, Falutz J, Theou O, Johnston BL, et al. Frailty in people aging with human immunodeficiency virus (HIV) infection. J Infect Dis. 2014;210(8):1170–1179. doi: 10.1093/infdis/jiu258. [DOI] [PubMed] [Google Scholar]
- 9.Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, et al. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–489. doi: 10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlandson KM, Plankey MW, Springer G, Cohen HS, Cox C, Hoffman HJ, et al. Fall frequency and associated factors among men and women with or at risk for HIV infection. HIV Med. 2016;17(10):740–748. doi: 10.1111/hiv.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz MA, Reske T, Cefalu C, Estrada J. Falls in HIV-infected patients: a geriatric syndrome in a susceptible population. J Int Assoc Provid AIDS Care. 2013;12(4):266–269. doi: 10.1177/2325957413488204. [DOI] [PubMed] [Google Scholar]
- 12.Sharma A, Hoover DR, Shi Q, Holman S, Plankey MW, Wheeler AL, et al. Falls among middle-aged women in the Women's Interagency HIV Study. Antivir Ther. 2016;21(8):697–706. doi: 10.3851/IMP3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smurzynski M, Collier AC, Koletar SL, Bosch RJ, Wu K, Bastow B, et al. AIDS clinical trials group longitudinal linked randomized trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9(4):269–282. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Erlandson KM, Wu K, Koletar SL, Kalayjian RC, Ellis RJ, Taiwo B, et al. frailty and components of the frailty phenotype are associated with modifiable risk factors and antiretroviral therapy. J Infect Dis. 2017;15(6):933–937. doi: 10.1093/infdis/jix063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masud T, Morris RO. Epidemiology of falls. Age Ageing. 2001;30(Suppl 4):3–7. doi: 10.1093/ageing/30.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 17.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(Suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 18.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology. 2010;21(5):658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 19.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 20.Evans SR, Ellis RJ, Chen H, Yeh TM, Lee AJ, Schifitto G, et al. Peripheral neuropathy in HIV: prevalence and risk factors. AIDS. 2011;25(7):919–928. doi: 10.1097/QAD.0b013e328345889d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis RJ, Evans SR, Clifford DB, Moo LR, McArthur JC, Collier AC, et al. Clinical validation of the NeuroScreen. J Neurovirol. 2005;11(6):503–511. doi: 10.1080/13550280500384966. [DOI] [PubMed] [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998. [Google Scholar]
- 23.Samper-Ternent R, Karmarkar A, Graham J, Reistetter T, Ottenbacher K. Frailty as a predictor of falls in older Mexican Americans. J Aging Health. 2012;24(4):641–653. doi: 10.1177/0898264311428490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lord SR, Ward JA, Williams P, Anstey KJ. Physiological factors associated with falls in older community-dwelling women. J Am Geriatr. 1994;42(10):1110–1117. doi: 10.1111/j.1532-5415.1994.tb06218.x. [DOI] [PubMed] [Google Scholar]
- 25.Pan X, Bai Jj. Balance training in the intervention of fall risk in elderly with diabetic peripheral neuropathy: A review. International Journal of Nursing Sciences. 2014;1(4):441–445. [Google Scholar]
- 26.Quach L, Galica AM, Jones RN, Procter-Gray E, Manor B, Hannan MT, et al. The nonlinear relationship between gait speed and falls: the Maintenance of Balance, Independent Living, Intellect, and Zest in the Elderly of Boston Study. J Am Geriatr. 2011;59(6):1069–1073. doi: 10.1111/j.1532-5415.2011.03408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]