Abstract
Objective
While prenatal 3D ultrasonography results in improved diagnostic accuracy, no data are available on biometric assessment of the fetal frontal lobe. This study was designed to assess feasibility of a standardized approach to biometric measurement of the fetal frontal lobe and to construct frontal lobe growth trajectories throughout gestation.
Study Design
A sonographic 3D volume set was obtained and measured in 101 patients between 16.1 and 33.7 gestational weeks. Measurements were obtained by two independent raters. To model the relationship between gestational age and each frontal lobe measurement, flexible linear regression models were fit using penalized regression splines.
Results
The sample contained an ethnically diverse population (7.9% Native Americans, 45.5% Hispanic/Latina). There was high inter-rater reliability (correlation coefficients: 0.95, 1.0, and 0.87 for frontal lobe length, width, and height; p-values < 0.001). Graphs of the growth trajectories and corresponding percentiles were estimated as a function of gestational age. The estimated rates of frontal lobe growth were 0.096 cm/week, 0.247 cm/week, and 0.111 cm/week for length, width, and height.
Conclusion
To our knowledge, this is the first study to examine fetal frontal lobe growth trajectories through 3D prenatal ultrasound examination. Such normative data will allow for future prenatal evaluation of a particular disease state by 3D ultrasound imaging.
Keywords: 3D ultrasound, obstetrics, fetal growth, frontal lobe, percentiles
INTRODUCTION
Neuroimaging studies, including both structural and functional brain imaging, have greatly advanced our understanding of brain-behavioral relationship in numerous disease states. The fetal frontal lobe is of great clinical interest due to the associated centers of motor, cognitive, and executive functioning in the child and adult. Several disease states, including, fetal alcohol spectrum disorders (FASD), schizophrenia, autism and attention deficit hyperactivity disorders have been associated with imaging changes in the frontal lobe.1–6 In the affected child and adult, pathologic findings on neuroimaging include diminished frontal lobe volume, cortical thinning, and abnormal gyrification.7, 8
At present, the standard antepartum ultrasound exam contains no specific biometric assessment of frontal lobe beyond global measures of head circumference and biparietal diameter (BPD) in calculating gestational age.9 Detailed neurosonography at specialized centers may also include assessment of structures within or adjacent to the frontal lobe, such as the cerebral ventricles, cava septum pellucidum, and corpus callosum.10,11 Even these detailed assessments, however, are limited to observations such as absence or gross dilatation of a structure. Such assessments do not address minor changes in frontal lobe size and morphology.
While three-dimensional (3D) ultrasonography results in a higher resolution and improved diagnostic accuracy of fetal facial anomalies over two-dimensional (2D) ultrasonography,12 to the best of our knowledge, no data are available on the normal development, growth trajectories, or reproducibility of biometric assessment of the fetal frontal lobe with 3D ultrasound. The purpose of this study was to establish the natural development of the fetal frontal lobe dimensions, construct frontal lobe ‘growth charts’, and to evaluate the reproducibility of these measurements.
MATERIALS AND METHODS
Study design and population
The study has been approved by the University of New Mexico (UNM) Human Research Review Committee (protocol: 13-031). Given that no personal identifiers were recorded, and the ultrasound exams were conducted as a part of the clinical evaluation (no patients were subjected to research-specific ultrasound examination), the study was granted a waiver of informed consent. Study participants included pregnant women receiving prenatal ultrasound examination at a community-based large perinatology practice in Albuquerque, NM. The practice provides prenatal care for a mix of public and private insurance patients in New Mexico and frequently performs 3D acquisition of the cranium for diagnostic purposes.
The 3D fetal brain imaging in 130 consecutive patients with singleton pregnancies was captured as part of a routine transabdominal anatomic survey and stored for later analysis by perinatologists on the study (SB and RH). Imaging was captured on the Voluson E8 Expert series ultrasound (GE, Schenectady, New York) and manipulated and measured using the software program 4D view (GE, Schenectady, New York). Measurements of the frontal lobe were obtained, as described below, entered into a web-based, secure and HIPAA compliant electronic data capture program without personal identifiers. Pregnancies were dated by evaluating the earliest available ultrasound examination and its relationship to menstrual dating, in accordance with AIUM and ACOG guidelines.13,14 For the purposes of this analysis, patients with major structural anomalies (n=4), missing data for the frontal lobe measures (n=4), and patients with maternal conditions known to affect fetal growth (n=21), i.e. gestational or type II diabetes mellitus and smoking, were excluded. The final sample size included 101 patients. The earliest gestational age of exam, included in the sample, occurred at 15.7 gestational weeks. The majority of exams took place during the second trimester (70.3%), and remaining 29.7% were conducted during the third trimester.
Ultrasound measures
Measurements of the fetal frontal lobe were obtained after each 3D volume set was manipulated in the following standardized manner to locate landmarks:
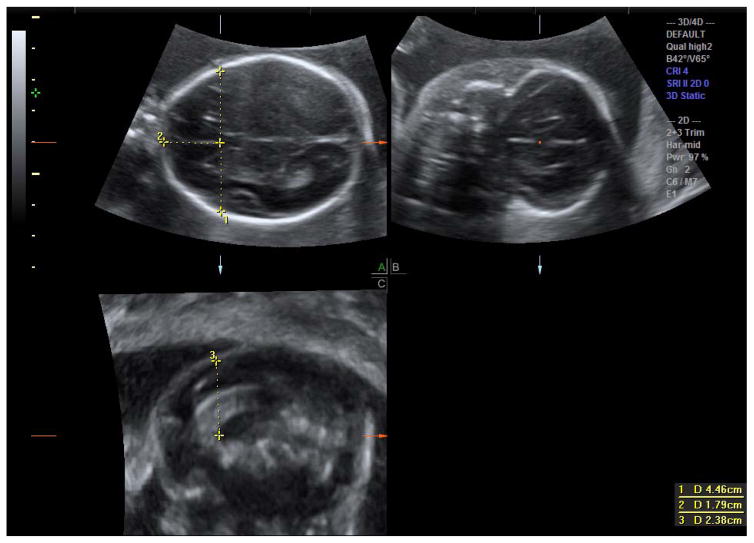
Acquire a 3D volume of the cranium utilizing the axial cut at the level of the BPD (with visualization of the thalami and cava septum pellucidum but not the cerebellar hemispheres)14 as the acquisition plane (A plane; top left image of a 4-box view, Figure 1a). This would make the B plane (top left image, Figure 1b) a coronal cut and the C plane (bottom left image; Figure 1c) a mid-sagittal cut. The bottom right image is the removed 3D rendered image.
Adjust the Z plane to make the falx horizontal in the axial and coronal planes. In the sagittal plane, rotate the Z plane to bring the corpus callosum and cava septum pellucidum into view.
Zoom to bring the cranium to the margins of the box.
-
The center of reference (CORef) should be placed at the:
Most anterior aspect of the genu in the sagittal plane
Midline and anterior in the corpus callosum in the axial plane
Midline and superior in the coronal plane
Measure the transverse width of the frontal lobe from the outer border of the cerebrum to the outer border of the cerebrum in the axial plane, passing through the CORef. (Figure 1a, measurement # 1).
Measure the length of the frontal lobe on the transverse plane from the outer cerebrum to the CORef (Figure 1a, measurement #2).
Measure the frontal lobe height on the sagittal plane from the CORef to the superior outer cerebrum, or alternatively, on the coronal plane passing through the CORef (Figure 1c, measurement #3).
Figure 1. 3D volume set of the fetal cranium.
Figure 1a (top left) represents an axial cut of the fetal cranium at the level of the corpus callosum/cava septum interface (where the two dotted lines intersect). The vertical dotted line (measurement #1) on the image represents the transverse width of the frontal lobe. The horizontal dotted line (measurement #2) represents the height of the frontal lobe from the corpus callosum to the superior inner calvarium.
Figure 1b (top right) represents the coronal plane image of the frontal lobe. The red dot is placed on the corpus callosum, which correlates with the intersection of the two dotted lines on Figure 1a.
Figure 1c (bottom left) represents the midline sagittal plane of the fetal cranium. The dotted vertical line (measurement #3) represents the height of the frontal lobe measured in a sagittal plane.
Images were then graded as ideal (all structural margins visible), adequate (structural margins visible on 2 out of 3 planes), or poor (structural margins visible on <2 planes). The ‘poor quality’ determination was based on either early gestational age disallowing presence of distinct anatomy or diminished quality of the 3D anatomy sweep.
Statistical analyses
The unit of analysis was the ultrasound exam. Standard summary statistics were conducted to describe the study sample. Means and standard deviations are presented for continuous variables (maternal age and gestational age). Counts and frequencies are shown for categorical variables. To establish inter-rater reliability, ultrasound images for a subset of the study population (27 subjects) were independently evaluated by two ultrasonographers (SB and RH). Readers were blinded to each other’s assessment. For each measure of the frontal lobe, Pearson correlation coefficient between the two readers and a corresponding p-value were estimated to assess inter-rated reliability. Correlation coefficients were used as a metric for inter-rater reliability because the growth measures are continuous variables. Inter-rater reliability was first estimated for all images, regardless of quality, and then again for a subset of images rated as ‘adequate’ or ‘good quality’ by both reviewers. To assess a relationship between the gestational age at exam (<18, 18–21.9, 22–32, and 32+ weeks) and the image quality (poor vs. adequate/good), a chi-square test was conducted.
To model the relationship between gestational age and each fetal frontal lobe growth measure (i.e., length, height, and width), linear regression models were fit using penalized regression splines. Regression splines model the relationship between an outcome and predictor using flexible basis functions that accommodate non-linearity in the outcome-predictor relationship.15 Penalized splines avoid the arbitrary selection of knots associated with many spline-based methods by using a large basis dimension and then shrinking basis coefficient estimates toward zero.15 For our linear regression models, we used a thin plate smoothing basis16 with basis dimension 15. The 10th, 50th, and 90th growth percentiles were estimated as a function of gestational age. The fitted means were transformed to percentiles using the transformation μ + σ ϕ(p), where μ is the fitted mean, σ is the estimated residual standard deviation, and ϕ(p) is the cut-off of the standard normal distribution at the pth percentile. The 95% confidence intervals were constructed for the growth percentiles by transforming confidence intervals for the fitted means from the regression model. Graphs of the growth trajectories as a function of gestational age were plotted for each measure of frontal lobe.
We compared the fitted models to a linear regression model to examine whether the growth measurements appear to change linearly with gestational age. To achieve this goal, the amount of structure being imposed by the fit of the spline model was quantified using the effective degrees of freedom (edf) of the smoother, which can be interpreted as the equivalent number of parameters in the model. For instance, an edf of 2 suggests only 2 parameters are needed to model the relationship between the predictor and outcome and this is essentially equivalent to a linear model.
In addition to presenting growth trajectories for a continuum of gestational ages in a figure format, percentiles and corresponding 95% confidence intervals (95% CI) were presented in a table format for a typical anatomic screen window (18–22 gestational weeks). Because the penalized spline fits suggested a linear relationship between gestational age and the frontal lobe growth measures, we calculated the average growth per week for each measure using simple linear regression. In addition, correlation coefficients (r) were estimated for an association between each measure of frontal lobe and head circumference. All data analyses were conducted in STATA v. 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) and the mgcv 17 package in R version 2.15.2 (R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.).
RESULTS
As shown in Table I, the study sample contained an ethnically diverse population (7.9% Native Americans, 45.5% Hispanic/Latina). The mean maternal age at enrollment was 29.0±6.6 years. The study captured ultrasound evaluations between 15.7 and 35.0 gestational weeks (mean gestational age 23.3±5.0). The most common indication for the ultrasound exam was anatomic survey (51.5%) followed by advanced maternal age (28.7%) and obesity (18.8%). The majority of images were either deemed of adequate (48.5%) or ideal (33.7%) quality.
Table I.
Description of the Study Sample (N=101)
| Maternal characteristics | Mean±SD |
|---|---|
| Maternal age (years) | 29.0±6.6 |
| Gestational age at exam (weeks) | 23.3±5.0 |
| Maternal race: | N (%) |
| White | 78 (77.2%) |
| Black or African American | 3 (3.0%) |
| American Indian or Alaskan Native | 8 (7.9%) |
| Asian/Asian American Islander | 4 (4.0%) |
| Other/not reported | 8 (7.9%) |
| Ethnicity: Hispanic or Latino | 46 (45.5%) |
| Indication for ultrasound exama: | |
| Advanced maternal age | 29 (28.7%) |
| Obesity | 19 (18.8%) |
| Thyroid disorder | 8 (7.9%) |
| Preterm labor/short cervix | 8 (7.9%) |
| Anatomic survey | 52 (51.5%) |
| Otherb | 13 (12.9%) |
| Image quality: | |
| Ideal | 34 (33.7%) |
| Adequate | 49 (48.5%) |
| Poor | 18 (17.8%) |
Some patients had multiple indications and the categories are not mutually exclusive
’Other’ indications include maternal disorders (e.g., ulcerative colitis, thrombophilia, kidney disorder, preeclampsia), family history of congenital anomaly, maternal history of adverse perinatal outcomes.
Inter-rater reliability for the frontal lobe measurements was generally high. For the 27 subjects whose ultrasound images were independently read by two raters, the Pearson correlation coefficients were 0.95, 1.0, and 0.87 for the frontal lobe length, width, and height, respectively (all p-values < 0.001). Image quality did not appear to influence the degree of inter-rater reliability since no qualitative differences in Pearson correlation coefficients were observed once the data were stratified by adequate/ideal versus poor image quality (data not shown). However, we found evidence of an association between poor image quality (according to the reviewer who read all images) and gestational age (p = 0.04). Only 3.1% (1 out of 32) of the images taken between 18 and 21.9 weeks were classified as poor quality, compared to 30.1% for <18 weeks, 20.8% for 22–31.9 weeks, and 37.5% for 32+ weeks.
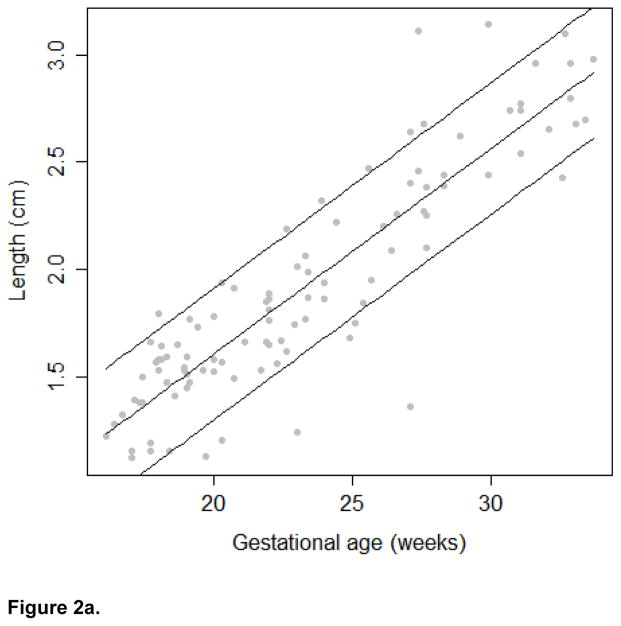
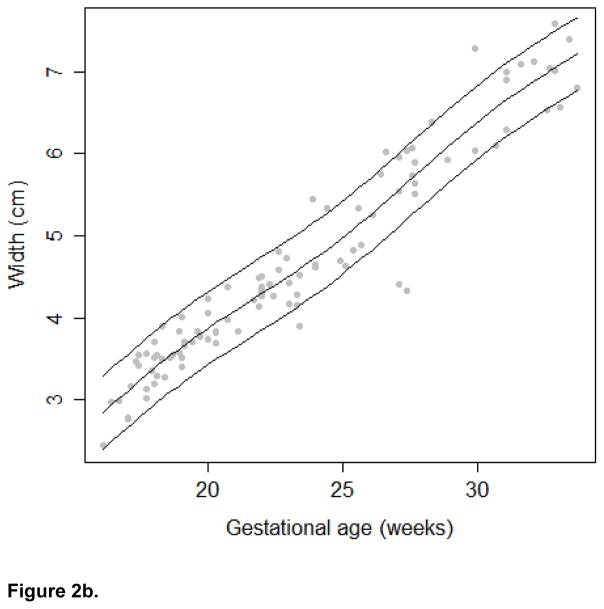
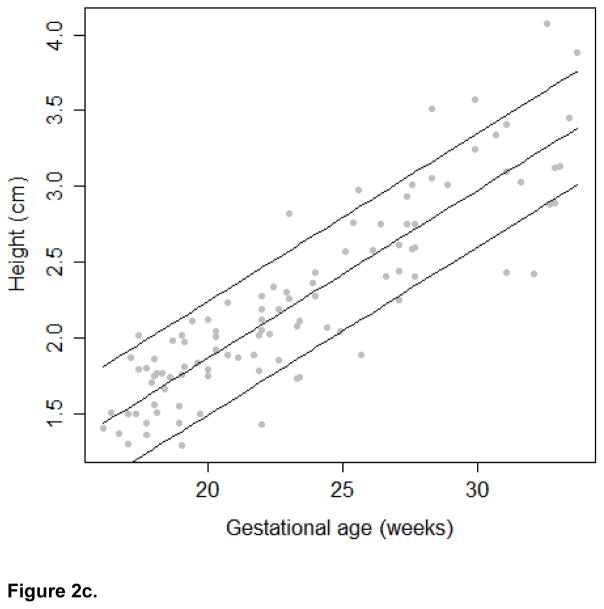
The growth trajectories and estimated 10th, 50th, and 90th percentiles for each growth measure are presented in Figures 2a, 2b, and 2c. In keeping with convention for other fetal biometric measures, we did not stratify by fetal gender. To assess linearity between the frontal lobe growth and gestational age, we calculated the effective degrees of freedom from the spline model. The edf for the frontal lobe length and height models was 2. Therefore, we conclude that the relationship between these growth measures and gestational age in our study population is best modeled using a linear relationship. The edf for the frontal lobe width model was 4.74, suggesting that the relationship between width and gestational age is better modeled using a non-linear function. In addition, a significant correlation was observed between the head circumference and frontal lobe length (r=0.69), width (r=0.69), and height (r=0.61; all p-values < 0.001)
Figure 2.
Figure 2a. Frontal Lobe Length Growth Trajectory
Figure 2b. Frontal Lobe Width Growth Trajectory
Figure 2c. Frontal Lobe Height Growth Trajectory
In addition to presenting growth trajectories across the span of gestational ages, Table II presents estimated percentiles and corresponding 95% confidence intervals for the growth measures at 18–22 gestational weeks. Confidence intervals were narrow for all measures and R-square values were high (.797 length, .925 width and .788 height). Assuming a linear relationship between gestational age and the growth measures, the estimated rate of growth for length is 0.096 cm/week (95% CI: 0.086; 0.105); for width is 0.247 cm/week (95% CI: 0.232; 0.261), and for height is 0.111 cm/week (95% CI: 0.099; 0.122). Sensitivity analysis revealed that after excluding 18 images of poor quality, qualitatively similar results were observed (data not shown).
Table II.
Frontal Lobe Percentiles by Gestational Age
| Gestational age | 50th percentile | 95% CI | 10th percentile | 95% CI | 90th percentile | 95% CI |
|---|---|---|---|---|---|---|
| Frontal Lobe Width | ||||||
| 18 | 3.38 | (3.26; 3.51) | 2.94 | (2.82; 3.06) | 3.83 | (3.71; 3.95) |
| 19 | 3.64 | (3.52; 3.76) | 3.19 | (3.08; 3.31) | 4.08 | (3.97; 4.20) |
| 20 | 3.87 | (3.75; 3.99) | 3.43 | (3.30; 3.55) | 4.32 | (4.19; 4.44) |
| 21 | 4.09 | (3.96; 4.21) | 3.64 | (3.51; 3.77) | 4.53 | (4.40; 4.66) |
| 22 | 4.30 | (4.17; 4.42) | 3.85 | (3.72; 3.98) | 4.74 | (4.61; 4.87) |
| Frontal Lobe Length | ||||||
| 18 | 1.41 | (1.34; 1.48) | 1.11 | (1.04; 1.18) | 1.72 | (1.65; 1.79) |
| 19 | 1.51 | (1.45; 1.57) | 1.20 | (1.14; 1.27) | 1.82 | (1.75; 1.88) |
| 20 | 1.60 | (1.55; 1.66) | 1.30 | (1.24; 1.36) | 1.91 | (1.85; 1.97) |
| 21 | 1.70 | (1.65; 1.75) | 1.39 | (1.34; 1.45) | 2.01 | (1.95; 2.06) |
| 22 | 1.80 | (1.75; 1.85) | 1.49 | (1.44; 1.54) | 2.10 | (2.05; 2.15) |
| Frontal Lobe Height | ||||||
| 18 | 1.65 | (1.56; 1.73) | 1.27 | (1.19; 1.36) | 2.02 | (1.94; 2.11) |
| 19 | 1.76 | (1.68; 1.83) | 1.38 | (1.31; 1.46) | 2.13 | (2.05; 2.21) |
| 20 | 1.87 | (1.80; 1.94) | 1.49 | (1.42; 1.56) | 2.24 | (2.17; 2.31) |
| 21 | 1.98 | (1.91; 2.04) | 1.60 | (1.54; 1.67) | 2.35 | (2.29; 2.42) |
| 22 | 2.09 | (2.03; 2.15) | 1.71 | (1.65; 1.77) | 2.46 | (2.40; 2.52) |
Note: Estimated frontal lobe percentiles by gestational age were based on the semiparametric regression model using 101 observations
DISCUSSION
Our findings support the feasibility of a standardized approach to biometric measurement of the fetal frontal lobe. We found good reproducibility among examiners and a high rate of ideal or adequate image acquisition. We also demonstrated the pattern of frontal lobe growth from 16 to 35 weeks. The advantage of 3D ultrasound assessment includes widespread availability, low cost to ultrasound units, and theoretically improved precision and reproducibility of measurements through use of the center of reference point and volume manipulation. Our technique also eliminates the contribution of the fetal cranium reflected in the standard biparietal diameter and head circumference measurements.
We are not aware of any previous studies which evaluated frontal lobe growth trajectories through 3D prenatal ultrasound exams. Before the relative recent advances in neuroimaging, including magnetic resonance imaging (MRI), normative brain growth data were supplied by postmortem studies.18 Perhaps the closest comparison to the prenatal 3D ultrasound imaging evaluation thus far is MRI studies in pediatric populations. The first reports of quantitative MRI studies in children emerged in the 1990s.19 These described the developmental trajectories of brain development for total cerebral volumes as well as specific brain structures, e.g. ventricles, corpus callosum, lobe-specific gray matter, cerebellum, for children and young adults (4–22 years of age).19, 20 Later studies, including the Brain Development Cooperative Group project, evaluated the link between structural brain MRI and behavioral development in healthy young children.21 In addition, a study by Nishida et al reported normative regional growth trajectories of cerebral structures for 12 neonates born between 31.1 and 42.6 weeks of gestation.22 Similar to our results, the frontal lobe volume increased linearly as a function of gestational age (R2 = 0.97) and demonstrated the fastest growth rate (2.93 ml/week) compared to other lobes.22
As mentioned earlier, a number of disorders which manifest by behavioral/neurocognitive deficits in the affected children have underlying neuro-pathological underpinnings, including structural changes in the frontal lobe. For example, in the field of FASD research, we have previously reported that 2D prenatal ultrasound identified specific markers (i.e., decreased frontothalamic distance, caval-calvarial distance, and orbital diameter) associated with prenatal alcohol exposure (PAE).23 Increasing evidence also indicates that in addition to a global effect of PAE on brain volumes resulting in microcephaly, PAE is associated with more subtle structural abnormalities of the brain,2, 4, 6 some of which can be visualized on 3D fetal sonograms,10, 11, 24 which allow for volumetric measures of intracranial structures.25 The 3D ultrasound is a preferred assessment tool during pregnancy over tomographic modalities, such as computerized tomography (CT) and magnetic resonance imaging (MRI), due to its safety and availability.26 Given that more than 90% of pregnant women in the U.S. undergo prenatal ultrasound examination, development of ultrasound markers of different disease states offers a unique opportunity to incorporate risk communication/reduction into existing genetic counseling networks. However, without the normative 3D sonographic growth data of specific brain structures in unaffected pregnancies, identifying disease-specific signatures is challenging.
As with many fetal anatomic structures, we found that the gestational age of the exam had an impact on the quality of assessment. We identified 18–22 weeks as the optimal age group for achieving an image quality rating of ideal (all structural margins visible) or adequate (structural margins visible on 2 out of 3 planes), while the quality of the images tended to be poorer for evaluations conducted earlier (<18 weeks) or later (≥ 32 weeks) in gestation. Factors which appeared to support this particular gestational window include the prominence of the cava septum, absence of fetal crowding associated with advanced gestational age and echogenicity of the fetal cranium. Posterior acoustic shadowing from the fetal cranium also played a role in the quality of imaging and inter-rater reliability of the frontal lobe height measurement. This particular measurement requires a clear interface between the most superior cerebral cortex and the echogenic calvarium. Posterior acoustic shadowing was one of the factors contributing to poorer image quality and higher degree of discrepancy between the two raters.
Several limitations of the current study need to be acknowledged. First, we used a semi-parametric linear regression model to estimate the growth percentiles. Less parametric approaches for growth curve estimation have been proposed, including the LMS method),27 quantile regression,28 and generalized additive models for location scale and shape (GAMLSS).29 These methods all rely on large sample sizes, while we chose a relatively more simple penalized spline model with normally distributed residuals to compromise between model flexibility and sample size limitations. The percentile estimates could be biased in the presence of violations of the normality assumption, such as heavier tails or a skewed distribution of growth measures at each age. However, based on model diagnostics, the assumptions of linear regression did not appear to be violated in our application.
Second, generalizability of the results might be limited due to a relatively small sample size; however, the sample appears to be representative with respect to typical indications for 3D ultrasound exam. It should be noted that ultrasound examination in the third trimester is typically conducted for clinical indications only. Ideally, to estimate normative data with respect to fetal growth, patients with uncomplicated pregnancies would need to be scheduled for the third trimester ultrasound. This would require an experimental rather than observational study design, which is always challenging with vulnerable populations such as pregnant women. To minimize the impact of maternal conditions on the estimated growth trajectories in the third trimester, we excluded conditions/exposures known to affect fetal growth (e.g. diabetes mellitus, smoking). Given that patients with conditions known to increase the risk of intrauterine growth restriction (e.g., smoking) were excluded, sample did not include patients with estimated fetal weight less than the 10th percentile for gestational age. To examine the effect of these maternal conditions further, a sensitivity analysis was conducted for a larger sample of 121 patients with those conditions (data not shown). Qualitatively, no differences in fetal frontal lobe growth trajectories were observed between the normative and larger samples.
Exclusion of the arachnoid and subarachnoid spaces could have provided further precision to our study. However, we anticipate any effect from this difference would be negligible. Furthermore, our measurement technique for the outer border of the frontal lobe was similar to (but more detailed than) that of a prior study estimating frontal lobe size on 2D sonography.30
An important strength of our study is the clinical applicability. Certainly, limitations of the 3D ultrasound did not allow for estimation of fetal frontal lobe ‘volume’ per se as in brain growth data produced by pediatric MRI studies mentioned earlier. While we could have used specialized software to reconstruct, outline, and precisely measure the frontal lobe, such measurements would have little clinical utility. Our approach to measurement of the frontal lobe can be performed on any ultrasound system with 3D technology.
Likewise, we felt it important to include images of all qualities as this more closely represents the quality of images the clinician will be interpreting in the office and is thus more generalizable. Additionally, images of poor quality would be more likely to be seen in women with obesity and other potential confounders; the exclusion of these could potentially bias our results. A sensitivity analysis excluding these data showed no significant difference. It should be noted that further imaging might have provided better quality images, such as a transvaginal exam for fetuses in cephalic presentations. However, given the retrospective nature of this study, we were not able to perform this.
Another strength of this study is the more exact measurement of the frontal lobe using 3D ultrasound. The frontal lobe is known to be more sensitive to environmental exposures such as alcohol and deficiencies in the frontal lobe may lead to significant lifelong impairments; accurately measuring the frontal lobe may be able to play a part in predicting these outcomes. Previous studies have evaluated the frontal lobe using BPD or other 2D measurements, which cannot provide a true volume assessment. On the other hand, our use of 3D sonography does provide an assessment of the volume of the frontal lobe. It is quite possible that certain conditions may lead to poor growth of the fetal frontal lobe, without affecting the BPD (which would be altered only in the most severe cases of global microcephaly and/or fetal growth restriction). Therefore, this novel approach to measurement has the potential to provide significantly better information regarding the developing fetal brain.
A unique strength of the study is broad representation of ethnic minorities largely underrepresented in research. It should also be noted that measures of inter-rater reliability were incorporated into this report to facilitate the assessment of reliability and to improve the accuracy of the estimated frontal lobe measurements in the study.
Results of this pilot study warrant future investigation into normative fetal brain growth trajectories, which should be evaluated in a larger sample. Reliable, representative, and readily available normative data will allow for future prenatal evaluation of a particular disease state by 3D ultrasound imaging. In addition, recent advances in prenatal laboratory screening methods (i.e., testing for cell-free fetal DNA in maternal peripheral blood) combined with improved resolution of 3D ultrasonography further highlight the need to have normative data for accurate risk assessment and counseling.
Acknowledgments
We would like to thank Dr. William Rayburn for critical review of the manuscript. We are also thankful to Shikhar Shrestha, M.S. and Indika Mallawaarachchi, MS for their assistance with the data management and analysis. Dr. Bakhireva’s effort is partially supported by the following grants from the NIH/NIAAA: R01 AA021771 and R15 AA022242.
References
- 1.Baribeau DA, Anagnostou E. A comparison of neuroimaging findings in childhood onset schizophrenia and autism spectrum disorder: a review of the literature. Front Psychiatry. 2013;4:175. doi: 10.3389/fpsyt.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bookstein FL, Connor PD, Covell KD, et al. Preliminary evidence that prenatal alcohol damage may be visible in averaged ultrasound images of the neonatal human corpus callosum. Alcohol. 2005;36:151–160. doi: 10.1016/j.alcohol.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Brun CC, Nicolson R, Lepore N, et al. Mapping brain abnormalities in boys with autism. Hum Brain Mapp. 2009;30:3887–3900. doi: 10.1002/hbm.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lebel C, Roussotte F, Sowell ER. Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev. 2011;21:102–118. doi: 10.1007/s11065-011-9163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li F, He N, Li Y, et al. Intrinsic Brain Abnormalities in Attention Deficit Hyperactivity Disorder: A Resting-State Functional MR Imaging Study. Radiology. 2014:514–523. doi: 10.1148/radiol.14131622. [DOI] [PubMed] [Google Scholar]
- 6.Riley EP, McGee CL, Sowell ER. Teratogenic effects of alcohol: a decade of brain imaging. Am J Med Genet C Semin Med Genet. 2004;127c:35–41. doi: 10.1002/ajmg.c.30014. [DOI] [PubMed] [Google Scholar]
- 7.Astley SJ, Aylward EH, Olson HC, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1671–1689. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, Lebel C, Lepage C, et al. Developmental cortical thinning in fetal alcohol spectrum disorders. Neuroimage. 2011;58:16–25. doi: 10.1016/j.neuroimage.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 9.AIUM practice guideline for the performance of obstetric ultrasound examinations. J Ultrasound Med. 2013;32:1083–1101. doi: 10.7863/ultra.32.6.1083. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo G, Pietrolucci ME, Mammarella S, et al. Assessment of cerebellar vermis biometry at 18–32 weeks of gestation by three-dimensional ultrasound examination. J Matern Fetal Neonatal Med. 2012;25:519–522. doi: 10.3109/14767058.2011.622006. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo G, Pietrolucci ME, Capponi A, Arduini D. Assessment of corpus callosum biometric measurements at 18 to 32 weeks’ gestation by 3-dimensional sonography. J Ultrasound Med. 2011;30:47–53. doi: 10.7863/jum.2011.30.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Kurjak A, Azumendi G, Andonotopo W, Salihagic-Kadic A. Three-and four-dimensional ultrasonography for the structural and functional evaluation of the fetal face. American journal of obstetrics and gynecology. 2007;196:16–28. doi: 10.1016/j.ajog.2006.06.090. [DOI] [PubMed] [Google Scholar]
- 13.American College of Obstetricians and Gynecologists. Committee Opinion no 611: method of estimating due date. Obstet Gynecol. 2014;124(4):863–6. doi: 10.1097/01.AOG.0000454932.15177.be. [DOI] [PubMed] [Google Scholar]
- 14.American Institute of Ultrasound in Medicine. AIUM Practice Parameter for the Performance of Obstetric Ultrasound Examinations. [Accessed January 19, 2016];AIUM website. 2013 Available at http://www.aium.org/resources/guidelines/obstetric.pdf.
- 15.Ruppert D, Wand MP, Carroll RJ. Semiparametric regression. Cambridge university press; 2003. [Google Scholar]
- 16.Wood SN. Thin plate regression splines. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2003;65:95–114. [Google Scholar]
- 17.Wood S. Generalized additive models: an introduction with R. CRC press; 2006. [Google Scholar]
- 18.McLennan J, Gilles F, Neff R. The Developing Human Brain. John Wright, PSG Inc; Boston, MA: 1983. A model of growth of the human fetal brain; pp. 43–58. [Google Scholar]
- 19.Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Giedd JN, Snell JW, Lange N, et al. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral cortex. 1996;6:551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 21.Almli CR, Rivkin M, McKinstry R. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35:308–325. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Makris N, Kennedy DN, et al. Detailed semiautomated MRI based morphometry of the neonatal brain: preliminary results. Neuroimage. 2006;32:1041–1049. doi: 10.1016/j.neuroimage.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Kfir M, Yevtushok L, Onishchenko S, et al. Can prenatal ultrasound detect the effects of in-utero alcohol exposure? A pilot study. Ultrasound in Obstetrics & Gynecology. 2009;33:683–689. doi: 10.1002/uog.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sotiriadis A, Dimitrakopoulos I, Eleftheriades M, Agorastos T, Makrydimas G. Thalamic volume measurement in normal fetuses using three-dimensional sonography. J Clin Ultrasound. 2012;40:207–213. doi: 10.1002/jcu.21888. [DOI] [PubMed] [Google Scholar]
- 25.Benavides-Serralde A, Hernandez-Andrade E, Fernandez-Delgado J, et al. Three-dimensional sonographic calculation of the volume of intracranial structures in growth-restricted and appropriate-for-gestational age fetuses. Ultrasound Obstet Gynecol. 2009;33:530–537. doi: 10.1002/uog.6343. [DOI] [PubMed] [Google Scholar]
- 26.Becker BG, Cosío FA, Huerta MEG, Benavides-Serralde J. Automatic segmentation of the cerebellum of fetuses on 3D ultrasound images, using a 3D Point Distribution Model. Engineering in Medicine and Biology Society (EMBC), 2010 Annual International Conference of the IEEE; IEEE; 2010. pp. 4731–4734. [DOI] [PubMed] [Google Scholar]
- 27.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Statistics in medicine. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 28.Wei Y, Pere A, Koenker R, He X. Quantile regression methods for reference growth charts. Stat Med. 2006;25:1369–1382. doi: 10.1002/sim.2271. [DOI] [PubMed] [Google Scholar]
- 29.Rigby R, Stasinopoulos D. Generalized additive models for location, scale and shape. Journal of the Royal Statistical Society: Series C (Applied Statistics) 2005;54:507–554. [Google Scholar]
- 30.Wass TS, Persutte WH, Hobbins JC. Am J Obstet Gynecol. 2001;185(3):737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]