Abstract
Chemoimmunotherapy with antibody-drug conjugates (ADC) is emerging as a promising therapy for solid tumors, while radioimmunotherapy (RAIT) of solid tumors has been relatively ineffective because of their resistance to radiation. We developed antibody-SN-38 conjugates that have significant anti-tumor activity in xenograft models at non-toxic doses. The goal of this study was to determine if an ADC could be combined with RAIT to enhance efficacy without a commensurate increase in host toxicity. Nude mice bearing human pancreatic cancer xenografts (Capan-1 and BxPC-3) were treated with a single dose of 90Y-labeled anti-mucin antibody (hPAM4; clivatuzumab tetraxetan) alone or in combination with an anti-Trop-2-SN-38 conjugate, typically administered twice weekly over 4 weeks. The combination, even at RAIT’s maximum tolerated dose, controlled tumor progression and cured established xenografts significantly better than the individual treatments, without appreciable toxicity. The ADC could be started 1 week after or up to 2 weeks before RAIT with similar enhanced responses, but delaying RAIT for 2 weeks after the ADC was less effective. A non-specific ADC provided additional benefit over using free drug (irinotecan), but the response was enhanced with the specific ADC. When targeting Capan-1 with ample mucin, hPAM4 could be used as the RAIT and the ADC agent without losing effectiveness, but in BxPC-3 with less mucin, targeting of different antigens was preferred. These studies show the feasibility of combining ADC and RAIT for improved efficacy without increased toxicity.
Keywords: antibody-drug conjugate, pancreatic cancer, radioimmunotherapy, SN-38, Trop-2
INTRODUCTION
Radioimmunotherapy (RAIT) is an effective treatment for non-Hodgkin lymphoma, but its effectiveness in solid tumors has been elusive (1). Difficulties in achieving meaningful anti-tumor responses in solid tumors is most likely related to their increased resistance to radiation, since there is no evidence that monoclonal antibodies (mAbs) used to target lymphoma have higher uptake or greater retention than those used for solid tumors (1–3). However, lymphoma therapy is undeniably aided by the therapeutic action of the unlabeled anti-CD20 antibody, including signaling events that enhance the cell’s sensitivity to radiation (4–7). Similarly, current efforts at developing antibody-drug conjugates (ADC) are promising in hematological malignancies, but also in solid tumors, such as breast cancer (8–10).
A number of anticancer drugs can enhance a tumor’s sensitivity to radiation, such as 5-fluorouracil, taxol and paclitaxel, and gemcitabine (GEM) (11–16). Most preclinical studies have combined the chemotherapeutic agent at a sub-therapeutic dose when given with RAIT, since if both treatments were given at therapeutically active doses, overlapping toxicities would likely require a reduction in the radiation dose, which could compromise the therapeutic response to RAIT. While improved responses have been noted in animal models, achieving substantial additional benefit in patients has been lacking (16–19).
Preclinical studies have indicated potent therapeutic responses when treating human pancreatic cancer xenografts with an anti-mucin antibody (PAM4 or clivatuzumab tetraxetan) labeled with 90Y, alone or in combination with GEM (20, 21). More recently, this antibody or an antibody to EGP-1 (also known as Trop-2) conjugated with SN-38, the active moiety of the prodrug, irinotecan, a topoisomerase-I inhibitor (22), was also found to be active against pancreatic cancer (23). 90Y-clivatuzumab tetraxetan has now advanced to clinical studies, where it is being administered in combination with radiosensitizing doses of GEM (24–26). Promising antitumor activity has been observed, and therefore we undertook this investigation to determine if an effective dose of an antibody-targeted drug could be combined with RAIT at its effective dose without increasing toxicity, but with improved efficacy. We were particularly concerned whether this combination can function best against two different antigen targets or if the same could be exploited. Also of interest was the temporal relationship in the administration of these two modalities. Our results show that two different immunoconjugates against the same or a different antigen target can have improved efficacy compared to the single-agent modalities, without increased host toxicity.
Materials and Methods
Antibodies, antibody conjugates, and cell lines
Capan-1 and BxPC-3 were purchased from the American Tissue Culture Collection (Manassas, VA). Capan-1 highly expresses the PAM-4 defined mucin, while BxPC-3 expresses less of this mucin. For example, immunohistology revealed >50% of Capan-1 xenografts stained intensely with the PAM4 antibody, with 5.6 mg/g of extractable antigen, while <5% of BxPC-3 xenografts stained weakly with PAM4, with a total extractable antigen of just 36 μg/g (27). Based on a cell-binding assay, where cell lines in culture plates were incubated with hRS7, which was then revealed with an anti-human IgG-horseradish peroxidase conjugate, both cell lines expressed similarly high levels of Trop-2 (i.e., ~20–30-fold higher than cell incubated with an irrelevant IgG).
Humanized PAM4 (hPAM4) and RS7 (hRS7) and their SN-38 conjugates were provided by Immunomedics, Inc. (Morris Plains, NJ). The hPAM4 and an irrelevant anti-CD20 antibody (veltuzumab) (28) was conjugated with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) and radiolabeled with 90Y (Perkin Elmer, Waltham, MA), according to a method published previously (29). Each radiolabeled product was tested for unbound 90Y (≤ 5%) and immunoreactivity by size-exclusion HPLC, using an anti-hPAM4 idiotype antibody (≥85% immunoreactive), as described previously (30).
Specific targeting hPAM4 and hRS-7-SN-38 conjugates (IMMU-134 and IMMU-132, respectively) and an irrelevant anti-CD20-SN-38 conjugate (IMMU-133), were prepared using the CL2A-SN-38 derivative as described by Cardillo et al. (23). The conjugates contained on average of 5–6 SN-38 moieties per IgG, with 1.0 mg of conjugate containing 16 μg of SN-38 equivalents. The conjugates were lyophilized and reconstituted in saline on the day of use.
Animal studies
Female athymic nude mice (Taconic, Germantown, NY) were implanted subcutaneously at 4–5 weeks of age with Capan-1 and BxPC-3 using a mixture of cells from tissue culture (1×107) and 0.2 mL from a 10% suspension taken from serially transplanted tumors. Tumors reached a size of ~0.3 to 0.6 cm3 (L × W × D) in 2–4 weeks, at which time animals were sorted into various groups, each having the same tumor-size range, and treatment was initiated.
The 90Y-hPAM4 (specific RAIT) or 90Y-anti-CD20 IgG (irrelevant RAIT) was injected intravenously (≤0.2 mL). Since most ADC treatments were given twice weekly for 4 weeks, the ADCs and irinotecan always were given intraperitoneally (≤0.3 mL). The specific dosage and treatment regimens of the ADC, RAIT, and GEM are provided for each experiment in the Results section. The standard ADC dosing was 0.5 mg/dose (~25 mg/kg; 0.4 mg/kg SN-38 equivalents). This treatment plan was devised to maintain a high level of the fully-loaded ADC in the blood based on the faster blood clearance of the ADC as compared to the unconjugated antibody, and the fact that the SN-38 gradually releases from the IgG (e.g., 50% cleaved within ~1 to 1.5 days) (23, 31). Irinotecan dosing assumed 1 mg of irinotecan yielded 0.575 mg of SN-38 equivalents (i.e., full conversion), since mice convert irinotecan to SN-38 much more efficiently than humans (32). When combination treatments were given on the same day, the ADC was injected 2 h before the 90Y-antibody to reduce personnel radiation exposure. GEM was given 2 days after the 90Y-hPAM4, which allowed time for the radiolabeled antibody to reach maximum levels in the tumor and clear somewhat from the blood and tissues.
Once treatments were initiated, animals were monitored daily, with once or twice weekly 3-dimensional measurements using a caliper and weight. Tumors were allowed to progress to 3.0 cm3, at which time the animals were removed from study and euthanized humanely. Animals showing any signs of morbidity or a decrease of more than 20% of their starting weight were also removed (censored).
Radioimmunoconjugate uptake in Capan-1 or BxPC-3 tumors was determined in animals that were necropsied 2 days after receiving 90Y-labeled DOTA-hPAM4 or DOTA-anti-CD20 IgG. The tissues were digested in a tissue solubilizer, and then counted in a scintillation cocktail, along with standards prepared from the injected product. These data are expressed as percent injected dose per gram (% ID/g).
Statistical analysis
Tumor sizes in the individual animals were plotted for each measurement. The time to progression to 3.0 cm3 (TTP) was considered the endpoint for a survival analysis performed using GraphPad Prism Software, version 5.0 (La Jolla, CA). The software calculated the median survival and statistical differences were based on the log-rank (Mantel-Cox) test using P ≤0.05.
Results
90Y-hPAM4 IgG RAIT with hRS7-SN-38 ADC
Previous studies had shown the hRS7 ADC given twice weekly for 4 weeks at 0.5 mg/dose did not result in any appreciable loss in weight, but significantly inhibited the growth of Capan-1 xenografts, but was not curative (23). 90Y-hPAM4 IgG cured most mice at its maximum tolerated dose (MTD) of 130 μCi (30). Therefore, the combination studies focused primarily on a reduced dose of only 75 μCi of 90Y-hPAM4 (~60% MTD) to assess the potential for therapeutic enhancement, but also with 130 μCi of 90Y-hPAM4 IgG to determine whether a therapeutically effective dose of ADC could be tolerated with the MTD of RAIT. Biodistribution studies confirmed that 90Y-hPAM4 IgG uptake in Capan-1 tumors measured 2 days after its injection was not affected by administering 0.5 mg of the hRS7-SN-38 two hours earlier (48.4 ± 16.4 vs. 45.3 ± 10.8 % ID/g, respectively; n = 3/group.
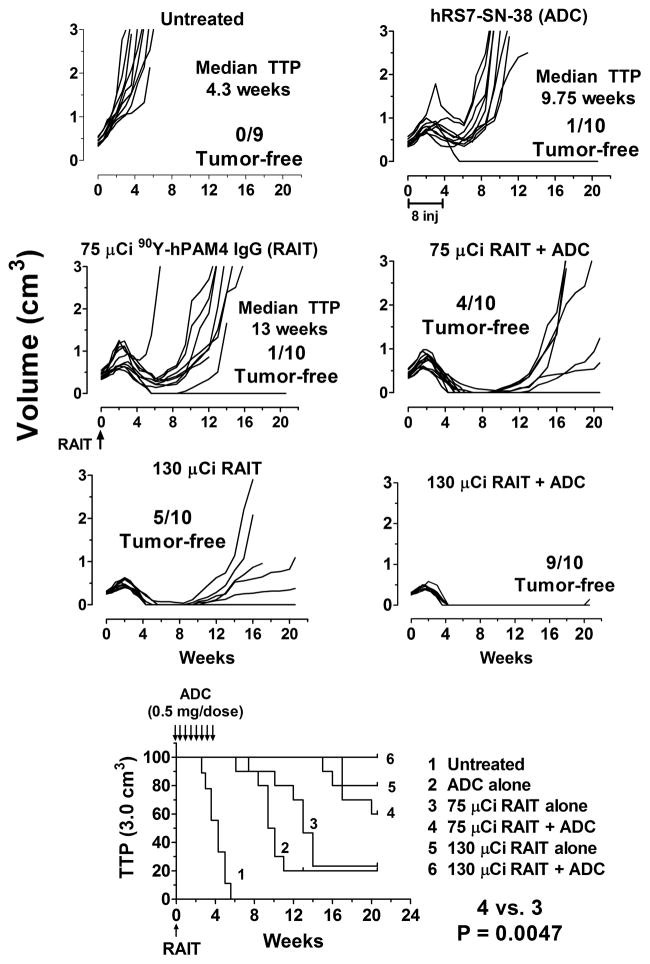
As shown in Figure 1, ADC alone improved the TTP from a median of 4.3 weeks in the untreated group to 9.75 weeks (P <0.001). The 75-μCi RAIT treatment alone increased the median survival to 13 weeks, and like the ADC-alone group, one animal was tumor-free after 21 weeks. In contrast, when ADC was initiated on the same day as 75 μCi RAIT, only 3 animals had tumors that progressed to 3.0 cm3 after 21 weeks, and 4 animals had no evidence of tumor, a significant improvement over RAIT alone (P = 0.005). The efficacy of this combination with the reduced RAIT dose was similar to the MTD of RAIT alone, but adding ADC to 130 μCi of RAIT improved efficacy, with 9/10 animals being tumor-free after 21 weeks. Importantly, the combination at RAIT’s MTD was safely tolerated in all animals with minimal additional weight loss over that seen with RAIT alone (Supplementary Fig. 1; no weight loss in the ADC-treated animals, not shown).
Figure 1. Enhanced therapeutic response by combining RAIT and ADC without substantial toxicity.
Nude mice (9–10 animals/group) bearing s.c. Capan-1 tumors averaging ~0.3 cm3 were given twice weekly injections of 0.5 mg (8 μg of SN-38) of the hRS7-SN-38 conjugate for 4 weeks starting on Day 0, or a single injection of 90Y-hPAM4 IgG at 75 μCi or 130 μCi (50 μg of hPAM4 IgG). Other groups of animals received the ADC treatment 2 h before the 2 RAIT treatments. Individual animal tumor growth curves are shown as well as a survival curve based on the time to progression (TTP) to 3.0 cm3. The median survival times and number of animals having no evidence of tumor at the conclusion of the study (21 weeks) are indicated.
Additional studies were performed to determine if there could be a temporal advantage by administering one treatment before the other. In this study group, the untreated animals had a median TTP of 3.4 weeks. The combination given on the same day again improved the median TTP (undefined after 18 weeks; 6/10 tumor-free at 18 weeks) significantly compared to RAIT alone (median TTP = 16.1 weeks; P = 0.039) or ADC alone (median TTP = 11.6 weeks; P = 0.023), with only 2 animals in the individual treatment groups being tumor-free at the conclusion of the study. No statistical difference in TTP was determined when ADC treatment was delayed 1 or 2 weeks after RAIT, and there were 8 animals tumor-free in each group. When RAIT was delayed 1 week after ADC, the anti-tumor effects were similar to when given on the same day (P = 0.456). However, when RAIT was delayed for 2 weeks after the start of ADC, only 4 animals were tumor-free at the conclusion of the study, with a median survival of 19.4 weeks, yielding significantly shorter TTP than when RAIT and ADC were given on the same day (P = 0.042), indicating that a lengthy delay in initiating RAIT after ADC can reduce therapeutic efficacy.
Another set of studies compared animals given RAIT (75 μCi 90Y-hPAM4) in combination with the twice-weekly ADC dosing regimen, giving 0.5 mg/dose for 2, 3, or 4 weeks (i.e., 4, 6, or 8 doses, respectively), and another group that received two, 2.0-mg doses in the same week as RAIT. In this set, untreated animals had a median TTP of 4.5 weeks and within 5 weeks all animals were censored because of tumor progression. The median TTP was not reached by the end of the study (16 weeks) in any of the other groups, but 2, 0, and 4 tumors were progressing in the groups receiving 1, 2, or 3 weeks of ADC dosing, respectively, at the termination of the study. The RAIT + ADC group that received 4 weekly injections had a better response than seen in the previous studies, rendering all animals tumor-free in <4 weeks, with one animal removed at week 12 because of a sudden excessive loss in weight, but it was tumor-free at the time. Thus, there was no evidence that shortening the number of ADC doses significantly reduced the response rate, but somewhat more tumors were progressing in those groups, while animals receiving 4 weeks of treatment were all tumor-free at the conclusion of the study.
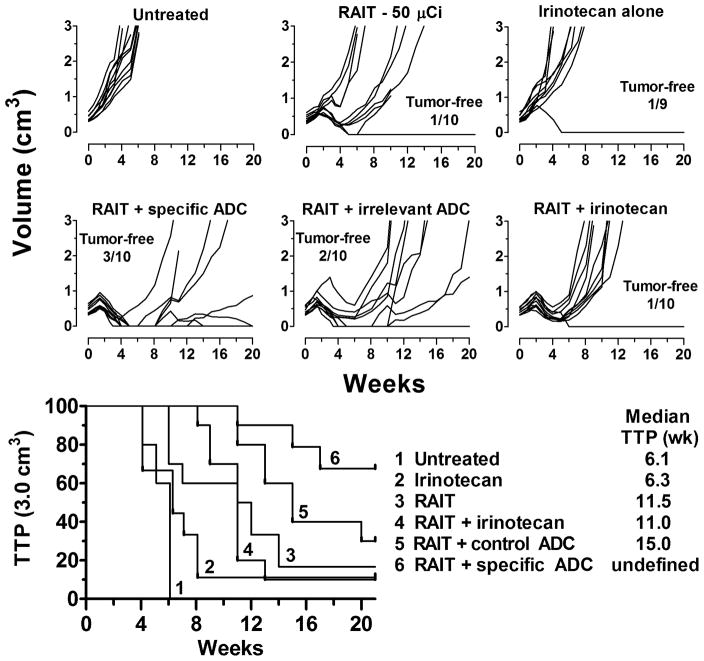
The efficacy of irinotecan alone and a control, non-targeted ADC (humanized anti-CD20-SN-38) was examined in comparison to the specific ADC. In this study, the RAIT dose was reduced to 50 μCi (50 μg IgG) in an effort to enhance the ability to assess the therapeutic benefits of the combinations. The irinotecan dose was adjusted so the same SN-38 mole equivalents were given as contained in the ADC. At this dose, irinotecan had a similar TTP as the untreated animals (6.3 vs. 6.1 weeks, respectively) (Figure 2). Adding this amount of irinotecan to RAIT provided no additional treatment benefit. Using an irrelevant IgG-SN-38 conjugate increased the median TTP to 15 weeks, a significant improvement over the combination with irinotecan (P = 0.0239). Adding the specific hRS7-SN-38 conjugate not only increased the median TTP (undefined after 21 weeks), but there was a more robust initial complete response in more animals. Thus, the irrelevant conjugate is a more effective SN-38-delivery agent than irinotecan, but the specific conjugate provided additional benefit.
Figure 2. Selective targeting by the ADC improves the combination response.
Nude mice bearing Capan-1 tumors (all groups starting with 10 animals) were given a single treatment of 90Y-hPAM4 IgG (50 μCi) on the same day treatment was initiated with irinotecan (13 μg, 7.5 μg SN-38 equivalents, twice weekly for 4 weeks), the specific (hRS7-SN-38; 0.5 mg/dose) or irrelevant (anti-CD20-SN-38; 0.5 mg/dose) conjugates given twice weekly for 4 weeks.
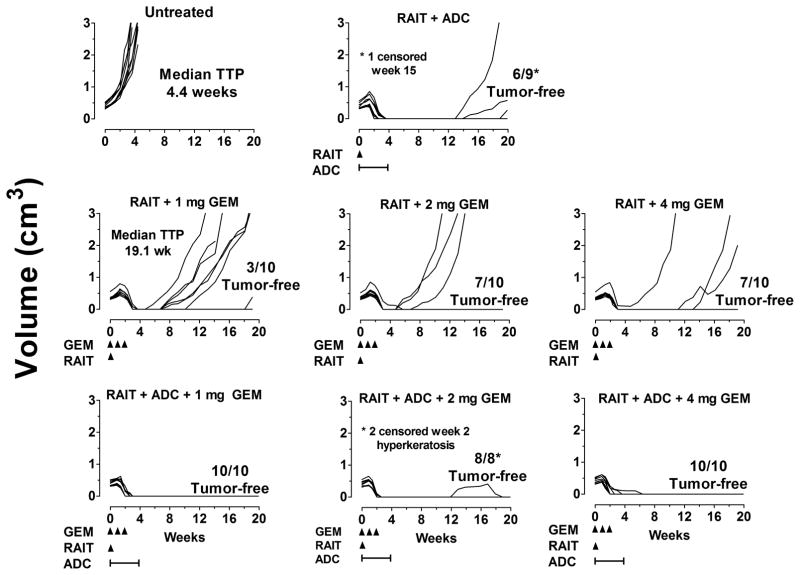
Since clinical studies are currently examining 90Y-hPAM4 IgG in combination with GEM, another study assessed the therapeutic efficacy of a single RAIT dose (75 μCi/50 μg) combined with 3 weekly GEM doses at varying amounts, with or without the hRS7-SN-38 ADC given (0.5 mg twice weekly x 4 weeks). The ADC treatment was started on the same day of RAIT, but GEM treatment was given 2 days after RAIT. GEM dosing ranged from 1 to 4 mg/dose, which extrapolates to a human equivalent dose of ~ 4 to 16 mg/kg. As indicated in Figure 3, the addition of 1 mg of GEM to RAIT was not as effective in controlling tumor growth as the RAIT + ADC combination, but with the higher doses of GEM, a similar number of animals were tumor-free at the study’s conclusion (20 weeks). When ADC was added to the combination of RAIT + GEM, irrespective of the GEM dose, all tumors were quickly eradicated, except one that initially progressed in the 2 mg GEM group 8 weeks after the cessation of the ADC treatment, but it eventually regressed. Two animals in this group were also censored just 2 weeks into the study because they developed hyperkeratosis. Animals in the treatment groups lost an average 2 to 6% of their weight 1 week the after treatments began, while untreated animals increased ~6% in weight, and while there was a trend for greater weight loss as the GEM dose increased, there were no significant differences among the various treatment groups.
Figure 3. RAIT, ADC, and GEM combination therapy.
Nude mice bearing Capan-1 tumors (all groups started with 10 animals) were given a single treatment of 90Y-hPAM4 IgG (75 μCi/50 μg) with GEM at the doses indicated once weekly for 3 weeks starting 2 days after the 90Y-hPAM4 treatment or with GEM plus the hRS7-SN-38 IgG conjugate (0.5 mg/dose, twice weekly for 4 weeks) that was initiated 2 h prior to the RAIT treatment.
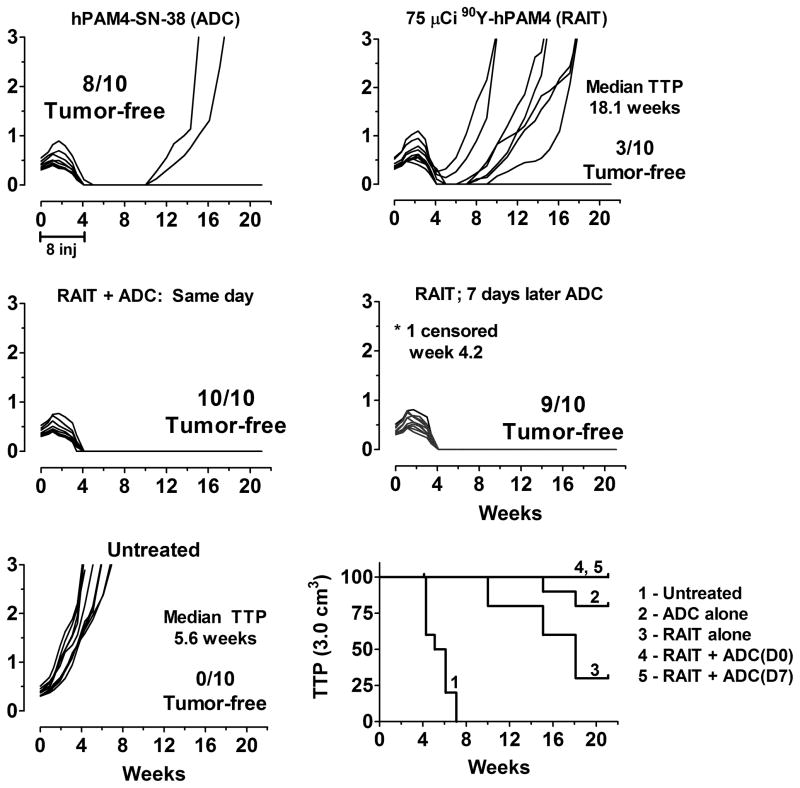
Collectively, these studies provided encouraging data that the combination of RAIT and ADC using antibodies targeting different antigens could enhance the response. We next examined the treatment of Capan-1 using RAIT and ADC targeting only the PAM4-defined mucin. Figure 4 shows the hPAM4-SN-38 conjugate was very effective in controlling tumor progression. In fact, when compared to all the earlier studies, it appeared to be more effective than the hRS7-SN-38 conjugate in this model. ADC was given either on the same day or 1 week later, with both combination groups providing an equal ability to enhance responses compared to RAIT alone.
Figure 4. RAIT and ADC combination therapy targeting the same antigen.
Nude mice bearing Capan-1 tumors were treated with 90Y-hPAM4 IgG alone (75 μCi/50 μg), hPAM4-SN-38 IgG (0.5 mg/dose, twice weekly for 4 weeks) alone, or they were given both treatments either on the same day (ADC given 2 h before RAIT) or delaying ADC treatment initiation for 1 week after RAIT.
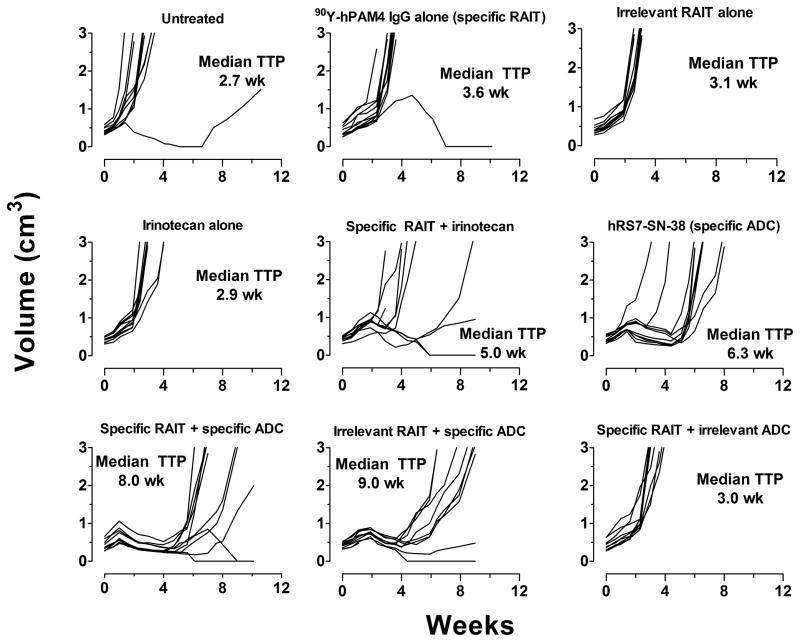
Since Capan-1 produces exceptionally high levels of the pancreatic mucin than most human pancreatic cancer cell lines (27), a second cell line, BxPC-3, which produced substantially less PAM4-reactive mucin, but had similar levels of Trop-2 as Capan-1, was examined. 90Y-hPAM4 uptake measured 2 days after injection averaged 18.1 ± 3.8% ID/g (n = 3) in BxPC-3 xenografts (0.42 ± 0.1 g), or ~2.5-fold less than the uptake in Capan-1, but ~2-fold higher than the uptake of the 90Y-labeled irrelevant anti-CD20 IgG (11.0 ± 4.5% ID/g; n = 3). 90Y-hPAM4 was less effective in controlling tumor progression in this model than in Capan-1, yet it significantly extended the median TTP to 3.0 cm3 from 2.7 weeks in the untreated animals to 3.6 weeks (P = 0.023) (Figure 5). Animals given an irrelevant 90Y-labeled humanized anti-CD20 IgG had a median TTP of 3.1 weeks. Irinotecan alone (dose normalized to same mole equivalents of SN-38 found in the ADC) was similar to the untreated group (2.9 weeks), but the combination of irinotecan and 90Y-hPAM4 RAIT enhanced the median TTP to 5.0 weeks (P = 0.0352). The hRS7-SN-38 conjugate alone had a median TTP of 6.3 weeks (hRS7-SN-38 vs. irinotecan, P <0.0001), and when combined with 90Y-hPAM4, it increased to 8.0 weeks (90Y-hPAM4 + hRS7-SN-38 vs. hRS7-SN-38, P = 0.0025). Based on the similarity in response between the 90Y-anti-CD20 IgG and 90Y-hPAM4, it was not surprising that the combination of the radiolabeled irrelevant IgG with the hRS7-SN-38 resulted in the same enhanced TTP as with the specific radiolabeled IgG (median TTP = 9.0 weeks), yet the combination of 90Y-hPAM4 with an irrelevant anti-CD20 IgG-SN-38 conjugate yielded no additional therapeutic benefit, with a median TTP of only 3.0 weeks. Thus, the specific hRS7-SN-38 was the main agent driving the response in this model, but adding a radioimmunoconjugate did improve the response.
Figure 5. Combination therapy in nude mice bearing the BxPC-3 human pancreatic cancer cell line.
Animals were given treatments of 90Y-hPAM4 IgG or the 90Y-labeled irrelevant anti-CD20 IgG alone (75 μCi/50 μg) or in combination with irinotecan (13 μg, twice weekly for 4 weeks), the specific hRS7-SN-38 IgG or the irrelevant anti-CD20-SN-38 conjugate (ADC given twice weekly for 4 weeks, 0.5 mg/dose).
DISCUSSION
Passive immunotherapy of cancer with unconjugated (naked) mAbs has gained an important role in the management of solid tumor, with agents such as cetuximab, panitumumab, trastuzumab, and bevacizumab, but most these mAbs are used to enhance the activity of other anticancer drugs, since alone, these unconjugated antibodies have limited ability to elicit meaningful and durable responses (33). Conjugating drugs, toxins or radionuclides to antibodies is intended to enhance the selectivity of drug delivery, but often these conjugates are used at their maximum tolerated dose in order to elicit an effective response, and thus they often are not combined with additional cytotoxic modalities unless other supportive measures are taken (e.g., 131I-tositumomab and chemotherapy given with autologous stem-cell support (34)), but they could be combined with unconjugated antibodies that are therapeutic, which should enhance the overall effects (35). Whether ADC and radioimmunoconjugates can be combined without increasing host toxicities, and therefore not compromising the therapeutic index of each, was addressed in this study, where we focused on pancreatic cancer because of our development of both ADC and RAIT agents for this disease.
90Y-labeled hPAM4 IgG is currently being evaluated in patients with advanced pancreatic cancer based on preclinical studies that found this agent was specific and effective in treating well-established pancreatic cancer xenografts (20, 21). The initial Phase I trial examining a single dose of the 90Y-hPAM4 IgG in patients who had received one prior therapy found 20 mCi/m2 was the MTD, but more importantly, there was evidence of measurable objective responses and disease stabilization in 5 patients (24). When measured against most of the earlier clinical trials in gastrointestinal cancers (19, 36–38), the initial results with radiolabeled PAM4 are encouraging; however, a more comprehensive approach is necessary. In this sense, since preclinical studies indicated that 90Y-hPAM4’s activity is enhanced when combined with GEM (20), clinical studies evaluating this combination are in progress as a frontline treatment, with initial results reporting an overall disease control rate was 55%, including 6 patients (16%) with partial responses and 15 (39%) with stabilization as best response (26).
In addition to the radioimmunoconjugate, we are examining the potential of ADCs in solid cancer therapy, focusing on SN-38 conjugated primarily to other anticancer mAbs, such as against CEACAM5 (31) and Trop-2 (23). We selected SN-38 because it is one of the most potent chemotherapeutic agents approved for clinical use in a water-soluble prodrug form, irinotecan (22). Irinotecan’s conversion to SN-38 is inefficient, with estimates suggesting that <5% is cleaved to the active SN-38 form (39). We reasoned that coupling SN-38 on an antibody would improve SN-38’s bioavailability in the tumor. Initial studies examined 6 conjugates prepared using different linkers and an anti-CEACAM5 antibody (31, 40). In vitro studies found SN-38 was gradually released from the IgG when incubated in human serum, with half-lives ranging from ~10 to 65 h. Animal studies revealed the best therapeutic activity occurred with a linkage designated CL2 that retained 50% of the SN-38 over ~35 h in human serum (31). The therapeutic activity of this conjugate was of particular interest because the CEACAM5 antibody did not internalize, a property generally thought to be essential for an effective ADC. We speculated that SN-38’s slow release from the ADC localized in the tumor allowed for locally higher concentrations of the free drug without the requirement for internalization of the intact conjugate. Since SN-38 is given clinically as a prodrug, its slow release from the antibody was not expected to have the same dire side effects that might occur with a more ultra-toxic cytotoxic agent, such as calicheamicin, auristatin, or maytansin, since these agents are too toxic to be given alone.
The initial testing of the hRS7 anti-Trop-2 IgG-SN-38 conjugate in mice and monkeys was reported recently (23). Trop-2 is an interesting cancer marker, often being associated with more aggressive forms of various cancers (41, 42); however, it is also expressed in a number of normal tissues, and thus testing in Cynomolgus monkeys that also express Trop-2 was imperative. They tolerated 120 mg/kg of hRS7-SN-38 (1.92 mg SN-38 equivalents/kg; human equivalent ~0.64 mg/kg SN-38 equivalents) given in 2 doses, 3 days apart, with minimal toxicity, but 240 mg/kg caused severe GI and hematologic toxicity (23). Mice, which do not express Trop-2, tolerated much higher doses than monkeys (3000 mg/kg or 48 mg/kg SN-38 equivalents; human equivalent 3.9 mg/kg SN-38 equivalents), but significant therapeutic responses occur at human equivalent dose ≤ 0.26 mg/kg SN-38 equivalents (23). Based on toxicity in monkeys and efficacy in mice, a therapeutic index of ≥ 3:1 is expected. This suggests that the SN-38 conjugate could be given at an effective dose with RAIT, making this an attractive combination for future clinical testing.
Other studies have cautioned that the practice of administering high doses of unlabeled anti-CD20 antibody therapy in advance of an anti-CD20 RAIT to ameliorate the CD20 antigen sink could reduce tumor uptake of the radioimmunoconjugate and compromise the therapeutic response in lymphoma (35, 43–45). By targeting with non-competing antibodies, we ensure that each conjugate can reach its maximum accretion level. However, it is important to emphasize that appreciable amounts of antibody are given in the ADC treatments. As we showed previously (23), it is possible to saturate antigen in small xenografts. Thus, in a repeat-dosing regimen, the later doses might not have the same level of specific localization and retention as the earlier doses, which can diminish specificity. Since less frequent dosing of the hRS7 ADC combined with RAIT also was as effective as using the full 4-week dosing schedule, the standard 4-week treatment regimen selected at the onset was unnecessary. Indeed, the therapeutic effect of the ADC at its MTD cannot be determined reliably in xenograft models because the protein dose would likely far exceed antigen saturation levels. Thus, while the ADC used in these studies appeared to be less effective than RAIT, RAIT was examined at its MTD and about 1.7-fold less than the MTD, while the cumulative amount of the ADC given to the mice was 15-times lower than its MTD in mice.
The specificity of the ADC treatment is also affected by the fact the CL2A linker allows SN-38 to be released slowly from the IgG, and therefore even an irrelevant IgG was a more effective means of delivering SN-38 than irinotecan. Indeed, other non-targeted SN-38 conjugates have been very effective in preclinical testing and early clinical trials underway (46–49). Even though the slow-release design reduces specificity, the conjugates are not reliant on internalization to exert an effect (50). Indeed, if internalization were a pre-requisite for potency, the hPAM4-SN-38 conjugate would not have been effective, since this antigen is primarily accessible in the extracellular pools of mucin, and not on the cell surface (RMS, unpublished data). However, since hRS7 internalizes, SN-38 could be carried inside the cell on the intact conjugate or as free SN-38 when released locally. The results in the BxPC-3 cell line also point to the value of having a dual targeting system. In this case, where PAM-4-reactive mucin levels were much lower than in Capan-1, the 90Y-hPAM4 was the less effective agent, but overall, the combination provided a better response than the individual agents.
As a topoisomerase I inhibitor, SN-38 exerts its optimal effects when cells are in the S-phase, and thus the 4-week treatment regimen provided sustained ADC exposure. However, because the combination had similar effects when the ADC was given in just one week suggests that the enhanced effects did not require an extended exposure. Temporal studies showed that response enhancement for the combination with RAIT could be achieved when the ADC was given as early as 2 weeks before RAIT to as late as 1 week after RAIT, but further delays in RAIT diminished the prospects for improvements to occur. Since GEM is currently being given clinically weekly for 4 weeks in combination with 90Y-hPAM4 administered weekly for 3 weeks, the hRS7-SN-38 IgG could either be substituted for the GEM or added with GEM, since even with the lowest dose of GEM, a significantly enhanced response was observed. However, the potential for additive toxicity of this trio combination at RAIT’s MTD was not examined.
In conclusion, the combination of RAIT with 90Y-hPAM4 and a non-competing SN-38 ADC, such as hRS7-SN-38, provides a complementary platform for improving therapeutic responses in xenografted human pancreatic cancer without substantial increases in toxicity. However, we appreciate that such xenograft models are limited in terms of the effects of undesired targeting by the mAbs in normal human tissues and different pharmacological disposition of the drugs and possibly ADCs in mice vs. humans. Nevertheless, just as preclinical studies of radiolabeled PAM4 alone and in combination with GEM appear to have been confirmed clinically, we are encouraged to pursue combination ADC-RAIT clinically.
Supplementary Material
Acknowledgments
Financial Support: This work was supported in part by PHS grant R01 CA115755.
We thank Ali Mostafa, Jayson Jebsen, and Louis Osorio for excellent technical assistance and Dr. S-J Moon and Fatma Tat for antibody-SN-38 conjugate preparations. This work was supported in part by PHS grant R01 CA115755.
Footnotes
Conflict of Interest Disclosure: SVG is an employee of Immunomedics, Inc. DMG has a financial interest in Immunomedics, Inc.
Presented at the ASCO 2011 Gastrointestinal Cancers Symposium in San Francisco, January 20–22, 2011 (J Clin Oncol 29: 2011 (suppl 4; abstr 206).
References
- 1.Sharkey RM, Goldenberg DM. Perspectives on cancer therapy with radiolabeled monoclonal antibodies. J Nucl Med. 2005;46(Suppl 1):115S–27S. [PubMed] [Google Scholar]
- 2.Dewaraja YK, Schipper MJ, Roberson PL, Wilderman SJ, Amro H, Regan DD, et al. 131I-tositumomab radioimmunotherapy: initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling. J Nucl Med. 2010;51:1155–62. doi: 10.2967/jnumed.110.075176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song H, Sgouros G. Radioimmunotherapy of solid tumors: searching for the right target. Curr Drug Deliv. 2011;8:26–44. doi: 10.2174/156720111793663651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez MC, Knox SJ. Radiobiology of radioimmunotherapy with 90Y ibritumomab tiuxetan (Zevalin) Semin Oncol. 2003;30:6–10. doi: 10.1053/j.seminoncol.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Honeychurch J, Cragg MS, Bayne M, Glennie MJ, Johnson PW, et al. Antibody-induced intracellular signaling works in combination with radiation to eradicate lymphoma in radioimmunotherapy. Blood. 2004;103:1485–94. doi: 10.1182/blood-2003-06-2037. [DOI] [PubMed] [Google Scholar]
- 6.Skvortsova I, Popper BA, Skvortsov S, Saurer M, Auer T, Moser R, et al. Pretreatment with rituximab enhances radiosensitivity of non-Hodgkin’s lymphoma cells. J Radiat Res (Tokyo) 2005;46:241–8. doi: 10.1269/jrr.46.241. [DOI] [PubMed] [Google Scholar]
- 7.Skvortsova I, Skvortsov S, Popper BA, Haidenberger A, Saurer M, Gunkel AR, et al. Rituximab enhances radiation-triggered apoptosis in non-Hodgkin’s lymphoma cells via caspase-dependent and - independent mechanisms. J Radiat Res. 2006;47:183–96. doi: 10.1269/jrr.47.183. [DOI] [PubMed] [Google Scholar]
- 8.Polson AG, Ho WY, Ramakrishnan V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin Investig Drugs. 2011;20:75–85. doi: 10.1517/13543784.2011.539557. [DOI] [PubMed] [Google Scholar]
- 9.Teicher BA. Antibody-drug conjugate targets. Curr Cancer Drug Targets. 2009;9:982–1004. doi: 10.2174/156800909790192365. [DOI] [PubMed] [Google Scholar]
- 10.Burris HA, 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 12.Wilson GD, Bentzen SM, Harari PM. Biologic basis for combining drugs with radiation. Semin Radiat Oncol. 2006;16:2–9. doi: 10.1016/j.semradonc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Burke PA, DeNardo SJ, Miers LA, Kukis DL, DeNardo GL. Combined modality radioimmunotherapy. Promise and peril. Cancer. 2002;94:1320–31. doi: 10.1002/cncr.10303. [DOI] [PubMed] [Google Scholar]
- 14.DeNardo SJ, Kroger LA, Lamborn KR, Miers LA, O’Donnell RT, Kukis DL, et al. Importance of temporal relationships in combined modality radioimmunotherapy of breast carcinoma. Cancer. 1997;80:2583–90. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2583::aid-cncr34>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Safran H, Rathore R. Paclitaxel as a radiation sensitizer for locally advanced pancreatic cancer. Crit Rev Oncol Hematol. 2002;43:57–62. doi: 10.1016/s1040-8428(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 16.Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, et al. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003;9:5842–52. [PubMed] [Google Scholar]
- 17.Richman CM, Denardo SJ, O’Donnell RT, Yuan A, Shen S, Goldstein DS, et al. High-dose radioimmunotherapy combined with fixed, low-dose paclitaxel in metastatic prostate and breast cancer by using a MUC-1 monoclonal antibody, m170, linked to indium-111/yttrium-90 via a cathepsin cleavable linker with cyclosporine to prevent human anti-mouse antibody. Clin Cancer Res. 2005;11:5920–7. doi: 10.1158/1078-0432.CCR-05-0211. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey RM, Hajjar G, Yeldell D, Brenner A, Burton J, Rubin A, et al. A phase I trial combining high-dose 90Y-labeled humanized anti-CEA monoclonal antibody with doxorubicin and peripheral blood stem cell rescue in advanced medullary thyroid cancer. J Nucl Med. 2005;46:620–33. [PubMed] [Google Scholar]
- 19.Shibata S, Raubitschek A, Leong L, Koczywas M, Williams L, Zhan J, et al. A phase I study of a combination of yttrium-90-labeled anti-carcinoembryonic antigen (CEA) antibody and gemcitabine in patients with CEA-producing advanced malignancies. Clin Cancer Res. 2009;15:2935–41. doi: 10.1158/1078-0432.CCR-08-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gold DV, Modrak DE, Schutsky K, Cardillo TM. Combined 90Yttrium-DOTA-labeled PAM4 antibody radioimmunotherapy and gemcitabine radiosensitization for the treatment of a human pancreatic cancer xenograft. Int J Cancer. 2004;109:618–26. doi: 10.1002/ijc.20004. [DOI] [PubMed] [Google Scholar]
- 21.Gold DV, Schutsky K, Modrak D, Cardillo TM. Low-dose radioimmunotherapy (90Y-PAM4) combined with gemcitabine for the treatment of experimental pancreatic cancer. Clin Cancer Res. 2003;9:3929S–37S. [PubMed] [Google Scholar]
- 22.O’Leary J, Muggia FM. Camptothecins: a review of their development and schedules of administration. Eur J Cancer. 1998;34:1500–8. doi: 10.1016/s0959-8049(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 23.Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: Preclinical studies in human cancer xenograft models and monkeys. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2939. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulec SA, Cohen SJ, Pennington KL, Zuckier L, Hauke RJ, Horne H, et al. Treatment of advanced pancreatic carcinoma with 90Y-clivatuzumab tetraxetan (humanized anti-pancreatic mucin antibody, hPAM4): A Phase I dose escalation trial. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2579. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pennington K, Guarion MJ, Serafini AN, Rocah-Lima C, Suppiah K, Schneider CJ, et al. Multicenter study of radiosensitizing gemcitabine combined with fractionated radioimmunotherapy for repeated treatment cycles in advanced pancreatic cancer. J Clin Oncol. 2009;27:231. (abst. 4620) [Google Scholar]
- 26.Ocean AJ, Guarino MJ, Pennington KL, Montero AJ, Bekaii-Saab T, Gulec SA, et al. Fractionated radioimmunotherapy with clivatuzumab tetraxetan combined with low-dose gemcitabine (Gem) is active in advanced pancreatic cancer (APC) J Clin Oncol. 2011;29 doi: 10.1002/cncr.27592. abst 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gold DV, Alisauskas R, Sharkey RM. Targeting of xenografted pancreatic cancer with a new monoclonal antibody, PAM4. Cancer Res. 1995;55:1105–10. [PubMed] [Google Scholar]
- 28.Goldenberg DM, Morschhauser F, Wegener WA. Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk Lymphoma. 2010;51:747–55. doi: 10.3109/10428191003672123. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths GL, Govindan SV, Sharkey RM, Fisher DR, Goldenberg DM. 90Y-DOTA-hLL2: an agent for radioimmunotherapy of non-Hodgkin’s lymphoma. J Nucl Med. 2003;44:77–84. [PubMed] [Google Scholar]
- 30.Karacay H, Sharkey RM, Gold DV, Ragland DR, McBride WJ, Rossi EA, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10-90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50:2008–16. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 31.Govindan SV, Cardillo TM, Moon SJ, Hansen HJ, Goldenberg DM. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009;15:6052–61. doi: 10.1158/1078-0432.CCR-09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morton CL, Wierdl M, Oliver L, Ma MK, Danks MK, Stewart CF, et al. Activation of CPT-11 in mice: identification and analysis of a highly effective plasma esterase. Cancer Res. 2000;60:4206–10. [PubMed] [Google Scholar]
- 33.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–27. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gopal AK, Rajendran JG, Gooley TA, Pagel JM, Fisher DR, Petersdorf SH, et al. High-dose 131I-tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or = 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25:1396–402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 35.Mattes MJ, Sharkey RM, Karacay H, Czuczman MS, Goldenberg DM. Therapy of advanced B-lymphoma xenografts with a combination of 90Y-anti-CD22 IgG (epratuzumab) and unlabeled anti-CD20 IgG (veltuzumab) Clin Cancer Res. 2008;14:6154–60. doi: 10.1158/1078-0432.CCR-08-0404. [DOI] [PubMed] [Google Scholar]
- 36.Sultana A, Shore S, Raraty MG, Vinjamuri S, Evans JE, Smith CT, et al. Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I131 KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma. BMC Cancer. 2009;9:66. doi: 10.1186/1471-2407-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tempero M, Leichner P, Baranowska-Kortylewicz J, Harrison K, Augustine S, Schlom J, et al. High-dose therapy with 90Yttrium-labeled monoclonal antibody CC49: a phase I trial. Clin Cancer Res. 2000;6:3095–102. [PubMed] [Google Scholar]
- 38.Tempero M, Leichner P, Dalrymple G, Harrison K, Augustine S, Schlam J, et al. High-dose therapy with iodine-131-labeled monoclonal antibody CC49 in patients with gastrointestinal cancers: a phase I trial. J Clin Oncol. 1997;15:1518–28. doi: 10.1200/JCO.1997.15.4.1518. [DOI] [PubMed] [Google Scholar]
- 39.Mathijssen RH, van Alphen RJ, Verweij J, Loos WJ, Nooter K, Stoter G, et al. Clinical pharmacokinetics and metabolism of irinotecan (CPT-11) Clin Cancer Res. 2001;7:2182–94. [PubMed] [Google Scholar]
- 40.Moon SJ, Govindan SV, Cardillo TM, D’Souza CA, Hansen HJ, Goldenberg DM. Antibody conjugates of 7-ethyl-10-hydroxycamptothecin (SN-38) for targeted cancer chemotherapy. J Med Chem. 2008;51:6916–26. doi: 10.1021/jm800719t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Day R, Dong Y, Weintraub SJ, Michel L. Identification of Trop-2 as an oncogene and an attractive therapeutic target in colon cancers. Mol Cancer Ther. 2008;7:280–5. doi: 10.1158/1535-7163.MCT-07-2003. [DOI] [PubMed] [Google Scholar]
- 42.Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–5. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112:830–5. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharkey RM, Karacay H, Johnson CR, Litwin S, Rossi EA, McBride WJ, et al. Pretargeted versus directly targeted radioimmunotherapy combined with anti-CD20 antibody consolidation therapy of non-Hodgkin lymphoma. J Nucl Med. 2009;50:444–53. doi: 10.2967/jnumed.108.058602. [DOI] [PubMed] [Google Scholar]
- 45.Kletting P, Meyer C, Reske SN, Glatting G. Potential of optimal preloading in anti-CD20 antibody radioimmunotherapy: an investigation based on pharmacokinetic modeling. Cancer Biother Radiopharm. 2010;25:279–87. doi: 10.1089/cbr.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koizumi F, Kitagawa M, Negishi T, Onda T, Matsumoto S, Hamaguchi T, et al. Novel SN-38-incorporating polymeric micelles, NK012, eradicate vascular endothelial growth factor-secreting bulky tumors. Cancer Res. 2006;66:10048–56. doi: 10.1158/0008-5472.CAN-06-1605. [DOI] [PubMed] [Google Scholar]
- 47.Sapra P, Zhao H, Mehlig M, Malaby J, Kraft P, Longley C, et al. Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin Cancer Res. 2008;14:1888–96. doi: 10.1158/1078-0432.CCR-07-4456. [DOI] [PubMed] [Google Scholar]
- 48.Guo Z, Wheler JJ, Naing A, Mani S, Goel S, Mulcahy M, et al. Clinical pharmacokinetics (PK) of EZN-2208, a novel anticancer agent, in patients (pts) with advanced malignancies: A phase I, first-in-human, dose-escalation study. J Clin Oncol. 2008;26 abstr 2556. [Google Scholar]
- 49.Hamaguchi T, Doi T, Eguchi-Nakajima T, Kato K, Yamada Y, Shimada Y, et al. Phase I study of NK012, a novel SN-38-incorporating micellar nanoparticle, in adult patients with solid tumors. Clin Cancer Res. 2010;16:5058–66. doi: 10.1158/1078-0432.CCR-10-0387. [DOI] [PubMed] [Google Scholar]
- 50.Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res. 2009;69:2358–64. doi: 10.1158/0008-5472.CAN-08-2250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.