Abstract
Psychosocial interventions for patients with chronic hepatitis C viral (HCV) infection are needed to attenuate the impact of extrahepatic symptoms, comorbid conditions, and treatment side effects on HCV health outcomes. We adapted empirically-supported interventions for similar patient populations to develop a Cognitive Behavioral Coping Skills group intervention for HCV patients (CBCS-HCV) undergoing treatment. The objectives of this paper are to describe the research activities associated with CBCS-HCV development and pilot testing, including: (1) formative work leading to intervention development; (2) preliminary study protocol; and (3) pilot feasibility testing of the intervention and study design. Formative work included a literature review, qualitative interviews, and adaption, development, and review of study materials. A preliminary study protocol is described. We evaluate the feasibility of conducting a randomized controlled trial (RCT) of the CBCS-HCV with 12 study participants in Wave 1 testing to examine: (a) feasibility of intervention delivery; (b) patient acceptability; (c) recruitment, enrollment, retention; (d) feasibility of conducting a RCT; (d) therapist protocol fidelity; and (e) feasibility of data collection. Numerous lessons were learned. We found very high rates of data collection, participant attendance, engagement, retention and acceptability, and therapist protocol fidelity. We conclude that many aspects of the CBCS-HCV intervention and study protocol were highly feasible. The greatest challenge during this Wave 1 pilot study was efficiency of participant enrollment due to changes in standard of care treatment. These findings informed two additional waves of pilot testing to examine effect sizes and potential improvements in clinical outcomes, with results forthcoming.
Keywords: Liver, Psychosocial, Lifestyle, Stress, Treatment, Direct acting antiviral (DAA), Psychological
1. Introduction
Chronic hepatitis C virus (HCV) is a global health epidemic that can lead to advanced liver disease, cirrhosis, and liver cancer and is associated with high morbidity, mortality, and healthcare utilization [1], [2]. Chronic HCV is also associated with diffuse HCV-related symptoms, poorer health-related quality of life (HRQOL), and other chronic conditions [3], [4], [5], [6]. Physical (e.g., fatigue, achiness) and neuropsychiatric symptoms (e.g., depression, insomnia, cognitive impairment) are common [3]. Causes underlying symptoms and poor HRQOL are multi-factorial, but may include chronic systemic inflammation of the central nervous system, comorbid conditions, health-related worry, and social stigma [3], [4], [5], [7], [8], [9], [10].
Treatment for HCV can also be difficult for patients to tolerate. Until 2014, treatment consisted of peginterferon/ribavirin (PegIFN/RBV) and caused numerous side effects, leading to nonadherence and premature treatment discontinuations [11], [12]. Since 2014, advances in treatment have virtually eliminated use of PegIFN in the U.S. and treatment now consists of newer, more effective directing acting antiviral (DAA) medicines [1]. However, some regimens still contain RBV, and while DAAs are more tolerable, they are still associated with side effects and decrements in HRQOL [13]. Moreover, higher risk patients (i.e. those with active psychiatric and addiction comorbidities) who were previously ineligible for treatment are now seeking treatment after waiting decades; these subgroups bring additional management challenges for providers [14].
Perhaps most importantly, the current repertoire of healthcare services available to people with chronic HCV is woefully limited to medical surveillance or curative, but expensive treatment. Many patients are denied access to treatment by insurance payors and are left with no other healthcare alternatives to improve their overall health and well-being. Psychosocial interventions could offer patients an alternative, holistic healthcare option: for some patients as they await treatment, for some to successfully undergo treatment, and for others, a non-medical service that may improve HRQOL beyond just viral cure.
Psychosocial interventions that include cognitive, behavioral, and lifestyle strategies may attenuate the negative impact of HCV symptoms and treatment side effects on HRQOL. Interventions that help patients cope with chronic illness have been developed for similar patient and treatment populations (e.g., HIV, cancer, chronic fatigue, chronic pain). Efficacy studies demonstrate improvements in psychological, physical, and immunological outcomes [15], [16], [17], [18], [19], [20], [21]. Despite the high prevalence of HCV and its individual and societal burden, few psychosocial interventions have been developed specifically for this large, underserved community [22], [23], [24].
Empirically-supported psychosocial interventions developed for other patient populations can be adapted for the needs of the HCV population [15], [17], [21], [25]. We developed a Cognitive Behavioral Coping Skills group intervention for HCV patients (CBCS-HCV) to optimize various health (e.g., HRQOL, psychological distress, symptoms) and treatment outcomes (e.g., adherence, viral cure). We used The Stage Model of Behavioral Therapies Research recommendations to guide intervention development [26]. The objectives of this paper are to describe the research activities associated with Stage 1a and 1b research of the CBCS-HCV, including: (1) the formative work that led to the development of the intervention; (2) the preliminary study protocol; and (3) preliminary pilot feasibility testing.
2. Methods
2.1. Guidance from The Stage Model of Behavioral Therapies Research
The Stage Model of Behavioral Therapies Research is a well-formulated, step-by-step plan created by NIH researchers to guide development of new or adapted behavioral treatments [26]. According to this model, research programs that are designed to scientifically evaluate behavioral/psychosocial treatments should progress through several specific stages, and within each stage, there are several essential steps to complete before moving on to the next stage. In this paper, we describe the steps we took during Stage 1a and Stage 1b to develop a new intervention for HCV patients. According to the stage model, the objective of Stage 1a research is to develop a preliminary treatment manual and study protocol. The objective of Stage 1b is to pilot test the preliminary intervention to evaluate feasibility of study design and aspects of the intervention. Performing these essential steps in Stage 1a and 1b research increases preparedness to conduct Stage 2 research, which is evaluation of an intervention in a future efficacy trial.
2.2. Stage 1a: Extensive formative work led to the development of the CBCS-HCV intervention study
The bulk of Stage 1a research activity was intervention development and manual writing, which culminated in two work products, the CBCS-HCV Patient Workbook and Therapist Manual. The formative work conducted to adapt and develop the CBCS-HCV included several activities which are elaborated below: (1) extensive literature review; (2) needs assessment of patients on HCV treatment; (3) drafting of the preliminary Therapist Manual and Patient Workbook; (5) review of these products by patients and clinical staff; (6) development of a provisional study protocol, including study design and methods; and (7) specification of provisional treatment outcomes and measures. Development of the CBCS-HCV intervention was also informed by several years of clinical experience that included conducting over 1000 psychological evaluations and brief interventions with HCV patients.
2.2.1. Literature review to inform intervention development
As part of Stage 1a research, to inform the content and structure of the intervention, we conducted an extensive literature review of (1) psychological, social, and behavioral aspects of living with chronic HCV and undergoing antiviral therapy and (2) empirically-based psychosocial interventions that could plausibly be adapted to improve HCV treatment and health outcomes. The findings from this literature review were published and directly informed several elements of CBCS-HCV [27]. First, we noted that patients complained of chronic, diffuse HCV-related symptoms prior to treatment, which could reflect extrahepatic manifestations of a chronic infectious, inflammatory disease or co-occurring conditions [3], [28], [29], [30]. These studies corroborated our clinical experience. We postulated that the development of cognitive behavioral and lifestyle skills to cope with HCV-related symptoms (e.g. fatigue, insomnia) impairing HRQOL needed to occur before treatment began in order to improve coping with pre-existing symptoms and prepare patients to deal with the unpleasant side effects of treatment. Second, we learned that somatic side effects (e.g., fatigue, insomnia, flu-like symptoms) occurred early in treatment and preceded and predicted later changes in cognitive-affective depressive symptoms (tearfulness, hopelessness, sadness) [31], [32]. Therefore, it seemed prudent that the CBCS-HCV be structured so that cognitive behavioral skills to cope with somatic symptoms (e.g., fatigue, insomnia) occurred early in therapy to reduce the risk of developing cognitive-affective components of depression later in treatment. Third, we noted that patients experienced feelings of social isolation and stigma [10]. They desired peer support that was often unavailable [33], leading to our decision to adapt a treatment that could be delivered in a group format versus individually. Collectively, these discoveries led us to review various cognitive behavioral interventions that previously demonstrated empirical support to improve outcomes in similar patient populations (e.g., HIV, cancer, osteoarthritis, insomnia, chronic fatigue) and which could be delivered in a group setting.
2.2.2. Patient needs assessment to inform intervention development
Simultaneous with the literature review, we conducted a qualitative needs assessment with patients undergoing antiviral treatment for HCV [34]. We sought to better understand patients' experiences during treatment, side effects, coping strategies, barriers and facilitators of medication adherence and persistence, and initial reactions to the idea of a group intervention that could provide skills training and peer support. Qualitative findings related to adherence were published and directly informed development of CBCS-HCV intervention material related to medication adherence [34]. For instance, facilitators of adherence included patient education, internal and social/external motivation, and practical behavioral strategies and routines. Barriers to adherence included changes in daily routine, being preoccupied with family or work responsibilities, and sleeping through dosing times. We also gathered patients' experiences with side effects of treatment and how lay people apply cognitive and behavioral skills to deal with side effects. Specifically, patients reported more difficulty coping with somatic symptoms, such a flu-like symptoms and exhaustion, rather than cognitive and emotional depressive symptoms; however, prolonged exposure to somatic symptoms led to depressive symptoms in some patients. These insights, combined with the literature review, confirmed our decision to address healthy lifestyle habits and somatic symptoms early in the intervention before patients start HCV treatment, to attenuate the risk of developing depressive symptoms later in treatment. Finally, most study participants indicated that a group setting would be acceptable.
Based on our literature review and formative studies, we identified several criteria that the ideal intervention would embody: 1) delivered in a group setting; 2) developed for patients undergoing a medical treatment that causes side effects; 3) teaching cognitive behavioral coping skills to manage physical and emotional symptoms and attenuate side effects such as fatigue, pain, depression, sleep disturbance; 4) improving healthy lifestyle habits as part of symptom prevention and management; 5) instilling stress management skills including coping with daily and interpersonal stressors; and 6) imparting medication adherence strategies.
2.2.3. Selection of evidence-based interventions to adapt for HCV
Several empirically-based psychosocial interventions for patients undergoing medical treatments were inspected during our literature review [15], [16], [17], [18], [19], [20], [21]. The Cognitive Behavioral Stress Management (CBSM) intervention [35], developed by psychologists at the University of Miami, is one such empirically-based intervention with over 20 years of rigorous testing and supportive evidence among populations of patients with medical illnesses such as cancer, HIV, and chronic fatigue syndrome, however not yet examined with HCV patients. The CBSM met several criterion and seemed highly suitable for adaptation to the HCV population. The original CBSM is a 10-session group-based cognitive-behavioral intervention focused on stress reduction, relaxation skills, coping skills, decreasing negative mood, improving HRQOL, and communication training. After our literature review [36], the CBSM was selected as the backbone on which to adapt the CBCS-HCV intervention. It met most of our requirements and had an impressive track record of clinical improvements in health and treatment outcomes for similar patient populations.
Meanwhile, we noted that the intervention needed to be modified to address specific issues and side effects unique to liver disease and HCV treatment which were not addressed in the CBSM. Therefore, we added health information about HCV and liver disease available online from the National Institute for Diabetes and Digestive and Kidney Diseases. We also reviewed other empirically-proven strategies and interventions that could address the needs of HCV patients and enhance treatment and health outcomes. For example, pre-existing depression, depressive side effects of treatment, and medication nonadherence were factors identified clinically and in the literature as frequently leading to worse treatment outcomes [37]. Therefore, we inspected empirically-supported interventions to address depression and adherence. The Cognitive-Behavioral Therapy intervention for Adherence and Depression (CBT-AD) which was developed for HIV + patients and includes modules on adherence and depression treatment, seemed highly suitable for HCV + patients undergoing treatment [21]. The HIV+ and HCV + patient populations share a number of similarities; both are chronic infectious diseases with similar symptomatology such as fatigue, both are highly stigmatized diseases, and both populations have a high prevalence of comorbid psychiatric and substance abuse histories. Furthermore, the treatment regimens both require high levels of adherence to daily medications that may involve challenging side effects and necessitate frequent follow-up visits with medical providers. Therefore, elements of the CBT-AD were incorporated into the CBCS-HCV. Likewise, strategies from other empirically-based psychological treatments were integrated into the CBCS-HCV to expand the comprehensiveness of the intervention for HCV patients: (1) the Cognitive Behavioral Treatment for Insomnia (CBT-I) protocol to target sleep disturbance with stimulus control, sleep hygiene, sleep restriction, and constructive worry techniques [18]; (2) Pain Coping Skills Training strategies, such as activity-rest cycles to cope with diffuse muscular and joint aches [19]; (3) Dialectical Behavioral Therapy and Anger Management strategies for coping with irritability, anger, interpersonal stress, and improving assertive communication [38], [39]; and (4) educational materials and strategies to promote healthy living to address nutrition, physical activity, and hydration, obtained from public access websites (https://www.choosemyplate.gov/ChoseMyPlate.gov; http://www.move.va.gov/). To summarize, the CBCS-HCV was adapted starting with CBSM modules as the primary backbone for intervention development, but we integrated material from other empirically-based interventions to develop a comprehensive psychosocial intervention relevant and useful for HCV patients.
2.2.4. Development of the preliminary CBCS-HCV intervention: structure and content
Based on the extensive literature review and needs assessment described above, the research team made several decisions that drove the structure and content of the CBCS-HCV group intervention. With regard to structure, the CBCS-HCV was developed to include 10 modules, similar to the structure of the CBSM, with several sections within each module to cover. Five weekly sessions occurred before starting HCV treatment to allow patients time to learn and practice new skills in order to have them readily available once treatment side effects developed. The other five sessions occurred during the first 12 weeks of HCV treatment concurrent to standard clinical follow-up visits (i.e., weeks 2, 4, 6, 8, 12 of treatment), to minimize patient burden and maximize fidelity to the intervention. The CBCS-HCV was designed for delivery in group format consisting of 6–8 participants, with each session anticipated to last approximately 90 min.
With regard to content in the modules, we adapted existing CBSM content for the CBCS-HCV when appropriate and thoughtfully integrated additional exercises, skill-building, and modules from other efficacious psychological treatments mentioned above [19], [21], [38], [39], [40]. The topics and skills covered in each of the 10 CBCS-HCV modules are listed in Table 1. Each module and group session was organized and delivered in the following order, similar to the format of CBSM modules, which has a proven record of patient satisfaction: (1) Introduction and practice of new relaxation techniques; 2) Review and application of the previous week's skills and homework exercises; and 3) Acquisition of new cognitive and behavioral skills. The first group session included an extensive discussion of confidentiality, group dynamics, and rules of conduct. Basic education about HCV, liver disease, and current HCV therapies were addressed in module 1. Positive lifestyle behaviors to promote liver health and coping with HCV symptoms were begun in module 1 and integrated into most of the earlier pre-treatment modules to help patients prepare for starting HCV treatment. Several CBT-based modules from the CBSM intervention were conducted before treatment began. Once treatment began, an emphasis was placed on depression prevention and healthy sleep hygiene, content that was added during adaptation, to work on skills to avoid these side effects commonly experienced during HCV treatment. Finally, the last few sessions were devoted to coping with interpersonal stressors applying various strategies from other interventions [38], [39]. Patients were asked to practice the new skills between sessions and return to the group with real-life examples of how these skills were applied and/or barriers to application. Patients had homework assignments to complete each week to encourage practice, tracking and application of new skills. Though HCV treatment could last 24 or 48 weeks for some patients, patients were encouraged to continue utilizing the CBCS skills during the remainder of treatment.
Table 1.
Content and structure of 10 CBCS-HCV group modules.
| Module | Specific Elements of Each Module |
||
|---|---|---|---|
| Relaxation Training | Review and Application of Previous Skills | Training in New Topic and Skills | |
| 1 | Progressive Muscle Relaxation | Introductions, Group Expectations | Intro & Overview, Positive Lifestyle Changes |
| 2 | Diaphragmatic Breathing | Positive Lifestyle Changes | Stress Awareness & Appraisal Lifestyle Changes |
| 3 | Autogenic Training | Stress & Appraisal, Lifestyle Changes | Negative Automatic Thoughts, Cognitive Distortions |
| 4 | Healing Wellness Imagery | Negative Automatic Thoughts, Cognitive Distortions | Cognitive Restructuring |
| 5 | Light Imagery | Cognitive Restructuring | Coping with Stress & Symptoms |
| 6 | Passive PMR | Coping with Stress & Symptoms | Cognitive-Behavioral Skills for Depression, Behavioral Activation, Pleasurable Activities |
| 7 | Immune System Guided Imagery | Cognitive-Behavioral Skills for Depression, Behavioral Activation, Pleasurable Activities | Activity-Rest Cycles, Sleep Hygiene |
| 8 | Self-Forgiveness Script | Activity-Rest Cycles, Sleep Hygiene | Anger Prevention/Management, Interpersonal Effectiveness |
| 9 | Mindfulness | Anger Prevention/Management, Interpersonal Effectiveness | Assertive Communication, Interpersonal Effectiveness |
| 10 | Group Choice | Assertive Communication, Interpersonal Effectiveness | Review of CBCS-HCV Program, Maintenance of Positive Changes |
2.2.5. Patient Workbook reviewed by patients and providers
The culmination of the Stage 1a research activities noted above were two work products: a preliminary 230-page, 10 module CBCS-HCV Patient Workbook and accompanying Therapist Manual. The final step in Stage 1a research was to have the CBCS-HCV intervention materials evaluated by patients and providers to ensure acceptability, readability, and useful content. The structure of the CBCS-HCV was discussed with two patient consultants. They were asked to provide feedback on the Patient Workbook by reading it carefully, making notes of their reactions, and completing a Patient Acceptability and Comprehension Survey after they reviewed each section of the 10 modules. After the review, the patient consultants were interviewed to discuss likes and dislikes of the Patient Workbook, usefulness of topics, readability of material presented, and additional missing strategies that were deemed important but not included. Each subsection of the Patient Workbook was rated on a 10-point Likert scale, from 0 = “Not at all useful” to 10 = “Extremely Useful”. The overall Patient Workbook received a score of 10 (extremely useful) from both patient reviewers. Subsections within each module ranged from “8” to “10.” A hepatology clinician also reviewed and provided feedback on the CBCS Patient Workbook regarding its content and whether the intervention provided accurate medical information and adequately addressed major psychological or behavioral issues. The patient and clinician feedback led to further refinement of the CBCS-HCV intervention and study materials prior to pilot-testing.
2.2.6. Selection of preliminary outcomes
For a future efficacy trial of the CBCS-HCV, we would be interested in evaluating general health outcomes, specific treatment outcomes, and mechanisms, therefore we cast a wide net to test the feasibility of data collection of these multiple outcomes. Specific treatment outcomes of interest in this pilot study included sustained virological response (SVR) 3 months after treatment, medication adherence and treatment persistence. Health outcomes of interest to test for feasibility of survey administration included HRQOL, perceived stress, HCV-associated symptoms, and treatment side effects. Mechanisms to test that might mediate clinical benefits included the active cognitive behavioral strategies learned, the nonspecific therapeutic environment of a group setting, and enhancing self-efficacy for HCV treatment.
2.3. Stage 1b: Description of study protocol to pilot test the CBCS-HCV
The main objective of Stage 1b research is to conduct a mini-pilot feasibility trial to determine study feasibility and refine elements of the intervention or study methods. Thus, we developed a detailed study protocol to pilot test. According to the Stage Model, stage 1b pilot feasibility testing should evaluate several elements listed in Table 2. We decided to address study elements A-G methodically using three “Waves” of study participants to allow us to refine elements of the intervention or study protocol in an iterative fashion. In the first wave of study participants (herein referred to as “Wave 1”), we address elements A-E. Based on Wave 1 pilot testing, we planned to refine the intervention and study methods and address elements F-G with separate participants in Wave 2 and 3 pilot testing.
Table 2.
Study elements to evaluate in Stage 1b pilot feasibility testing.
| A |
B |
C |
D |
E |
F |
G |
|
|---|---|---|---|---|---|---|---|
| Patient acceptability | Recruit/enroll participants | Intervention delivery | Therapist protocol fidelity | Data collection; measurement of outcomes | Clinically significant improvement | Effect size estimates | |
| Wave 1 | X | X | X | X | X | – | – |
| Wave 2&3 | X | X |
Below, we describe the preliminary study protocol written to conduct Wave 1 pilot testing of the CBCS-HCV, and then we present the results of the actual pilot and feasibility testing with Wave 1.
2.3.1. Study design
The study team determined that the most appropriate control condition for the pilot study was standard of care (SC), as opposed to no control condition, a waitlist or an attentional control, so that we could evaluate feasibility of randomization and data collection during SC. This pilot feasibility study was designed to evaluate the feasibility of conducing a two-arm RCT with study participants randomized to CBCS-HCV or SC. The intention was to enroll a total of 14 participants in Wave 1, with 7 randomized to CBCS-HCV and 7 randomized to SC. This study was approved by the UNC IRB Committee prior to recruitment.
2.3.2. Study methods
2.3.2.1. Participants and clinical setting
Patients with HCV invited to participate were being seen in an outpatient tertiary care hepatology center associated with a large academic medical center in the U.S. South serving residents of a primarily rural state. Patients were eligible to participate who were (1) English-speaking adults (age 21 or older); (2) infected with genotype 1; (3) deemed eligible for HCV therapy by a hepatologist by standard clinical criteria; and (4) waitlisted to start HCV treatment. Both patients naïve to HCV treatment and treatment-experienced were eligible. All participants gave written informed consent prior to data collection.
Exclusion criteria were as follows: (1) unable to provide written informed consent; (2) currently participating in a pharmaceutical clinical trial of HCV therapeutics; (3) co-infected with HIV or Hepatitis B; (4) evidence of illicit substance abuse (excluding marijuana) in the prior 6 months as reported during screening or noted in the medical record; (5) current elevated suicidal ideation reported during screening or noted in medical record; (6) current significant personality disorder or features reported during screening or noted in patient's medical record judged to be potentially detrimental to the group therapeutic setting for other participants; (7) could not make personal commitment to attend study visits and/or CBCS groups; and (8) otherwise medically or psychiatrically contraindicated to proceed with HCV therapy at the time of study enrollment as determined by their provider.
2.3.2.2. Recruitment process
Potentially eligible patients were pre-screened from an active waitlist of patients awaiting HCV treatment initiation or directly referred by providers. Eligible patients were then contacted and scheduled to attend a research visit which began with the informed consent process. Consented patients were screened for potential substance abuse or psychiatric issues using a brief psychiatric screening tool and a follow-up psychological interview if needed, to determine appropriateness for a group intervention.
2.3.2.3. Randomization
The protocol dictated that when a group of 14 eligible patients were consented and enrolled, we would randomize participants to CBCS-HCV (n = 7) and SC (n = 7). A computer-based randomization procedure was developed to conduct randomization with the plan to contact patients and inform them of group assignment.
2.3.2.4. Standard of care condition
The protocol dictated that 7 participants would be randomized to the SC condition, and proceed with starting HCV treatment and followed by the clinical hepatology team per clinical management for HCV. During the recruitment phase, standard HCV therapy for genotype 1 patients was response-guided triple therapy with PegIFN/RBV plus teleprevir or boceprevir for 24 or 48 weeks [41]. Per standard clinical procedures, patients attended regularly scheduled follow-up visits for safety and efficacy monitoring at 2 or 4-week intervals (e.g., treatment weeks 2, 4, 6, 8, 12). Viral response was monitored by clinicians to evaluate drug efficacy, and treatment could be discontinued at week 4 or 12 if viral reduction was insufficient. Data collection for SC participants occurred on the same day as their regular clinic visits to reduce patient burden.
2.3.2.5. Study therapist
The study therapist was the lead investigator (D.M.E.), a PhD-level licensed clinical psychologist with several years of clinical experience conducting psychological evaluations with HCV patients undergoing antiviral therapy.
2.3.2.6. CBCS-HCV treatment condition
The protocol dictated that participants randomized to the CBCS-HCV condition would attend 5 weekly in-person CBCS sessions prior to starting HCV treatment. Group participants would start taking their medications within the same 2 week time frame, and then return for the 6th CBCS-HCV session on the same day as their follow-up clinic appointment at week #2 during treatment. Patient Workbook materials for each module were provided at each session. A research coordinator was present to audio-record each session, complete measures of observed therapist adherence and competency, and collect participant treatment acceptability and comprehension surveys after each session. The therapist did not review these measures until after the study was complete.
2.3.2.7. Data collection assessment schedule
Table 3 lists the feasibility and outcome data collected at each assessment period.
Table 3.
Data collection and assessment schedule.
| Assessment Time Period | T1 | T2 | T3 | T4 | ||
|---|---|---|---|---|---|---|
| HCV Treatment Time point |
Baseline Pre-Treatment |
Start of HCV Treatment | Week 12 HCV Treatment | Week 24 HCV Treatment |
||
| CBCS-HCV Time point |
Baseline Start CBCS |
CBCS sessions 1–4 |
Session 5 | CBCS sessions 6–10 |
Final CBCS session 10 | 3-month Post-CBCS |
| Study Feasibility | ||||||
| Ratio of screens/enrollees | X | |||||
| RCT Study Design | X | |||||
| Retention Rates | X | X | X | X | X | X |
| Study Visit Attendance | X | X | X | X | ||
| CBCS Session Attendance | X | X | X | X | ||
| Missing Data | X | X | X | X | X | X |
| Patient Acceptability | X | X | X | X | ||
| Protocol Fidelity | ||||||
| Therapist's Adherence | X | X | X | X | ||
| Therapist's Competence | X | X | X | X | ||
| Demographics/Clinical | X | |||||
| Psychosocial Surveys | ||||||
| FACIT HRQOL | X | X | X | X | ||
| PSS Stress Severity | X | X | X | X | ||
| Depression | X | X | X | X | ||
| Anger | X | X | X | X | ||
| Anxiety | X | X | X | X | ||
| Fatigue | X | X | X | X | ||
| Sleep Disturbance | X | X | X | X | ||
| Sleep Impairment | X | X | X | X | ||
| Pain Intensity | X | X | X | X | ||
| Pain Interference | X | X | X | X | ||
| Medication Adherence | ||||||
| MEMS Caps | X | X | X | |||
| Pill Counts | X | X | X | |||
| Self-Report Adherence | X | X | X | |||
| Medical | ||||||
| Treatment Completion | X | |||||
| Virological Response | X | X | ||||
| Process Measures | ||||||
| Group Processes | X | |||||
| MOCS | X | X | X | |||
| Treatment Self-Efficacy | X | X | X | |||
T1: Baseline assessment after consent and screening.
T2: For participants in the CBCS-HCV condition, after CBCS session #5, immediately before starting HCV treatment. SC participants completed T2 immediately before starting HCV treatment.
T3: At HCV Treatment Week 12 for all study participants. Immediately following the last CBCS-HCV session for the CBCS-HCV participants.
T4: Three months post-intervention, at treatment week 24 for patients still on HCV treatment.
2.3.2.8. Remuneration
Study participants were compensated $25 for completion of each of the four assessments. CBCS-HCV participants received $25 for attendance at each of the 10 CBCS-HCV group sessions.
2.3.3. Measures
2.3.3.1. Study feasibility measures
The protocol was designed to evaluate the feasibility of the following study elements in the following ways: (a) feasibility of randomization procedure by whether we were able to enroll and randomize a block of 14 participants; (b) recruitment efforts by the proportion of patients contacted for screening versus those who attended the follow-up baseline screen; (c) enrollment by the proportion of patients consented versus those enrolled who began the CBCS-HCV intervention or treatment; (d) retention in the CBCS-HCV group by the number of CBCS-HCV sessions attended and the proportion who started and finished the CBCS-HCV intervention; and (e) the feasibility of data collection by the average of data points completed at each assessment period.
2.3.3.2. Therapist protocol fidelity measures
To develop means to evaluate the therapist's adherence to the intervention manual and competency to deliver the intervention [26], we implemented the following processes which will be needed for a future efficacy trial.
2.3.3.3. Audio-recordings
All CBCS group sessions were audio-recorded to assess feasibility of doing so in a larger trial, and enable the therapist to review the sessions to improve future performance and train future therapists to deliver the CBCS-HCV. Qualitative data from the interviews were not analyzed for pilot testing with Wave 1.
2.3.3.4. Therapist's adherence to the CBCS-HCV protocol
Study staff observed the delivery of each module and completed an observer-rating form of the therapist's adherence to the CBCS protocol. Using a scale of 0–100%, the observer rated the proportion of each subsection of the Therapist Manual that was completed. Length of time required to cover each subsection was tracked for future refinement.
2.3.3.5. Therapist's competency in delivering CBCS modules
Study staff observed the delivery of each CBCS-HCV module and completed an observer-rating form of the therapist's competency. Ratings were made on 14 items based on the following Likert scale: 1 = Not At All; 2 = A little; 3 = moderately; and 4 = A lot/Extremely. This scale was developed by researchers at the University of Miami to capture CBSM therapist's competency to manage group dynamics and demonstrate awareness of the nonspecific therapeutic processes (i.e., empathic listening, trust) which impact treatment outcomes [42].
2.3.4. Patient acceptability and comprehension measures
Critical to Stage 1b work is to demonstrate patient acceptability of the psychosocial intervention, which was measured by the following:
2.3.4.1. Patient acceptability and comprehension scale
CBCS-HCV participants completed a 14-item brief survey administered by a research coordinator at the end of each of the 10 sessions to rate the session on acceptability, usefulness, comprehension, and group process. Each item is rated on a five-point Likert scale from “Not At All” to “A lot/Extremely”. The survey was adapted from one developed by interventionists at the University of Miami to examine the CBSM intervention [42]. The therapist did not review these data until after the study was complete.
2.3.4.2. Exit interview
One month after the last CBCS-HCV group session, participants were invited to attend an exit focus group conducted by the lead PI/therapist and a psychology postdoctoral fellow who was not involved in intervention delivery. An interview guide was developed to facilitate a discussion about comprehension of the information provided in the modules, usefulness of each module, opinion about the length and number of sessions, group size, structure, and overall topics or strategies that were most useful or most needed improvement. The lead PI participated in the interview related to content, but left the room during discussion of therapist performance. The interview was audio-recorded to inform future modifications of the CBCS-HCV.
2.3.5. Preliminary outcome and process measures
One objective for Wave 1 pilot testing was to determine the feasibility of data collection for a future RCT. Listed below are several outcomes and the surveys used to measure them, which could potentially be utilized in a future efficacy study.
2.3.5.1. Health-related quality of life (HRQOL)
The Functional Assessment of Cancer Therapy-General Population (FACT-GP) was used to evaluate HRQOL. The FACT-GP is an instrument derived from the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system to measure HRQOL during the management of chronic illness [43]. The FACT-GP is a 21-item survey that assesses four HRQOL domains: Physical well-being; Social/Family well-being; Emotional well-being; and Functional well-being. Items are rated on a five-category response system ranging from 0 (not at all) to 4 (very much). The FACIT instruments have been shown to be reliable, valid, and sensitive to change in clinical trial and observational settings [43], [44].
2.3.5.2. Perceived stress
The Perceived Stress Scale (PSS) is a widely used survey to measure stress perception. The scale includes 10 items, rated using a 5-point Likert scale, from 0 (never) to 4 (very often) where patients report the frequency of symptoms in the past month. The PSS has been shown to have good reliability and validity [45].
2.3.5.3. HCV symptoms & treatment side effects
Several of the NIH Patient-Reported Outcomes Information System (PROMIS) measures (www.nihpromis.org) were used to measure commonly reported HCV-associated symptoms which may worsen during HCV treatment, or new onset side effects of HCV treatment [46]. We utilized eight PROMIS short forms to measure a precise HCV symptom or common treatment side effect: 1) Depression Short Form (SF)-8 items; 2) Anger SF-8 items; 3) Anxiety SF-4 items; 4) Fatigue SF-8 items; 5) Sleep Disturbance SF-8 items; 6) Sleep Related Impairment SF-8 items; 7) Pain Intensity SF-3 items; 8)Pain Interference SF-4 items.
2.3.6. Medication adherence
The protocol dictated that we would assess the feasibility of using various forms of measurement of medication adherence to determine which was most feasible and associated with fewer missing values.
2.3.6.1. Medication event monitoring system (MEMs; AARDEX, Switzerland)
Electronic pill caps were used to measure daily adherence to taking RBV pills dosed twice a day. Patients were instructed at baseline in the use of the MEMs cap. The MEMs caps recorded the precise date and time of each cap opening and data from the MEMs caps were downloaded at each clinic visit. PegIFN injections and other pills, which came in thrice daily bubble-pack strips, did not lend themselves to MEMs measurement. The MEMs caps were measured in the CBCS-HCV condition only.
2.3.6.2. Pill counts
RBV pill counts were obtained at each treatment visit from week 2–12. Number of pills remaining in the RBV bottle were monitored to determine ideal vs actual proportion of pills taken between clinic visits.
2.3.6.3. Self-report adherence
Patient-reported adherence for the last 7 days prior to a clinic visit was measured using a brief staff-administered survey, adapted from a previous NIH-funded trial on HIV adherence [47].
2.3.7. Active CBCS skills
The Measure of Current Status (MOCS) was developed to evaluate the potential active ingredients of the CBSM intervention. Thirteen items from the MOCS Part A were used to assess participants' self-perceived status on several skills that are acquired and practiced during the CBCS intervention, such as relaxation, assertiveness, awareness of tension, and confidence in coping ability [48]. The MOCS Part A was administered to participants in both conditions.
2.3.8. Self-efficacy for HCV treatment
A modified version of the HCV-Treatment Self-Efficacy Scale (HCV-TSE) measures patients' confidence to perform specific activities related to HCV treatment. A total score and four underlying factors can be calculated: patient communication, general physical coping, general psychological coping, and adherence self-efficacy. The measure was found to have satisfactory reliability and validity [49]. The HCV-TSE was administered to participants in both conditions.
2.3.9. Group experience
We adapted items from other reliable and validated surveys that were relevant to the CBCS-HCV group intervention, incorporating three items from the Working Alliance Inventory-Client Short Form [50], four items from the Group Climate Questionnaire [51], and 11 items from the Therapeutic Factors Inventory-Short Form [52]. The final survey included 18 items to evaluate nonspecific therapeutic processes of group psychotherapy.
3.0. Results of pilot study in wave 1: implementation and feasibility testing
Below we describe the feasibility of conducting a small RCT of the CBCS-HCV group intervention with Wave 1 study participants, with an emphasis on feasibility of study design and methods, protocol fidelity, and patient acceptability.
3.1. Feasibility of study design elements
3.1.1. Feasibility of recruitment and enrollment
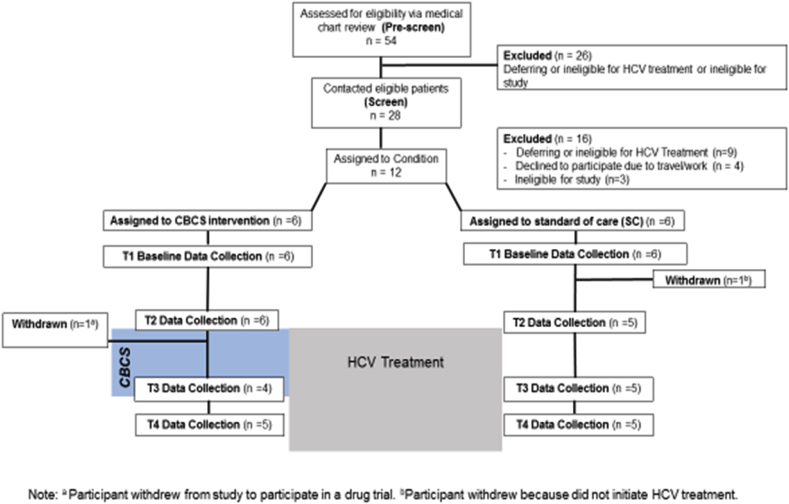
The study flowchart depicted in Fig. 1 shows the number of patients initially pre-screened from the treatment waitlist (n = 54), those contacted and briefly screened over the phone (n = 28) and, finally, those consented, enrolled, and assigned to one of the two conditions (n = 12). Of the 54 pre-screened, 52% (28/54) were eligible for a follow-up phone screen, and of those phone screened, 43% (12/28) were eligible and agreed to participate. We encountered significant challenges during recruitment due to the rapidly evolving landscape of HCV treatment which led hepatologists and patients to defer treatment during the study period until newer, less toxic medications were available. As a result, the number of patients ready to start treatment during the recruitment phase was drastically lower than we had anticipated, taking 3 months to consent 12 patients. Other identified recruitment barriers were travel distance for research visits and overall ineligibility for HCV treatment.
Fig. 1.
Study flowchart.
3.1.2. Feasibility of randomization
As a result of extremely slow enrollment that took 3 months to complete, we discovered that the initial RCT study design and intention to randomize when a block of 14 participants were enrolled, was not feasible. Patients who had already received their HCV medications from the pharmacy were eager to begin taking them and did not want to further postpone. Due to difficulty in retaining enough enrolled subjects required for randomization, we made the decision to switch to an observational intervention trial. Participants who were willing and interested in the CBCS-HCV intervention (n = 6) were assigned to the treatment condition, while participants willing to participate in the study by doing surveys (n = 6), were assigned to the SC condition. This study design still allowed us to obtain valuable information about other aspects of the CBCS intervention and study design elements. Although recruitment presented unexpected challenges, patient feedback regarding a group psychosocial intervention was positive. In patients who were contacted about the study, the most common reason for declining to participate was travel distance and transportation barriers. No patient declined to participate due to lack of interest. Many commented that a group intervention for patients undergoing HCV treatment would be very valuable, but that in-person sessions before HCV treatment began were impractical.
3.1.3. Feasibility of study retention
Of the 12 patients enrolled in the study, retention in both the CBCS and SC conditions was high (10/12; 83%). Out of 6 participants assigned to CBCS condition, one participant attended the first 5 CBCS sessions and completed T1 and T2 assessments, but then was recruited into a drug clinical trial before HCV therapy began (See Fig. 1). Another CBCS patient attended 7 CBCS sessions and underwent HCV treatment for 6 weeks, but was discontinued from HCV treatment due to a combination of severe neuropsychiatric side effects and lack of viral response. This patient remained in the study, missed T3 assessment, completed T4, and missed CBCS sessions 8–11. Out of 6 participants assigned to SC, one participant decided to defer HCV treatment based on personal issues and was withdrawn from the study after T1 but before T2. In total, 10 of the 12 assigned participants completed the study and the majority of study assessments (T1-T4). Of these 10 participants, almost half were discontinued from HCV treatment at some point by providers or patients due to lack of viral response or medical/psychiatric side effects, but all 10 remained in the study and completed follow-up assessments.
3.1.4. Feasibility of retention in the CBCS-HCV intervention
Of the 6 participants originally enrolled in the CBCS group intervention, attendance at all CBCS sessions was high, with an average attendance across 6 participants and 11 modules of 93.7% (range 80%–100%).
3.1.5. Feasibility of delivering CBCS-HCV intervention
Early into the CBCS group sessions, it was determined that it was not feasible to cover all of the intervention material in 10 sessions of 90 min duration. The CBCS group members encouraged the therapist to expand the duration and number of sessions, therefore Wave 1 participants attended a total of 11 sessions; sessions 1–4 lasted 90 min and sessions 5–11 lasted 2 h each. All CBCS group members supported these changes to allow for more in-depth discussion and application of CBCS skills. The therapist was able to cover a majority of the material in 2 h. Content not covered in a session due to time constraints was carried over to the next session or assigned as reading homework.
3.1.6. Feasibility of data collection
Data collection of outcome measures at T1 through T4 assessment periods was high; we obtained 97.5% data completion for all patients at all time points (range 75%–100%). Even patients who were discontinued from HCV treatment remained in the study to complete T3 and T4. Overall, the logistics of collecting patient-reported outcome surveys in pencil/paper format in a busy liver clinic or via mail were manageable. Allocated space for research activities and support from providers, clinic staff, and participants made the data collection process feasible.
3.2. Baseline patient characteristics
We enrolled 12 participants from November 2012 to February 2013 to participate in Wave 1 pilot testing. Characteristics of the study sample are presented in Table 4.
Table 4.
Sample characteristics.
| Characteristic | CBCS |
SC |
Total |
|---|---|---|---|
| n = 6 | n = 6 | n = 12 | |
| Age (SD) | 52.8 (6.8) | 48.2 (13.1) | 50.5 (10.2) |
| Male | 5 (83.3%) | 4 (66.7%) | 9 (75.0%) |
| Race | |||
| Caucasian | 4 (68%) | 5 (83%) | 9 (75%) |
| Black/African-American | 1 (17%) | 1 (17%) | 2 (17%) |
| Hispanic/Latino | 1 (17%) | 0 (0%) | 1 (8%) |
| Educational Attainment | |||
| High school diploma or GED | 3 (50%) | 2 (33%) | 5 (42%) |
| Post-high school education | 3 (50%) | 4 (67%) | 7 (58%) |
| Employment Status | |||
| Working part-time or full-time | 3 (50%) | 2 (33.3%) | 5 (41.5%) |
| Disabled | 3 (50%) | 2 (33.3%) | 5 (41.5%) |
| Unemployed | 0 (0%) | 2 (33.3%) | 2 (17%) |
| Insurance Status | |||
| Private insurance | 1 (17%) | 2 (33%) | 3 (25%) |
| Medicaid or Medicare | 2 (33%) | 2 (33%) | 4 (33%) |
| Uninsured | 1 (17%) | 1 (17%) | 2 (17%) |
| Combination/other | 2 (33%) | 1 (0%) | 3 (25%) |
| Treatment naïve | 6 (100%) | 5 (83%) | 11 (92%) |
| Evidence of Cirrhosis | 2 (33%) | 3 (50%) | 5 (42%) |
3.3. Protocol fidelity
3.3.1. Therapist adherence
On average, the observer ratings of the therapist's adherence to the CBCS manual (i.e., how much of each subsection was covered) demonstrated coverage of 76% of all materials, with a range from 0% to 100%. Typically, 100% of the earlier and larger subsections of a session were covered (i.e., relaxation technique, review of previous skills), and later subsections (i.e., new skills) were covered less, due to time constraints of a 90–120 min session. Review of these subsections were given as homework or were covered at the next session.
3.3.2. Therapist competency
Table 5 provides the median for each of the 14 items across all 11 modules. The overall median observer rating was 3.64 (range 2–4).
Table 5.
Observer ratings of therapist competency areas.
| Raw Item/Content Area | Median (SD) Across 11 Modules |
|---|---|
| Awareness dynamics | 3.0 (0.52) |
| Maintains dynamics | 3.0 (0.70) |
| Maintains engagement | 4.0 (0.00) |
| Working alliance | 4.0 (0.50) |
| Confidence | 4.0 (0.30) |
| Articulate | 4.0 (0.47) |
| Active listener | 4.0 (0.52) |
| Warm | 4.0 (0.30) |
| Genuine | 4.0 (0.30) |
| Interest | 4.0 (0.47) |
| Empathic | 4.0 (0.50) |
| Dynamic | 3.0 (0.45) |
| Specific feedback | 3.0 (0.52) |
| Challenges | 3.0 (0.60) |
Note. Rating scale ranged from 1 = “Not at all” to 4 = “A lot/extremely”.
3.4. Patient acceptability
Table 6 lists the median patient acceptability and comprehension ratings collected from all CBCS participants after each of the 11 sessions. The average median rating across all sessions and all patients was 4.2 out of 5 (SD = 0.16), indicating a very high level of acceptability and comprehension.
Table 6.
Patient Acceptability and comprehension ratings.
| Item Content | Ratings Across 11 CBCS Sessions Median (SD) |
|
|---|---|---|
| 1 | We had a good session today | 4.7 (0.30) |
| 2 | People seemed to genuinely care about each other today | 4.4 (0.39) |
| 3 | People bonded today | 4.0 (0.59) |
| 4 | I felt understood today | 4.0 (0.31) |
| 5 | I felt comfortable today | 4.4 (0.31) |
| 6 | The content was informative today | 4.3 (0.30) |
| 7 | The content was relevant to me today | 4.3 (0.41) |
| 8 | I understood most of the material we discussed | 4.0 (0.26) |
| 9 | Today's material was easy to follow | 4.0 (0.26) |
| 10 | I thought there was too much to cover** | 3.8 (0.61) |
| 11 | I thought that we did not have enough time for discussion and review today** | 3.8 (0.55) |
| 12 | I can use what I learned today | 4.5 (0.30) |
| 13 | I am looking forward to the next session | 4.5 (0.29) |
| 14 | I intend to remain in this program | 4.8 (0.18) |
Note. Range: 1 = “Not At All” to 5 = “Extremely. **Items reversed coded.
4.0. Discussion
The objectives of this paper were to describe Stage 1a and Stage 1b research activities to develop and test a psychosocial intervention for patients with chronic HCV undergoing antiviral therapy. During Stage 1a we conducted formative work that led to the development of the intervention, Patient Workbook and Therapist Manual, and in Stage 1b we conducted a small pilot feasibility study to evaluate the feasibility of the study methods and intervention. We learned numerous lessons from pilot testing in Wave 1 with regard to successful features of the study methods and intervention, as well as the most challenging areas in need of consideration to move the CBCS-HCV research program forward. The major challenge during this Wave 1 pilot study was that recruitment and enrollment impediments prevented us from randomizing participants. However, patient acceptability ratings of the CBCS-HCV group intervention were very promising, and aside from recruitment issues, the majority of other study elements were found feasible.
In this paper, we thoroughly described the formative work leading to the development of the CBCS-HCV group intervention, including the literature review, a patients' needs assessment, drafting and beta-testing of a preliminary Patient Workbook and Therapist Manual. We also delineated details of the preliminary study design and testing of methods used with Wave 1 study participants to determine if a full scale RCT efficacy trial would be doable. It was critical to test the feasibility of conducting a two-armed RCT study design and other study procedure implementation, as well as pilot test the CBCS-HCV group intervention to ensure patients found it acceptable and useful.
Recruitment was substantially impaired by the rapid changes in HCV treatment regimens and patients or providers deferring treatment to await easier treatments. This drastically reduced the number of patients available to participate. As a result of slow enrollment, it took 3 months to recruit 12 patients (of 54 screened); therefore, we had to forego the infeasible RCT study design. Instead, we conducted an observational study, assigning willing patients to each condition, which still allowed us to evaluate all aspects of the CBCS-HCV intervention as well as the feasibility of implementing several other study elements. In the future, an RCT will still be the preferred approach to minimize confounding and bias. Recruitment will be much more feasible in future studies when a larger number of patients are initiating treatment and there is a greater chance of rapid enrollment of a block of patients required for randomization. Transportation and travel cost to attend the five CBCS-HCV sessions prior to HCV treatment initiation posed additional patient-level barriers. During the recruitment process, some patients reported interest and value in attending a group intervention, but could not commit due to transportation and travel issues for research-only visits. In the future, innovative delivery methods, such as telehealth videoconferencing may need to be considered to disseminate and implement the CBCS–HCV on a wider scale, particularly in rural states where travel to optimal healthcare is a salient patient-level obstacle [50], [51].
Also during Wave 1 pilot testing, several patients were discontinued from HCV treatment due to viral nonresponse or side effects. Premature discontinuation occurred equally in both conditions, but all study participants were committed to remaining in the study and completed most subsequent assessments. Under newer regimens and treatment standards, stopping rules due to viral nonresponse are no longer recommended and therefore will not affect future CBCS-HCV studies.
Despite these challenges, we had very high rates of participant engagement, attendance in the study and CBCS intervention, study retention, and enthusiasm for the CBCS-HCV intervention. Patient acceptability ratings were very high. Data collection across time points was very high, likely attributable to aligning the majority of data collection time points with patients' regularly scheduled medical visits. Finally, all aspects of implementing the intervention sessions and data collection were smoothly integrated within a busy liver outpatient clinic, with high levels of support and flexibility from clinical staff.
Enthusiasm for the CBCS-HCV group intervention as assessed by patient acceptability measures was high and seemed related to several therapeutic factors. Participants rated nonspecific therapeutic factors, such as group cohesiveness and an engaging therapist, as very high. They also rated the modules and material covered in the Patient Workbook as highly relevant, informative, and useful. During the exit interview, participants noted that the CBCS skills that they acquired and practiced (e.g., relaxation, stress management, cognitive appraisal, assertiveness training) were applicable and useful not only for undergoing HCV treatment, but also for coping with a wide range of daily life stressors that diminish quality of life, well-being, and interpersonal relationships. This patient feedback suggests that the CBCS-HCV group intervention may have even broader applicability to the larger untreated HCV population who suffer from significantly worse physical, social and mental health functioning, compared to the general U.S. population [4], [52].
The patient acceptability findings also provide insight into ways in which to improve future iterations of the CBCS-HCV materials and intervention. Relative to other high scores, patients indicated that there was too much material to cover in each session and inadequate time for discussion and review. They also found some material less easy to understand. Future studies may need to determine the health literacy of all session materials to revise complicated language. Extending the number of sessions was recommended by the participants during the exit interview and would provide more adequate coverage and opportunity for discussion. The findings also suggest that therapists need to pay attention to group dynamics and bonding while balancing coverage of all materials to ensure protocol fidelity. Increasing the number of sessions would allow more time for group discussion and facilitate group cohesiveness.
Based on Wave 1 pilot testing, lessons learned, and patient feedback, we followed recommendations of the Stage Model and revised some aspects of the study methods and group intervention to conduct further pilot testing with a small number of Wave 2 and Wave 3 study participants. With Wave 2 and 3, we will continue to evaluate feasibility, but will specifically address the last few elements of Stage 1b research, a) to estimate effect sizes and b) demonstrate clinically significant improvement in some of the outcome measures to inform a future efficacy trial. The results of Wave 2 and 3 are forthcoming.
To conclude, we present the formative work, preliminary study protocol, and feasibility testing for the CBCS-HCV group intervention in Wave 1 study participants. A mini-RCT was not feasible due to treatment deferral at the time of recruitment for Wave 1, as patients and providers were awaiting IFN-free treatment. A RCT study design will be re-evaluated with Wave 2 and 3 participants and those results are forthcoming. The majority of study elements were found to be feasible and the intervention was smoothly implemented. Importantly, the CBCS-HCV received extremely positive reviews from participants who were enthusiastic about the group setting and content of the intervention. Participants reported that the CBCS-HCV would be useful for all patients with HCV dealing with daily stressors, symptoms and quality of life issues, not only those initiating HCV treatment. Based on further pilot testing with Wave 2 and 3, it may be beneficial in the future to examine the CBCS-HCV group intervention in patients undergoing all-oral HCV treatment, as the medications still induce side effects such as fatigue, headaches, and nausea [53], [54], [55]. It may also be useful to consider the CBCS-HCV as an alternative healthcare service for patients awaiting HCV treatment or for those who are cured but still suffer from poor physical, mental, and social functioning. A final consideration moving forward may be to evaluate the CBCS-HCV group intervention delivered via telehealth videoconferencing, as a way to reduce enrollment barriers in an efficacy study, and in the long run, to provide easier access to a potentially useful psychosocial intervention for the broader HCV population [50], [51].
Funding
Support for this study was provided to Donna Evon (K23DK089004-04), Carol Golin (K24HD06920), Michael Fried (K24DK066144).
Disclosures
Carol Golin and Rachel Ruffin have no conflict of interests to disclose. Donna Evon has served as an ad hoc consultant and receives grant funding from Gilead. Michael Fried has received research funding from and served as a consultant for AbbVie, BMS, Gilead, and Merck. He serves as consultant to TARGET PharmaSolutions.
Writing assistance
None.
Author contributions
Donna Evon, Carol Golin, and Michael Fried developed the study concept and design. Rachel Ruffin was involved in data management, data collection, data entry, and monitoring of therapist fidelity to the protocol. Donna Evon, Rachel Ruffin, Carol Golin were involved in data analysis. All of the authors were involved with drafting or revising the manuscript for important intellectual content. All authors have given approval of the final manuscript.
Acknowledgements
We would like to thank Drs. Jason Bonner and Daria Ebneter for contributing to the adaptation of the CBCS-HCV by developing modules and incorporating public-access intervention materials. We would like to thank Teodora Stoica and Dr. Rachel Jones for data collection and entry. Tremendous gratitude is extended to Dr. Suzanne Lechner and investigators at the University of Miami for assistance with adaption of the CBSM modules for development of the CBCS-HCV. We extend our gratitude to study participants who participated in pilot testing of the CBCS-HCV intervention.
References
- 1.AASLD-IDSA Recommendations for Testing m, and Treating Hepatitis C. http://www.hcvguidelines.org (Accessed 9 May 2016)
- 2.Ghany M.G., Strader D.B., Thomas D.L., Seeff L.B. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lang C.A., Conrad S., Garrett L., Battistutta D., Cooksley W.G., Dunne M.P. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J Pain Symptom Manag. 2006;31(4):335–344. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Kallman J., O'Neil M.M., Larive B., Boparai N., Calabrese L., Younossi Z.M. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. DigDisSci. 2007;52(10):2531–2539. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- 5.Younossi Z., Kallman J., Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45(3):806–816. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 6.Basseri B., Yamini D., Chee G., Enayati P.D., Tran T., Poordad F. Comorbidities associated with the increasing burden of hepatitis C infection. Liv. Int. Off. J. Int. Assoc. Study Liv. 2010;30(7):1012–1018. doi: 10.1111/j.1478-3231.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 7.Dan A.A., Martin L.M., Crone C., Ong J.P., Farmer D.W., Wise T. Depression, anemia and health-related quality of life in chronic hepatitis C. J. Hepatol. 2006;44(3):491–498. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Fontana R.J., Bieliauskas L.A., Back-Madruga C., Lindsay K.L., Kronfol Z., Lok A.S. Cognitive function in hepatitis C patients with advanced fibrosis enrolled in the HALT-C trial. J. Hepatol. 2005;43(4):614–622. doi: 10.1016/j.jhep.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Foster G.R., Goldin R.D., Thomas H.C. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27(1):209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- 10.Zickmund S., Ho E.Y., Masuda M., Ippolito L., LaBrecque D.R. 'They treated me like a leper': stigmatization and the qualify of life of patients with hepatitis C. J. General Intern. Med. 2003;18(10):835–844. doi: 10.1046/j.1525-1497.2003.20826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried M.W. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 Suppl 1) doi: 10.1053/jhep.2002.36810. S237–S44. [DOI] [PubMed] [Google Scholar]
- 12.Fried M.W., Shiffman M.L., Reddy K.R., Smith C., Marinos G., Goncales F.L., Jr. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. NEnglJMed. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 13.Younossi Z.M., Stepanova M., Zeuzem S., Dusheiko G., Esteban R., Hezode C. Patient-reported outcomes assessment in chronic hepatitis C treated with sofosbuvir and ribavirin: the VALENCE study. J. Hepatol. 2014;61(2):228–234. doi: 10.1016/j.jhep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Grebely J., Robaeys G., Bruggmann P., Aghemo A., Backmund M., Bruneau J. Recommendations for the management of hepatitis C virus infection among people who inject drugs. Int. J. Drug Policy. 2015;26(10):1028–1038. doi: 10.1016/j.drugpo.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen B.L., Farrar W.B., Golden-Kreutz D.M., Glaser R., Emery C.F., Crespin T.R. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22(17):3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoni M.H. Stress management effects on psychological, endocrinological, and immune functioning in men with HIV infection: empirical support for a psychoneuroimmunological model. Stress. 2003;6(3):173–188. doi: 10.1080/1025389031000156727. [DOI] [PubMed] [Google Scholar]
- 17.Given C., Given B., Rahbar M., Jeon S., McCorkle R., Cimprich B. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. JClinOncol. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 18.Edinger J.D., Olsen M.K., Stechuchak K.M., Means M.K., Lineberger M.D., Kirby A. Cognitive behavioral therapy for patients with primary insomnia or insomnia associated predominantly with mixed psychiatric disorders: a randomized clinical trial. Sleep. 2009;32(4):499–510. doi: 10.1093/sleep/32.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keefe F.J., Caldwell D.S. Cognitive behavioral control of arthritis pain. Med. Clin. North Am. 1997;81(1):277–290. doi: 10.1016/s0025-7125(05)70515-0. [DOI] [PubMed] [Google Scholar]
- 20.Lopez C., Antoni M., Penedo F., Weiss D., Cruess S., Segotas M.C. A pilot study of cognitive behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. JPsychosomRes. 2011;70(4):328–334. doi: 10.1016/j.jpsychores.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safren S.A., O'Cleirigh C., Tan J.Y., Raminani S.R., Reilly L.C., Otto M.W. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groessl E.J., Ho S.B., Asch S.M., Stepnowsky C.J., Laurent D., Gifford A.L. The hepatitis C self-management program: sustainability of primary outcomes at 1 year. Health Educ. Behav. 2013;40(6):730–740. doi: 10.1177/1090198113477112. [DOI] [PubMed] [Google Scholar]
- 23.Silberbogen A.K., Ulloa E., Mori D.L., Brown K. A telehealth intervention for veterans on antiviral treatment for the hepatitis C virus. PsycholServ. 2012;9(2):163–173. doi: 10.1037/a0026821. [DOI] [PubMed] [Google Scholar]
- 24.Ramsey S.E., Engler P.A., Stein M.D., Brown R.A., Cioe P., Kahler C.W. Effect of CBT on depressive symptoms in methadone maintenance patients undergoing treatment for hepatitis C. J. Addict. Res. Ther. 2011;2(2):2–10. doi: 10.4172/2155-6105.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoni M.H., Lechner S.C., Kazi A., Wimberly S.R., Sifre T., Urcuyo K.R. How stress management improves quality of life after treatment for breast cancer. J. Consult Clin. Psychol. 2006;74(6):1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rounsaville B.J., Carroll K.M., Onken L.S. A stage model of behavioral therapies research: getting started and moving on from stage I. Clin. Psychol. Sci. Pract. 2001;8(2):133–142. [Google Scholar]
- 27.Evon D.M., Golin C.E., Fried M.W., Keefe F.J. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? J. Consult Clin. Psychol. 2013;81(2):361–374. doi: 10.1037/a0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammad A., Carey J.J., Storan E., Scarry M., Coughlan R.J., Lee J.M. Prevalence of fibromyalgia among patients with chronic hepatitis C infection: relationship to viral characteristics and quality of life. JClinGastroenterol. 2012;46(5):407–412. doi: 10.1097/MCG.0b013e3182485528. [DOI] [PubMed] [Google Scholar]
- 29.Carlson M.D., Hilsabeck R.C., Barakat F., Perry W. Role of sleep disturbance in chronic hepatitis C infection. Curr. Hepat. Rep. 2010;9(1):25–29. doi: 10.1007/s11901-010-0030-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sockalingam S., Abbey S.E., Alosaimi F., Novak M. A review of sleep disturbance in hepatitis C. JClinGastroenterol. 2010;44(1):38–45. doi: 10.1097/MCG.0b013e3181b314ea. [DOI] [PubMed] [Google Scholar]
- 31.Wichers M.C., Koek G.H., Robaeys G., Praamstra A.J., Maes M. Early increase in vegetative symptoms predicts IFN-alpha-induced cognitive-depressive changes. PsycholMed. 2005;35(3):433–441. doi: 10.1017/s0033291704003526. [DOI] [PubMed] [Google Scholar]
- 32.Robaeys G., De B.J., Wichers M.C., Bruckers L., Nevens F., Michielsen P. Early prediction of major depression in chronic hepatitis C patients during peg-interferon alpha-2b treatment by assessment of vegetative-depressive symptoms after four weeks. World JGastroenterol. 2007;13(43):5736–5740. doi: 10.3748/wjg.v13.i43.5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treloar C., Rance J., Bath N., Everingham H., Micallef M., Day C. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: the ETHOS study, Australia. Int. J. Drug Policy. 2015;26(10):992–998. doi: 10.1016/j.drugpo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Evon D.M., Golin C.E., Bonner J.E., Grodensky C., Velloza J. Adherence during antiviral treatment regimens for chronic hepatitis C: a qualitative study of patient-reported facilitators and barriers. J. Clin. Gastroenterol. 2015;49(5):e41–50. doi: 10.1097/MCG.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antoni M., Ironson G., Schneiderman N. Oxford University Press; New York: 2007. Cognitive-behavioral Stress Management. [Google Scholar]
- 36.Evon D.M., Golin C.E., Fried M.W., Keefe F.J. Chronic hepatitis C and antiviral treatment regimens: where can psychology contribute? J. Consult. Clin. Psychol. 2013;81(2):361–374. doi: 10.1037/a0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evon D.M., Esserman D.A., Bonner J.E., Rao T., Fried M.W., Golin C.E. Adherence to PEG/ribavirin treatment for chronic hepatitis C: prevalence, patterns, and predictors of missed doses and nonpersistence. J. Viral Hepat. 2013;20(8):536–549. doi: 10.1111/jvh.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Linehan M.M. Guilford Press; New York, NY: 1993. Skills Training Manual for Treatment of Borderline Personality Disorder. [Google Scholar]
- 39.Reilly P.M., Shopshire M.S. Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Adminstration; Rockville, MD: 2002. Anger Management for Substance Abuse and Mental Health Clients: a Cognitve Behavioral Therapy Manual. [Google Scholar]
- 40.Edinger J.D., Wohlgemuth W.K., Krystal A.D., Rice J.R. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. ArchInternMed. 2005;165(21):2527–2535. doi: 10.1001/archinte.165.21.2527. [DOI] [PubMed] [Google Scholar]
- 41.Butt A.A., Kanwal F. Boceprevir and telaprevir in the management of hepatitis C virus-infected patients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012;54(1):96–104. doi: 10.1093/cid/cir774. [DOI] [PubMed] [Google Scholar]
- 42.Lechner S.C., Whitehead N.E., Vargas S., Annane D.W., Robertson B.R., Carver C.S. Does a community-based stress management intervention affect psychological adaptation among underserved black breast cancer survivors? J. Natl. Cancer Inst. Monogr. 2014;2014(50):315–322. doi: 10.1093/jncimonographs/lgu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Webster K., Cella D., Yost K. The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual. Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cella D., Nowinski C.J. Measuring quality of life in chronic illness: the functional assessment of chronic illness therapy measurement system. Arch. Phys. Med. Rehabil. 2002;83(12 Suppl 2):S10–S17. doi: 10.1053/apmr.2002.36959. [DOI] [PubMed] [Google Scholar]
- 45.Cohen S., Kamarck T., Mermelstein R. A global measure of perceived stress. J. Health Soc. Behav. 1983;24(4):385–396. [PubMed] [Google Scholar]
- 46.Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J. Clin. Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golin C.E., Earp J., Tien H.C., Stewart P., Porter C., Howie L.A. 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. JAcquirImmuneDeficSyndr. 2006;42(1):42–51. doi: 10.1097/01.qai.0000219771.97303.0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carver C.S. 2006. Measure of Current Status.http://www.psy.miami.edu/faculty/ccarver/sclMOCS.html Available from: [Google Scholar]
- 49.Bonner J.E., Esserman D.A., Golin C.E., Evon D.M. Self-efficacy and adherence to antiviral treatment for chronic hepatitis C. J. Clin. Gastroenterol. 2015;49(1):76–83. doi: 10.1097/MCG.0000000000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marziali E. E-health program for patients with chronic disease. TelemedJEHealth. 2009;15(2):176–181. doi: 10.1089/tmj.2008.0082. [DOI] [PubMed] [Google Scholar]
- 51.Palyo S.A., Schopmeyer K.A., McQuaid J.R. Tele-pain management: use of videoconferencing technology in the delivery of an integrated cognitive-behavioral and physical therapy group intervention. PsycholServ. 2012;9(2):200–202. doi: 10.1037/a0025987. [DOI] [PubMed] [Google Scholar]
- 52.Spiegel B.M., Younossi Z.M., Hays R.D., Revicki D., Robbins S., Kanwal F. Impact of hepatitis C on health related quality of life: a systematic review and quantitative assessment. Hepatology. 2005;41(4):790–800. doi: 10.1002/hep.20659. [DOI] [PubMed] [Google Scholar]
- 53.Lawitz E., Poordad F.F., Pang P.S., Hyland R.H., Ding X., Mo H. Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet. 2014;383(9916):515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 54.Zeuzem S., Ghalib R., Reddy K.R., Pockros P.J., Ben Ari Z., Zhao Y. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection: a randomized trial. Ann. Intern Med. 2015;163(1):1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 55.Everson G.T., Towner W.J., Davis M.N., Wyles D.L., Nahass R.G., Thuluvath P.J. Sofosbuvir with velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 hepatitis C virus infection: a randomized trial. Ann. Intern Med. 2015;163(11):818–826. doi: 10.7326/M15-1000. [DOI] [PubMed] [Google Scholar]