Abstract
Background
The number of brain metastases (BM) plays an important role in the decision between stereotactic radiosurgery (SRS) and whole brain radiation therapy (WBRT).
Methods
We analyzed the survival of 5750 SRS-treated BM patients as a function of BM number. Survival analyses were performed using Kaplan-Meier analysis as well as univariate and multivariate Cox proportional hazards models.
Results
BM patients were first categorized as those with 1, 2–4, and 5–10 BM based on the scheme proposed by Yamamoto et al (Lancet Oncology 2014). Median overall survival for patients with 1 BM was superior to those with 2–4 BMs (7.1 mo v. 6.4 mo, p=0.009), and survival of patients with 2–4 BMs did not differ from those with 5–10 BMs (6.4 mo v. 6.3 mo, p=0.170). The median survival of patients with >10 BMs was lower than those with 2–10 BMs (6.3 mo v. 5.5 mo, p=0.025). In a multivariate model that accounted for age, Karnofsky Performance Score (KPS), systemic disease status, tumor histology, and cumulative intracranial tumor volume (CITV), we observed a ~10% increase in hazard of death when comparing patients with 1 versus 2–10 BM (p<0.001) or 10 versus >10 BM (p<0.001). When BM number was modeled as a continuous variable rather than using the Yamamoto classification, we observed a step-wise 5% increase in the hazard of death for every increment of 5–6 BM (p<0.001).
Conclusions
The contribution of BM number to overall survival is modest, and should be considered as one of the many variables considered in the decision between SRS and WBRT.
Keywords: Stereotactic radiosurgery, brain metastasis, radiation, survival, prognostication
INTRODUCTION
An estimated 25–45% of all cancer patients develop brain metastases (BM), which is uniformly fatal if left untreated1. Because of the blood-brain barrier, most chemotherapy agents fail to achieve therapeutic concentration in the central nervous system. Thus, radiation remains the mainstay for BM patients who are poor surgical candidates1,2. Radiation can be given as whole brain radiation therapy (WBRT), where cumulative dose is fractionated in time, or stereotactic radiosurgery (SRS), where cumulative dose is fractionated in space3. Generally speaking, both modalities are effective in controlling tumor growth.4 The available data suggests that the radiation modality does not significantly impact survival of BM patients5–7, as systemic disease control remains the major determinant of overall survival8–10. However, with increased recognition of the deleterious neuro-cognitive effects of WBRT11,12, increased utilization of SRS has been noted in recent studies13,14.
One important criterion in the deliberation between WBRT and SRS involves the number of metastases15. WBRT is generally used as treatment for patients with a high number of BM, while SRS is typically considered for patients with a limited number of BM16,17. The threshold for this determination remains an area of active research. The early randomized controlled studies of SRS were designed to test efficacy in patients with <4 BM4,5, with the implication that WBRT should be considered for patients with >4 BM. This framework was challenged by the landmark study by Yamamoto et al.18, demonstrating similar survival patterns for SRS-treated BM patients with 2–4 versus 5–10 BM. Despite these studies, the published literature addressing this matter remains limited. Here, we examined the issue using a collated database of 5750 consecutive BM patients. Our results indicate that the contribution of BM number to survival is modest and should be considered as one of the many variables in the decision between SRS and WBRT.
METHODS
Study Cohort
All data was collected under Institutional Review Board (IRB) approved retrospective reviews at all involved institutions. Data was compiled by the four co-senior authors (AH, RS, MY, and CCC) and represents consecutive BM patients treated with SRS from 1994–2014. Each patient had confirmed histologic diagnosis and underwent SRS without surgical resection based on the recommendation of a multidisciplinary brain tumor board. Patients who underwent surgical resection of BM were not included in this study. In total, 124 patients were collected by AH (Melanoma Institute of Australia, Wollstonecraft, Australia), 3067 patients by TS (Tsukiji Neurological Clinic, Tokyo, Japan), 2504 by MY (Katsuta Hospital Mito GammaHouse, Hitachinaka, Japan), and 1180 by CCC (University of California, San Diego, USA).
All patients in our retrospective study were deceased at the time of data compilation. Follow-up on vital status in the US cohort was performed using the social security death index. Only patients with cancer of the breast, gastrointestional tract (GI), lung, kidney, and melanoma were included in the study. The compiled dataset includes a total of 5750 patients. Missing information was found in 408 patients (7% of the studied population).
Radiosurgery Technique
Radiosurgery was performed as previously described19,20. In brief, patients underwent Magnetic Resonance Imaging (MRI) of the brain with either the Toshiba America Medical Systems MRI machine (Tustin, California) at 2 mm slices or the GE Healthcare MRI machine (Milwaukee, Wisconsin) using 1 mm slices in conjunction with the Leksell stereotactic head frame. MRI was performed using axial and coronal T1-weighted pre- and post-contrast sequences. Following the initial MR radiography, cases underwent review by a team consisting of at least one of each of the following specialists: neurosurgeon, radiation oncologist, and medical physicist. Planning of the radiation dose for each of the metastases was performed using the Elekta Gamma Plan software. The calculated dose was given to the 50% isodose line in all patients. Generally speaking, dosing was in line with the RTOG 95-08 clinical trial4. Patients’ clinical presentation/status, number of metastases, tumor volume, and prior or planned WBRT were all weighed prior to the final determination of dosage. Doses to particularly sensitive areas of the brain such as the optic nerve and brainstem were limited to 10 Gy and 18 Gy respectively with no single dose of radiation exceeding 24 Gy.
Clinical parameters and modeling
Patient and BM characteristics (age, Karnofsky Performance status [KPS], systemic disease status, tumor histology, cumulative intracranial tumor volume [CITV], and number of metastasis) were collected at the time of presentation for the initial radiosurgery. Overall survival was determined from the time of the initial SRS to the time of death. Based on the classification scheme utilized by the previous prospective study by Yamamoto et al.18, patients were initially stratified as presenting with 1, 2–4, 5–10, or >10 BM. For each cohort, Kaplan-Meier survival plots were created and median overall survival times were calculated. Univariate Cox proportional hazards models were then created to examine the influence of age, KPS, CITV, systemic disease control, tumor histology, and number of metastases (1, 2–4, 5–10, >10) on survival. Because overall survival did not significantly differ between patients who presented with 2–4 and 5–10 BM, these categories were ultimately collapsed into a single category of 2–10 BM. A multivariate cox proportional hazards model was then used to test whether the risk of death differed between patients who presented with 1, 2–10, and >10 BM after accounting for age, KPS, CITV, systemic disease control, and tumor histology.
Statistical analysis
All statistical analyses and figure generation were performed using R version 3.3.1 with all p-values < 0.05 considered significant. The R package “Survival” was used in order to create Kaplan-Meier (KM) plots, as well as to perform the corresponding multivariate Cox proportional hazards (CPH) model regression. The R package “tableone” was used to create the demographics table.
RESULTS
Patient demographics and tumor characteristics
Patient characteristics stratified by primary tumor histology are provided in Table 1. The predominant cancer type in this study was lung (n=3746), followed by breast (n=710), GI (n=692), renal cell carcinoma (n=321), and melanoma (n=282). There were 1753 patients with 1 BM, 1872 patients with 2–4 BM, 1144 patients with 5–10 BM, and 981 patients with >10 BM. Of the four centers participating in this study, we found that patients treated in the U.S. center were more likely to harbor single BM relative to the Japanese and Australian Centers (Supplemental Table 1). The distribution of age (p=0.138) and KPS (p=0.130) were similar across BM number categories. Gender, systemic disease status, and primary tumor pathology differed between each of the metastasis groupings (p<0.001). Lastly, CITV increased with the number of BM (p<0.001).
Table 1.
Demographics by primary tumor histology
| Breast | GI | Lung | Melanoma | RCC | p | |
|---|---|---|---|---|---|---|
| n | 710 | 692 | 3745 | 282 | 321 | |
| Sex = M (%) | 5 (0.7) | 464 (67.1) | 2556 68.3) | 186 (66.0) | 199 (62.0) | <0.001 |
| Age (mean (sd)) | 55.72 (11.79) | 65.89 (10.09) | 65.29 (10.66) | 57.95 (15.34) | 62.40 (12.24) | <0.001 |
| KPS (%) | <0.001 | |||||
| <70 | 81 (11.7) | 135 (19.6) | 289 (7.8) | 36 (14.4) | 58 (18.5) | |
| 70–80 | 257 (37.1) | 313 (45.4) | 1092 (29.5) | 122 (48.8) | 121 (38.5) | |
| 90–100 | 355 (51.2) | 242 (35.1) | 2324 (62.7) | 92 (36.8) | 135 (43.0) | |
| Systemic disease control (%) | 166 (27.6) | 47 (7.3) | 352 (9.8) | 16 (5.7) | 24 (7.9) | <0.001 |
| CITV (median [IQR]) | 6.90 [2.50, 16.45] |
9.17 [4.40, 16.44] |
3.75 [1.27, 9.58] |
1.99 [0.65, 5.52] |
5.35 [2.20, 10.30] |
<0.001 |
| Number of metastases (%) | <0.001 | |||||
| 1 | 201 (28.3) | 263 (38.0) | 1003 (26.8) | 155 (55.0) | 131 (40.8) | |
| 2–4 | 202 (28.5) | 262 (37.9) | 1200 (32.0) | 96 (34.0) | 112 (34.9) | |
| 5–10 | 156 (22.0) | 119 (17.2) | 792 (21.1) | 24 (8.5) | 53 (16.5) | |
| 10+ | 151 (21.3) | 48 (6.9) | 750 (20.0) | 7 (2.5) | 25 (7.8) |
Note: Percentages refer to percent of individual strata e.g. 0.7% of breast cancer patients were male.
Median survival of SRS-treated patients
The median follow-up of the cohort was 6.4 months. Kaplan-Meier (KM) plots were generated for patients with 1, 2–4, 5–10, and >10 BM (Figure 1). Median overall survival for patients with 1 BM was superior to those with 2–4 BMs (7.1 mo v. 6.4 mo, respectively, p=0.009) (Table 2). The median survival of patients with 2–4 BMs did not significantly differ from those with 5–10 BMs (6.4 mo v. 6.3 mo, respectively, p=0.170), while the median survival of patients with >10 BMs was lower than that of patients with either 2–4 or 5–10 BMs (6.3 mo v. 5.5 mo, respectively, p=0.025). Based on these initial observations, we performed all subsequent analyses with both the original groupings and the new groupings combining the 2–4 and 5–10 categories. A similar set of KM plots was created for the new metastasis groupings (Figure 2).
Figure 1.
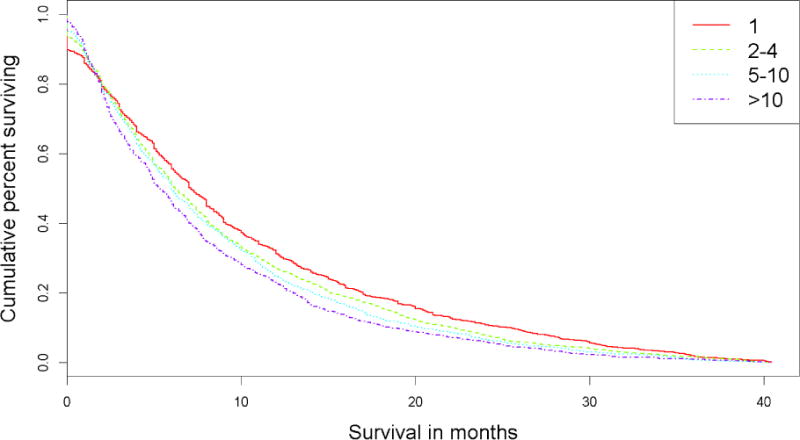
Kaplan-Meier survival plot for original groupings
Table 2.
Median overall survival time by metastasis grouping
| Original number of metastasis groupings | 1 | 2–4 | 5–10 | >10 |
|---|---|---|---|---|
| Median Survival in Months | 7.1 | 6.4 | 6.3 | 5.5 |
| Collapsed number of metastasis groupings | 1 | 2–10 | >10 | |
| Median Survival in Months | 7.1 | 6.4 | 5.5 | |
Figure 2.
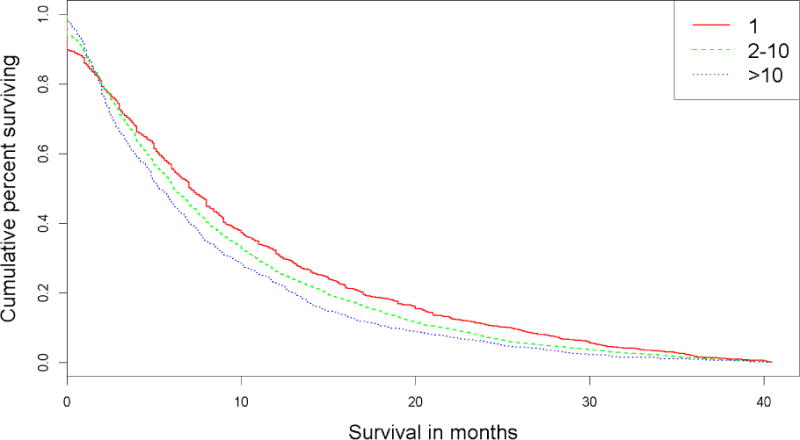
Kaplan-Meier survival plot for condensed groupings
We next imposed the selection criteria previously imposed by Yamamoto et al in the landmark prospective study18 and repeated our analysis after excluding patients that did not fulfill these criteria. This repeat analysis revealed survival estimates whose confidence intervals overlap those reported by Yamamoto et al. (Table 3).
Table 3.
Median overall survival of patients who satisfy inclusion criteria by Yamamoto et al.18
| Number of Brain metastases | Current cohort | Yamamoto et al 201418 |
|---|---|---|
| 1 | 11.5 (10.5–12.2) | 13.9 (12.0–15.6) |
| 2–4 | 10.7 (10.0–11.4) | 10.8 (9.4–12.4) |
| 5–10 | 10.5 (9.9–11.4) | 10.8 (9.1–12.7) |
Median overall survival shown with 95% confidence interval in parentheses
Univariate survival analysis
In a univariate Cox proportional hazards model (Table 4), we found that patient age, KPS, systemic disease status, CITV, and tumor histology were each independently associated with overall survival. In terms of BM, the hazard ratio (HR) of death for patients with 1 BM significantly differed from those with 2–4 BM (p=0.010). When comparing patients with 2–4 and 5–10 BM, the risk of death was similar (p=0.170). Finally, the HR of death was lower in patients who presented with 5–10 BM relative to those with >10 BM (p=0.025).
Table 4.
Univariate Cox proportional hazards model for both metastasis groupings
| Original Groupings | New groupings | |||||
|---|---|---|---|---|---|---|
| Reference | Hazard Ratio | P value | Reference | Hazard Ratio | P value | |
| Number of Metastases | 1 vs 2–4 | 1.090 | 0.010 | 1 vs 2–10 | 1.111 | <.001 |
| 2–4 vs 5–10 | 1.053 | 0.170 | 2–10 vs >10 | 1.139 | <.001 | |
| 5–10 vs >10 | 1.102 | 0.025 | ||||
| Age (years) | 1.005 | <.001 | 1.005 | <.001 | ||
| KPS (points) | 0.977 | <.001 | 0.977 | <.001 | ||
| CITV (cc) | 1.015 | <.001 | 1.015 | <.001 | ||
| Systemic disease status | uncontrolled | 0.511 | <.001 | uncontrolled | 0.511 | <.001 |
| Breast vs. other histology | Other histology | 0.885 | 0.002 | Other histology | 0.885 | 0.002 |
Note: Categories with no reference group were continuous variables, and their corresponding hazard ratios refer to an increment of 1 of their respective units.
Multivariate survival analysis
We next created a multivariate Cox proportional hazards model to determine whether BM-number category remains a significant predictor of survival after accounting for patient age, KPS, systemic disease status, CITV, and tumor histology. Given that the median overall survival analysis and univariate Cox models both suggested no significant difference between patients who presented with 2–4 and 5–10 BM, these two categories were combined in the multivariate analysis (right columns of Table 5). As shown in Table 5, all variables that were significant in univariate analysis remain statistically significant in the multivariate model. We found that the hazard of death for patients with 2–10 BM was increased by 11% relative to those with 1 BM (p<0.001). The hazard of death for patients with 2–10 BM was increased by 14% relative to those with >10 BM (p<0.001).
Table 5.
Multivariate Cox proportional hazards model for both metastasis groupings
| Original Groupings | New groupings | |||||
|---|---|---|---|---|---|---|
| Reference | Hazard Ratio | P value | Reference | Hazard Ratio | P value | |
| Number of Metastases | 1 vs 2–4 | 1.103 | 0.005 | 1 vs 2–10 | 1.110 | 0.001 |
| 2–4 vs 5–10 | 1.015 | 0.692 | 2–10 vs >10 | 1.128 | 0.002 | |
| 5–10 vs >10 | 1.117 | 0.014 | ||||
| Age (years) | 1.003 | 0.015 | 1.003 | 0.015 | ||
| KPS (points) | 0.979 | <.001 | 0.979 | <.001 | ||
| CITV (cc) | 1.007 | <.001 | 1.007 | <.001 | ||
| Systemic disease Status | uncontrolled | 0.582 | <.001 | uncontrolled | 0.582 | <.001 |
| Breast vs other histology | Other histology | 0.838 | 0.000 | Other histology | 0.839 | <.001 |
| AIC | 80238 | 80203 |
Note: Categories with no reference group were continuous variables, and their corresponding hazard ratios refer to an increment of 1 of their respective units.
Model comparison
We wished to use an established statistical metric to assess how collapsing the 2–4 and 5–10 groupings into a single category would impact the prognostic utility of our model. Akaike Information Criteria (AIC) is a commonly used metric in this regard. It determines how well a model explains the data, while penalizing incorporation of variables that do not contribute to this process. Typically, an AIC difference of ≥2 between models implies that the model with the lower AIC is superior with statistical significance21. As shown in Table 5, the model with the old groupings had an AIC of 80238 and the new grouping model had an AIC of 80203, with a difference of 35. As the only change performed was the grouping of the middle two categories, we can attribute the improvement directly to the combination of BM categories.
Analysis by primary cancer histology
We wished to determine whether the survival association with BM number remains robust after stratification by the primary cancer histology. Kaplan-Meier survival analysis was separately performed for SRS-treated BM patients suffering from lung, melanoma, renal cell, GI, and breast cancer (Fig 3). In lung, melanoma, renal cell, and GI patients, the survival association with BM number (classified based on the Yamamoto scheme) remained robust. However, no significant survival difference was observed between breast cancer patients suffering from 1, 2–10, and > 10 BM.
Figure 3.
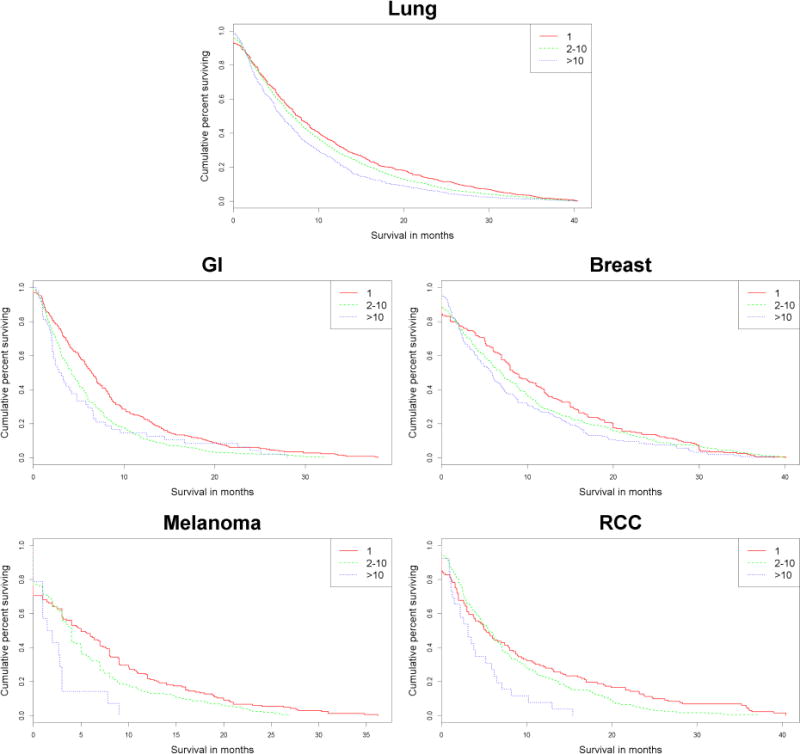
Histology specific Kaplan-Meier survival plots
Analysis of hazard ratio as a function of BM number
We next examined the HR of death as a function of BM number treated as a continuous variable rather than based on the Yamamoto scheme18. We performed a multivariate Cox proportional hazards model to determine the risk of death by increasing BM after accounting for patient age, KPS, systemic disease status, CITV, and tumor histology. Since we did not find robust association between BM number and overall survival in breast cancer patients, we excluded the breast cancer patient cohort in this analysis. In Figure 4, we plotted the increase in HR for the number of BM relative to the previous number of BM. For instance, the HR indicated by two on the abscissa is the relative risk of death for patients with 2 or fewer BM relative to those suffering from 3 or greater BM. In this analysis, we did not observe a continuous increase in the hazard of death for every increment of one BM. Instead, we observed a step-wise increase in the hazard of death, with an ~4% increase for every increment of 6–7 BM (p<0.001).
Figure 4.
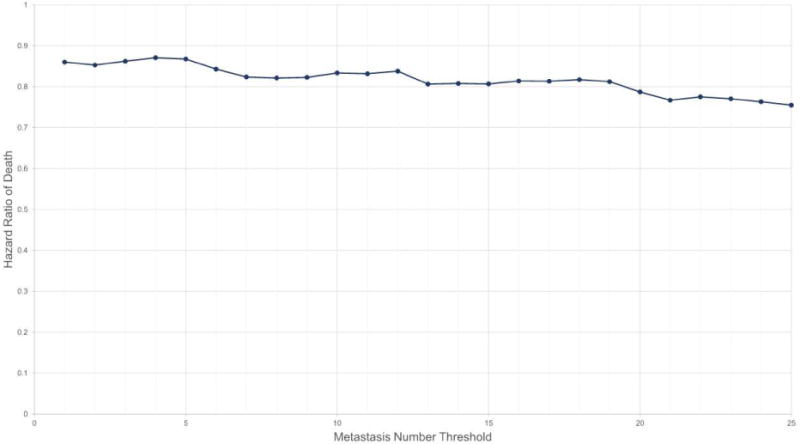
Cox proportional hazards analysis by stepwise increments of metastasis number
DISCUSSION
In clinical practice, the number of metastases plays a key role in the determination of optimal radiation modality for an individual BM patient. Here, using rigorous statistical methodologies, we show that the contribution of BM number to survival is relatively modest and constitutes only one of the many variables that should be considered in the decision between SRS and WBRT. In our collated dataset of 5750 SRS-treated BM patients, we observed an approximate ~10% increase in hazard of death when comparing patients with 1 versus 2–10 BM (p<0.001) or 2–10 versus >10 BM (p<0.001). When BM number is modeled as a continuous variable rather than based on the Yamamoto classification of 1, 2–10, and >10, we observed stepwise 4% increase in the hazard of death for every increment of 6–7 BM (p<0.001). Importantly, these survival effects were rigorously calculated after controlling for key clinical variables previously shown to influence survival, including age, KPS, systemic disease status, and CITV. Since the contribution of BM number to survival is modest, it should be considered in the greater clinical context in the decision between SRS and WBRT. The considerations for an 80-year-old lung cancer patient with 10 BM, KPS <50, uncontrolled systemic disease, and a CITV of 20cc who has exhausted all medical options are different relative to another patient with 10 BM who is a 40-year-old afflicted with HER2+ breast cancer with KPS of 100, CITV of <4cc, and systemically controlled cancer.
Consistent with results reported by Yamamoto et al.18, we found that the relative survival pattern of patients afflicted with a single metastasis is more favorable than those afflicted with 2–4, 5–10, and >10 metastases. Also consistent was our finding that the survival patterns of SRS-treated patients with 2–4 and 5–10 metastases were comparable. While the absolute survival interval differed between our study and the Yamamoto study, these differences were entirely due to patient selection. Our study examined the outcome of all patients treated by the authors during the study period, while the Yamamoto study18 prospectively enrolled patients with, for instance, KPS>70, CITV<15cc, and a reasonable survival expectation. When we repeated analysis of our data after imposing the criteria of the Yamamoto study, the median overall survivals obtained were comparable (Table 3). The apparent difference in the survival intervals between these two studies highlights the challenges in generalizing data from trials involving selected cohorts to the general patient population that clinicians treat on a daily basis22,23.
In order to address the interaction between the number of metastases and CITV, we employed a statistical model that examined both variables and found that both independently contribute to survival. In broad strokes, this finding translates into the following. In terms of tumor volume, a SRS-treated patient with 10 “small” BM is expected to exhibit better survival than a SRS-treated patient with 10 “big” BM. In terms of tumor number, a SRS-treated patient with two BM is expected to exhibit improved survival relative to a SRS-treated patients with six BM. Most interestingly, the number of BM continues to be an important prognosticator after controlling for CITV. This finding implies that given two patients with the same CITV, a patient with 2 BM is expected to survive longer than another patient with 10 BM. The biologic basis for this finding remains unclear.
In our study, we did not observe a robust association between the number of BM and survival in breast cancer patients. This finding is consistent with the published disease-specific graded prognostic scale (ds-GPA) for SRS-treated breast cancer patients, where the number of BM was not a prognostic variable.9 In contrast, the number of BM was a prognostic variable for SRS-treated BM patients suffering from lung cancer, melanoma, and renal cell cancer in ds-GPA. Robust association between BM number and survival were observed in our study as well (Figure 2, Tables 4 and 5).
Significant survival gains have been made in recent years for a small subset of patients because of targeted therapy24 or immunotherapy25,26. In non-small cell lung cancer, for instance, it is estimated that <25 % of all patients derive genuine benefit from these therapies27–29. While there is significant interest in studying the impact of SRS in these patients, we should not lose sight of the clinical need to study the population who does not derive benefit from targeted or immunotherapy. Our study is important in this regard. Only the tail end of our study period overlaps with the routine use of targeted or immunotherapy30,31, and nearly all of our study population was not exposed to these therapies. As such, this study provides insights into the survival prognostication for majority of the BM patients who are not treated with targeted therapy or immunotherapy.
There are several inherent limitations to a retrospective multinational cohort study involving patients treated by different practitioners. The remarkable agreement between our results and those observed in a prospective observation study18, however, supports the robustness of the reported findings. It is likely that pooling cohorts from multiple institutions and practitioners minimizes the impact of the inter-institutional variation in criteria of patient selection. The large sample size of this study should also mitigate the impact of patient specific variables on the reported results32,33. The lack of neuro-cognitive11 and quality of life measures34 in our study represents another limitation. While overall survival is an important outcome measure, considerations should also be given to the anticipated neuro-cognitive and quality of life deficits, as well as the risk for the development of distant metastasis12,14,35. Finally, though our study provides useful information for the majority of SRS-treated BM patients who are not treated with targeted or immunotherapy, there is a critical need to study the interaction between SRS and these agents. Unfortunately, our dataset provides little insight in this regard.
CONCLUSION
In our analysis of 5750 SRS-treated BM patients, we found a step-wise 4% increase in the hazard of death for every increment of 6–7 BM (p<0.001). This quantitative survival association should be considered in the broader clinical context to personalize radiosurgery care for BM patients.
Supplementary Material
Highlights.
Here we present an analysis of a multi-institutional international cohort (n=5750) consisting of patients undergoing SRS for one or more brain metastases.
The survival analyses performed include: Kaplan-Meier analysis, univariable and multivariable Cox proportional hazards, and Akaike Information Criteria comparison.
There are different survival patterns for SRS-treated patients afflicted with 1, 2–10, and >10 BM, with an approximate 10% increment in the hazard ratios of death between these BM categories.
On continuous analysis, our findings demonstrate a 4% increase in hazard of death for every increase of 6–7 metastases.
Acknowledgments
This manuscript is not under consideration for publication in any other journal, and all authors have approved the enclosed manuscript. No relevant financial/funding disclosures.
Abbreviation list in order of appearance
- SRS
stereotactic radiosurgery
- BM
brain metastasis(es)
- WBRT
whole brain radiation therapy
- RCC
renal cell carcinoma
- KPS
Karnofsky Performance Score
- CITV
cumulative intracranial volume
- IRB
institutional review board
- MRI
magnetic resonance imaging
- RTOG
Radiation Therapy Oncology Group
- Gy
Gray
- KM
Kaplan-Meier
- AIC
Akaike Information Criteria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interests.
References
- 1.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 2.Eichler AF, Loeffler JS. Multidisciplinary management of brain metastases. The oncologist. 2007;12:884–898. doi: 10.1634/theoncologist.12-7-884. [DOI] [PubMed] [Google Scholar]
- 3.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet (London, England) 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama H, Hiroki S, Tago M, et al. Stereotactic Radiosurgery Plus Whole-Brain Radiation Therapy vs Stereotactic Radiosurgery Alone for Treatment of Brain Metastases. Jama. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 6.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rades D, Janssen S, Dziggel L, et al. A matched-pair study comparing whole-brain irradiation alone to radiosurgery or fractionated stereotactic radiotherapy alone in patients irradiated for up to three brain metastases. BMC Cancer. 2017;17(1):30. doi: 10.1186/s12885-016-2989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70(2):510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 9.Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. International journal of radiation oncology, biology, physics. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 10.Ebner DK, Gorovets D, Rava P, et al. Patients with long-term control of systemic disease are a favorable prognostic group for treatment of brain metastases with SRS alone. World Neurosurg. 2016 doi: 10.1016/j.wneu.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.McDuff SG, Taich ZJ, Lawson JD, et al. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry. 2013;84(12):1384–1391. doi: 10.1136/jnnp-2013-305166. [DOI] [PubMed] [Google Scholar]
- 12.Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA. 2016;316(4):401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatiboglu MA, Tuzgen S, Akdur K, Chang EL. Treatment of high numbers of brain metastases with Gamma Knife radiosurgery: a review. Acta Neurochir (Wien) 2016;158(4):625–634. doi: 10.1007/s00701-016-2707-6. [DOI] [PubMed] [Google Scholar]
- 14.Soliman H, Das S, Larson DA, Sahgal A. Stereotactic radiosurgery (SRS) in the modern management of patients with brain metastases. Oncotarget. 2016;7(11):12318–12330. doi: 10.18632/oncotarget.7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ree AH, Redalen KR. Personalized radiotherapy: concepts, biomarkers and trial design. Br J Radiol. 2015;88(1051):20150009. doi: 10.1259/bjr.20150009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patil CG, Pricola K, Sarmiento JM, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2012;(9):CD006121. doi: 10.1002/14651858.CD006121.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue J, Kubicek GJ, Grimm J, et al. Biological implications of whole-brain radiotherapy versus stereotactic radiosurgery of multiple brain metastases. J Neurosurg. 2014;121(Suppl):60–68. doi: 10.3171/2014.7.GKS141229. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. The Lancet Oncology. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 19.Marcus LP, Marshall D, Hirshman BR, et al. Cumulative Intracranial Tumor Volume (CITV) Enhances the Prognostic Value of the Lung-Specific Graded Prognostic Assessment (GPA) Model. Neurosurgery. 2016;79:246–252. doi: 10.1227/NEU.0000000000001123. [DOI] [PubMed] [Google Scholar]
- 20.Marshall DC, Marcus LP, Kim TE, et al. Management patterns of patients with cerebral metastases who underwent multiple stereotactic radiosurgeries. Journal of neuro-oncology. 2016;128:119–128. doi: 10.1007/s11060-016-2084-2. [DOI] [PubMed] [Google Scholar]
- 21.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. New York, NY: Springer New York; 2004. [Google Scholar]
- 22.Park HS, Wang EH, Rutter CE, Corso CD, Chiang VL, Yu JB. Changing practice patterns of Gamma Knife versus linear accelerator-based stereotactic radiosurgery for brain metastases in the US. J Neurosurg. 2016;124(4):1018–1024. doi: 10.3171/2015.4.JNS1573. [DOI] [PubMed] [Google Scholar]
- 23.Lester-Coll NH, Dosoretz AP, Magnuson WJ, Laurans MS, Chiang VL, Yu JB. Cost-effectiveness of stereotactic radiosurgery versus whole-brain radiation therapy for up to 10 brain metastases. J Neurosurg. 2016;125(Suppl 1):18–25. doi: 10.3171/2016.7.GKS161499. [DOI] [PubMed] [Google Scholar]
- 24.Moraes FY, Taunk NK, Marta GN, Suh JH, Yamada Y. The Rationale for Targeted Therapies and Stereotactic Radiosurgery in the Treatment of Brain Metastases. Oncologist. 2016;21(2):244–251. doi: 10.1634/theoncologist.2015-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franceschini D, Franzese C, Navarria P, et al. Radiotherapy and immunotherapy: Can this combination change the prognosis of patients with melanoma brain metastases? Cancer Treat Rev. 2016;50:1–8. doi: 10.1016/j.ctrv.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Cohen JV, Kluger HM. Systemic Immunotherapy for the Treatment of Brain Metastases. Frontiers in oncology. 2016;6:49. doi: 10.3389/fonc.2016.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Cang S, Liu D. Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol. 2016;9:34. doi: 10.1186/s13045-016-0268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters S, Adjei AA, Gridelli C, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 29.Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–595. doi: 10.1200/JCO.2006.07.3585. [DOI] [PubMed] [Google Scholar]
- 30.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 31.Soria J-C, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK -rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet. 2017;389(10072):917–929. doi: 10.1016/S0140-6736(17)30123-X. [DOI] [PubMed] [Google Scholar]
- 32.Button KS, Ioannidis JP, Mokrysz C, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 33.Lachin JM, Foulkes MA. Evaluation of Sample Size and Power for Analyses of Survival with Allowance for Nonuniform Patient Entry, Losses to Follow-Up, Noncompliance, and Stratification. Biometrics. 1986;42(3):507. [PubMed] [Google Scholar]
- 34.Chow R, Tsao M, Pulenzas N, et al. Do patients with brain metastases selected for whole brain radiotherapy have worse baseline quality of life as compared to those for radiosurgery or neurosurgery (with or without whole brain radiotherapy)? Ann Palliat Med. 2016;5(1):1–12. doi: 10.3978/j.issn.2224-5820.2015.11.01. [DOI] [PubMed] [Google Scholar]
- 35.Marsh JC, Gielda BT, Herskovic AM, Abrams RA. Cognitive Sparing during the Administration of Whole Brain Radiotherapy and Prophylactic Cranial Irradiation: Current Concepts and Approaches. J Oncol. 2010;2010:198208. doi: 10.1155/2010/198208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


