Abstract
Guided by the main tenets of contemporary models of the developmental origins of health and disease, this study evaluated whether individual differences in reactivity of the hypothalamic-pituitary-adrenal (HPA) axis and Sympathetic Nervous System (SNS) moderate the effect of prenatal exposure to trauma on internalizing and externalizing behaviors during infancy. Participants were a community sample of 182 mothers (M age = 25 years, 43% Caucasian, 33% Black/ African American, 24% Biracial/Other) and their infants (59% girls; M age = 11.8 months). Each mother completed questionnaires that assessed IPV experienced during pregnancy and also reported on her infant’s behavior problems. Infant saliva samples (later assayed for cortisol and sAA) were collected before and after a frustrating task (i.e., arm restraint) . Results revealed that the association between in utero IPV and infant internalizing behaviors was most pronounced for infants with asymmetrical HPA-SNS (i.e., high-cortisol and low-sAA) reactivity to frustration, and least pronounced for infants with symmetrical HPA-SNS (i.e., low-cortisol and low-sAA or high-cortisol and high-sAA) reactivity to frustration. Higher levels of externalizing behavior, in contrast, were associated with higher levels of prenatal IPV but unrelated to either cortisol or sAA reactivity to stress. Findings replicate documented associations between maternal IPV exposure during pregnancy and offspring risk. Moreover, findings advance our understanding of individual differences in the developmental origins of health and disease and provide additional evidence that assessing multiple stress biomarkers contributes to a more comprehensive understanding of individual vulnerability to adversity.
Keywords: intimate partner violence, prenatal stress, cortisol, sAA, internalizing, externalizing
1. Introduction
A large body of literature highlights the detrimental effects of negative gestational experiences, including exposure to prenatal stress, on many aspects of development1. Of note, some studies identify intimate partner violence (IPV) that mothers experience during pregnancy as a particularly adverse stressor for their children2, 3, 4, 5. IPV creates a stressful, unpredictable, and dangerous environment for pregnant women that includes both acute traumatic events and chronic anticipation of abusive behaviors. Only a few investigators have examined its effects on offspring, despite the fact that IPV occurs frequently during pregnancy6 and that its prenatal effects may be even more pronounced and long lasting than those of milder or less chronic pregnancy stressors (e.g., stressful life events)7. These studies document a significant association between prenatal IPV exposure and birth outcomes (low birth weight)2, stress response alterations4, temperamental difficulties3 and internalizing (e.g., sad, inhibited) and externalizing (e.g., oppositional, aggressive, rule-breaking, impulsive) behaviors during infancy4 and childhood8.
The fields studying neural, psychological and behavioral development are just now beginning to understand individual differences in young children’s outcomes after prenatal stress exposure (i.e., multifinality)9. Contemporary models of development propose that biological predispositions can make individuals more or less sensitive to environmental inputs, and that distinct profiles of physiological reactivity and regulation are most beneficial in different contexts10, 11. In theory, children who are more biologically susceptible to environmental influences have the worst outcomes in high-risk environments, but show the best outcomes in supportive and enriched environments10, 11. Aligned with these predictions, research has documented interactions between indices of stress response activity and environmental adversity, including family and sociodemographic risk12, 13. Emerging research on prenatal stress is also consistent with the tenets of these models, such that biological (i.e., genetic) predispositions moderate the effect of maternal stress during pregnancy on infant negative emotionality14 and childhood externalizing problems15. Although interrelated, prenatal exposure to IPV and high physiological arousal during early life may similarly interact to shape infant behavioral outcomes
The psychobiology of the stress response is multi-faceted and involves coordination among several physiological systems, including but not limited to the Hypothalamic-Pituitary-Adrenal (HPA) axis activity and Sympathetic branch of the Autonomic Nervous System (SNS)16, 17. The SNS orchestrates the “fight or flight” response to stress through the effects of catecholamines (i.e., adrenaline, noradrenaline) and is thought to represent a defensive reaction in response to situations where the individual is effectively and appropriately mobilizing resources to deal with challenge. Activation of this system leads to a fast response across multiple systems in the body, including enhanced cardiovascular tone, respiratory flow, blood flow to muscles, and elevated blood glucose18. Salivary alpha amylase (sAA), an enzyme released in response to sympathetic activation, is widely used as a valid peripheral marker of SNS activity19. The Hypothalamic-Pituitary-Adrenal (HPA) axis is a relatively slower, but longer acting system, that is thought to reflect a “defeat” reaction (HPA activity increase in response to circumstances where the individual is overwhelmed, withdraws, and is distressed). Threat recognition leads to secretion and release of the corticotropin-releasing hormone (CRH) by the hypothalamus, which stimulates release of adrenocorticotropin hormone (ACTH) by the pituitary, and leads to cortisol release by the adrenal cortex. Cortisol exerts its effects throughout the brain and body inducing enhanced glucose metabolism, immunosuppression, and changes in cognition, memory, and emotion20. Some evidence suggests a normative dampening of the cortisol response during the first year of life21; however, studies document that infants exposed to psychosocial risk display cortisol mobilization to stressors22, 23, 24 and that infant cortisol reactivity is relatively stable across laboratory stressors and in the short term25. Less is known about the infant sAA response, but research documents increases 5 to 10 minutes post-stress among 12 month old infants26.
Despite having somewhat different functions, the SNS and HPA axis are highly interconnected: hypothalamic CRH neurons and noradrenargic neurons can become co-activated, as they respond to the same neurochemicals and can also modulate each other’s activity through reciprocal neural connections18. Thus, a multi-system measurement approach is needed to more accurately operationalize the activity of the biological components of the stress response27, 28. Bauer, Quas, & Boyce18 delineated two competing models of HPA/ANS coordination. The “additive” effects model assumes joint activity that “totals” moderate levels of arousal is optimal, such that a stress response characterized by moderate activation of both systems, or high activation of one system accompanied by low activation of another represents an adaptive response, while joint high activation or de-activation that leads to excessive or not enough arousal, respectively, is maladaptive. The second model proposes “interactive” effects, so that the systems have complimentary functions and disassociations in activity (i.e., activation of only one system) reflect inefficient coordination.
A few studies have tested these alternative models of coordination by measuring salivary levels of cortisol and alpha-amylase, which can be collected using non-invasive methods, are relatively inexpensive to assay, and have strong associations with more direct indices of SNS and HPA axis activity29, 30. However, the results of these studies have been inconsistent and difficult to interpret because the study designs, participants, behavioral measures, and saliva sampling strategies (e.g., diurnal rhythm, stress-reactivity, basal levels) are very different. Three studies suggest that “asymmetrical” activity between these two stress response systems is associated with better cognitive and behavioral outcomes, while “symmetrical” activation is generally associated with psychosocial problems31, 32, 33. However, these studies differ on the specific patterns that confer risk for internalizing and externalizing behaviors (e.g., low cortisol paired with low sAA reported by Chen et al.31, and Gordis et al.33; high cortisol paired with high sAA reported by El-Sheikh et al.32). In contrast, one study34 found that high-cortisol combined with low-sAA reactivity was associated with more attention, anxiety, depressive symptoms, and social problems among school-aged children and adolescents. As a result, the association between HPA and SNS functioning and internalizing or externalizing behaviors during childhood is poorly understood. This is particularly true for infants, as no study to date has evaluated links between HPA and SNS multi-system activationto a stressor and infant socioemotional outcomes.
Interactions between chidlren’s physiological stress reactivity and their environment may help explain some of these discrepant findings. However, only two studies to date have evaluated associations between multi-system coordination as moderators of environmental risk and no study has focused on early childhood. Koss et al.35 evaluated the interaction between marital conflict, HPA and SNS reactivity among a community sample of second graders. Results showed that children with a profile of high sAA and low cortisol activity in response to a stressor (viewing conflict vignettes) were most affected by marital discord; cortisol/sAA coordination moderated the effect of marital conflict on children’s concurrent internalizing, and later (7th grade) internalizing and externalizing problems. Similarly, Chen et al.36 evaluated cortisol/sAA coordination as a moderator of the effect of parental harsh discipline on child internalizing and externalizing behaviors among a large sample of inner-city 11–12 year olds. Results showed that for boys, harsh discipline was associated with more internalizing and externalizing problems for those with asymmetric HPA-SNS activity (i.e., high-cortisol and low-sAA or low-cortisol and high-sAA). These findings suggest that specific profiles of HPA-SNS activity may enhance susceptibility to environmental risk, but it is hard to tease apart the effects of stress system activity and environmental risks, as HPA and SNS functioning are themseleves shaped by environmental inputs throughout childhood21.
A focus on the prenatal context and infant outcomes can help minimize these bidirectional effects that child biological sensitivity and environmental factors may exert on each other over time. Although infant socioemotional problems remain understudied, it is important to address internalizing and externalizing problems among this age group, as infants growing up in high risk environments can experience clinically significant impairment37. One study found that 35% of 12- to 18-month-olds referred to protective services for child maltreatment allegations have clinically significant behavioral and emotional problems38. Moreover, high levels of internalizing and externalizing problems during infancy persist in the short term (1 year later) 39, 40 and predict later emotional and behavioral disorders (school age)41, particularly among children exposed to high levels of family stress42.
Present Study
The present study evaluated the combined effects of prenatal adversity as well as stress-related HPA axis and SNS reactivity as predictors of internalizing and externalizing behaviors when infants were about 12-month-old infants. With only a handful of studies with older children to guide predictions35, 36, we expected a significant interaction between prenatal IPV exposure and cortisol/sAA coordination, such that asymmetrical coordination combined with high levels of prenatal IPV would be associated with more infant internalizing and externalizing behaviors. Because previous studies reported gender effects on HPA and SNS links to behavioral outcomes among older children36, infant sex was assessed as a potential covariate. Other covariates evaluated were smoking, alcohol use, and substance use during pregnancy (as these are associated with infant behavioral outcomes43); maternal education, income, mental health problems, and reports of stressful life events during the postpartum year, as these are associated with infant early adaptation44; and postnatal exposure to IPV, due to significant continuity between pregnancy and postpartum partner violence45.
Methods
2.1 Participants and Procedure
Participants were 182 mother-infant dyads, recruited from two metropolitan areas in the Midwestern United States. Infants (M age = 11.8 months) were 59% girls and 41% boys. The sample was ethnically diverse and primarily low income. Sample characteristics are summarized in Table 1.
Table 1.
Demographic Characteristics
| Infant | |
|---|---|
| Gender | 59% girls, 41% boys |
| Age | M = 11.8 months, Range = 11 to 13 months |
| Ethnicity | 29% Black/African American; 28% White/Caucasian; 37% Multiracial; 6% Other |
| Number of siblings | 50% only child; 25% one sibling; 13% two siblings; 12% 3 or more |
|
| |
| Mother | |
|
| |
| Age | M = 24.5 years, SD = 4.81 |
| Ethnicity | 43% White/Caucasian; 33% Black/African American; 9% Latino/Hispanic; 15% Multiracial |
| Education | 22% No high school; 31% High school degree or GED; 49% Post-high school education |
| Employment | 34% Unemployed; 19% Student; 47% Employed |
| Monthly Income | M = $1,170, SD = $961; 85% qualified for Medicaid |
| Relationship Status | 21% Married and living with partner; 28% Not married but living with partner; 50% Not living with a partner |
Participants were recruited through fliers posted in community social service agencies that assist women with young children, domestic violence organizations, and local businesses. Flyers were also electronically posted in Craigslist™ and Facebook™. Inclusion criteria were: (1) English-speaking, (2) 18 to 34 years old, (3) involved in a heterosexual romantic relationship for at least 6 weeks during their pregnancy, and (4) willing to not breast feed their infants for 2 hours prior to assessment. Exclusion criteria were: (1) currently pregnant, (2) endocrine or other disorders associated with abnormal glucocorticoid release (Cushings, Addisons Disease, cancer), and (3) premature delivery (i.e., < 37 weeks). During a phone screening, women were classified as exposed to IPV exposure during pregnancy and/or postpartum if they reported experienced threats or actions of moderate and severe violence. All women who met the inclusion criteria and had experienced IPV pre- and/or postpartum were invited to participate in the study. Women who met inclusion criteria but had no IPV exposure were enrolled to match the demographic characteristics of the IPV-exposed group (i.e., race/ethnicity, income, marital status, age, and educational status). Based on the phone screening, 18.7% of women were classified as experiencing only pregnancy IPV, 6.6% reported only postpartum IPV, 42.9% reported pregnancy and postpartum IPV, and 31.9% were classified as controls.
All dyads completed in-person assessments scheduled to start between 12:30 and 13:00 hr. All assessments were started around the same time of day to account for the circadian rhythm of cortisol and sAA production, as normative increases and decreases in hormone levels throughout the day can make it hard to ascertain the magnitude of the cortisol or sAA stress response46. To ensure the quality of saliva samples, mothers were instructed not to feed their babies or brush their teeth 1 hour prior to the assessment and to avoid highly acidic or sugary drinks 20 min prior to the visit. Interviews were administered by two trained graduate and/or undergraduate students and took approximately three hours to complete. Upon arrival to the lab, mothers completed informed consent, a demographic questionnaire, and questions about their baby’s health and recent sleeping, eating, and drinking. Babies were encouraged to explore and play with age-appropriate toys. The first saliva sample was collected (about 30 min after arrival to the lab). Afterwards, dyads completed the challenge task (see below), and saliva samples were collected 5 min, 20 min, and 40 min after the end of the challenge task. In between samples, the mother completed the additional questionnaires while the interviewer played with the infant. Mothers were financially compensated, and the infants received a small toy.
2.2 Measures
Severity of Violence against Women Scales (SVAWS)47
Women’s exposure to IPV was assessed with this 46-item questionnaire. Items include threats of violence, physically violent behaviors, and sexual violence (e.g., “punched you,” “demanded sex whether you wanted to or not,”) and are rated using a 4-point scale, from “Never” to “Many Times.” Women completed the SVAWS twice: once for experiences of IPV endured from male partners during pregnancy (prenatal IPV exposure) and once for IPV experienced since the child’s birth (postnatal IPV exposure). To improve accuracy of this retrospective measure, we used an event calendar. Compared to standard methods of interviewing, this method leads to increased accuracy of reporting retrospective events, including IPV48. All items were summed into a pregnancy IPV (M = 20.72, SD = 28.34, Range = 0 to 126) and a postnatal IPV (M = 12.85, SD = 21.98, Range = 0 to 138) score. Seventy-nine percent of women reported at least one incident of violence (physical, psychological, or sexual) during pregnancy, and 64% reported at least one IPV experience during the postpartum year. The scale has demonstrated good psychometric properties in other samples49 and in the present study (α = .97 during pregnancy; α = .98 postpartum).
Postnatal Cumulative Risk
This variable was created to control for demographic and postnatal environmental factors that affect child outcomes44, 50. This approach, recommended when a large number of risk factors are assessed in a relatively small sample51, was the sum of 5 binary variables: income (below Medicaid poverty cut-off = 1; above Medicaid poverty cut-off = 0), marital status (single = 1; living with a partner = 0), negative life events experienced [total Life Experiences Survey]52 score on the highest 25% percentile = 1; lowest 75% percentile = 0], and clinically significant levels of depression, anxiety, or PTSD, obtained using recommended cut off scores using the Edinburgh Perinatal Depression Scale53, the Modified PTSD Symptom Scale – Self Report54 and the GAD-755 (any mental health problem that is clinically significant = 1; all three scores below clinical cut offs = 0). The cumulative risk score ranged from 0 to 5 (M = 2.21; SD = 1.14).
Perinatal Risk Assessment Monitoring Survey (PRAMS)56 were used to assess smoking (i.e., cigarettes smoked in an average week), drinking (i.e., drinks consumed in an average day) and other drug use during pregnancy (yes/no), including marihuana, cocaine/crack, heroin, hallucinogens, sedatives, tranquilizers, amphetamines, pain killers, and inhalants. Higher scores indicate less tobacco, alcohol, and drug use. Sixty six percent of women reported that they did not smoke during pregnancy, 26% reported smoking less than one to five cigarettes per day, and 8% smoked 6 or more cigarettes daily. Ninety two percent of women reported they did not drink during their pregnancy, 6% reported drinking less than one to five drinks per week, and 1% of women drank 6 drinks or more per week. Seventy eight percent of women reported they did not use drugs during their pregnancy, 17% used one substance (primarily marihuana, pain killers, sedatives, or tranquilizers), and 5% used 2 or more substances.
Infant Social and Emotional Assessment – Revised Short Form (ITSEA)57
This 99-item parent-report questionnaire assesses social and emotional problems and competencies. Mothers read a list of behaviors and rated how true each was of her child using a 3-point scale from “Not true/rarely” to “Very true/often.” Mean scores ranging from 0 to 2 were calculated for the internalizing (e.g., “looked unhappy or sad without any reason,” “been afraid when s/he should not be;” M = .42, SD = .19, Range = .06 to 1.03) and externalizing (e.g., “acted bossy,” “is disobedient or defiant;” M = .56, SD = .30, Range = .03 to 1.55) domains. Alpha reliability coefficients were .89 and .78 for the externalizing and internalizing domain scales respectively. These scales have good concurrent and prospective correlations with the Child Behavior Checklist for children ages 1.5 to 558,59.
Emotion Elliciting Challenge Task
Following Berry et al.60 and Eiden et al.61 infant’s saliva was collected before and after participation in a Modified Lab-TAB Arm Restraint procedure62. Infants were placed in a highchair and given a fun toy to play with for two min. Restraint was then administered. In the original version, the mother conducts the restraint, but in the modified version of the task the restraint was conducted by the interviewer while the mother watched while sitting behind and slightly to the left of the infant, so the infant could not see her. Reaching from behind, the interviewer gently placed her hands on the infant’s forearms, moved them to the infant’s side, and continued to hold them gently, yet firmly enough so that the infant could not pull free for 2 min. If the child cried hard for 20 consecutive sec, the restraint was terminated early. After the restraint, the infant was removed from the chair and returned to his/her mother for a short recovery period. Ninety-six percent of children showed some visible distress during the task and 70% had early terminations.
Collection and Determination of Salivary Analytes
Following Granger et al.,63 saliva was collected from infants using hydrocellulose microsponges placed in their mouths (BD Visitec 7 cm Eye Sponge). Problems with saliva collection (infant crying, infant ate or drank water shortly before sample) were recorded for each sample. Samples were temporarily refrigerated at 4 °C immediately after collection and then frozen and stored at −80 °C. To prevent sublimation during storage, saturated microsponges were later thawed and centrifuged for 15 min at 1300 rpm to extract saliva.
Cortisol
Saliva from baseline, 20 min post-challenge, and 40 min post-challenge samples was assayed for cortisol using a commercially available enzyme immunoassay (EIA) kit specifically designed for use with saliva using the manufacturer’s recommended protocol (Salimetrics, Carlsbad, CA). The assay is 510K cleared (US FDA) as a diagnostic measure of adrenal function; the range of detection is from 0.003 to 3.0 μg/dl. The intra- and inter-assay coefficients of variation from our samples in this study were found to be 7.9% and 9.8%, respectively. Scores that were 3 standard deviations above the mean were windsorized to minimize the effect of outliers. Raw cortisol levels were .31 ug/dL (SD = .53) at baseline, .33 ug/dL (SD = .47) 20-min post-challenge, and .39 ug/dL (SD = .48) 40-min post-challenge. Previous research has proposed an increase of 20% in cortisol levels (about twice the frequently reported coefficients of variation) constitute a mobilization of the cortisol response4, 64. Following these guidelines, 60% of infants were responders. Per conventional practices, cortisol measures were log-transformed for use in statistical analyses. Area under the Curve from ground (AUCg) was calculated using the trapezoid formula with log-transformed values65. AUCg is a robust measure, as it integrates multiple samples and both the magnitude and the pattern of the cortisol response.
sAA
Saliva from baseline, 5 min post-challenge, and 20 min post-challenge samples was assayed for sAA using a commercially available kinetic reaction assay kit (Salimetrics, LLC) that has intra- and inter-assay coefficients of variation of 7.5 % and 6.0%, respectively. All samples were assayed in duplicate. Scores that were 3 standard deviations above the mean were windsorized to minimize the effect of outliers. Mean raw sAA levels were 43.50 U/mL (SD = 31.78) at baseline, 45.29 U/mL (SD = 34.98) 5-min post-challenge, and 46.19 U/mL (SD = 34.92) 20-min post-challenge. Using a 20% increase in sAA levels as an index of sAA response, 46% of infants were responders. Following conventional practices, sAA values were square-root transformed for statistical analyses. Area under the Curve from Ground (AUCg) was calculated using the trapezoid formula with square-root transformed values65.
3. Results
3.1 Preliminary Analyses
Five percent of data points were missing and the MCAR test revealed the data were Missing Completely at Random, Little’s MCAR Chi-square = 86.49, p = 1.00. Thus, data were imputed using the Estimation Maximization Likelihood method on SPSS 22 and the imputed data set was used for analyses. See Table 2 for correlations between cortisol, sAA, IPV exposure, and infant internalizing and externalizing behaviors. Prenatal and postnatal IPV were strongly correlated (r = .70, p < .05) and additional analyses were conducted without postnatal IPV to avoid issues of multicollinearity. Sampling time of day, time since eating or drinking, time since sleeping, baby’s current mood, baby’s current health, immunizations, and medication use in the last 2 days were not associated with infant AUCg scores for cortisol or sAA. Correlations between potential covariates and key study variables were also explored (See Table 3). Infant sex and ethnicity were not associated with internalizing behaviors, but males and children of ethnic minority background had higher scores for externalizing behaviors (Kendall’s tau = .15, p < .05, and Kendall’s tau = .13, p < .0, respectively). Prenatal exposure to drugs was not associated with infant cortisol and sAA reactivity or behavioral outcomes; however, maternal smoking during pregnancy was associated with internalizing and externalizing behaviors (r = −.18, p <.05, and r = −.22, p <.05, respectively) and alcohol use during pregnancy was associated with cortisol AUCg (r = −.18, p <.05). Only covariates that were significantly associated with the key variables used in each model were included as covariates for hypothesis testing.
Table 2.
Bivariate Correlations between Key Study Variables
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Prenatal IPV | .71* | .34* | .37* | −.01 | .05 | .11 | .04 | .19* | .22* | .15* | .18* |
| 2. Postnatal IPV | .20* | .20* | .01 | .04 | .02 | .00 | .03 | .06 | .01 | .04 | |
| 3. Externalizing | .59* | .02 | .01 | .08 | .01 | .12 | .10 | .03 | .07 | ||
| 4. Internalizing | −.06 | −.03 | .02 | .00 | .08 | .08 | .05 | .04 | |||
| 5. Baseline cortisol | .69* | .71* | .85* | .13 | .11 | .11 | .12 | ||||
| 6. 20-min post cortisol | .71* | .94* | .13 | .12 | .10 | .09 | |||||
| 7. 40-min post cortisol | .87* | .16* | .19* | .16* | .19* | ||||||
| 8. Cortisol AUCg | .17* | .16 | .15 | .16* | |||||||
| 9. Baseline sAA | .89* | .85* | .92* | ||||||||
| 10. 5-min post sAA | .89* | .98* | |||||||||
| 11. 20-min post sAA | .96* | ||||||||||
| 12. sAA AUCg |
Table 3.
Correlations between Key Variables and Potential Covariates
| Gendera | Ethnic Minoritya | Pregnancy Smoking | Pregnancy Alcohol | Pregnancy Drugs | Cumulative Postnatal Risk | |
|---|---|---|---|---|---|---|
| Externalizing | .15* | .13* | −.22* | −.07 | .09 | .40* |
| Internalizing | .04 | .07 | −.18* | −.01 | .09 | .31* |
| Cortisol AUC | .04 | .12 | .06 | −.18* | −.05 | .09 |
| sAA AUC | .05 | .12 | −.04 | .03 | −.14 | .02 |
| Pregnancy IPV | .06 | .09 | −.10 | .02 | .04 | .47* |
Correlation is significant at the 0.05 level (2-tailed).
Kendall’s Tau used for correlations between one dichotomous and one continuous variable. Pearson’s reported for all other correlations.
3.2 Main Analyses
The combined effects of HPA and SNS reactivity and prenatal IPV exposure were evaluated using two step-wise multiple regressions with 1000 Bootstrap samples to predict infants’ internalizing or externalizing behaviors. Bootstrapping is recommended for analyses with moderately sized samples to obtain more accurate standard errors that are not dependent on the assumption of a normal distribution66. The first step of the models included the covariates assessed. Based on bivariate correlations, the internalizing model included pregnancy smoking and cumulative risk (associated with internalizing problems), as well as pregnancy alcohol use (associated with cortisol AUCg). The externalizing model included infant sex, ethnicity, pregnancy smoking, and cumulative risk (associated with externalizing problems), as well as pregnancy alcohol use (associated with cortisol AUCg). The first step of each model also included the mean-centered main effect of prenatal IPV, cortisol AUCg, and sAA AUCg. The second step included the mean-centered 2-way interactions: IPV-by-cortisol, IPV-by-sAA, and cortisol-by-sAA. The last step included the mean-centered 3-way interaction of IPV-by-cortisol-by-sAA.
Internalizing Behaviors
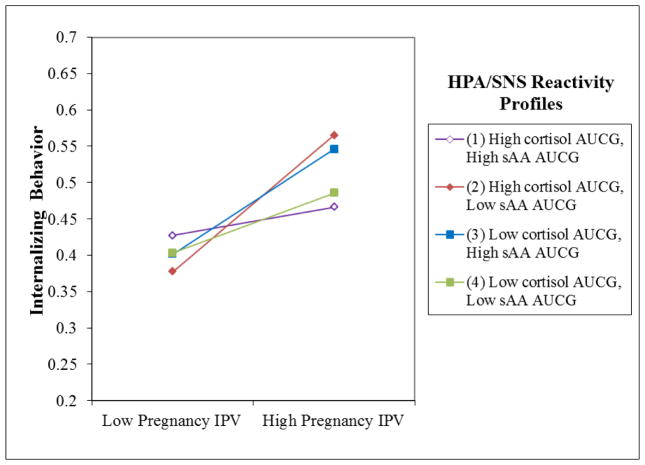
The model explained 20% of the variance of infant internalizing behaviors. Internalizing behaviors were associated with prenatal IPV exposure, Standardized B = .33, Bootstrapped p = .00, in the first step. Furthermore, the IPV-by-cortisol-by-sAA interaction was a significant predictor of internalizing behaviors, Standardized B = −.17, Bootstrapped p = .047 (Table 4). Following Aiken and West67, this interaction was plotted to represent infant internalizing levels one standard deviation above and below the mean prenatal IPV levels for four groups of children: high-cortisol and high-sAA, low-cortisol and low-sAA, high-cortisol and low-sAA, and low-cortisol and high-sAA. Infants with high-cortisol and low-sAA were most at risk for internalizing behaviors when they experienced high levels of prenatal IPV, but not when they experienced low levels of prenatal IPV (See Figure 1). Slope difference tests indicated that the effect of prenatal IPV among children with high-cortisol and low-sAA was stronger than the effect of prenatal IPV for children with low-cortisol and low-sAA (t = −1.737, p = .08) or with high cortisol and high sAA (t = 1.772, p = .08). None of the covariates were significant predictors of internalizing problems.
Table 4.
Three Way Interaction Predicts Infant Internalizing Behavior
| Std B | B | S.E. | p | 95% CI | |
|---|---|---|---|---|---|
| Pregnancy IPV | .325 | .002 | .001 | .001 | .001 – .003 |
| Cortisol AUCg | .050 | .000 | .003 | .932 | −.006 – .007 |
| sAA AUCg | .006 | .000 | .000 | .928 | −.001 – .001 |
| Cumulative Risk | .144 | .018 | .010 | .068 | −.002 – .037 |
| Pregnancy Smokinga | −.138 | −.022 | .012 | .069 | −.048 – .001 |
| Pregnancy Alcohola | .023 | .009 | .022 | .599 | −.039 – .050 |
| Pregnancy IPV X Cortisol AUCg | .103 | .000 | .000 | .212 | .000 – .001 |
| Pregnancy IPV X sAA AUCg | −.060 | .000 | .000 | .388 | .000 – .000 |
| Cortisol AUCG X sAA AUCg | −.079 | .000 | .000 | .252 | .000 – .000 |
| Pregnancy IPV X Cortisol AUCg X sAA AUCg | −.167 | −.000004 | .000 | .047 | .000 – .000 |
Higher values indicate less smoking and alcohol use during pregnancy.
Figure 1.
HPA/SNS Coordination Moderates the Relationship between Pregnancy IPV and Infant Internalizing Behaviors
Externalizing Behaviors
The model explained 23% of the variance of infant externalizing behaviors. Externalizing behaviors were predicted by prenatal IPV exposure, Standardized B = .33, p = .00, but not by either cortisol AUCg or sAA AUCg. Also, the 2- and 3-way interactions did not significantly predict externalizing outcomes (Table 5). In addition, more cigarette exposure during pregnancy (Standardized B = −.19, p = .01) and being male (Standardized B = .24, p = .00) predicted more externalizing behaviors.
Table 5.
Pregnancy IPV Predicts Infant Externalizing Behavior
| Std B | B | S.E. | p | 95% CI | |
|---|---|---|---|---|---|
| Pregnancy IPV | .228 | .002 | .001 | .009 | .001 – .004 |
| Cortisol AUCg | .008 | .001 | .006 | .909 | −.009 – .014 |
| sAA AUCg | .002 | .000 | .001 | .983 | −.001 – .001 |
| Cumulative Risk | .245 | .048 | .015 | .001 | .018 – .078 |
| Pregnancy Smokinga | −.189 | −.047 | .015 | .003 | −.078 – .018 |
| Pregnancy Alcohola | .022 | .014 | .044 | .714 | −.093 – .085 |
| Infant Gender | .188 | .113 | .037 | .003 | .037 – .190 |
| Infant Ethnicity | .092 | .062 | .042 | .141 | −.021 – .146 |
| Pregnancy IPV X Cortisol AUCg | .146 | .000 | .000 | .141 | .000 – .001 |
| Pregnancy IPV X sAA AUCg | −.096 | .000 | .000 | .195 | .000 – .000 |
| Cortisol AUCG X sAA AUCg | .000 | .000 | .000 | .996 | .000 – .000 |
| Pregnancy IPV X Cortisol AUCg X sAA AUCg | −.077 | .000 | .000 | .480 | .000 – .000 |
Higher values indicate less smoking and alcohol use during pregnancy.
4. Discussion
The study’s findings indicate that the association between prenatal IPV exposure and internalizing behavior at 12 months of age was moderated by patterns of multi-system physiological reactivity to stress. That is, the effects of in utero IPV on internalizing behaviors was most pronounced for infants with high-cortisol and low-sAA reactivity, and least pronounced for infants with symmetrical cortisol and sAA reactivity. This pattern of findings was independent of the potentially confounding effects of gender, prenatal drug, alcohol, and tobacco exposure, as well as postnatal environmental risk. In contrast, higher levels of externalizing behaviors were associated with higher levels of prenatal IPV but unrelated to the main or combined (symmetrical or asymmetrical) effects of infant stress reactivity. These findings are novel, highlight the robust association between prenatal IPV exposure and early socioemotional outcomes, and emphasize the importance of using a multi-system approach to study relationships between stress systems and behavior. The results are also consistent with contemporary theories that focus on individual differences in biological sensitivity and susceptibility to adversity and have implications for our understanding of the developmental origins of health and disease.
Our results are generally consistent with the prenatal stress literature and the handful of studies that have evaluated links between maternal exposure to IPV during pregnancy and infant outcomes2,3,4. A growing literature now documents that maternal stress and trauma during pregnancy can lead to a range of negative developmental and psychosocial outcomes, including internalizing and externalizing problems during infancy and childhood1, mirroring findings with youth exposed to other prenatal teratogens (smoking, alcohol and substance use, restricted nutritional intake61, 68. Associations between prenatal IPV and internalizing or externalizing problems were robust and remained when other prenatal insults (i.e., cigarette, alcohol, and drugs) and multiple postnatal risks were taken into account. This suggests a direct link between prenatal IPV and infant outcomes potentially mediated by changes in the uterine environment that shape fetal brain development, including set-points and thresholds of reactivity for the HPA axis and the SNS4, 21, 69, 70.
Our results also extend previous research by suggesting that patterns of stress reactivity can help explain which infants are particularly susceptible to the effects of prenatal IPV, and echo the findings of moderation of environmental risk by genetic predisposition14, 15, and stress system activity-by-environment interactions that have been reported by others12, 13. An asymmetrical pattern, characterized by high-cortisol and low-sAA stress reactivity emerged as a potential endophenotype for increased sensitivity; this pattern of reactivity was associated with higher levels of infant internalizing behaviors, but only among infants who had been exposed to high levels of prenatal IPV. Laurent et al.28 propose that the HPA and SNS systems respond to psychosocial stress in unique ways, such that the sympathetic branch of the SNS reflects both approach- and withdrawal-related arousal, while HPA axis activity is associated with negative valence states, such as distress. This hypothesis is generally consistent with assumptions forwarded by Henry71 that the HPA axis response reflects a “defeat” reaction (i.e., cortisol elevates in response to circumstances where the individual is overwhelmed, withdraws, and is distressed), whereas the SNS response is considered a “defense” reaction (i.e., sAA elevates in response to situations where the individual is effectively and appropriately mobilizing resources to deal with the challenge, “fight or flight”). Based on our findings and these theoretical perspective, low sAA and high cortisol may represent a particularly detrimental coordination style that reflects too little approach- related arousal and too much negativity.
On the other hand, children with symmetrical cortisol and sAA reactivity displayed consistently low levels of internalizing behaviors regardless of their prenatal IPV exposure. In a prior study, youth with this stress-reactivity profile were also minimally susceptible to the effects of harsh parenting practices36. A symmetrical cortisol and sAA reactivity profile may reflect the “dandelion” endophenotype proposed by Ellis and colleagues13. They posit that some children are less physiologically reactive to environmental input, which is advantageous in that they are likely to maintain adequate functioning in either low-stress or high-stress circumstances. This response profile is also theorized to be least responsive to enriched environments, but we were unable to test this assuptiom because the environmental index we used, exposure to IPV, did not accurately capture strengths of the prenatal environment.
Notably, we found that prenatal IPV was the only predictor of externalizing behaviors. The strong negative influence of prenatal IPV is not surprising, as other studies of prenatal IPV exposure have found associations with early externalizing and difficult temperament3, 4 and family violence is a strong predictor of child externalizing behaviors72. It is possible that for these very young children the detrimental influence of prenatal IPV generally “overrides” temperamental or biological susceptibility, leading to increases in externalizing behaviors regardless of the infants’ stress response patterns. Even among infants who are less reactive to the environment, prenatal exposure to IPV may affect other brain systems associated with behavioral control (e.g., prefrontal cortex)73. In the context of a lifespan developmental perspective, our findings are generally consistent with the developmental origins of health and disease (DOHaD) conceptual framework, which proposes that nutrition, chemical exposures, and stressors are particularly impactful during windows of developmental plasticity and increase susceptibility to later illness74, 75.
Neither the main effect of stress reactivity nor the interactions among cortisol reactivity, sAA reactivity, and prenatal IPV exposure predicted infant levels of externalizing behavior. This was somewhat surprising given Berry et al.76 found that HPA/SNS coordination was associated with effortful control among toddlers, a precursor to externalizing problems77. However, other studies have reported that externalizing problems are not reliably associated with stress reactivity78. In addition, levels of externalizing behaviors are lower among 12-month-old children as compared to older children, and the correlates associated with externalizing also show some variability at different developmental stages37. The developmental stage of the children we assessed may help explain this null finding.
Our findings need to be interpreted in light of the retrospective nature of the prenatal IPV exposure data, and concurrent assessment of infant internalizing and externalizing behaviors and stress response indices, obtained when the infants were 12 months old. However, it is important to note that an event calendar was used in order to minimize the limitations of retrospective methods79. Also, we used maternal reports to assess infant behavioral outcomes; a combination of maternal reports and observer ratings could enhance the validity of this infant outcome. Last, only about half of all infants displayed mobilization of the cortisol and sAA in response to the Arm Restraint Task; future research that integrates tasks of varying “stressfulness” can help elucidate potential differences between the sensitivity of the stress response (or the likelihood to show increases to milder tasks), the intensity of HPA and SNS responses, and their associations with young children’s behavioral outcomes. Despite these limitations, the study has significant strengths and addresses important gaps in this field. To our knowledge, this study is the first to evaluate the interaction between the multi-system HPA/SNS coordination and contextual adversity very early in life. Evaluating this association early on is vital for a better understanding of the interplay between biological and environmental influences before they have significantly altered each other over time (i.e., environmental calibration of the stress response). A prospective longitudinal study of symmetry and asymmetry in the patterns of HPA and SNS stress-reactivity is an important next step to advance our understanding of sensitivity to the environment at different developmental stages. Also, the study is the first to integrate prenatal exposure to IPV as a relevant contextual moderator to understand internalizing and externalizing behaviors. Despite it being a common stressor for pregnant women, IPV has rarely been evaluated in studies of infant biobehavioral outcomes. Last, ethnic diversity was a strength of our sample, and 72% of infants belonged to ethnic minority group.
Conclusion
Exposure to prenatal IPV was associated with increased levels of externalizing behaviors at 12 months of age, but its effects on internalizing behaviors were moderated by patterns of cortisol and sAA coordination: the association between prenatal IPV and infant internalizing behaviors was most potent for infants with low-sAA and high-cortisol reactivity, while prenatal IPV was not significantly associated with internalizing behaviors among infants with a profile of symmetrical cortisol and sAA reactivity. The findings suggest that the relationships between prenatal IPV, infant HPA-SNS coordination, and behavioral outcomes are complex and additional studies to disentangle the effects of prenatal IPV on multi-system stress reactivity and early behavioral outcomes would be worthwhile. The present research sets the stage for future longitudinal research to explore interactions between multi-system stress reactivity and contextual risk in order to identify the environmental influences that are most relevant at different developmental stages, as well as the changing associations between biological susceptibility and child functioning.
Highlights.
Interest in investigating the developmental origins of disease by understanding the role of the prenatal period is growing.
Two major stress systems are involved in mediating the effects of prenatal stress on childhood functioning
Little is known about the effects on children of the interaction between these two stress systems, i.e. the hypothalamic-pituitary-adrenal (HPA) axis and the Sympathetic Nervous System (SNS).
Findings showed that the symmetry or asymmetry of these systems was differentially related to infant internalizing behaviors.
Findings suggest that assessing multiple stress biomarkers leads to a more comprehensive understanding of individual vulnerability to adversity.
Acknowledgments
Funding: This work was supported by the National Institutes of Health [R03HD058868-02, 2009] and Blue Cross Blue Shield of Michigan [1388.II, 2008].
Footnotes
Disclosure Statement: In the interest of full disclosure, DAG is founder and chief scientific and strategy advisor at Salimetrics LLC and SalivaBio LLC, and these relationships are managed by the policies of the committees on conflict of interest at Johns Hopkins University School of Medicine and at the University of California, Irvine. No other authors have information to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Talge NM, Neal C, Glover V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? Journal of Child Psychology and Psychiatry. 2007 Mar 1;48(3–4):245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy CC, Schei B, Myhr TL, Du Mont J. Abuse: a risk factor for low birth weight? A systematic review and meta-analysis. Canadian Medical Association Journal. 2001 May 29;164(11):1567–72. [PMC free article] [PubMed] [Google Scholar]
- 3.Burke JG, Lee LC, O’Campo P. An exploration of maternal intimate partner violence experiences and infant general health and temperament. Maternal and Child Health Journal. 2008 Mar 1;12(2):172–9. doi: 10.1007/s10995-007-0218-z. [DOI] [PubMed] [Google Scholar]
- 4.Levendosky AA, Bogat GA, Lonstein JS, Martinez-Torteya C, Muzik M, Granger DA, Von Eye A. Infant adrenocortical reactivity and behavioral functioning: relation to early exposure to maternal intimate partner violence. Stress. 2016 Jan 2;19(1):37–44. doi: 10.3109/10253890.2015.1108303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Torteya C, Bogat GA, Levendosky AA, Von Eye A. The influence of prenatal intimate partner violence exposure on hypothalamic–pituitary–adrenal axis reactivity and childhood internalizing and externalizing symptoms. Development and psychopathology. 2016 Feb 1;28(01):55–72. doi: 10.1017/S0954579415000280. [DOI] [PubMed] [Google Scholar]
- 6.Taillieu TL, Brownridge DA. Violence against pregnant women: Prevalence, patterns, risk factors, theories, and directions for future research. Aggression and Violent Behavior. 2010 Feb 28;15(1):14–35. [Google Scholar]
- 7.Bergman K, Sarkar P, O'connor TG, Modi N, Glover V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. Journal of the American Academy of Child & Adolescent Psychiatry. 2007 Nov 30;46(11):1454–63. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Torteya C, Muzik M, McGinnis EW, Rosenblum KL, Bocknek EL, Beeghly M, DeCator D, Abelson JL. Longitudinal examination of infant baseline and reactivity cortisol from ages 7 to 16 months. Developmental psychobiology. 2015 Apr 1;57(3):356–64. doi: 10.1002/dev.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996 Sep 1;8:597–600. [Google Scholar]
- 10.Belsky J. Differential susceptibility to rearing influence. Origins of the social mind: Evolutionary psychology and child development. 2005:139–63. [Google Scholar]
- 11.Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, Van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and psychopathology. 2011 Feb 1;23(01):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- 12.Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006 Dec 31;45(12):1510–20. doi: 10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- 13.Obradović J, Bush NR, Stamperdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child development. 2010 Jan 1;81(1):270–89. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pluess M, Velders FP, Belsky J, van IJzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VW, Hofman A, Arp PP, Verhulst FC, Tiemeier H. Serotonin transporter polymorphism moderates effects of prenatal maternal anxiety on infant negative emotionality. Biological psychiatry. 2011 Mar 15;69(6):520–5. doi: 10.1016/j.biopsych.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Zohsel K, Buchmann AF, Blomeyer D, Hohm E, Schmidt MH, Esser G, … Laucht M. Mothers' prenatal stress and their children's antisocial outcomes–a moderating role for the Dopamine D4 Receptor (DRD4) gene. Journal of child psychology and psychiatry. 2014;55(1):69–76. doi: 10.1111/jcpp.12138. [DOI] [PubMed] [Google Scholar]
- 16.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. Jama. 1992 Mar 4;267(9):1244–52. [PubMed] [Google Scholar]
- 17.Weiner H. Perturbing the organism: The biology of stressful experience. University of Chicago Press; 1992. Jun 1, [Google Scholar]
- 18.Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002 Apr 1;23(2):102–13. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009 May 31;34(4):486–96. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 20.Meyer SE, Chrousos GP, Gold PW. Major depression and the stress system: a life span perspective. The Science of Mental Health: Compulsive disorder and Tourette's syndrome. 2001:143. doi: 10.1017/s095457940100308x. [DOI] [PubMed] [Google Scholar]
- 21.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002 Feb 28;27(1):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 22.Hibel LC, Granger DA, Blair C, Cox MJ. Maternal sensitivity buffers the adrenocortical implications of intimate partner violence exposure during early childhood. Development and Psychopathology. 2011 May 1;23(2):689. doi: 10.1017/S0954579411000010. [DOI] [PubMed] [Google Scholar]
- 23.Jansen J, Beijers R, Riksen-Walraven M, de Weerth C. Cortisol reactivity in young infants. Psychoneuroendocrinology. 2010 Apr 30;35(3):329–38. doi: 10.1016/j.psyneuen.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Towe- Goodman NR, Stifter CA, Mills- Koonce WR, Granger DA. Interparental aggression and infant patterns of adrenocortical and behavioral stress responses. Developmental psychobiology. 2012 Nov 1;54(7):685–99. doi: 10.1002/dev.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12-to 18-month-old infants: Stability over time, location, and stressor. Biological Psychiatry. 2003 Oct 1;54(7):719–26. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- 26.Davis EP, Granger DA. Developmental differences in infant salivary alpha-amylase and cortisol responses to stress. Psychoneuroendocrinology. 2009 Jul 31;34(6):795–804. doi: 10.1016/j.psyneuen.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. Journal of Adolescence. 2012 Aug 31;35(4):1081–95. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Laurent HK, Powers SI, Granger DA. Refining the multisystem view of the stress response: Coordination among cortisol, alpha-amylase, and subjective stress in response to relationship conflict. Physiology & behavior. 2013 Jul 2;119:52–60. doi: 10.1016/j.physbeh.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz EB, Granger DA, Susman EJ, Gunnar MR, Laird B. Assessing salivary cortisol in studies of child development. Child development. 1998 Dec 1;69(6):1503–13. [PubMed] [Google Scholar]
- 30.West SG, Granger DA, Kivlighan KT, Psota TL, Hurston KL. Salivary alpha-amylase response to the cold pressor is correlated with cardiac markers of sympathetic activation. Inannual meeting of the American Psychosomatic Society; Denver, CO. 2006. [Google Scholar]
- 31.Chen FR, Raine A, Soyfer L, Granger DA. Interaction of adrenocortical activity and autonomic arousal on children’s externalizing and internalizing behavior problems. Journal of abnormal child psychology. 2015a Jan 1;43(1):189–202. doi: 10.1007/s10802-014-9900-y. [DOI] [PubMed] [Google Scholar]
- 32.El-Sheikh M, Erath SA, Buckhalt JA, Granger DA, Mize J. Cortisol and children’s adjustment: The moderating role of sympathetic nervous system activity. Journal of Abnormal Child Psychology. 2008 May 1;36(4):601–11. doi: 10.1007/s10802-007-9204-6. [DOI] [PubMed] [Google Scholar]
- 33.Gordis EB, Granger DA, Susman EJ, Trickett PK. Asymmetry between salivary cortisol and α-amylase reactivity to stress: Relation to aggressive behavior in adolescents. Psychoneuroendocrinology. 2006 Sep 30;31(8):976–87. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Allwood MA, Handwerger K, Kivlighan KT, Granger DA, Stroud LR. Direct and moderating links of salivary alpha-amylase and cortisol stress-reactivity to youth behavioral and emotional adjustment. Biological psychology. 2011 Sep 30;88(1):57–64. doi: 10.1016/j.biopsycho.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koss KJ, George MR, Davies PT, Cicchetti D, Cummings EM, Sturge-Apple ML. Patterns of children's adrenocortical reactivity to interparental conflict and associations with child adjustment: a growth mixture modeling approach. Developmental psychology. 2013 Feb;49(2):317. doi: 10.1037/a0028246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen FR, Raine A, Rudo-Hutt AS, Glenn AL, Soyfer L, Granger DA. Harsh discipline and behavior problems: The moderating effects of cortisol and alpha-amylase. Biological psychology. 2015b Jan 31;104:19–27. doi: 10.1016/j.biopsycho.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Van Zeijl J, Mesman J, Stolk MN, Alink LR, Van IJzendoorn MH, Bakermans- Kranenburg MJ, Juffer F, Koot HM. Terrible ones? Assessment of externalizing behaviors in infancy with the Child Behavior Checklist. Journal of Child Psychology and Psychiatry. 2006 Aug 1;47(8):801–10. doi: 10.1111/j.1469-7610.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Horwitz SM, Hurlburt MS, Heneghan A, Zhang J, Rolls-Reutz J, Fisher E, Landsverk J, Stein RE. Mental health problems in young children investigated by US child welfare agencies. Journal of the American Academy of Child & Adolescent Psychiatry. 2012 Jun 30;51(6):572–81. doi: 10.1016/j.jaac.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briggs-Gowan MJ, Carter AS, Bosson-Heenan J, Guyer AE, Horwitz SM. Are infant-toddler social-emotional and behavioral problems transient? Journal of the American Academy of Child & Adolescent Psychiatry. 2006 Jul 31;45(7):849–58. doi: 10.1097/01.chi.0000220849.48650.59. [DOI] [PubMed] [Google Scholar]
- 40.Mathiesen KS, Sanson A. Dimensions of early childhood behavior problems: Stability and predictors of change from 18 to 30 months. Journal of Abnormal Child Psychology. 2000 Feb 1;28(1):15–31. doi: 10.1023/a:1005165916906. [DOI] [PubMed] [Google Scholar]
- 41.Briggs-Gowan MJ, Carter AS. Social-emotional screening status in early childhood predicts elementary school outcomes. Pediatrics. 2008 May 1;121(5):957–62. doi: 10.1542/peds.2007-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell SB, Shaw DS, Gilliom M. Early externalizing behavior problems: Toddlers and preschoolers at risk for later maladjustment. Development and psychopathology. 2000 Sep 1;12(03):467–88. doi: 10.1017/s0954579400003114. [DOI] [PubMed] [Google Scholar]
- 43.Kearney MH, Munro BH, Kelly U, Hawkins JW. Health behaviors as mediators for the effect of partner abuse on infant birth weight. Nursing research. 2004 Jan 1;53(1):36–45. doi: 10.1097/00006199-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 44.Hooper SR, Burchinal MR, Roberts JE, Zeisel S, Neebe EC. Social and family risk factors for infant development at one year: An application of the cumulative risk model. Journal of Applied Developmental Psychology. 1998 Jan 1;19(1):85–96. [Google Scholar]
- 45.Martin SL, Mackie L, Kupper LL, Buescher PA, Moracco KE. Physical abuse of women before, during, and after pregnancy. Jama. 2001 Mar 28;285(12):1581–4. doi: 10.1001/jama.285.12.1581. [DOI] [PubMed] [Google Scholar]
- 46.Klimes-Dougan BO, Hastings PD, Granger DA, Usher BA, Zahn-Waxler CA. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Development and psychopathology. 2001 Sep 1;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- 47.Marshall LL. Development of the severity of violence against women scales. Journal of family violence. 1992 Jun 1;7(2):103–21. [Google Scholar]
- 48.Yoshihama M, Gillespie B, Hammock AC, Belli RF, Tolman RM. Does the life history calendar method facilitate the recall of intimate partner violence? Comparison of two methods of data collection. Social Work Research. 2005 Sep 1;29(3):151–63. [Google Scholar]
- 49.Huth-Bocks AC, Levendosky AA, Semel MA. The direct and indirect effects of domestic violence on young children's intellectual functioning. Journal of family violence. 2001 Sep 1;16(3):269–90. [Google Scholar]
- 50.Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: The influence of social and family risk factors. Child development. 1993 Feb 1;64(1):80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 51.Burchinal MR, Roberts JE, Hooper S, Zeisel SA. Cumulative risk and early cognitive development: a comparison of statistical risk models. Developmental psychology. 2000 Nov;36(6):793. doi: 10.1037//0012-1649.36.6.793. [DOI] [PubMed] [Google Scholar]
- 52.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of consulting and clinical psychology. 1978 Oct;46(5):932. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 53.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. The British journal of psychiatry. 1987 Jun 1;150(6):782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 54.Coffey SF, Gudmundsdottir B, Beck G, Palyo SA, Miller L. Screening for PTSD in motor vehicle accident survivors using the PSS-SR and IES. Journal of traumatic stress. 2006 Feb;19(1):119. doi: 10.1002/jts.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Archives of internal medicine. 2006 May 22;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 56.Gilbert BC, Shulman HB, Fischer LA, Rogers MM. The Pregnancy Risk Assessment Monitoring System (PRAMS): methods and 1996 response rates from 11 states. Maternal and Child Health Journal. 1999 Dec 1;3(4):199–209. doi: 10.1023/a:1022325421844. [DOI] [PubMed] [Google Scholar]
- 57.Briggs-Gowan MJ, Carter AS, Skuban EM, Horwitz SM. Prevalence of social-emotional and behavioral problems in a community sample of 1-and 2-year-old children. Journal of the American Academy of Child & Adolescent Psychiatry. 2001 Jul 31;40(7):811–9. doi: 10.1097/00004583-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Carter AS, Briggs-Gowan MJ, Jones SM, Little TD. The infant–toddler social and emotional assessment (ITSEA): Factor structure, reliability, and validity. Journal of abnormal child psychology. 2003 Oct 1;31(5):495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- 59.Briggs-Gowan MJ, Carter AS, Irwin JR, Wachtel K, Cicchetti DV. The Brief Infant-Toddler Social and Emotional Assessment: screening for social-emotional problems and delays in competence. Journal of pediatric psychology. 2004 Mar 1;29(2):143–55. doi: 10.1093/jpepsy/jsh017. [DOI] [PubMed] [Google Scholar]
- 60.Berry D, Blair C, Willoughby M, Granger DA Family Life Project Key Investigators. Salivary alpha-amylase and cortisol in infancy and toddlerhood: Direct and indirect relations with executive functioning and academic ability in childhood. Psychoneuroendocrinology. 2012 Oct 31;37(10):1700–11. doi: 10.1016/j.psyneuen.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Eiden RD, Granger DA, Schuetze P, Veira Y. Child behavior problems among cocaine-exposed toddlers: Indirect and interactive effects. Development and psychopathology. 2011 May 1;23(02):539–50. doi: 10.1017/S0954579411000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldsmith HH. Studying temperament via construction of the Toddler Behavior Assessment Questionnaire. Child development. 1996 Feb 1;67(1):218–35. [PubMed] [Google Scholar]
- 63.Granger DA, Kivlighan KT, El-Sheik MO, Gordis EB, Stroud LR. Salivary α-amylase in biobehavioral research. Annals of the New York Academy of Sciences. 2007 Mar 1;1098(1):122–44. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- 64.Martinez-Torteya C, Dayton CJ, Beeghly M, Seng JS, McGinnis E, Broderick A, Rosenblum K, Muzik M. Maternal parenting predicts infant biobehavioral regulation among women with a history of childhood maltreatment. Development and psychopathology. 2014 May 1;26(02):379–92. doi: 10.1017/S0954579414000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003 Oct 31;28(7):916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 66.Fox J. An R and S-PLUS Companion to Applied Regression: A Web Appendix to the Book. Sage; Thousand Oaks, CA: 2002. Jan, Bootstrapping regression models. URL http://cran.r-project.org/doc/contrib/Fox-Companion/appendix-bootstrapping.pdf. [Google Scholar]
- 67.Aiken LS, West SG, Reno RR. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- 68.Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7- month- old infants. Developmental psychobiology. 2008 Dec 1;50(8):819–34. doi: 10.1002/dev.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological psychiatry. 2005 Aug 1;58(3):211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 70.Taylor ZE, Spinrad TL, VanSchyndel SK, Eisenberg N, Huynh J, Sulik MJ, Granger DA. Sociodemographic risk, parenting, and effortful control: Relations to salivary alpha- amylase and cortisol in early childhood. Developmental psychobiology. 2013 Dec 1;55(8):869–80. doi: 10.1002/dev.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Henry JP. Biological basis of the stress response. Integrative physiological and behavioral science. 1992 Jan 1;27(1):66–83. doi: 10.1007/BF02691093. [DOI] [PubMed] [Google Scholar]
- 72.Evans SE, Davies C, DiLillo D. Exposure to domestic violence: A meta-analysis of child and adolescent outcomes. Aggression and violent behavior. 2008 Apr 30;13(2):131–40. [Google Scholar]
- 73.Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and teratology. 2002 Jun 30;24(3):385–95. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- 74.Barker DJ. Mothers, babies and health in later life. Elsevier Health Sciences; 1998. [Google Scholar]
- 75.Hanson MA, Gluckman PD. Developmental origins of health and disease: new insights. Basic & clinical pharmacology & toxicology. 2008 Feb 1;102(2):90–3. doi: 10.1111/j.1742-7843.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 76.Berry D, Blair C, Willoughby M, Granger DA Family Life Project Key Investigators. Salivary alpha-amylase and cortisol in infancy and toddlerhood: Direct and indirect relations with executive functioning and academic ability in childhood. Psychoneuroendocrinology. 2012 Oct 31;37(10):1700–11. doi: 10.1016/j.psyneuen.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 77.Spinrad TL, Eisenberg N, Gaertner B, Popp T, Smith C, Kupfer A, Greving K, Liew J, Hofer C. Relations of maternal socialization and toddlers' effortful control to children's adjustment and social competence. Developmental Psychology. 2007 Sep;43(5):1170–1186. doi: 10.1037/0012-1649.43.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alink LR, van IJzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: Mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Developmental psychobiology. 2008 Jul 1;50(5):427–50. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- 79.Belli RF. The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory. 1998;6:383–406. doi: 10.1080/741942610. [DOI] [PubMed] [Google Scholar]