Abstract
Background
Post-extraction alveolar bone loss, mostly affecting the buccal plate, occurs despite regenerative procedures. To better understand possible determinants, this prospective case series assessed gingival blood perfusion and tissue molecular responses in relation to post-extraction regenerative outcomes.
Methods
Adults scheduled to receive bone grafting in maxillary, non-molar, single tooth extraction site were recruited. Clinical documentation included probing pocket depth (PD), keratinized tissue width (KT), tissue biotype (TB), plaque (P) and bleeding. Wound closure was clinically evaluated. Gingival blood perfusion was measured by Laser Doppler Flowmetry (LDF). Wound fluid (WF) and gingival biopsies were analyzed for protein levels and gene expression, respectively, of relevant molecular markers. Bone healing outcomes were determined radiographically (Cone Beam Computerized Tomography; CBCT). Healing was followed for 4 months.
Results
Data from 15 patients (50 ± 5 years, 8 males) are reported. Postoperatively, neither complications nor changes in PD, KT or TB were observed. Postoperatively, LDF revealed decreased perfusion followed by hyperemia that persisted 1 month (p≤0.05). WF levels of angiopoietin-2, interleukin-8, tumor necrosis factor-α, and vascular endothelial growth factor peaked on day 6 (p≤0.05) and decreased thereafter. Only interleukin-8 and tumor necrosis factor-α exhibited increased gene expression. Linear bone changes were negligible. Volumetric bone changes were minimal but statistically significant, with more bone loss when membrane was used (p=0.05).
Conclusion
Gingival blood perfusion following post-extraction bone regenerative procedures follows an ischemia-reperfusion model. Transient increases in angiogenic factor levels and prolonged hyperemia characterize the soft tissue response. These soft tissue responses do not determine radiographic bone changes.
KEY WORDS (MESH TERMS): Alveolar bone loss, Gingiva, Guided tissue regeneration, Tooth extraction, Wound healing
Post-extraction alveolar ridge dimensional changes are well documented, 1–4 with greater width than height loss, more pronounced on the vestibular aspect.1–4 Consequently, bone preservation and/or augmentation techniques have been used to prevent/regenerate bone loss.5–10 However, alveolar width loss occurs despite these procedures,11–13 a fact that calls for better understanding of the factors influencing early wound healing following such procedures.
Soft tissue biotype has been linked to regenerative surgery outcomes,14–15 partly attributed to differences in vascular supply, inflammatory response and ability to overcome surgery-related transient ischemia, properties critical for optimal wound healing. Surgical trauma, including tooth extraction, flap elevation, vertical incisions and suturing, may impede blood circulation to the surgical site.16 In addition, biomaterial properties (chemical and structural composition, morphology, absorption process and timing) affect healing outcomes.13 Bone and membrane placement underneath the flap and physiological post-osseous tissue exposure changes can affect early postoperative soft tissue blood flow recovery and bone regeneration. 17–19
Flap blood perfusion can be monitored non-invasively by using techniques such as Orthogonal Polarization Spectral (OPS) Imaging20–22 and Laser Doppler Flowmetry (LDF).23–28 While OPS imaging provides direct monitoring of microcirculatory changes20–22, LDF allows evaluation of microcirculatory blood flow and monitoring of circulatory recovery after various interventions.23–28
Sample size limitations generally hamper analyses of wound-associated tissues. Laser Capture Microdissection (LCM) is a method allowing specific area/interface isolation and molecular (DNA and RNA) analysis of limited tissue samples (e.g., punch biopsy), in addition to regular histology. Thus LCM provides specific localized gene expression information for a particular cell or tissue. Despite the advantages of LCM in studying tissue/cell-specific biology, the use of this technique has been limited in clinical periodontal studies.29, 30
The purpose of the present prospective case series was to evaluate changes in gingival blood perfusion and tissue biomarker response following post-extraction bone regeneration procedures, in relation to bone fill as clinical outcome. Wounds of similar size at similaranatomical locations were chosen and tooth extraction and flap elevation was performed, followed by either socket preservation (SP; bone graft and wound dressing) or guided bone regeneration (GBR; bone graft and absorbable barrier membrane), depending on buccal bone integrity.
MATERIALS AND METHODS
Study Design
The study was a prospective case series (observational trial). Clinical examination and sampling were conducted prior to surgery and at 3, 6, 9 days, 1 month and 4 months post-surgery on patients receiving extraction prior to implant placement at single non-molar maxillary sites. Clinical parameters, wound healing measures, gingival crevicular fluid (GCF) and wound fluid (WF) samples, LDF readings and gingival biopsies were obtained. Clinical and radiographic measurements and fluid/tissue sampling were performed by a single trained examiner. The study protocol (#2014H0150) was approved by The Ohio State University (OSU) Institutional Review Board and all patients provided written informed consent prior to treatment.
Subject Population
Patients referred to the OSU Advanced Periodontics Clinics for pre-implant tooth extraction in a single, tooth-bound non-molar maxillary site were recruited between August 2014 and December 2015. Based on standard of care, infection - including periodontitis - was treated prior to regenerative procedures. Active infection at extraction site was a contraindication for immediate bone regeneration. Thus, inclusion criteria were: adults (18–65 years old) with stable periodontal and systemic health (ASA I or II). Exclusion criteria were: smoking, pregnancy, uncontrolled periodontal or systemic disease.
Surgical Procedures
All surgeries were performed by OSU periodontal residents under direct faculty (BL and DNT) supervision. All surgeons were trained for surgical protocol. Clinical and radiographic measurements and sampling during surgical and follow-up appointments were conducted by single trained clinician (LA). Routine surgical protocols including local anesthesia (Lidocaine 2% with 1:100,000 epinephrine), atraumatic extraction, socket debridement and saline§§ irrigation were applied. Following tooth extraction, buccal flap was elevated to assess buccal plate integrity. When socket walls were intact (four-wall residualdefect), socket preservation (SP) was performed using allograft bone material*** and absorbable collagen wound dressing††† to seal the socket entrance. When buccal ridge deficiency was noted, guided bone regeneration (GBR) was performed using the same allograft material and resorbable collagen membrane‡‡‡ placed under the flap, covering the buccal bony defect and sealing the socket (see supplementary figures 1 and 2 in online Journal of Periodontology). Flaps were approximated with absorbable sutures§§§ without effort to obtain primary closure. Patients received antibiotic (5–7 days; Amoxicillin 500mg or Clindamycin 300mg, tid) and analgesic (3–10 days; Ibuprofen 600mg or Acetaminophen 325mg every 4–6 hrs, as needed) medication and antimicrobial rinse (0.12% chlorhexidine gluconate; 3 times daily for 2 weeks) prescriptions per standard clinical protocol.
Clinical Measures
The following clinical parameters were recorded: pocket depth (PD), measured on the two adjacent teeth (6 surfaces/tooth, using UNC-15 periodontal probe); keratinized tissue width (KT) on mid-buccal of treatment site (using periodontal probe); PD and KT were recorded prior to surgery and at 4 months (immediately prior to reentry); tissue biotype (TB), assessed by measuring tissue thickness on mid-buccal of flap after elevation (at 3 mm apical to flap margin, using a non-tension wax caliper), and classified as thick if thickness > 1 mm, and thin if ≤ 1 mm (modified from Muller et al.31); TB was recorded during initial surgery and at 4 months (during re-entry); and plaque level (P), quantified as percentage of plaque-positive among tooth surfaces on the surgical sextant, recorded at baseline (prior to surgery) and all five postoperative visits.
Clinical wound closure was documented immediately after surgery and during all postoperative visits. In addition, hydrogen peroxide (HP) test was used to determine complete wound closure.32, 33
Clinical wound healing categories (Mature wound healing, Erythema, Bleeding, Graft Mobility, Suppuration, Necrosis) were used to evaluate early clinical outcomes (modified from Kloostra et al.32). Each category was scored as absent/present (0/1). Mature wound healing was defined as complete wound closure with no other significant findings/complications. Erythema was defined as increase in redness compared to adjacent non-operated sites. Bleeding was considered present when spontaneous bleeding was detected at the wound site. Graft mobility was evaluated by gentle palpation of the site to detect any loose sub-gingival material. Suppuration was evaluated by detection of discharge. Any visually determined soft and/or hard tissue necrosis was considered present.
LDF Measurements
A custom surgical stent was fabricated for each patient, using 0.06″ thermo-formed material. A 2.5 mm diameter hole was created on the mid-buccal surface to stabilize LDF sleeve and the probe**** at a standardized position perpendicular to the tissue surface and at a distance of 0.5–1 mm from the gingiva (distance from gingival surface to probe). LDF signals were recorded for 120 seconds in arbitrary Perfusion Units (PU)††††. Measurements were obtained before surgery, immediately postoperatively, and at all five postoperative visits. Changes in blood flow were calculated as percent PU difference (ΔPU%) between perfusion at specific site at a specific time point (PUt) and the corresponding baseline value (PU0), according to the formula:ΔPU%= (PUt − PU0/PU0) × 100 26,34 LDF instrument was calibrated following manufacturer’s protocol. Prior to study initiation, pilot LDF assessments were conducted on pristine tooth sites, sites receiving non-surgical periodontal treatment, and sites receiving simple flap elevation, to optimize stent fabrication and LDF probe positioning (data not shown).
Crevicular/Wound Fluid Sampling and Multiplex Assays
GCF and WF samples were obtained from adjacent teeth at baseline and from wound area edges at each postoperative visit until clinical wound closure, using a sterile paper strip‡‡‡‡. A total of 6 samples (30 seconds sampling time/strip) were collected at each time point. GCF/WF volume was immediately determined using a calibrated electronic volume quantification unit§§§§. Samples were then stored (−20°C, sterile vials) until further processing. Fluid elution was performed as previously detailed.35 A commercially available panel ***** for multiplex assays was used to determine molecular markers, including Angiopoietin-2 (Ang-2), interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and vascular endothelial growth factor (VEGF).
Soft Tissue Biopsies, Laser Capture Microdissection (lcm) and Quantitative Polymerase Chain Reaction (q-pcr) Assays
Soft tissue punch biopsies (3 mm diameter, 2 mm thickness, obtained from disto-palatal corner of wound bed) were taken from treatment site during surgery, at 9 days and at 4 months (immediately prior to reentry). Because one case was missing follow-up samples, due to clinical sampling difficulties, the sample size for LCM and Q-PCR analyses was 14. Biopsies were immediately frozen in liquid nitrogen and then stored in −20°C. LCM36 was used to isolate tissue facing bone (see supplementary Figure 3 in online Journal of Periodontology). Briefly, serial sections (10 μm thickness) were cut from OCT embedded frozen specimens. The sections were stained using a LCM compatible modified hematoxylin quick staining procedure37. The sections were mounted on a PEN (polyethylene napthalate) membrane glass slides†††††treated with RNaseOUT™‡‡‡‡‡ and UV, followed by cutting and catapulting as described earlier 36–38. Tissue facing bone (see supplementary Figure 3 in online Journal of Periodontology) was collected in RNA lysis solution38 and RNA was extracted using Picopure RNA Isolation Kit,§§§§§ following manufacturer’s instructions.37,38 mRNA extraction and quantitative polymerase chain reaction (Q-PCR) were performed as described36–38 to determine the expression levels of Angiopoietin-2, IL-8, TNF- α and VEGF mRNA.
Cone-beam computed tomography (CBCT)
CBCT******images were obtained at 1–3 days postoperatively and at 4 months, using the aforementioned LDF stent as guide. Because one case was missing follow-up samples, due to clinical sampling difficulties, the sample size for CBCT analyses was 14. The stent was used with radiopaque marker placed on the buccal aspect as reference point for linear measurements. The standard CBCT protocol was modified to reduce radiation while obtaining a detailed image of the limited surgical area [8X8 cm FOV with 14.7 seconds exposure time allowing 200 μm voxel size]. Linear and 3-dimensional (3D) measurements were conducted on the DICOM data set and reconstructed using specific software††††††. To calculate possible CBCT image distortion, a stable structure (gutta-percha incorporated into stent) was used and measured in each CBCT. The mean difference between two time points for this specific measurement was 0.02±0.17 mm (p=0.279). Calibration for CBCT parameters was performed by repeating linear measurements on three de-identified CBCT scans showing intra-examiner reliability between readings (p=0.144).
Data Management and Statistical Analysis
Sample size was determined by using a prioritest, to detect a 50 PU difference between two time points for LDF readings at p=0.05 level.26,27
Data were analyzed using statistical software‡‡‡‡‡‡. Repeated measures mixed model with Bonferroni adjustments was used to compare time dependent differences for clinical, radiographic findings and changes in wound fluid amount, content as well as soft tissue gene expression. Sandwich estimator was used to control the correlation due to dependence of the observations among repeated measurements. Generalized linear mixed model was used for the analysis of repeated measured categorical wound healing parameters. Q-PCR data (fold difference from baseline) between groups (membrane vs non membrane) was analyzed by using unpaired t-test. Differences between CBCT readings were analyzed by paired t-test. Pearson correlation coefficients were calculated to reveal the association between various parameters for the data obtained from days 3, 6 and 9 (specifically focusing on wound fluid content). Differences were accepted as statistically significant at p≤0.05 level.
RESULTS
Study Population
Table 1 presents study population demographics. 20 subjects were recruited and 15 (8 males) completed the study. Two subjects were excluded because of changes in surgical needs and 3 due to failure to comply with protocol visits. Each subject contributed a single site; all sites were maxillary and 80% were anterior (Table 1). 53% (n=8) of sites were classified as having thick TB (Table 1, p>0.05). Following uneventful tooth extraction, 9 patients received SP while 6 received GBR. In 3 GBR cases, a delayed augmentation procedure was performed due to soft tissue deficiency, by allowing post-extraction soft tissue healing (6 weeks); for these cases, as for all other cases, sample timing started on the day of GBR procedure. There was no statistically significant difference between SP and GBR subgroups with respect to age, gender, or anatomic location (p>0.05; data not shown).
Table I.
Study Population Demographics
| Age (years) | 50±5* | |
| Healing time (days) | 135±4* | |
| Gender | Female | 7 |
| Male | 8 | |
| Anatomical location | Anterior | 10 |
| Premolar | 5 | |
| Surgical protocols | SP | 9 |
| GBR | 6 | |
| PD (mm) | Pre | 2.3±0.1* |
| Post | 2.0±0.1* | |
| KT (mm) | Pre | 5.6±0.3* |
| Post | 5.4±0.3* | |
| Tissue biotype | Thin | 7 |
| Thick | 8 | |
Mean±SE
Wound Healing Clinical Outcomes
There was no statistically significant difference in PD (adjacent teeth) or KT (surgical site) between baseline and follow-up measurements (Table 1, p>0.05). Plaque decreased from pre-surgical levels up to 9 days, with negligible time and site related differences (data not shown; p>0.05). The initial TB classification changed at re-entry surgery in 8 cases; six changed from thin to thick TB and two from thick to thin TB (p>0.05).
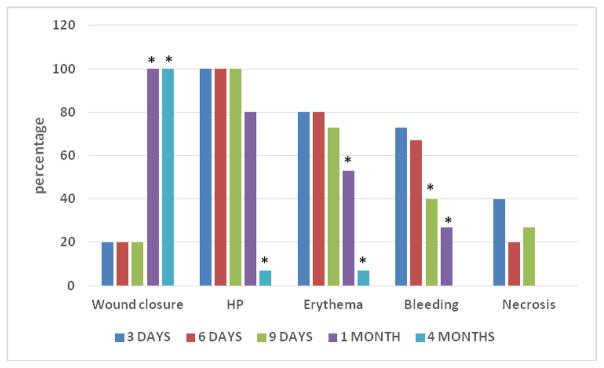
Given the chosen surgical approach (no effort towards primary flap closure), most wounds (80%) were exposed at surgery completion and during early healing times. Although all wounds were clinically closed at 1 month (Fig. 1), 80% of wounds were still positive with the HP-test; at 4 months all but one tested HP-negative (Fig. 1). Erythema was prevalent during early healing, with 53% of sites still positive at 1 month (Fig. 1). The majority of sites exhibited bleeding during early healing, decreasing over time (Fig. 1). Wound margin necrosis was noticeable in <50% of sites during early healing and only up to day 9 (Fig. 1). Graft mobility was noted only in one case, at day 9.
Figure 1.
Wound Healing Clinical Outcomes
For all parameters and all time points, percentages are calculated based on n=15.
HP: sites testing positive with H2O2 test (see text for details).
*p≤0.05 (compared to 3 days post-operative period)
Blood Perfusion and GCF/WF Volume
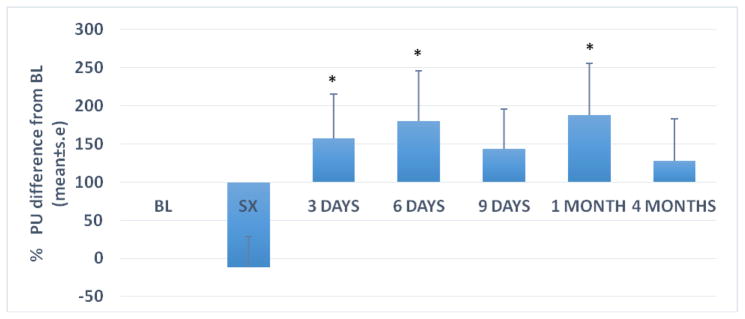
Figure 2 shows LDF results over time. At baseline, before local anesthesia, LDF readings were 126 ± 20 PU (mean±se). Overall, blood flow decreased non-significantly at the end of surgery (−12±41ΔPU%; P>0.05). Blood flow increased by 150% on average during early healing and persisted throughout the observation period (188±68ΔPU% at 1 month; p=0.006; Fig. 2).
Figure 2.
Gingival Blood Perfusion (Laser Doppler Flowmetry)
Bars represent mean+se. For all time points n=15.
BL: Immediately prior to surgery, prior to local anesthesia
SX: Immediately following surgery
*p<0.05; percent change from baseline
Analysis of ΔPU% by TB revealed no statistically significant differences between biotypes (Table 2; p>0.05). Despite GBR sites having more than 2-fold higher ΔPU% values, ΔPU% was not statistically significantly different between sites where membrane was present (GBR) or absent (SP) under the flap (Table 2; p>0.05). In contrast, ΔPU% showed statistically significant variation based on wound closure, whether clinically determined or through HP test (p<0.01; Table 2).
Table II.
Gingival Blood Perfusion by Surgical conditions
| meanΔ PU%±s.e | p-value | ||
|---|---|---|---|
| Primary wound closure (at surgery) | Yes (n=3) | 31±24 | <0.01 |
| No (n=12) | 154±34 | ||
| Wound Exposure (all time points) | Yes | 175±28 | <0.01 |
| No | 50±28 | ||
| HP test (all time points) | Positive | 162±31 | 0.01 |
| Negative | 73±27 | ||
| Thin biotype (at surgery) | Yes (n=7) | 42 ± 55 | 0.666 |
| No (n=8) | 77 ± 37 | ||
| Surgical protocol (all time points) | SP (n=9) | 89±18 | 0.057 |
| GBR (n=6) | 193±32 |
GBR: guided bone regeneration; HP: H2O2; SP: socket preservation;
difference from perfusion immediately prior to surgery (prior to local anesthesia)
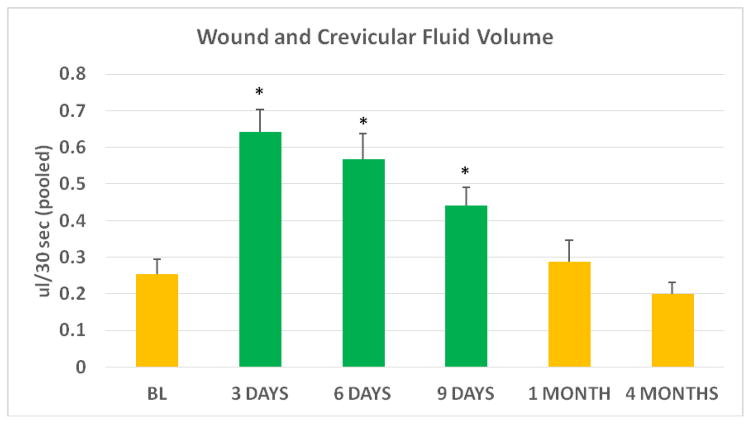
WF volume increased more than 3-fold during the first 3 days of healing, relative to adjacent teeth GCF volume at baseline (p<0.01; Figure 3), followed by a consistent decrease during early wound healing, until wound closure (Figure 3). Relative to baseline, GCF volumes were unchanged at 1 and 4 months postoperatively. (p>0.05).
Figure 3.
Wound and Crevicular fluid volume
Wound fluid (green bars) collected from wound edge is reported for postoperative days 3, 6, and 9. Reported fluid volumes at BL, 1 month and 4 months represent crevicular fluid (yellow bars) obtained from adjacent teeth (at BL there was no wound, and at 1 and 4 months all wounds were clinically closed).
Bar represents mean±s.e. For all data points n=15
BL: Immediately prior to surgery, prior to local anesthesia
*p≤0.01 (difference from BL)
Molecular Markers: WF Protein Content and Tissue Biopsy Gene Expression
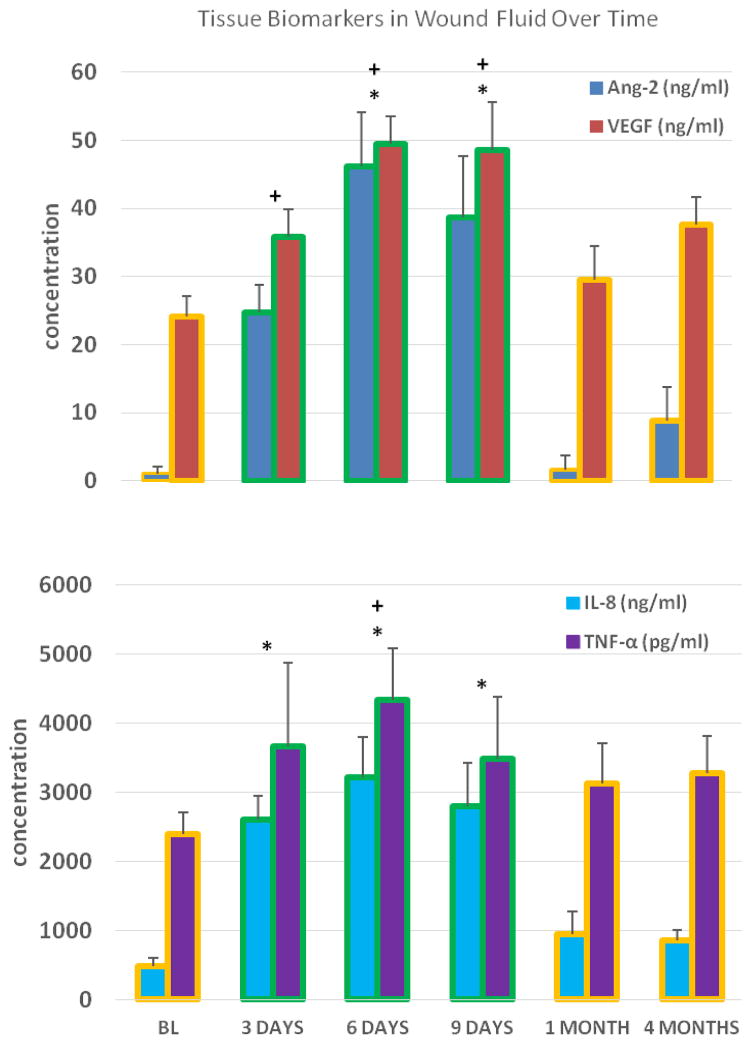
Both Ang-2 and VEGF levels in WF increased significantly from baseline GCF levels during early healing (p<0.05; Fig 4A). Ang-2 and VEGF levels in WF peaked on day 6 (46±8 ng/ml and 49±4 ng/ml, respectively; p<0.0001) and decreased thereafter, returning to baseline (GCF) levels at 1 month (Fig 4A).
Figure 4.
Figures 4A and 4B. Tissue Biomarkers in Wound Fluid Over Time
Crevicular fluid collected from adjacent teeth (BL; baseline, 1 and 4 months) and wound fluid from wound edge (days 3, 6 and 9); n= 15 for all data points. Bars with yellow outline represent crevicular fluid data and bars with green outline represent wound fluid data
* p≤0.01 (Ang-2 and IL-8 gene expression; difference from baseline)
+ p≤0.05 (VEGF and TNF-α gene expression; difference from baseline)
IL-8 and TNF-α levels also increased significantly during early healing (p<0.05; Fig 4B). Similar to Ang-2 and VEGF, IL-8 and TNFα levels peaked on day 6 (3214±581 ng/ml and 4338±749 pg/ml, respectively; p≤0.01) and decreased thereafter, returning to baseline levels at day 9 (TNFα) and at 1 month (IL-8) (Fig 4B).
Changes in ΔPU% were not directly associated with specific WF protein content. However, among the four analyzed molecules, IL-8 and VEGF concentrations were negatively correlated with collected WF amount (p=0.002; r=−0443 and p=0.01; r=−0.366, respectively; samples from days 3, 6 and 9). In addition, WF IL-8 concentration was negatively correlated with membrane presence (p=0.02; r=−0.334).
Gene expression of the same biomarkers was examined in tissue biopsies using LCM, focusing on the aspect of the tissue facing bone. The data revealed that while Ang-2 and VEGF gene expression did not change from baseline, IL-8 and TNF-α gene expression increased >3-fold at 9 days (see supplementary Fig. 4 in online Journal of Periodontology; p>0.05). At 4 months, IL-8 gene expression was as high as on day 9, while TNF-α expression was further increased (see supplementary Fig. 4 in online Journal of Periodontology). These differences became statistically significant both for IL-8 (p=0.04) and TNFα(p=0.02) only when membrane was present. Increases in gene expression for any of the four molecules was not associated with ΔPU% changes within healing gingiva (p>0.05). Similarly, there was no direct association between WF protein content and corresponding soft tissue gene expression for any of the analyzed biomarkers.
CBCT Outcomes
CBCT scans obtained after surgery and at 4 months post-operative period were compared to determine hard tissue dimensional changes. For linear measurements (from standard mark on the guide to buccal bone), the mean change was −0.84±0.15 mm (median=−0.77 mm; range: −1.83–+0.02 mm), without differences between SP and GBR sites (see supplementary Table 1 in online Journal of Periodontology; p>0.05). For volumetric measurements, the mean change was −0.16±0.04 cm3 (median= −0.09 cm3; range: −0.42–+0.01 cm3), with SP sites exhibiting significantly less volumetric changes than GBR sites (see supplementary Table 1 in online Journal of Periodontology; p=0.05).
There was no correlation between soft tissue perfusion changes (ΔPU%) and bone linear or volumetric changes (r≤0.16; p≥0.11). Similarly, changes in bone linear or volumetric changes did not correlate with wound exposure in bucco-lingual dimension (p≥0.074; data not shown). However, there was weak but statistically significant negative correlation (r=−0.064; p=0.046) between wound exposure and volumetric bone changes, with larger exposure associated with less gain in volumetric hard tissue dimensions (less bone fill).
DISCUSSION
Based on current evidence, buccal bone remodeling and loss following extraction occurs despite application of bone grafting procedures.1–4,8–12 This prospective case series was designed to determine gingival blood perfusion, specific protein and gene expression levels, and early clinical soft tissue healing outcomes, as well as radiographic outcomes of alveolar bone regeneration, in a continuing pursuit to better understand the factors that possibly influence early wound healing following such procedures. To the best of our knowledge this is the first clinical study to assess gingival blood perfusion or to use laser capture microdissection in relation to alveolar bone grafting procedures. The results indicate that hyperemic soft tissue conditions remain up to 4 months postoperatively, despite clinically complete soft tissue wound healing and regardless of absorbable membrane use; however, the level of the early postoperative hyperemic response was increased in the presence of wound exposure/incomplete healing, a finding consistent with routine clinical observations. Ang-2, VEGF, IL-8 and TNF-α protein levels within wound fluid were transiently elevated postoperatively, while only IL-8 and TNF-α gene expression was elevated, persistently, especially when absorbable membrane was present. The post-extraction soft tissue perfusion and molecular marker changes did not correlate with radiographic bone regenerative outcomes. These findings suggest that soft tissue response to acute surgical trauma involves a chronic recovery phase, especially in the presence of routine biomaterials such as absorbable membrane. The extent to which this protracted recovery period may affect the long-term quality and/or quantity of the regenerated bone remains to be determined.
LDF, a non-invasive method to follow changes in gingival blood flow, has been used in periodontal research to investigate gingival and gingival flap perfusion in response to various conditions and interventions.25–28 Decreased perfusion immediately following surgery has been consistently reported, attributed to the vasoconstrictive effect of local anesthetics and the traumatic/disruptive effect of incisions on vascularization.39 Previously, only one pilot dog study reported LDF-based assessment of gingival flap perfusion for the first 3 days following GBR, using a polylactic-acid membrane without bone graft;19 the authors reported lower LDF values on the membrane side and when membranes were exposed. The present results, i.e., delayed return of gingival blood flow to baseline levels following bone grafting - independent of membrane use - and greater increase of gingival blood flow in cases of wound/membrane exposure, are contradicting the aforementioned animal study results. These discrepancies can be attributed to the significant methodological differences(host species, materials, flap design) between the two studies. The persistent hyperemic postoperative response observed in the present study, especially with incomplete wound closure (Table 2), may or may not have a direct effect on bone fill but is likely impacting soft tissue quality, especially during the early healing period. The present study findings also suggest that gingival flap healing, as assessed by blood perfusion, differs between routine periodontal surgery for periodontitis treatment,27,28 where perfusion returned to baseline levels within 2 weeks, and the post-extraction grafting procedures studied here. Whether such differences should be attributed to the presence of graft material or the anticipated tissue remodeling following tooth loss remains to be determined.
Among the other parameters investigated in relation to gingival blood flow, neither tissue biotype nor procedure (SP vs GBR) had a significant effect (Table 2), although membrane presence (GBR) showed a trend for higher postoperative blood flow.
In addition to clinical parameters and LDF measurements, wound fluid parameters (volume, protein concentration) and gingival tissue gene expression were studied to detect molecular changes relative to perfusion changes. Wound fluid volume and specific protein content increased during early healing time, as expected, and returned to baseline levels mostly by 1 month. In terms of gene expression within the tissue interface facing the bone, statistically non-significant increases in Ang-2, VEGF, IL-8 and TNF-α were found. Ang-2, which is highly induced at sites of vascular remodeling,40 was increased in wound fluid during early wound healing with minimal gene expression increases. VEGF, an endothelial cell mitogen, chemotactic agent and inducer of vascular permeability,41 reached peak wound fluid concentration at 6 and 9 days, also with minimal gene expression changes. IL-8, a pro-inflammatory leukocyte chemo-attractant and important regulator in wound healing inflammation phase,42 was dete cted at high concentrations within the wound fluid during early days of wound healing with approximately 4-fold increase (statistically non-significant) in soft tissue gene expression which persisted up to 4 months. TNF-α, a potent pro-inflammatory cytokine that stimulates bone resorption and protease production by fibroblasts and osteoblasts,43 exhibited protein and gene expression changes similar to IL-8, with elevated gene expression up to 4 months. Collectively, a primed and longer-lasting inflammatory response was observed at both wound fluid protein and soft tissue gene expression level for specific physiologically relevant proteins; this molecular response is consistent with the longer hyperemic response observed through LDF.
As mentioned above, open wound was associated with higher and longer-lasting hyperemic gingival response in the present study. However, wound exposure seems to have a marginal effect on bone fill as CBCT-determined volume changes are minimally negatively affected while linear changes at buccal bone location are not affected at all. A 3-dimensional volume measurement provides a better understanding of post-treatment changes than linear measurements, because it reflects changes in the area as a whole rather than at specific point or surface. In the present study, the volumetric area of interest included the surrounding bone and teeth due to difficulty in discriminating between graft material and native bone. However, any possible underestimation of changes - because of increases in total volume area - was mitigated by performing subject-based analysis. A linear measurement, using a guide-embedded marker, was also made, to identify bone remodeling specifically affecting buccal bone surface. The present study results are in agreement with previous studies where SP/GBR could not totally prevent post-extraction bone resorption,2, 6–9,12 although bone loss was minimal in the current study.
The present study is not without limitations. The sample size, although calculated based on published data on LDF findings following gingival flap surgery,27,28 is small. The current study, based on 4.7 ± 0.9 months of healing, has shorter follow-up than other SP/GBR studies;13 this time point was chosen to match routine implant placement time following bone regeneration with specific allograft. In the present study only single tooth sites were included, a fact that may explain the minimal buccal plate loss observed compared to similar studies that report significant post-extraction ridge dimensional changes occurring at the expense of the buccal plate, when treating extensively resorbed alveolar ridges.2, 13 Therefore, it appears that by limiting wound size and healing time, the pre-extraction buccal bone wall can be preserved, at least until implant placement surgery. Because sites other than maxillary anterior ones were excluded, due to lip/cheek movement interfering with LDF probe stabilization, and because of the predictable ridge deficiency without post-extraction grafting in maxillary anterior sites, the inclusion of control sites, i.e., without SP or GBR, was not possible.
In conclusion, and within the limits of this prospective case series, following post-extraction bone regeneration procedures gingival blood perfusion levels are protractedly elevated and are accompanied by transient specific angiogenic/pro-inflammatory protein concentration increases in wound fluid and by moderate but sustained IL-8 and TNF-α gene expression increases at the soft tissue-bone interface. In addition, gingival blood perfusion is significantly affected by lack of wound closure, which may represent a longer/chronic inflammatory response. Future studies of longer follow-up duration and with larger sample size should help determine the possible impact of physiological/molecular gingival changes on bone regenerative procedure outcomes.
Acknowledgments
The authors declare no conflicts of interest with this study. The study was supported by a seed grant from OSU College of Dentistry (2014) and by a grant from American Academy of Implant Dentistry Foundation (2015), both to the senior author (BL).
Footnotes
0.9% Sodium Chloride- Salvin Dental Specialties, Inc., Charlotte, NC, USA
FDBA, Straumann, Andover, MA, USA
Collagen Plug, Zimmer Biomet, Carlsbad, CA, USA
Biomend Extend, Zimmer Biomet, Carlsbad, CA, USA
coated VICRYL® (poly(lactic-co-glycolic acid), Ethicon LLC, Cincinnati, OH, USA
Periflux System 5000 PF 5010 LDPM, Perimed AB, Sweden
Perisoft software, PSW 2, version 2.5.5, Perimed AB, Ardmore PA, USA
Periopaper®, Oraflow Inc., Hewlett, NY, USA
Periotron 8000®, Oraflow Inc., Hewlett, NY, USA
Bio-Plex ProHuman Cancer Biomarker panel 2, Bio-Rad Life Sciences, Hercules, CA, USA
Carl Zeiss Microscopy, Kanigsallee 9–21, 37081 Gőttingen, Germany
Ambion, ThermoFisher Scientific, Waltham, MA, 02451 USA
Life Technologies, ThermoFisher Scientific, Waltham, MA, 02451 USA
i-CAT system, Imaging Services International, Hatfield, PA, USA
OsiriX Lite v.7.0.2, Pixmeo, Geneva, Switzerland
GraphPad Prism 5, GraphPad Software, Inc., La Jolla, CA, 92037 USA, and Statistical Analysis Software (SAS PROC GENMOD), version 9.3, SAS Institute Inc., Cary, NC, USA
References
- 1.Araujo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. Journal of clinical periodontology. 2005;32:212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 2.Johnson K. A study of the dimensional changes occurring in the maxilla following tooth extraction. Aust Dent J. 1969;14:241–244. doi: 10.1111/j.1834-7819.1969.tb06001.x. [DOI] [PubMed] [Google Scholar]
- 3.Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: a clinical and radiographic 12-month prospective study. Int J Periodontics Restorative Dent. 2003;23:313–323. [PubMed] [Google Scholar]
- 4.Leblebicioglu B, Hegde R, Yildiz VO, Tatakis DN. Immediate effects of tooth extraction on ridge integrity and dimensions. Clin Oral Invest. 2015;19:1777–1784. doi: 10.1007/s00784-014-1392-1. [DOI] [PubMed] [Google Scholar]
- 5.Aimetti M, Romano F, Griga FB, Godio L. Clinical and histologic healing of human extraction sockets filled with calcium sulfate. Int J Oral Maxillofac Implants. 2009;24:902–909. [PubMed] [Google Scholar]
- 6.Barone A, Aldini NN, Fini M, Giardino R, Calvo Guirado JL, Covani U. Xenograft versus extraction alone for ridge preservation after tooth removal: a clinical and histomorphometric study. Journal of periodontology. 2008;79:1370–1377. doi: 10.1902/jop.2008.070628. [DOI] [PubMed] [Google Scholar]
- 7.Hoad-Reddick G, Grant AA, McCord JF. Osseoretention? Comparative assessment of particulate hydroxyapatite inserted beneath immediate dentures. Eur J Prosthodont Restor Dent. 1994;3:61–65. [PubMed] [Google Scholar]
- 8.Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. Journal of periodontology. 2003;74:990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 9.Lekovic V, Camargo PM, Klokkevold PR, et al. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. Journal of periodontology. 1998;69:1044–1049. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- 10.Lekovic V, Kenney EB, Weinlaender M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. Journal of periodontology. 1997;68:563–570. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 11.Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012;23(Suppl 5):22–38. doi: 10.1111/j.1600-0501.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 12.Leblebicioglu B, Salas M, Ort Y, Johnson A, Yildiz VO, Kim DG, Agarwal S, Tatakis DN. Determinants of alveolar ridge preservation differ by anatomic location. Journal of clinical periodontology. 2013;40:387–395. doi: 10.1111/jcpe.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morjaria KR, Wilson R, Palmer RM. Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res. 2014;16:1–20. doi: 10.1111/j.1708-8208.2012.00450.x. [DOI] [PubMed] [Google Scholar]
- 14.Baldi C, Pini-Prato G, Pagliaro U, et al. Coronally advanced flap procedure for root coverage. Is flap thickness a relevant predictor to achieve root coverage? A 19-case series. J Periodontol. 1999;70:1077–1084. doi: 10.1902/jop.1999.70.9.1077. [DOI] [PubMed] [Google Scholar]
- 15.Evans CD, Chen ST. Esthetic outcomes of immediate implant placements. Clinical oral implants research. 2008;19:73–80. doi: 10.1111/j.1600-0501.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 16.Mormann W, Ciancio SG. Blood supply of human gingiva following periodontal surgery. A fluorescein angiographic study. J Periodontol. 1977;48(11):681–692. doi: 10.1902/jop.1977.48.11.681. [DOI] [PubMed] [Google Scholar]
- 17.Azzi R, Etienne D, Takei H, Carranza F. Bone regeneration using the pouch-and-tunnel technique. Int J Periodontics Restorative Dent. 2009;29:515–21. [PubMed] [Google Scholar]
- 18.Schemitsch EH, Weinberg JA, McKee MD, Richards RR. The relative importance of intramedullary, intracortical and extraosseous soft-tissue blood flow to the repair of devascularized canine tibia cortex. Ann Plast Surg. 1997;38:623–631. doi: 10.1097/00000637-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Vergara JA, Quinones CR, Nasjleti CE, Caffessee RG. Vascular response to guided tissue regeneration procedures using nonresorbable and bioabsorbable membranes in dogs. J Periodontol. 1997;68:217–224. doi: 10.1902/jop.1997.68.3.217. [DOI] [PubMed] [Google Scholar]
- 20.Lindeboom JA, Mathura KR, Harkisoen S, Van den Akker HP, Ince C. Effect of smoking on the gingival capillary density: assessment of gingival capillary density with orthogonal polarization spectral imaging. J Clin Periodontol. 2005;32:1208–1212. doi: 10.1111/j.1600-051X.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindeboom JA, Mathura KR, Ramsoekh D, Harkisoen S, Aartman IH, Van den Akker HP, Ince C. The assessment of the gingival capillary density with orthogonal spectral polarization (OPS) imaging. Archives of Oral Biology. 2006;51:697–702. doi: 10.1016/j.archoralbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Milstein DMJ, Mathura KR, Lindeboom JAH, Ramsoekh D, Lindeboom R, Ince C. The temporal course of mucoperiosteal flap revascularization at guided bone regeneration-treated implant sites: a pilot study. J Clin Periodontol. 2009;36:892–897. doi: 10.1111/j.1600-051X.2009.01470.x. [DOI] [PubMed] [Google Scholar]
- 23.Svensson H, Pettersson H, Svedman P. Laser Doppler flowmetry and laser photometry for monitoring free flaps. Scand J Plast Reconstr Surg. 1985;19:245–249. doi: 10.3109/02844318509074510. [DOI] [PubMed] [Google Scholar]
- 24.Yuen JC, Feng Z. Monitoring free flaps using the laser Doppler flowmeter: five-year experience. Plast Reconstr Surg. 2000;105:55–61. doi: 10.1097/00006534-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Baab DA, Oberg PA, Holloway GA. Gingival blood flow measured with a laser Doppler flowmeter. Journal of periodontal research. 1986;21:73–85. doi: 10.1111/j.1600-0765.1986.tb01440.x. [DOI] [PubMed] [Google Scholar]
- 26.Donos N, D’Aiuto F, Retzepi M, Tonetti M. Evaluation of gingival blood flow by the use of laser Doppler flowmetry following periodontal surgery. A pilot study. Journal of periodontal research. 2005;40:129–137. doi: 10.1111/j.1600-0765.2005.00777.x. [DOI] [PubMed] [Google Scholar]
- 27.Retzepi M, Tonetti M, Donos N. Comparison of gingival blood flow during healing of simplified papilla preservation and modified Widman flap surgery: a clinical trial using laser Doppler flowmetry. Journal of clinical periodontology. 2007;34:903–911. doi: 10.1111/j.1600-051X.2007.01119.x. [DOI] [PubMed] [Google Scholar]
- 28.Retzepi M, Tonetti M, Donos N. Gingival blood flow changes following periodontal access flap surgery using Laser Doppler flowmetry. Journal of Clinical Periodontology. 2007;34:437–443. doi: 10.1111/j.1600-051X.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 29.Guyodo H, Meuric V, Le Pottier L, Martin B, Faili A, Pers JO, Bonnaure-Mallet M. Colocalization of Porphyromonas gingivalis with CD4+ T cells in periodontal disease. FEMS Immunol Med Microbiol. 2012 Mar;64(2):175–83. doi: 10.1111/j.1574-695X.2011.00877.x. [DOI] [PubMed] [Google Scholar]
- 30.Li M, Firth JD, Putnins EE. Keratinocyte growth factor-1 expression in healthy and diseased human periodontal tissues. J Periodontal Res. 2005 Apr;40(2):118–28. doi: 10.1111/j.1600-0765.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 31.Muller HP, Heinecke A, Schaller N, Eger T. Masticatory mucosa in subjects with different periodontal phenotypes. Journal of clinical periodontology. 2000;27:621–626. doi: 10.1034/j.1600-051x.2000.027009621.x. [DOI] [PubMed] [Google Scholar]
- 32.Kloostra PW, Eber RM, Wang HL, Inglehart MR. Surgical versus non-surgical periodontal treatment: psychosocial factors and treatment outcomes. Journal of periodontology. 2006;77:1253–1260. doi: 10.1902/jop.2006.050302. [DOI] [PubMed] [Google Scholar]
- 33.Marucha PT, Kiecolt-Glaser JK, Favagehi M. Mucosal wound healing is impaired by examination stress. Psychosom Med. 1998;60:362–365. doi: 10.1097/00006842-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Zannetta-Barbosa D, Klinge B, Svensson H. Laser Doppler flowmetry of blood perfusion in mucoperiosteal flaps covering membranes in bone augmentation and implant procedures. A pilot study in dogs. Clin Oral Implants Res. 1993;4:35–38. doi: 10.1034/j.1600-0501.1993.040105.x. [DOI] [PubMed] [Google Scholar]
- 35.Emecen-Huja P, Eubank TD, Shapiro V, Yildiz V, Tatakis DN, Leblebicioglu B. Peri-implant versus periodontal wound healing. J Periodontol. 2013;40(8):816–824. doi: 10.1111/jcpe.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Datta S, Malhotra L, Dickerson R, Chaffee S, Sen CK, Roy S. Laser Capture Microdissection: Big data from small samples. Histol Histopathol. 2015;30(11):255–69. doi: 10.14670/HH-11-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuhn DE, Roy S, Raditke J, Gupta S, Sen CK. Laser microdissection and pressure-catapulting technique to study gene expression in the reoxygenated myocardium. Am J Physiol Heart Circ Physiol. 2006;290(6):H2625–32. doi: 10.1152/ajpheart.01346.2005. [DOI] [PubMed] [Google Scholar]
- 38.Roy S, Patel D, Khanna S, Gordillo GM, Biswas S, Friedman A, Sen CK. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc Natl Acad Sci U S A. 2007;104(36):14472–7. doi: 10.1073/pnas.0706793104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn J, Pogrel MA. The effects of 2% lidocaine with 1:100,000 epinephrine on pulpal and gingival blood flow. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:197–202. doi: 10.1016/s1079-2104(98)90426-7. [DOI] [PubMed] [Google Scholar]
- 40.Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Laboratory investigation; a journal of technical methods and pathology. 2001;81:361–373. doi: 10.1038/labinvest.3780244. [DOI] [PubMed] [Google Scholar]
- 41.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rennekampff HO, Hansbrough JF, Kiessig V, Dore C, Sticherling M, Schroder JM. Bioactive interleukin-8 is expressed in wounds and enhances wound healing. J Surg Res. 2000;93:41–54. doi: 10.1006/jsre.2000.5892. [DOI] [PubMed] [Google Scholar]
- 43.Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–117. doi: 10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]