Abstract
Purpose
It has been reported previously that the combination of bevacizumab with hypofractionated stereotactic re-irradiation (HFSR) with 30Gy (6GyX5 fractions) was safe and efficacious in recurrent glioblastomas and high-grade gliomas (HGG). Because most recurrences are local, developing intensified HFSR dosing schedules remains of interest. We hypothesized that in combination with bevacizumab, HFSR doses could be further escalated, and designed a prospective phase I trial to establish the maximum tolerated dose (MTD) of a 3-fraction HFSR delivered concomitantly with standard doses of bevacizumab.
Materials and Methods
Patients with recurrent HGG with KPS ≥ 60, history of standard fractionated initial radiation, tumor volume at recurrence ≤ 40cc and absence of brainstem or corpus callosum involvement were eligible. A standard 3+3 Phase I dose escalation trial design was utilized, with dose-limiting toxicities (DLT) defined as any grade 3–5 toxicities possibly, probably, or definitely related to radiation. Bevacizumab was given at a dose of 10mg/kg every two weeks. HFSR was initiated after two bevacizumab doses, delivered in 3 fractions every other day, starting at 9 Gy/fraction.
Results
A total of 3 patients were enrolled at the 9GyX3 dose level cohort, 5 enrolled in the 10GyX3 cohort and 7 in the 11GyX3 cohort. One DLT of grade 3 fatigue and cognitive deterioration possibly related to HFSR was observed in the 11GyX3 cohort, and this dose was declared the MTD in combination with bevacizumab. Although no symptomatic radionecrosis was observed, substantial treatment-related effects and necrosis were observed in resected specimens. The intent-to-treat median overall survival (OS) was 13 months.
Conclusions
Re-irradiation using a 3-fraction schedule with bevacizumab support is feasible and reasonably well tolerated. Dose-escalation was possible up to 11GyX3, which achieves a near doubling in the delivered biological equivalent dose to normal brain, in comparison to our previous 6GyX5 schedule. Promising OS warrants further investigation.
Introduction
Chemoradiotherapy with temozolomide is widely accepted as the standard initial treatment for newly diagnosed high-grade gliomas.1,2 However, tumor progression or recurrence remains the norm, and salvage therapy options are limited, typically achieving median overall survival (OS) of 6–9 months.3–5 With the main pattern of failure remaining local recurrence,6 improved survival requires optimized local control. Historically, the use of salvage re-irradiation has been limited by concerns of toxicity and potential injury to functional brain. More recently, advances in stereotactic radiation have significantly reduced the potential toxicity of re-irradiation, as high precision targeting allows for better delineation of radiation margins and maximal sparing of normal brain tissue. A variety of regimens have been used, with total doses from 25 to 36 Gy, delivered in fraction sizes ranging from 3 to 9 Gy.7 While prior studies all demonstrated the feasibility and safety of re-irradiation in high-grade gliomas, in-depth clinical comparison of outcomes is difficult due to differences in patient selection, previous pre-treatment profiles, and variable use of concomitant chemotherapy, in addition to the variability in radiation treatment techniques, total dose, and dose per fraction. As such, the optimal total radiation dose and fractionation regimen remains unclear.8
The premise for this phase I trial was based on a previous study investigating hypofractionated stereotactic re-irradiation (HFSR) of 30 Gy delivered in 5 fractions in combination with bevacizumab for patients with HGG.9 The regimen was well-tolerated and demonstrated promising efficacy, with a median OS of 12.5 months. Within that trial, the majority of patients still had local recurrence within the field of re-irradiation.10 This raised the question of whether higher doses of radiation would be safe and, if so, more effective in preventing local tumor recurrence. Importantly, none of the patients in the previous trial had developed symptomatic radiation necrosis. While the advantage of adding an anti-vascular endothelial growth factor (VEGF) drug such as bevacizumab to re-irradiation has not been fully elucidated, there is mounting evidence supporting its role in mitigating the risk of symptomatic radiation necrosis and allowing for more aggressive HFSRT doses.9,11–14 Based on the hypothesis that HFSRT could be further escalated when used with bevacizumab, we designed this phase I trial with the primary objective of establishing the maximum tolerated dose of a 3-fraction HFSR schedule when delivered with concomitant bevacizumab to treat recurrent high-grade gliomas.
Methods
Patients
This investigator-initiated prospective study protocol was registered at clinicaltrials.gov (NCT #xxxxxx- anonymized). The protocol and informed consent were approved by each participating institution’s Institutional Review Board (IRB). Written informed consent was obtained from all patients. Roche provided bevacizumab and partial funding.
The inclusion criteria included histologically confirmed grade III or IV glioma with imaging or pathologic evidence of recurrence, and tumor volume ≤ 40 cc. Patients must have received prior treatment of approximately 60 Gy of radiotherapy, and have KPS ≥ 60, age ≥ 18, and adequate bone marrow and organ function. Exclusion criteria included multicentric disease, disease infiltrating the corpus callosum or the brainstem, prior use or contra-indication to the use of bevacizumab, prior treatment with radiosurgery, and suspected or documented radionecrosis. Therapeutic anticoagulation (e.g. for venous thromboembolism) was allowed.
Treatment
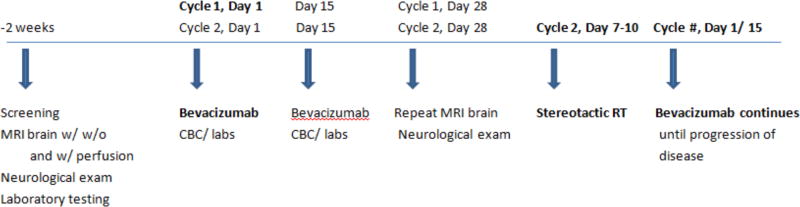
The study treatment schema is shown in Figure 1. Patients received bevacizumab at a dose of 10mg/kg IV once every two weeks on days 1(+/− 3 days) and 15(+/− 3 days) of each 28-day cycle. On day 28 (−4 days) the MRI was repeated for HFSR planning. If tumor size remained within study parameters, HFSR was started within days 7–10 of cycle 2, with a total of 3 treatments given on an every-other-day schedule (e.g. Mon/Wed/Fri). IMRT was utilized, with the initial re-irradiation dose consisting of 9 Gy×3 fractions, estimated to deliver a biologically equivalent dose only moderately higher in comparison to the 6 Gy×5 fraction regimen given in the prior phase II study.9
Figure 1.
Treatment schema (CBC = complete blood count; RT = radiotherapy)
MRI scans were performed at baseline, then at the end of cycle 1 for radiation treatment planning, at the end of cycle 2, and then every second cycle thereafter until disease progression. T1 post contrast and T2 FLAIR images from post-cycle 1 MRI were fused to the treatment planning CT to define the gross tumor volume (GTV), which encompassed T-1 post contrast enhancing disease; at the discretion of the treating radiation oncologist, mass-like T2/FLAIR abnormality could be included in the GTV, provided other treatment parameters were followed. The planning treatment volume (PTV) consisted of the GTV plus a 2–5mm margin at the discretion of the treating radiation oncologist. The prescription dose covered a minimum of 95% of the PTV.
Blood pressure was monitored every other week. Complete blood count with differential was obtained every other week for the first two cycles and then once every cycle. Urine protein/creatinine ratio and chemistries including BUN, creatinine, sodium, potassium, SGOT, SGPT, total bilirubin and total protein were measured every cycle until disease progression. Neurological and physical exams with KPS were performed post cycles 1 and 2, then after every 2 cycles.
Statistical Design
A standard 3+3 statistical design was employed. Dose limiting toxicities were defined as any grade 3–5 radiation injury or any non-hematologic toxicity felt to be possibly, probably, or definitely related to radiation or the combination of radiation and bevacizumab, identified within the evaluation period of 3 months following the completion of radiotherapy. Patients were deemed evaluable for DLT assessment if they received at least one dose of re-irradiation. Toxicities felt solely due to bevacizumab were not considered DLTs.
Because of the possibility of late toxicities developing after the 3-month DLT evaluation period, a safety stopping rule was also applied. If during the course of the trial more than two grade 3 or higher late toxicities occur at, or below the highest radiation dose assigned at that point, accrual would be stopped, and the institutional Data and Safety Monitoring Committee would be consulted. Upon review of all toxicities, one or more of the following decisions would be made: 1) To proceed with the 3+3 design, 2) To discontinue accrual to the dose associated with the late toxicity or any dose above it, 3) To suspend the study and allow for a sufficiently long followup period to monitor late toxicities, 4) To evaluate a lower dose.
Exploratory efficacy analyses consisted of response rate utilizing RANO criteria,15 as well as progression-free survival (PFS) and OS utilizing Kaplan-Meier methodology.
Results
Patient characteristics
Table 1 illustrates the characteristics of the fifteen patients with recurrent high-grade gliomas enrolled in the study. Eighty percent of the patients were men; median age was 63, median KPS was 90 and 67% had glioblastoma. Methylation status was known in eight cases; 75% of these were unmethylated. Mean enhancing tumor size at largest diameter was 2.65 cm. Patients had received a median of 2 prior lines of treatment, with 60% having experienced two or more recurrences. Median time from initial diagnosis to study enrollment was 14.3 months. At the time of study enrollment, 5 patients were 7–12 months from initial diagnosis, 4 patients were 12.1 – 18 months from initial diagnosis, 6 patients were > 18 months from initial diagnosis. Though it was not feasible to collect detailed prior radiation treatment plans for review, the recurrences for all patients were local and would have been at least partially within the original high-dose field.
Table 1.
Patient Characteristics
| Characteristics (N = 15) | N (%) | Range |
|---|---|---|
|
| ||
| Gender | ||
| Men | 12 (80) | |
| Women | 3 (20) | |
|
| ||
| Age (years) | ||
| Median (years) | 63 | [50–73] |
| <60 years | 5 (33) | |
| ≥60 years | 10 (67) | |
|
| ||
| Histology | ||
| Glioblastoma | 10 (67) | |
| Anaplastic astrocytoma | 5 (33) | |
|
| ||
| KPS | ||
| Median score | 90 | [70–100] |
|
| ||
| MGMT methylation status | ||
| Unknown | 7 (47) | |
| Unmethylated | 6 | |
| Methylated | 2 | |
|
| ||
| Prior salvage chemotherapies | ||
| Median | 2 | [1–3] |
| 1 prior treatment | 6 | |
| 2 prior treatments | 8 | |
| 3 prior treatments | 1 | |
|
| ||
| Mean tumor size at largest diameter (cm) | 2.65 | [1.8 – 5.37] |
Treatment and toxicity
Three patients were enrolled into Cohort 1 (9 Gy × 3), and all were evaluable, with no DLTs observed. A total of 5 patients were enrolled into Cohort 2 (10 Gy × 3), two of whom were deemed non-evaluable for DLT at the end of Cycle 1 due to disease progression that exceeded the tumor volume limit of 40 cc, preventing the use of HFSR. For the 3 evaluable patients treated with HFSR, there were no DLTs. Four patients were enrolled in Cohort 3 (11 Gy × 3); one patient came off study at the end of Cycle 1, prior to initiating HFSR, due to a grade 1 ischemic stroke observed on the MRI. Of the 3 patients evaluable for DLT, one patient had grade 3 fatigue and cognitive deterioration, which was deemed a DLT. As per the 3+3 design, that cohort was expanded with three additional patients but no other DLTs were observed. Of note, one of the patients treated at this dose level developed a grade 2 ischemic stroke within the original radiation field but outside the re-irradiation field. This grade 2 toxicity was felt potentially related to bevacizumab but not directly related to re-irradiation, and was not considered a DLT. However, from unanimous decision among investigators, further dose escalation was not attempted, and the 11 Gy × 3 was deemed the MTD.
For those patients who received HFSR on study, reasons for being removed from study included disease progression in eight cases, toxicity in two, one case of a prolonged treatment hold, and another patient who decided to withdraw due to logistic reasons but who continued to receive bevacizumab as per protocol locally and was still followed for efficacy.
Toxicities associated with bevacizumab were in line with studies of single-agent bevacizumab. A summary of all grade 3–5 toxicities is shown in Table 2.
Table 2.
Grades 3 and 4 toxicities deemed definitely, possibly or likely related tostudy treatment (N=15). No grade 5 toxicities were observed.
| Grade 3 | Grade 4 | |
|---|---|---|
| Fatigue | 2 | 0 |
| Hypertension | 1 | 1 |
| CNS necrosis | 1 | 0 |
| Meningitis | 1 | 0 |
| Leukopenia | 1 | 0 |
| Lymphopenia | 1 | 0 |
| Neutropenia | 1 | 0 |
| Hyponatremia | 1 | 0 |
| Skin Infection | 1 | 0 |
| Infections and other infestations | 1 | 0 |
| Muscle weakness | 1 | 0 |
Exploratory Efficacy Analysis and Patterns of Recurrence
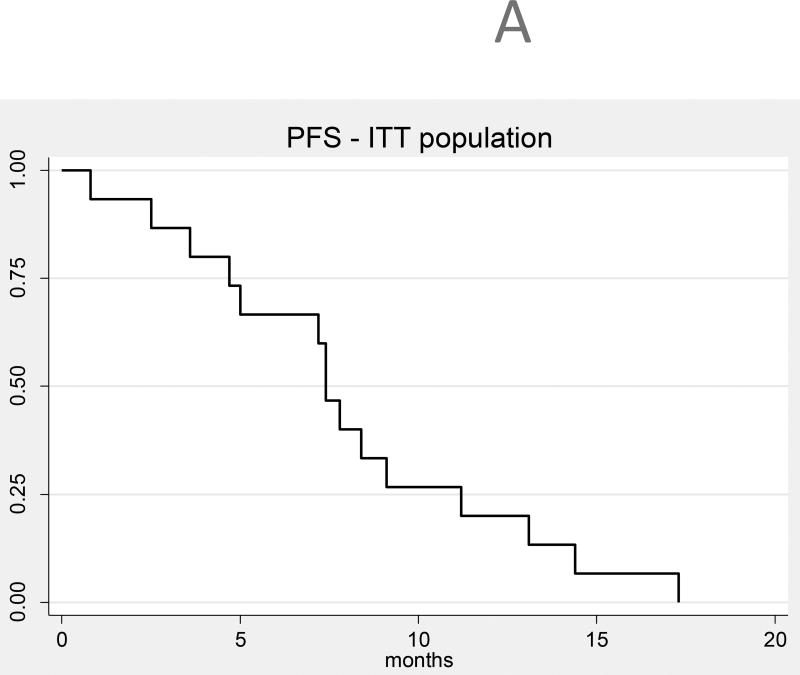
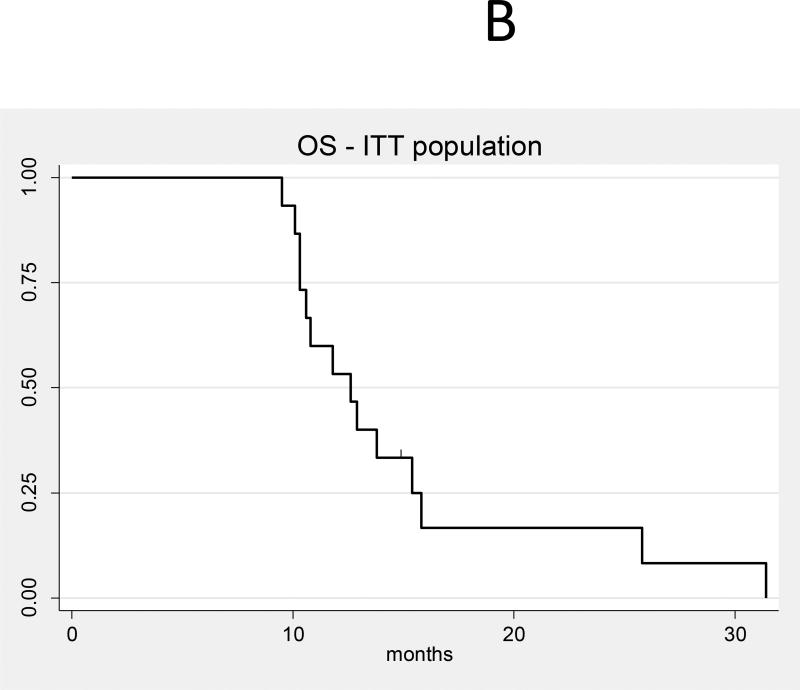
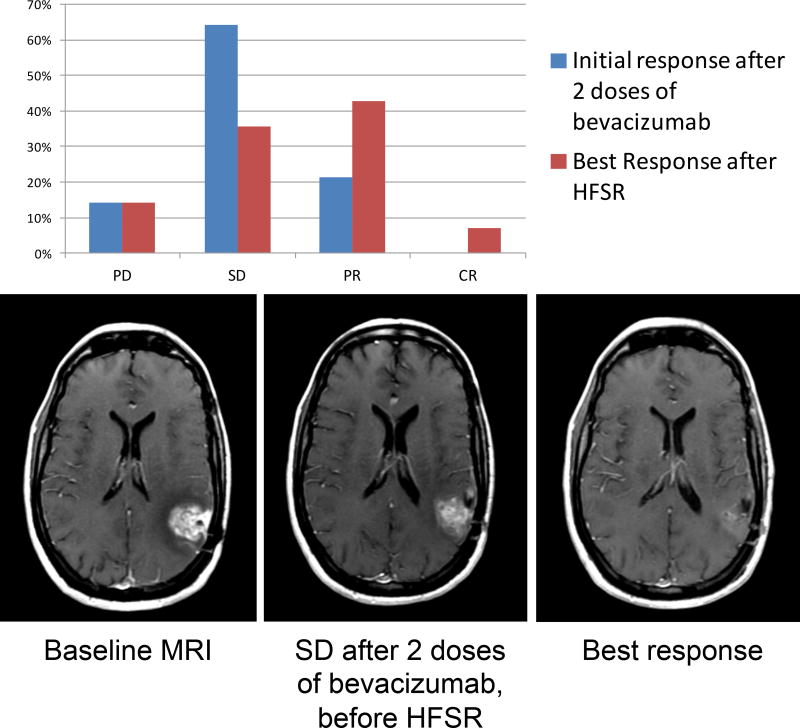
Given the small number of patients, analysis of efficacy is exploratory only; response assessment was performed using the RANO criteria.15 In the intent-to-treat basis (N=15), the median OS (Figure 2a) from time of treatment initiation was 13 months (95% CI 10–15) and the median PFS (Figure 2b) was 7 months (95% CI 4,9). Figure 3 shows response rate after initial bevacizumab treatment, and best response after HFSR for the 12 evaluable patients for response. The objective response rate (complete response [CR] + partial response [PR]) was 21% prior to HFSRT (3 partial responses), and increased to a best response after HFSR of 50% (6 PR and 1 CR). Among the 12 patients who received re-irradiation on study, 4 (33%) had distant recurrences and 8 (67%) had local recurrences. Three patients underwent surgical resection for suspicion of tumor progression after HSFR. All patients had persistent tumor, although substantial treatment-related changes and necrosis were also present on histologic examination in all three. In one of those patients, necrosis accounted for more than 90% of the sample, and this patient was deemed to have a treatment-related CNS necrosis, CTCAE grade3.
Figure 2.
A: Progression-free survival (all patients, intent-to-treat population, N = 15)
B: Overall survival (all patients, intent-to-treat population, N = 15)
Figure 3.
Response rates after initial bevacizumab treatment and following HFSR (N=12), and example of a patient with radiographic response seen on T1 post-contrast MRI following HFSR. PD: progressive disease; SD: stable disease; PR: partial response; CR: complete response.
Discussion
Recurrent high-grade gliomas carry a poor prognosis, and currently there are limited salvage treatment options. None of the frequently used therapies including single-agent bevacizumab, nitrosoureas nor tumor treatment fields have been shown to improve survival.3,4 While the efficacy of radiotherapy in gliomas remains undisputable, historical consensus has been against re-irradiation for these patients because of associated risks of neurotoxicity16 deriving from conventional external beam techniques. However, recent advances in radiotherapy techniques, particularly IMRT stereotactic technology, have allowed for more accurate spatial targeting and delivery of higher biologic doses to the infiltrating tumor, particularly in hypofractionated schedules. The widespread use of such techniques has revived interest in HFSR as an attractive treatment option for both newly diagnosed14 and recurrent gliomas.
The rationale for hypofractionation is based on increased tumor cell kill as a direct result of higher radiation dose per fraction, reduction in tumor cell repopulation and enhanced activity on glioma stem cells. Moreover, the convenience of a condensed treatment schedule is an important consideration, especially in patients with recurrent glioma who often have poor performance status, limited mobility, and a short expected life span. Overall, data from studies employing re-irradiation at disease recurrence, alone17–30 or in combination with temozolomide31–37, nitrosourea38, sorafenib39, sunitinib,40 gefitinib,41 panobinostat42 or bevacizumab12,13,38,43 support a therapeutic benefit with minimal toxicity. In particular, most of these studies have consistently reported a median OS in excess of 10 months, which compares favorably to available salvage chemotherapy or biologic therapies that typically achieve median OS of 7–9 months. However, it must be noted that with few exceptions,9,40,41 the vast majority of these studies is retrospective, therefore carrying inherent limitations and potential selection bias.
Maximizing the dose of HFSR at re-irradiation must be weighed against the risk of injury to normal brain tissue, with substantial sequelae associated with resulting radiation necrosis. Here lies the potential role of adding an anti-VEGF drug such as bevacizumab, with the intent of preventing symptomatic radiation necrosis and minimizing corticosteroid use. Bevacizumab, a monoclonal antibody against VEGF, was granted accelerated approval by the Food and Drug Administration in May 2009 for the treatment of recurrent glioblastoma, and is commonly used for recurrent disease.44,45 It has been hypothesized that the addition of bevacizumab has the potential to produce an advantageous balance between normal brain protection from radiation injury through improvements in brain tolerance to radiotherapy and enhanced cytotoxicity of radiation on tumor cells and their neovasculature. It is theorized that the synergy of this combined therapy sensitizes the tumor endothelia to the delivered radiation, disrupts paradoxical angiogenesis, and induces apoptosis through the disinhibition of VEGF.9,13 Antitumor effects on cancer stem cells and their peri-vascular niche have also been studied.11,13 Recent phase 3 studies in newly diagnosed glioblastoma adding bevacizumab to standard radiotherapy44,46 have failed to improve survival, and therefore it remains unclear if the synergistic effects observed in pre-clinical models exist in humans, or if higher radiotherapy doses are required for triggering such effects. Perhaps more importantly, bevacizumab prevents symptomatic edema by decreasing vascular permeability, which seems to be the principal component of its radioprotective effects. When comparing hypofractionated re-irradiation regimens that include bevacizumab to those that do not, bevacizumab appears to decrease rates of radiographically detectable radionecrosis.11,13 It is however noteworthy that tissue analysis of some patients re-operated following HFSR in this and other studies have shown abundant tissue necrosis, which often develops without accompanying symptomatic edema or contrast-enhancement on MRI. This suggests that radiotherapy-related tissue destruction seems to still occur, and while mostly asymptomatic, caution should be exerted when treating tumors located within eloquent areas of the brain; the use of functional MRI techniques to select patients for treatment may be of interest in such cases.
Challenges encountered during the study included the 3+3 trial design with a long DLT evaluation period, which is not an optimal means of conducting a trial in which late toxicities are expected. A continuous reassessment method may have been preferable, which would have expedited accrual and results. In addition, throughout treatment, and similar to daily neuro-oncology practice, it was difficult to differentiate tumor progression from radiation treatment effects, further exemplifying the need for novel neuroimaging tools. As with previous trials involving re-irradiation, our patient population was restricted to those that had a tumor volume less than 40cc and were free of multi-focal disease; this patient population may have an intrinsically better prognosis than unselected recurrent HGG populations.23 It remains unclear if re-irradiation of larger tumor volumes, inclusive of larger pools of patients, would be feasible with this aggressive fractionation regimen.47 Finally, this study did not formally evaluate quality of life or changes in neurocognitive function to fully assess the effect of our treatment regimen.
The study reported here demonstrates that for patients with recurrent high-grade gliomas, HFSR at doses up to 33 Gy delivered over 3 fractions in combination with bevacizumab is associated with an acceptable toxicity profile, and may achieve outcomes that are at least comparable to similar regimens delivered over five to six fractions. The resulting MTD represents a near doubling in the biologically equivalent dose to normal brain, as compared to our previous regimen of 30 Gy in 5 fractions. Further insight on the role of HFSR in recurrent glioblastoma will be provided by an ongoing randomized phase II study (RTOG 1205), which has a target accrual of 178 patients that are assigned to receive bevacizumab alone or in combination with re-irradiation using 35 Gy in 10 fractions. Comparison of the toxicity profiles and outcome data will be of interest. Other planned or ongoing trials will investigate the combination with immune-checkpoint inhibitors, supported by pre-clinical studies showing synergistic effects between hypofractionated radiotherapy and these agents. In the meantime, re-irradiation strategies, particularly when combined with bevacizumab, remain a reasonable consideration for treatment of patients with recurrent HGG that have few or no other therapeutic options.
Summary.
Contemporary radiotherapy techniques allow for highly accurate targeting of infiltrating tumor and more aggressive dosing regimens. Recent trials have explored the safety and feasibility of hypofractionated stereotactic reirradiation (HFSR) for recurrent gliomas. Taking advantage of improved radiotherapy tolerance afforded by the combination with bevacizumab, we explored escalating doses of HFSR, and found that doses up to 33 Gy delivered in 11 GyX3 fractions were reasonably safe and well-tolerated, with promising overall survival of 13 months.
Acknowledgments
Funding
This was an investigator-initiated prospective multicenter phase II trial sponsored by Memorial Sloan Kettering Cancer Center, supported in part by NIH grant P30 CA 008748. Genentech provided bevacizumab and additional partial financial support.
The authors report the following compensated activities, all outside of the current work: Employment: IB is currently an employee of Enlitic, Inc.
Scientific Advisory Board: LD (Roche, Sapience, Juno Therapeutics); ABL (Genentech, Astra Zeneca, Abbvie, Sapience, Bioclinica, VBI Vaccines, Cortice, Oxigene, prime Oncology, Regeneron, Novartis, Heron, Foundation Medicine, Midatech, Celgene); AO (BMS, Merck, Stemline, Inovio, AstraZeneca, Alexion, Oxigene, CarThera, Novartis, Juno Therapeutics).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinicaltrials.gov registration: NCT01392209
Disclosures
The remainder of the authors have nothing to disclose.
Preliminary results previously presented at the American Academy of Neurology Annual Meeting, 2015 and at the 21st International Conference on Brain Tumor Research and Therapy, 2016.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Amelio D, Amichetti M. Radiation therapy for the treatment of recurrent glioblastoma: an overview. Cancers (Basel) 2012;4:257–80. doi: 10.3390/cancers4010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician's choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310:1842–50. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SM, Kim JH, Kim SJ, et al. Hypofractionated intensity-modulated radiotherapy using simultaneous integrated boost technique with concurrent and adjuvant temozolomide for glioblastoma. Tumori. 2013;99:480–7. doi: 10.1177/030089161309900407. [DOI] [PubMed] [Google Scholar]
- 7.Taunk NK, Moraes FY, Escorcia FE, et al. External beam re-irradiation, combination chemoradiotherapy, and particle therapy for the treatment of recurrent glioblastoma. Expert Rev Anticancer Ther. 2016;16:347–58. doi: 10.1586/14737140.2016.1143364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt B, Lee HJ, Ryeom S, et al. Combining Bevacizumab with Radiation or Chemoradiation for Solid Tumors: A Review of the Scientific Rationale, and Clinical Trials. Curr Angiogenes. 2012;1:169–179. doi: 10.2174/2211552811201030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–63. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro LQ, Beal K, Goenka A, et al. Patterns of failure after concurrent bevacizumab and hypofractionated stereotactic radiation therapy for recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2013;85:636–42. doi: 10.1016/j.ijrobp.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 11.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niyazi M, Ganswindt U, Schwarz SB, et al. Irradiation and bevacizumab in high-grade glioma retreatment settings. Int J Radiat Oncol Biol Phys. 2012;82:67–76. doi: 10.1016/j.ijrobp.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Cuneo KC, Vredenburgh JJ, Sampson JH, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82:2018–24. doi: 10.1016/j.ijrobp.2010.12.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20:5023–31. doi: 10.1158/1078-0432.CCR-14-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–72. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 16.Omuro AM, Martin-Duverneuil N, Delattre JY. Complications of radiotherapy to the central nervous system. Handb Clin Neurol. 2012;105:887–901. doi: 10.1016/B978-0-444-53502-3.00030-6. [DOI] [PubMed] [Google Scholar]
- 17.McKenzie JT, Guarnaschelli JN, Vagal AS, et al. Hypofractionated stereotactic radiotherapy for unifocal and multifocal recurrence of malignant gliomas. J Neurooncol. 2013;113:403–9. doi: 10.1007/s11060-013-1126-2. [DOI] [PubMed] [Google Scholar]
- 18.Fokas E, Wacker U, Gross MW, et al. Hypofractionated stereotactic reirradiation of recurrent glioblastomas : a beneficial treatment option after high-dose radiotherapy? Strahlenther Onkol. 2009;185:235–40. doi: 10.1007/s00066-009-1753-x. [DOI] [PubMed] [Google Scholar]
- 19.Henke G, Paulsen F, Steinbach JP, et al. Hypofractionated reirradiation for recurrent malignant glioma. Strahlenther Onkol. 2009;185:113–9. doi: 10.1007/s00066-009-1969-9. [DOI] [PubMed] [Google Scholar]
- 20.Grosu AL, Weber WA, Franz M, et al. Reirradiation of recurrent high-grade gliomas using amino acid PET (SPECT)/CT/MRI image fusion to determine gross tumor volume for stereotactic fractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:511–9. doi: 10.1016/j.ijrobp.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 21.Vordermark D, Kolbl O, Ruprecht K, et al. Hypofractionated stereotactic re-irradiation: treatment option in recurrent malignant glioma. BMC Cancer. 2005;5:55. doi: 10.1186/1471-2407-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudes RS, Corn BW, Werner-Wasik M, et al. A phase I dose escalation study of hypofractionated stereotactic radiotherapy as salvage therapy for persistent or recurrent malignant glioma. Int J Radiat Oncol Biol Phys. 1999;43:293–8. doi: 10.1016/s0360-3016(98)00416-7. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd SF, Laing RW, Cosgrove VP, et al. Hypofractionated stereotactic radiotherapy in the management of recurrent glioma. Int J Radiat Oncol Biol Phys. 1997;37:393–8. doi: 10.1016/s0360-3016(96)00455-5. [DOI] [PubMed] [Google Scholar]
- 24.Yazici G, Cengiz M, Ozyigit G, et al. Hypofractionated stereotactic reirradiation for recurrent glioblastoma. J Neurooncol. 2014;120:117–23. doi: 10.1007/s11060-014-1524-0. [DOI] [PubMed] [Google Scholar]
- 25.Ogura K, Mizowaki T, Arakawa Y, et al. Efficacy of salvage stereotactic radiotherapy for recurrent glioma: impact of tumor morphology and method of target delineation on local control. Cancer Med. 2013;2:942–9. doi: 10.1002/cam4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciammella P, Podgornii A, Galeandro M, et al. Hypofractionated stereotactic radiation therapy for recurrent glioblastoma: single institutional experience. Radiat Oncol. 2013;8:222. doi: 10.1186/1748-717X-8-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizumoto M, Okumura T, Ishikawa E, et al. Reirradiation for recurrent malignant brain tumor with radiotherapy or proton beam therapy. Technical considerations based on experience at a single institution. Strahlenther Onkol. 2013;189:656–63. doi: 10.1007/s00066-013-0390-6. [DOI] [PubMed] [Google Scholar]
- 28.Maranzano E, Anselmo P, Casale M, et al. Treatment of recurrent glioblastoma with stereotactic radiotherapy: long-term results of a mono-institutional trial. Tumori. 2011;97:56–61. doi: 10.1177/030089161109700111. [DOI] [PubMed] [Google Scholar]
- 29.Patel M, Siddiqui F, Jin JY, et al. Salvage reirradiation for recurrent glioblastoma with radiosurgery: radiographic response and improved survival. J Neurooncol. 2009;92:185–91. doi: 10.1007/s11060-008-9752-9. [DOI] [PubMed] [Google Scholar]
- 30.Combs SE, Thilmann C, Edler L, et al. Efficacy of fractionated stereotactic reirradiation in recurrent gliomas: long-term results in 172 patients treated in a single institution. J Clin Oncol. 2005;23:8863–9. doi: 10.1200/JCO.2005.03.4157. [DOI] [PubMed] [Google Scholar]
- 31.Minniti G, Scaringi C, De Sanctis V, et al. Hypofractionated stereotactic radiotherapy and continuous low-dose temozolomide in patients with recurrent or progressive malignant gliomas. J Neurooncol. 2013;111:187–94. doi: 10.1007/s11060-012-0999-9. [DOI] [PubMed] [Google Scholar]
- 32.Fogh SE, Andrews DW, Glass J, et al. Hypofractionated stereotactic radiation therapy: an effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–53. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aktan M, Koc M, Kanyilmaz G. Survival following reirradiation using intensity-modulated radiation therapy with temozolomide in selected patients with recurrent high grade gliomas. Ann Transl Med. 2015;3:304. doi: 10.3978/j.issn.2305-5839.2015.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osman MA. Phase II trial of temozolomide and reirradiation using conformal 3Dradiotherapy in recurrent brain gliomas. Ann Transl Med. 2014;2:44. doi: 10.3978/j.issn.2305-5839.2014.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinez-Carrillo M, Tovar-Martin I, Zurita-Herrera M, et al. Salvage radiosurgery for selected patients with recurrent malignant gliomas. Biomed Res Int. 2014;2014:657953. doi: 10.1155/2014/657953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti A, Pontoriero A, Arpa D, et al. Efficacy and toxicity of CyberKnife re-irradiation and "dose dense" temozolomide for recurrent gliomas. Acta Neurochir (Wien) 2012;154:203–9. doi: 10.1007/s00701-011-1184-1. [DOI] [PubMed] [Google Scholar]
- 37.Combs SE, Bischof M, Welzel T, et al. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J Neurooncol. 2008;89:205–10. doi: 10.1007/s11060-008-9607-4. [DOI] [PubMed] [Google Scholar]
- 38.Minniti G, Agolli L, Falco T, et al. Hypofractionated stereotactic radiotherapy in combination with bevacizumab or fotemustine for patients with progressive malignant gliomas. J Neurooncol. 2015;122:559–66. doi: 10.1007/s11060-015-1745-x. [DOI] [PubMed] [Google Scholar]
- 39.Den RB, Kamrava M, Sheng Z, et al. A phase I study of the combination of sorafenib with temozolomide and radiation therapy for the treatment of primary and recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 2013;85:321–8. doi: 10.1016/j.ijrobp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wuthrick EJ, Curran WJ, Jr, Camphausen K, et al. A pilot study of hypofractionated stereotactic radiation therapy and sunitinib in previously irradiated patients with recurrent high-grade glioma. Int J Radiat Oncol Biol Phys. 2014;90:369–75. doi: 10.1016/j.ijrobp.2014.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwer AL, Kavanagh BD, McCammon R, et al. Radiographic and histopathologic observations after combined EGFR inhibition and hypofractionated stereotactic radiosurgery in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;73:1352–7. doi: 10.1016/j.ijrobp.2008.06.1919. [DOI] [PubMed] [Google Scholar]
- 42.Shi W, Palmer JD, Werner-Wasik M, et al. Phase I trial of panobinostat and fractionated stereotactic re-irradiation therapy for recurrent high grade gliomas. J Neurooncol. 2016;127:535–9. doi: 10.1007/s11060-016-2059-3. [DOI] [PubMed] [Google Scholar]
- 43.Flieger M, Ganswindt U, Schwarz SB, et al. Re-irradiation and bevacizumab in recurrent high-grade glioma: an effective treatment option. J Neurooncol. 2014;117:337–45. doi: 10.1007/s11060-014-1394-5. [DOI] [PubMed] [Google Scholar]
- 44.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334–40. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 46.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 47.Back M, Gzell CE, Kastelan M, et al. Large volume re-irradiation with bevacizumab is a feasible salvage option for patients with refractory high-grade glioma. Neurooncol Pract. 2015;2:48–53. doi: 10.1093/nop/npu031. [DOI] [PMC free article] [PubMed] [Google Scholar]