Abstract
Purpose
Fractionated radiotherapy is commonly used to treat benign meningiomas (BM) however the optimal dose and late adverse effects are not well defined. We aimed to assess the outcomes of BM treated to two radiation dose levels.
Methods and Materials
We randomly assigned patients (1:1) with incompletely resected or recurrent BM to two radiation doses, 55.8 Gy(RBE) and 63.0 Gy(RBE) of fractionated combined proton-photon radiotherapy. The primary endpoint was local control with hypothesis of improved tumor control with higher dose. Secondary endpoints included progression-free survival (PFS), overall survival (OS), and rates of treatment-related toxicities.
Results
Between 1991 and 2000, 47 patients were randomized. Three patients were excluded for non-benign histology therefore 44 patients were analyzed, 22 who received 55.8 Gy(RBE) and 22 who received 63.0 Gy(RBE). The median follow-up was 17.1 years. Local control for the entire cohort was 98% at 10 years and 90% at 15 years. Of the five patients with local recurrence, 4 occurred after 10 years of follow-up and 3 were in the lower dose group (p=0.322). In the modified intention to treat analysis, there was no difference in PFS (p=0.234) and OS (p=0.271) between arms. A total of 26 patients (59%) experienced a Grade 2 or higher late toxicity including 9 patients (20%) incurring a cerebrovascular accident (CVA), seven of which were deemed at least possibly attributable to irradiation. The median time between completion of radiation therapy and CVA was 5.6 years (range 1.4–14.0).
Conclusion
Fractionated combined proton-photon radiotherapy is effective for BM with no apparent benefit in dose escalation. Further investigation is needed to better define the risk of late toxicities including CVA after cranial radiation therapy.
Keywords: meningioma, proton radiotherapy, cerebrovascular accident, late effects
INTRODUCTION
Meningiomas are the most common primary central nervous system tumor and comprise approximately 1/3 of all primary brain and spinal tumors (1). Although 80% are benign and have a slow growth rate, they can cause serious morbidity and even mortality. Complete surgical removal remains the mainstay of treatment, however not all benign meningioma (BM) can be safely resected. Prior retrospective studies have demonstrated that fractionated radiation therapy (RT) increases local control rates for incompletely resected BM from approximately 70% to greater than 90% at five and 10 years thus RT is often used for subtotally resected and routinely used to manage recurrent BM (2–4). However, with improved control rates and cause specific survival of upwards of 90% at 10–15 years (5), patients who have received RT for BM are at risk for late effects which can result in significant morbidity such as visual or hearing impairment, hypopituitarism and neurocognitive deficits.
Radiotherapy, either conventionally fractionated or delivered in a single session, is frequently used in the management of BM at risk for recurrence or progression. Multiple retrospective series have suggested that dose escalation beyond 60 Gy is correlated with improved long-term tumor control in patients with atypical or malignant meningiomas (6–8). In addition, prior series have reported varying rates of toxicity and have suggested that recurrent tumors portend a worse prognosis than de novo BM (9, 10). The majority of these studies however are retrospective in nature and there have been no prior trials with significant long term follow-up in radiotherapy or neurosurgery, which is crucial for conditions such as BM that have a propensity for late failures, since actuarial analyses are unable to capture changing relapse patterns over time. As a result, the optimal dose for treatment with fractionated RT is unclear and the long-term toxicities and patterns of failure remain undefined. We address this research gap in our prospective study, which assesses long-term outcomes of patients with incompletely excised or recurrent BM randomized to two radiation dose levels of combined proton-photon RT.
METHODS AND MATERIALS
Study Design
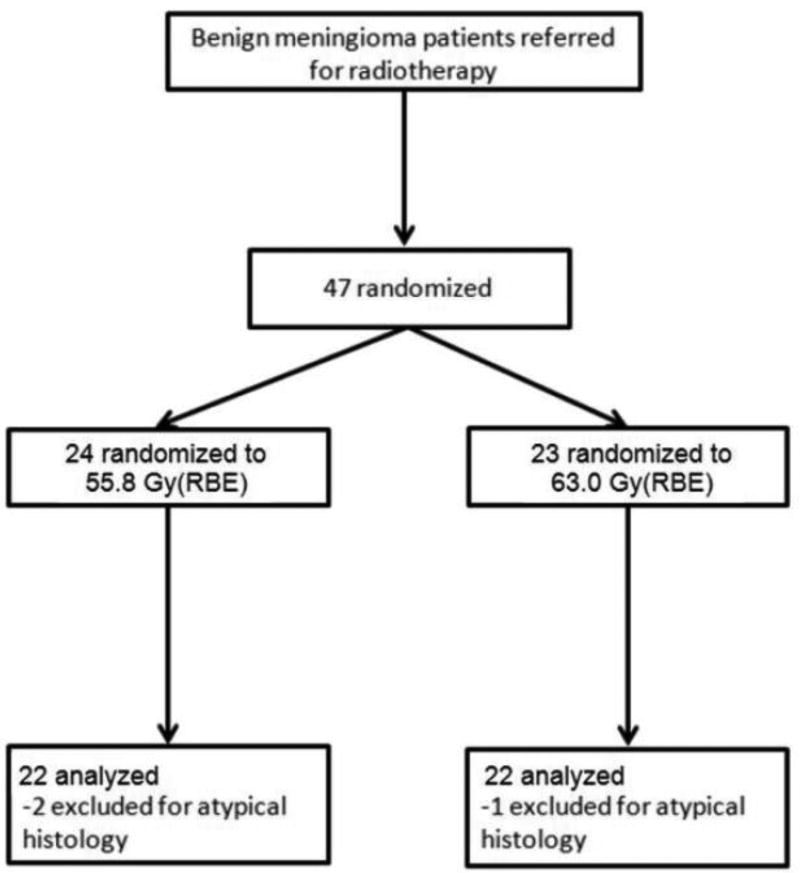
This prospective 1:1 non-blinded randomized controlled trial compared radiation doses of 55.8 Gy(RBE) and 63.0 Gy(RBE) delivered by fractionated combined proton-photon external beam radiotherapy (Figure 1). Treatment assignment was allocated with equal probability using randomized permuted blocks of size 4 within strata. Randomization was stratified by (i) tumor volume of ≤100 ml versus >100 ml. and (ii) treatment indication of subtotal resection (STR) with immediate adjuvant postoperative radiotherapy versus recurrent (or progressive) tumor. Patient registration and randomization were managed by the Quality Control Center at *XXX*, independent of the study staff. The original sample size of 120 patients included an allowance for 10% ineligibility. The primary hypothesis was an improvement in the local control rate from 80% to 93% at five years with 80% power at a one-sided 0.05 level. The secondary endpoints included progression-free survival (PFS), overall survival (OS), and acute and late treatment-related toxicities. However, the study was closed early prior to reaching the target sample size due to slow accrual. This study was approved by our institutional review board.
Figure 1.
CONSORT diagram for the trial
Study Population and Follow-up
Eligibility criteria included patients of age ≥ 18 with Karnofsky performance status ≥ 70 who had incompletely resected or recurrent BM with both histologic and radiographic documentation. All patients provided informed consent. To assess local control, magnetic resonance imaging (MRI) was performed six months after completion of treatment and annually thereafter. Tumor control was defined as absence of radiologic progression showing tumor margin(s) extending in any direction at least five mm beyond that seen on baseline scans. Patients were seen in follow-up at one month, every three months during the first year with intervals increasing to annually at five years and after. Acute and late toxicities were recorded during all visits and then rescored based on the CTCAE v4.0 toxicity scale (11). All follow-up MRIs for patients who were noted to have symptoms suggestive of a cerebrovascular accident (CVA) were reviewed by a neuroradiologist. Cause of death for patients lost to follow-up was determined via the Social Security Index or other national registries of death.
Treatment Planning
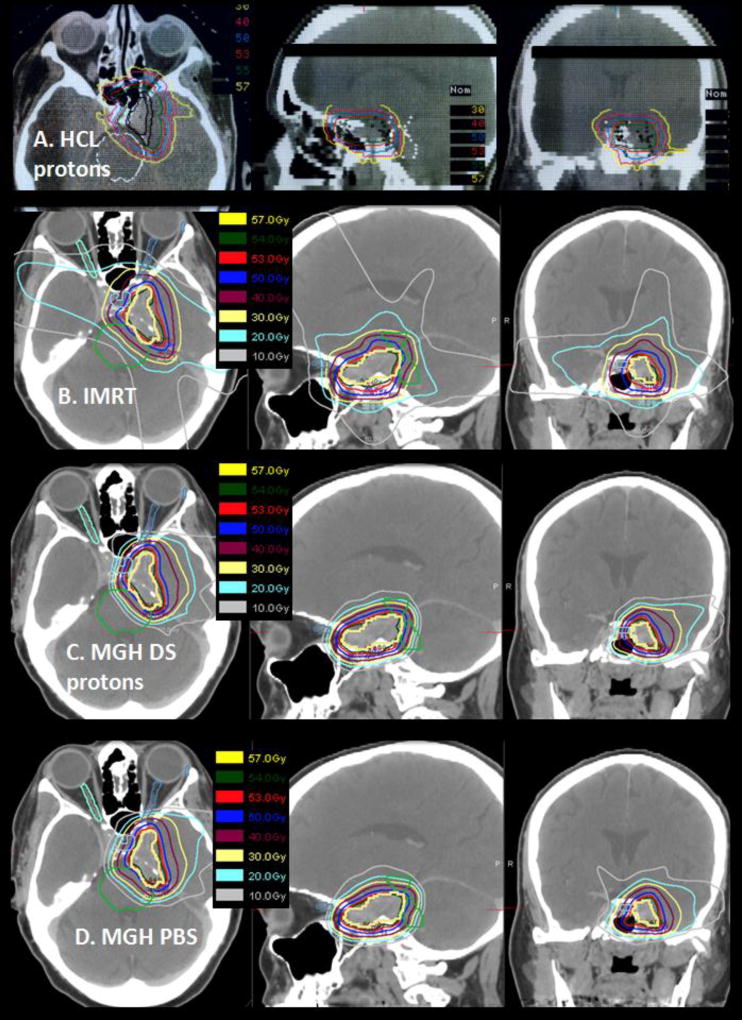
Treatment planning was performed on a 3D CT image-based planning system developed specifically for the proton treatment program (12–13). Gross tumor volume (GTV) and clinical target volume (CTV), including possible regions of microscopic spread, were outlined on serial sections of a contrast-enhanced planning CT scan. Dose constraints included 53 Gy(RBE) to the brainstem center, 63 Gy(RBE) to the brainstem surface ≤1cm from the GTV, 54 Gy(RBE) to the optic nerve and chiasm and 63 Gy(RBE) to the medial temporal lobes. A treatment plan used for one of the study patients is shown in Figure 2.
Figure 2.
(A) Actual proton radiotherapy plan for a patient with recurrent meningioma treated to 55.8 Gy(RBE) with representative axial, sagittal and coronal images are shown. The treatment used an antiquated passive scattering proton therapy system. (B, C, D) A similar clinical case planned with photon IMRT (B), passive scattering proton therapy (C), and pencil beam scanning proton therapy (D) were created for dosimetric comparison. Target coverage was similar in all plans however dose to surrounding normal tissues differed significantly. Compared with the patient’s actual treatment plan, the photon IMRT plan is the least conformal and the contemporary passive scatter and pencil beam scanning plans are more conformal.
Radiotherapy
Proton therapy was utilized in this study as a means for dose escalation while minimizing surrounding normal tissue collateral exposure. Patients were treated four times weekly with the 160 MeV proton beam at the *XXX*. Due to limited availability at the *XXX* however, patients were treated once weekly with 4- or 10-MV photons at *XXX* in Boston, MA. Daily radiation fraction size was planned to be 1.8 Gy(RBE) for both protons and photons. After initiation of the study, the *XXX* proton beam was recalibrated using an ion chamber technique which demonstrated that the prior Faraday cup technique employed was 8% higher in dose output (14). At the time of the change in calibration practice, 15 patients had been entered into the protocol and had received a daily proton dose of 1.94 Gy(RBE). It was decided to continue the protocol using the daily proton dose of 1.94 Gy(RBE) that had been employed in the 15 patients already treated. Photon daily dose continued to be 1.8 Gy.
Statistical Analysis
All comparisons between arms were analyzed by modified intention to treat, excluding patients who were found to be ineligible. Fisher’s exact test was used to compare the distribution of patient characteristics, acute and late toxicities when the data were binary or categorical. Wilcoxon rank-sum test was used to analyze continuous covariates and change in symptoms and toxicity coded as multinomial categories. Time to local recurrence, PFS and OS were measured from the starting date of radiation therapy. PFS was measured until the earlier, date of death or development of radiologic progression, and was otherwise censored at the last follow-up for progression-free patients still alive. OS was measured until the date of death or was censored at the time of latest follow-up. PFS and OS rates were estimated by the Kaplan-Meier method with the 95% confidence interval (95% CI) based on the log-log transformation and compared using the log-rank test. Median follow-up was calculated by applying the Kaplan-Meier method to OS with death as the censoring event. The cumulative risks of local recurrence and CVA were estimated by considering death as a competing risk of failure. The 95% CI was based on the pointwise confidence interval for the cumulative incidence function, and the arms were compared using Gray’s test. Data analysis was performed using SAS 9.4 (SAS Inst Inc, Cary, NC). StatXact 6 (Cytel Software Corp, Cambridge, MA) was used to compute exact p-values when the data were sparse, in particular, change in symptoms and toxicities as well as survival outcomes in the recurrent subgroup, as only a single patient had failed. All p-values are based on a two-sided hypothesis test, except as noted for the primary hypothesis.
RESULTS
Patient Characteristics
Between 1991 and 2000, 47 patients were enrolled in the study and randomized to receive either 55.8 Gy(RBE) or 63.0 Gy(RBE). Three patients were later found to have atypical histology and were excluded, thus a total of 44 patients are included in the analyses, 23 of whom were treated for STR. Median follow-up was 17.1 years, with 25 patients alive between 3.1 and 20.2 years. Follow-up of 10 years or longer was obtained for all but 4 of the surviving patients. Last follow-up date was in March, 2015. Baseline patient characteristics are described in Table 1.
Table 1.
Demographic and Clinical Characteristics
| Randomized arm | 55.8 Gy(RBE) (N=22) |
63.0 Gy(RBE) (N=22) |
p-value |
|---|---|---|---|
|
| |||
| Age (years) | 0.004 | ||
| Median | 61 | 50.5 | |
| Range | 31–77 | 38–76 | |
|
| |||
| Sex | 1.000 | ||
| Female | 14 (64%) | 15 (68%) | |
| Male | 8 (36%) | 7 (32%) | |
|
| |||
| Tumor location | 0.280 | ||
| Sphenoid wing | 7 (32%) | 8 (36%) | |
| Cavernous sinus | 3 (14%) | 8 (36%) | |
| Sella/Parasella | 6 (23%) | 2 (9%) | |
| Cerebellar pontine angle | 3 (14%) | 1 (5%) | |
| Clival/prepontine space | 3 (14%) | 1 (5%) | |
| Olfactory groove | 1 (5%) | 0 | |
| Optic canal | 0 | 1 (5%) | |
| Tentorium | 0 | 1 (5%) | |
|
| |||
| Smoking status* | 0.200 | ||
| Yes | 9 (45%) | 13 (68%) | |
| No | 11 (55%) | 6 (32%) | |
|
| |||
| Comorbidities | |||
| Hypertension | 3 (14%) | 6 (27%) | 0.457 |
| Diabetes mellitus | 0 | 4 (18%) | 0.108 |
| Hyperlipidemia | 1 (5%) | 0 | 1.000 |
| Coronary artery disease | 1 (5%) | 0 | 1.000 |
| One or more of the above | 4 (18%) | 6 (27%) | 0.721 |
|
| |||
| Radiation indication | 1.000 | ||
| STR, postoperative | 11 (50%) | 12 (55%) | |
| Recurrent disease | 11 (50%) | 10 (45%) | |
|
| |||
| GTV (cc)* | 0.107 | ||
| Median | 39.7 | 13.2 | |
| Range | (5.6–243.0) | (3.0–313.0) | |
|
| |||
| CTV (cc)* | 0.237 | ||
| Median | 49.0 | 28.0 | |
| Range | (9.0–464.0) | (6.5–150.7) | |
STR, subtotal resection; GTV, gross tumor volume; CTV, clinical target volume
Missing data on smoking status (5 patients), GTV (4 patients), CTV (14 patients)
Radiation Therapy Delivered
Twenty-two patients were randomized to each study arm. All patients were treated per protocol without a break in radiotherapy, except one patient initially randomized to the 63.0 Gy(RBE) arm who due to concerns regarding potential visual toxicity, received 55.8Gy (RBE). All pre-specified dose constraints were met. Compared to pure photon radiotherapy with an IMRT plan, a greater volume of normal brain tissue was spared with the combined proton-photon modality (Figure 2B). However, the early passive scatter modality employed in the study (Figure 2A) is less conformal than current treatments with passive scattering protons (Figure 2C) and far less conformal than pencil beam scanning protons (Figure 2D) which is becoming the standard of care for proton radiotherapy in many institutions.
Local Control
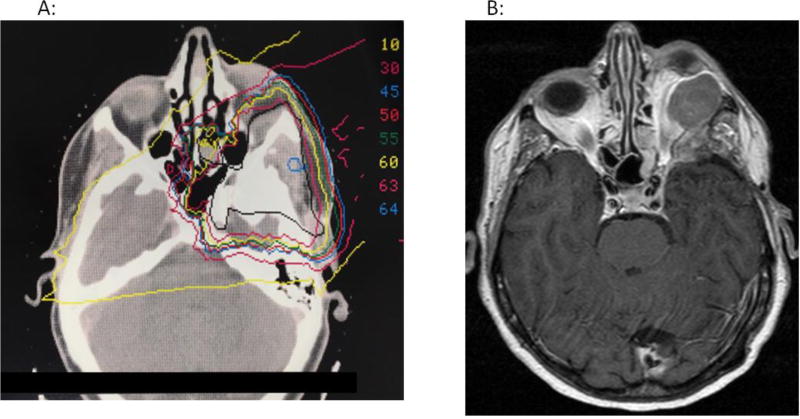
Three patients had local recurrence in the 55.8 Gy(RBE) arm and two in the 63 Gy(RBE) arm resulting in tumor control rates at 15 years of 85% (95% CI: 67–97%) and 95% (95% CI: 79–100%), respectively (one-sided p=0.322). Local failure after upfront postoperative treatment occurred in one patient following 63 Gy(RBE). In contrast, 4 patients treated for tumor recurrence experienced failures, 3 after 55.8 Gy(RBE)and 1 after 63 Gy(RBE). Four of the five failures occurred more than 10 years after initiation of radiotherapy. All five recurrences were either marginal and/or in-field. Figure 4 shows an example of a patient who had a marginal recurrence.
Figure 4.
This patient was initially diagnosed with a left sphenoid wing and left orbital meningioma in 1982, then underwent multiple resections for recurrence in 1982, 1989 and 1999. She received radiotherapy to 63 Gy(RBE) in 1999. Her radiotherapy plan is shown in Figure 4A, with the black line representing her clinical target volume (CTV) and all other colors representing isodose lines. She had no evidence of progression until 2010 when she developed proptosis and was found to have tumor progression in the left orbit (Figure 4B). This late recurrence was treated with near total resection.
Survival
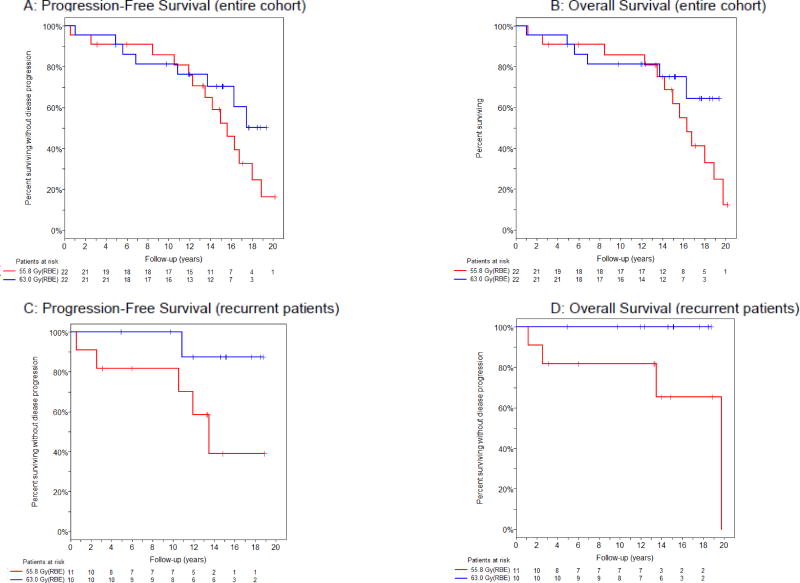
For the cohort as a whole, there was no difference in PFS (p=0.234) or OS (p=0.271), as shown in Table 2. When stratified by pre-irradiation disease status, the trend towards survival benefit of the higher dose was seen only in patients who were treated for recurrent disease. Specifically, for this subgroup, PFS at 15 years was 87% (95% CI: 39–98%) in the higher dose group and 39% (95% CI: 7–71%) in the lower dose group (p=0.061). The difference in OS at 15 years was similar, favoring the higher dose, with estimates of 100% (95% CI not obtainable) vs. 65% (95% CI: 24–88%) (p=0.150) (Figure 3). None of these trends were statistically significant. Nineteen patients died, 14 in the lower and 5 in the higher dose arm.
Table 2.
Progression-Free Survival and Overall Survival
| Randomized arm | 55.8 Gy(RBE) | 63.0 Gy(RBE) | p-value |
|---|---|---|---|
|
| |||
| Progression-Free Survival (95% CI) | |||
|
| |||
| Entire Cohort | 0.234 | ||
| 5 years | 91% (68–98%) | 91% (68–98%) | |
| 10 years | 86% (62–95%) | 81% (58–93%) | |
| 15 years | 52% (28–72%) | 70% (45–86%) | |
| Median (years) | 15.6 (12.3–18.0) | NR | |
|
| |||
| Subtotal Resection | 0.820 | ||
| 5 years | 100%* | 83% (48–96%) | |
| 10 years | 91% (51–99%) | 67% (34–86%) | |
| 15 years | 64% (30–85%) | 57% (25–80%) | |
| Median (years) | 16.3 (12.3–18.9) | 16.3 (4.9-NR) | |
|
| |||
| Recurrent | 0.061 | ||
| 5 years | 82% (45–95%) | 100%* | |
| 10 years | 82% (45–95%) | 100%* | |
| 15 years | 39% (7–71%) | 87% (39–98%) | |
| Median (years) | 13.5 (2.6-NR) | NR | |
|
| |||
| Overall Survival (95% CI) | |||
|
| |||
| Entire cohort | 0.271 | ||
| 5 years | 91% (68–98%) | 91% (68–98%) | |
| 10 years | 86% (62–95%) | 81% (58–93%) | |
| 15 years | 62% (35–80%) | 75% (50–89%) | |
| Median (years) | 16.3 (13.5–18.9) | NR | |
|
| |||
| Subtotal Resection | 0.975 | ||
| 5 years | 100%* | 83% (48–96%) | |
| 10 years | 91% (51–99%) | 67% (34–86%) | |
| 15 years | 64% (30–85%) | 57% (25–80%) | |
| Median (years) | 16.3 (12.3–18.9) | 16.3 (4.9-NR) | |
|
| |||
| Recurrent | 0.150 | ||
| 5 years | 82% (45–95%) | 100%* | |
| 10 years | 82% (45–95%) | 100%* | |
| 15 years | 65% (24–88%) | 100%* | |
| Median (years) | 19.8 (2.6–19.8) | NR | |
95% CI: 95% confidence interval; NR: not reached;
No failure events were observed
Figure 3.
Kaplan-Meier estimate of progression-free survival (A,C) and overall survival (B,D) by randomized arm for the entire cohort (A,B) and among patients treated for recurrent meningioma (C,D).
Toxicity
Acute toxicities occurring during radiation therapy and within the first 6 months of treatment completion were predominantly Grade 1 and transient (supplemental Table 1) although 12 (27%) of patients experienced an acute toxicity that was Grade 2 or higher. In contrast, 26 (59%) of patients experienced a late toxicity that was Grade 2 or higher (Table 3) including 7 patients (16%) with late vision loss. Of these 7 patients, 5 were treated to 55.8 Gy(RBE) and 2 to 63 Gy(RBE) and 5 and 2 had subtotally resected vs. recurrent meningioma, respectively. Three of the patients, all of whom had central tumors adjacent to or abutting the optic chiasm, developed bilateral vision loss while the other 4 patients’ symptoms were ipsilateral to the side of the treated meningioma. Follow-up MRIs of 3 of the patients demonstrated optic nerve enhancement suggestive of radiation-induced optic neuropathy and follow-up MRI of another patient revealed atrophy in the brainstem, cerebellum and optic chiasm also suggestive of radiation effect. The median time to development of vision loss was 27 months (range 13–61 months). There was a single instance of symptomatic Grade 4 cerebral edema in the lower dose arm. Asymptomatic brain necrosis occurred in one case in the 55.8 Gy(RBE) arm and in two cases in the 63 Gy(RBE) arm. There were no statistically significant differences in the rate of any of acute or late toxicities among treatment arms.
Table 3.
Late Effects
| Randomized arm | 55.8 Gy(RBE) | 63.0 Gy(RBE) | p-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | * | |
|
| |||||||||||
| Vision loss | 0 | 2 (9%) | 1 (5%) | 2 (9%) | 5 (23%) | 1 (5%) | 0 | 1 (5%) | 0 | 2 (9%) | 0.412 |
| Visual field deficit | 4 (18%) | 1 (5%) | 0 | 0 | 5 (23%) | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 0.185 |
| Diplopia | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 2 (9%) | 0 | 1 (5%) | 0 | 3 (14%) | 0.607 |
| Exophthalmos | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 2 (9%) | 0.488 |
| Eye, other | 1 (5%) | 2 (9%) | 0 | 0 | 3 (14%) | 2 (9%) | 0 | 0 | 0 | 2 (9%) | 1.000 |
| Hearing loss | 4 (18%) | 1 (5%) | 1 (5%) | 0 | 6 (27%) | 2 (9%) | 1 (5%) | 1 (5%) | 0 | 4 (18%) | 0.721 |
| Tinnitus | 2 (9%) | 0 | 0 | 0 | 2 (9%) | 1 (5%) | 2 (9%) | 0 | 0 | 3 (14%) | 1.000 |
| Olfactory alteration | 0 | 0 | 0 | 0 | 0 | 2 (9%) | 0 | 0 | 0 | 2 (9%) | 0.488 |
| Gustation alteration | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Dysphasia | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Neuromotor deficit | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Weakness | 0 | 1 (5%) | 0 | 0 | 1 (5%) | 2 (9%) | 0 | 0 | 0 | 2 (9%) | 1.000 |
| Facial numbness | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 3 (14%) | 1 (5%) | 0 | 0 | 4 (18%) | 0.345 |
| Facial weakness | 3 (14%) | 1 (5%) | 0 | 0 | 4 (18%) | 1 (5%) | 1 (5%) | 0 | 0 | 2 (9%) | 0.664 |
| Ataxia | 1 (5%) | 1 (5%) | 0 | 0 | 2 (9%) | 1 (5%) | 2 (9%) | 0 | 0 | 3 (14%) | 1.000 |
| Fall | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 2 (9%) | 0 | 0 | 3 (14%) | 0.233 |
| Dysarthria | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Headache | 4 (18%) | 1 (5%) | 0 | 0 | 5 (23%) | 2 (9%) | 1 (5%) | 0 | 0 | 3 (14%) | 0.698 |
| Dizziness | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Vertigo | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Depression | 0 | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 1 (5%) | 1.000 |
| Neurocog deficit | 4 (18%) | 2 (9%) | 1 (5%) | 0 | 7 (32%) | 6 (27%) | 0 | 0 | 0 | 6 (27%) | 1.000 |
| Endocrine deficit | 0 | 6 (27%) | 0 | 1 (5%) | 7 (32%) | 0 | 10 (45%) | 0 | 0 | 10 (45%) | 0.537 |
| Osteoporosis | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Cerebral edema | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 0 | 0 | 0 | 1.000 |
| Brain atrophy | 0 | 0 | 0 | 0 | 0 | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Skin changes | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1 (5%) | 0 | 0 | 0 | 1 (5%) | 1.000 |
| Alopecia | 0 | 0 | 0 | 0 | 0 | 2 (9%) | 0 | 0 | 0 | 2 (9%) | 0.488 |
| Fatigue | 4 (18%) | 0 | 0 | 0 | 4 (18%) | 2 (9%) | 1 (5%) | 0 | 0 | 3 (14%) | 1.000 |
Comparison of any grade
Of the 44 patients included in our analyses, a total of nine patients (20%) experienced a CVA confirmed by both MRI and clinical notes, corresponding to a long-term cumulative incidence of 22% (95% CI: 10–35%) (eFigure 1). Three of these patients received the higher dose of radiation (p=0.284). Seven of these CVAs, all ischemic, were deemed possibly, probably or definitely due to radiation based on their timing and location in the vascular distribution encompassed by the high dose radiotherapy volume. In all cases, CVAs occurred within a major vessel, most commonly the middle cerebral artery, and often in context of gross disease. The median time between completion of radiation therapy and CVA was 5.6 years (range 1.4–14.0). Two cases were deemed unlikely to be related to radiation. One unlikely CVA occurred on the contralateral side of the tumor nine months after completion of radiation therapy. The other had resection of recurrent tumor six months after completion of radiation, and suffered a CVA on the second postoperative day which was attributed to perioperative morbidity. Of the seven patients whose CVAs were at least possibly related to radiotherapy, three were Grade 4, three were Grade 3, and one was Grade 2 toxicity.
DISCUSSION
Although fractionated radiotherapy has been used in the treatment of BM for years, its efficacy, dosing and toxicity profile have been established on the basis of retrospective studies rather than randomized trial data. To the best of our knowledge, this is the first prospective randomized study on the treatment of BM. With a median follow-up of 17.1 years among surviving patients, four of five observed failures occurred 10 or more years after completion of treatment. Our study underscores the need for long-term follow-up in BM patients, which has also been suggested in prior retrospective series with long follow-up, including a Swedish cohort followed for 25 years in which there was a high rate of relapse (47%) with nearly half of patients (48%) dying from BM (15). In contrast, studies where patients were followed for 10 years or shorter have frequently shown local control rates of approximately 90%, which may be due to failure to capture late recurrences (16, 17). Our belief is that BM patients should be followed via physical exam and MRI for life, albeit with increasing intervals between scans as they progress further out from completion of treatment.
We found a trend for improved PFS and OS in the 63 Gy(RBE) arm as compared to the 55.8 Gy(RBE) arm for patients who were treated for recurrent disease. This finding is in line with prior retrospective series which have shown that recurrent disease, regardless of upfront treatment, portends more aggressive tumor behavior (9, 10). Larger studies would be important to validate these trends and possibly to provide statistical significance. Also it should be noted that the two dose arms in this study are not markedly different and both are above current common dose range for BM of 50 to 54 Gy; thus, excellent local control is expected with both doses. It is possible therefore that a larger study comparing a wider dose difference, such as 50 Gy(RBE) vs. 63 Gy(RBE), may provide the statistical power to detect a clinically significant benefit for dose escalation. Unfortunately, we were unable to obtain the cause of death for the majority of patients in our study; however we suspect that the observed higher number of deaths in the low dose arm (14 vs. 5) may be due at least in part to the older age of patients randomized to the lower dose. Therefore, it is conceivable that the trends for improved overall survival and improved local control with higher dose are unrelated. Again, further research is needed to better elucidate whether BM benefits from radiotherapy dose escalation.
Existing literature on fractionated radiotherapy for BM lacks long-term, comprehensive reporting on clinical symptoms. Several prior retrospective studies have focused on risk factors for peritumoral edema (18, 19) and another large series of over 300 patients treated with fractionated radiotherapy for BM showed low acute and late toxicity rates with no new neurologic deficits occurring after treatment (17). Our study adds to the literature by inclusion of detailed documentation of a wide range of toxicities following radiotherapy. Proton therapy decreases radiation exposure to surrounding normal tissues as compared to photon radiotherapy thus the primary theoretical advantage of proton radiotherapy is reduction in treatment-related toxicity. At 59%, the rate of Grade 2 or higher late toxicity in our cohort is relatively high. Notably, 16% of patients incurred late vision loss and 55% experienced a late visual toxicity of any grade despite meeting all pre-specified dose constraints. We hypothesize that this may be because both arms in our study were treated to doses higher than 54 Gy(RBE), which is the dose constraint often used for many intracranial critical structures, such as the brainstem, optic nerves and chiasm. Other possibilities include a higher actual daily dose of 1.94 Gy(RBE) given re-calibration or planning uncertainty with older radiotherapy techniques; however, given that the treatment plans are not currently available electronically, we are unable to determine the exact dose received by the visual structures. This study does not compare proton to photon radiotherapy so it cannot provide direct support for the use of proton over photon radiotherapy for treatment of BM on the basis of tumor control or potential reduction in late treatment effects.
We were also surprised to find that nine patients (20%) in our cohort experienced a CVA in follow up and that seven of these cases (16% of all patients) were at least possibly related to radiation. This crude rate is significantly higher than in the general population where the lifetime cumulative CVA rate is approximately 2% for individuals between the age of 40 and 59 and rises to approximately 6% between the ages of 60 and 79 (20). Radiation-induced atherosclerosis leading to cardiac disease is a well recognized phenomenon in survivors of Hodgkin lymphoma and breast cancer; however, recognition of increased CVA risk due to radiation exposure, has only recently has been gaining widespread acknowledgement. For example, three studies have demonstrated an increased incidence of CVA in head and neck cancer patients treated with radiotherapy (21–23) and another series found suggestion of elevated CVA risk in breast cancer survivors (24). Data on the association of brain irradiation with increased CVA risk in adults is also emerging including a recent SEER analysis of 19,565 patients diagnosed with a primary brain tumor showing that patients with tumors near central arterial circulations were more likely to experience CVA-specific mortality after radiotherapy (25). In addition, a large single institution study of childhood cancer survivors treated with cranial irradiation showed that the risk of CVA was as high as 1 in 8 (26). We hypothesize that radiotherapy, possibly in conjunction with presence of gross tumor, is associated with cerebral vascular events, and the availability of rare high quality long term prospective data is detecting a treatment sequelae that is frequently under appreciated. Whether a dose threshold of the doses used in this study of 55.8 and 63 Gy(RBE) is sufficient alone to increase the risk of CVA or whether this is additive in context to mass effect or other attribute of tumor presence in unknown. Alternative reasons to radiation exposure for why such a high proportion of our cohort of BM patients experienced a CVA may have been related to underlying risk factors: of the seven patients whose CVAs were possibly associated with radiotherapy, four were smokers including one with known coronary artery disease. In fact, in our entire study population, 10 patients (23%) had either baseline hypertension, hyperlipidemia, coronary artery disease, and/or diabetes, thus our cohort may have been at higher risk for CVA than the general U.S. population. Our findings highlight the need for further research on risk of cerebrovascular disease in all patients treated with brain radiotherapy, especially those with benign conditions such as meningiomas, who are projected to have excellent long-term survival.
Strengths of our study include its randomized design, long follow-up and detailed collection of symptoms. Limitations include small patient numbers and lack of patient-specific information such as cause of death. In particular, given that only 44 patients were randomized whereas the established sample size for the trial was 120 patients, findings in our study are inconclusive and ideally should be validated prior to adoption into clinical practice. In addition, although all patients in our study were treated with 3D-based treatment proton and photon therapy, the treatment planning software and delivery techniques available in the 1990s were more limited in their ability to achieve dose conformality as compared to modern planning tools, thus patients treated today are likely to have improved ratio of superior target coverage with sparing of surrounding non-target normal tissues, possibly translating to superior outcomes than those reported in our study.
CONCLUSIONS
Our study demonstrates that fractionated proton-photon radiotherapy is an effective treatment option for intracranial BM, although long-term follow-up is required to monitor for late failures. In addition, we would urge clinicians to continue to remain vigilant about possible long-term toxicities, including the risk of CVA.
Supplementary Material
Acknowledgments
We acknowledge Tarin Norkum’s assistance in obtaining details regarding randomization and assistance with clinical trial registration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- Beow Y. Yeap: Risk of Ovarian Cancer Algorithm (ROCA®) (Stock or Other Ownership), AstraZeneca (Honoraria)
- Jay S. Loeffler: Advanced Oncology (Leadership), Richard Schiller (Expert Testimony)
- Helen A. Shih: Genentech (Consultant or Advisory Role, past), UpToDate (Writer), International Journal of Radiation, Biology and Physics (Editor, past)
eFigure 1. Cumulative risk of cerebrovascular accident for the entire cohort.
eFigure 2: Cumulative local recurrence for the entire cohort, stratified by treatment arm.
References
- 1.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307–14. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldsmith BJ, Wara WM, Wilson CB, Larson DA. Postoperative irradiation for subtotally resected meningiomas. A retrospective analysis of 140 patients treated from 1967 to 1990. J Neurosurgery. 1994;80(2):195–201. doi: 10.3171/jns.1994.80.2.0195. [DOI] [PubMed] [Google Scholar]
- 3.Mendenhall WM, Morris CG, Amdur RJ, Foote KD, Friedman WA. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98(7):1473–82. doi: 10.1002/cncr.11645. [DOI] [PubMed] [Google Scholar]
- 4.Korah MP, Nowlan AW, Johnstone PA, Crocker IR. Radiation therapy alone for imaging-defined meningiomas. Int J Radiat Oncol Biol Phys. 2010;76(1):181–6. doi: 10.1016/j.ijrobp.2009.01.066. [DOI] [PubMed] [Google Scholar]
- 5.Tanzler E, Morris CG, Kirwan JM, Amdur RJ, Mendenhall WM. Outcomes of WHO Grade I meningiomas receiving definitive or postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(2):508–13. doi: 10.1016/j.ijrobp.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Chan AW, Bernstein KD, Adams JA, Parambi RJ, Loeffler JS. Dose escalation with proton radiation therapy for high-grade meningiomas. Technol Cancer Res Treat. 2012;11(6):607–14. doi: 10.7785/tcrt.2012.500267. [DOI] [PubMed] [Google Scholar]
- 7.Madani I, Lomax AJ, Albertini F, Trnkova P, Weber DC. Dose-painting intensity-modulated proton therapy for intermediate- and high-risk meningioma. Radiat Oncol. 2015;10:72. doi: 10.1186/s13014-015-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald MW, Plankenhorn DA, McMullen KP, et al. Proton therapy for atypical meningiomas. J Neurooncol. 2015;123(1):123–8. doi: 10.1007/s11060-015-1770-9. [DOI] [PubMed] [Google Scholar]
- 9.Condra KS, Buatti JM, Mendenhall WM, Friedman WA, Marcus RB, Jr, Rhoton AL. Benign meningiomas: primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39(2):427–36. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 10.Engenhart-Cabillic R, Farhoud A, Sure U, et al. Clinicopathologic features of aggressive meningioma emphasizing the role of radiotherapy in treatment. Strahlenther Onkol. 2006;182:641–6. doi: 10.1007/s00066-006-1555-3. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0 NCI, NIH, DHHS. 2009 May 29; NIH publication # 09-7473. [Google Scholar]
- 12.Goitein M, Abrams M. Multi-dimensional treatment planning: I. Delineation of anatomy. Int J Radiat Oncol Biol Phys. 1983;9(6):777–87. doi: 10.1016/0360-3016(83)90002-0. [DOI] [PubMed] [Google Scholar]
- 13.Urie M, Goitein M, Wagner M. Compensating for heterogeneities in proton radiation therapy. Phys Med Biol. 1984;29(5):553–66. doi: 10.1088/0031-9155/29/5/008. [DOI] [PubMed] [Google Scholar]
- 14.Vatnitsky S, Siebers J, Miller D, et al. Proton dosimetry intercomparison. Radiother Oncol. 1996;41(2):169–77. doi: 10.1016/s0167-8140(96)01800-2. [DOI] [PubMed] [Google Scholar]
- 15.Pettersson-Segerlind J, Orrego A, Lönn S, Mathiesen T. Long-term 25-year follow-up of surgically treated parasagittal meningiomas. World Neurosurg. 2011;76(6):564–71. doi: 10.1016/j.wneu.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Lee JY, Niranjan A, McInerney J, Kondziolka D, Flickinger JC, Lunsford LD. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- 17.Fokas E, Henzel M, Surber G, Hamm K, Engenhart-Cabillic R. Stereotactic radiation therapy for benign meningioma: long-term outcome in 318 patients. Int J Radiat Oncol Biol Phys. 2014;89:569–75. doi: 10.1016/j.ijrobp.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 18.Patil CG, Hoang S, Borchers DJ, 3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63(3):435–40. doi: 10.1227/01.NEU.0000325257.58684.92. discussion 440–2. [DOI] [PubMed] [Google Scholar]
- 19.Girvigian MR, Chen JC, Rahimian J, Miller MJ, Tome M. Comparison of early complications for patients with convexity and parasagittal meningiomas treated with either stereotactic radiosurgery or fractionated stereotactic radiotherapy. Neurosurgery. 2008;62(5 Suppl):A19–27. doi: 10.1227/01.neu.0000325933.34154.cb. discussion A2792013;8. [DOI] [PubMed] [Google Scholar]
- 20.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 21.Dorresteijn LD, Kappelle AC, Boogerd W, et al. Increased risk of ischemic stroke after radiotherapy on the neck in patients younger than 60 years. J Clin Oncol. 2002;20(1):282–8. doi: 10.1200/JCO.2002.20.1.282. [DOI] [PubMed] [Google Scholar]
- 22.Haynes JC, Machtay M, Weber RS, Weinstein GS, Chalian AA, Rosenthal DI. Relative risk of stroke in head and neck carcinoma patients treated with external cervical irradiation. Laryngoscope. 2002;112(10):1883–7. doi: 10.1097/00005537-200210000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Arthurs E, Hanna TP, Zaza K, Peng Y, Hall SF. Stroke after radiation therapy for head and neck cancer: what is the risk? Int J Radiat Oncol Biol Phys. 2016;96(3):589–96. doi: 10.1016/j.ijrobp.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Jagsi R, Griffith KA, Koelling T, Roberts R, Pierce LJ. Stroke rates and risk factors in patients treated with radiation therapy for early-stage breast cancer. J Clin Oncol. 2006;24(18):2779–85. doi: 10.1200/JCO.2005.04.0014. [DOI] [PubMed] [Google Scholar]
- 25.Aizer AA, Du R, Wen PY, Arvold ND. Radiotherapy and death from cerebrovascular disease in patients with primary brain tumors. J Neurooncol. 2015;124(2):291–7. doi: 10.1007/s11060-015-1839-5. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk IW, van der Pal HJ, van OS RM, et al. Risk of symptomatic stroke after radiation therapy for childhood cancer: a long-term follow-up cohort analysis. Int J Radiat Oncol Biol Phys. 2016;96(3):597–605. doi: 10.1016/j.ijrobp.2016.03.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.