Abstract
Purpose
The recommended first-line treatment for young children infected with human immunodeficiency virus (HIV) includes the liquid formulation of the co-formulated protease inhibitors lopinavir/ritonavir (Kaletra™). Clinical reports indicate that some children readily accept the taste of Kaletra, whereas others strongly reject it, which can deter therapeutic adherence and outcomes.
Methods
As a proof-of-concept approach, we used a sensory panel of genotyped adults to document the range of individual differences in the taste and palatability (hedonics) of the liquid formulation of Kaletra and other taste stimuli, including common excipients. Panelists rated taste sensations using generalized labelled magnitude scales to determine genotype-phenotype relationships. Several months later, they were retested to assess response reliability.
Findings
Not all panelists had the same sensory experience when tasting Kaletra. Palatability ratings varied widely, from moderate like to strongest imaginable dislike, and were reliable over time. The more irritating and bitter Kaletra tasted, the more disliked by the panelist. The more they disliked the taste of Kaletra, the more they disliked the taste of its excipient ethanol and the bitter stimulus denatonium. Those who experienced less bitter and sweeter taste sensations had a different genetic signature than the others. Bitterness and irritation ratings of Kaletra varied by the orphaned bitter receptor gene, TAS2R60, whereas sweetness ratings of Kaletra varied by the cold receptor gene, TRPM8 which is activated by menthol, an excipient of Kaletra. Neither genotype related to ratings for ethanol or denatonium, however.
Implications
The use of a sensory panel holds promise as a first step in determining the nature of individual differences in the palatability of existing pediatric drug formulations and sources of variation. In this era of personalized medicine, the need is great to develop psychophysical tools to determine which drugs will show variation in acceptance by children and whether patterns of individual variation in taste as assessed by adults mirror those of young patients.
Keywords: pediatric drug, liquid formulation, taste, hedonics, candidate genes, adult sensory panel
Introduction
The World Health Organization estimates that half of all pediatric patients do not take their medicines correctly1, in part because of the lack of “child-friendly,” good-tasting formulations2,3. It has been argued that the bitter taste of a drug is a sensory expression of its pharmacological activity4. And the more bitter its taste, the more likely children will reject it, especially when they are unable to swallow solid oral dosage forms (e.g., pills, capsules) which have the advantage of encapsulating the taste of the active pharmaceutical ingredients (APIs)1 and instead are treated with liquid formulations. However, recent discoveries shed light on why solid oral dosage forms do not completely bypass the negative effects of liquid formulations5. Beyond conscious taste and smell6, G protein-coupled receptors (GPCRs) are located in extraoral tissues, including upper airways7,8, throat9, lungs10, gut11 and skin12. While we have no conscious access to flavor information arising from these sites, relevant cell types in these tissues sense chemical stimuli leading to common side effects such as nausea, vomiting and skin irritation13,14.
To address the lack of adequate pediatric formulations, legislation was passed in the United States and European Union to create incentives for testing in children15,16 and collaborative initiatives highlighted gaps in knowledge, one of which is the lack of palatability assessments2,17–19. Because taste is the key problem in children’s acceptance of medicine2,3,20,21, authorities recommended that “palatability” assessments involve children whenever possible or taste-screening approaches (e.g., adult panels), provided the alternative method is examined for result transferability to children2,22–26.
A fundamental challenge to this global research priority is the lack of validated and universally agreed upon protocols for assessing the taste of medicines, from the earliest Phase 1 clinical trials when human volunteers can taste the medicine for the first time to FDA-approved drug use in clinical care. Furthermore, the expectation that a given medicine or formulation should taste good for an entire population of children fails to recognize the highly personal nature of taste, highlighting the importance of the inherent variation that makes each child a unique patient, commonly referred to as precision medicine27,28. While personal variation among adults exists in the taste of some drugs (e.g., propylthiouracil (PTU)29, quinine30,31, ibuprofen32), a fundamental challenge remains in assessing individual differences when the patients are newborns or young children, for whom there are no validated methods.
We conducted a proof-of-principle study to establish whether the taste of a pediatric medicine varied among a sensory panel of genotyped adults. To represent the ‘gestalt’ experience of pediatric patients, the medicine evaluated was the liquid formulation of the co-formulated protease inhibitors lopinavir/ritonavir (LPV/r, Kaletra) which is part of the first-line HIV treatment for children under three years of age33—the first dose can be delivered as early as two weeks of life34. We chose this drug because clinically experienced pediatric HIV care providers report that some, but not all, infants accept or tolerate the taste of LPV/r35, as evidenced by their refusal to take the medicine or by involuntary reactions such as gagging, nausea, and vomiting36. Likewise, some of their older pediatric (and adult) patients claim to like the taste of LPV/r, while others strongly dislike its taste34. To probe individual differences further, we genotyped for selected candidate genes involved in taste, chemical irritation and temperature perception.
Methods
Panelists
We recruited unrelated women (N=84) who were enrolled as adult sensory panelists in a research study on bitter taste perception and the efficacy of classic bitter blockers. We clarify that the use of a sensory panel in academic research can differ from its application in the food industry37. Like in other academic research32,38,39, the sensory panel in the present study is comprised of individuals who are trained in the use of psychophysical tools (gLMS, hedonic gLMS) that allow for valid across-group comparisons40,41. The sensory evaluation of the product, in this case a pediatric medicine, focuses on various dimensions of taste (e.g., bitterness) as well as palatability, and the output is the perception of the individual panelist. The Office of Regulatory Affairs at the University of Pennsylvania and the Institutional Review Board of the Children’s Hospital of Philadelphia approved all procedures and consent forms and the study complies with the Declaration of Helsinki for Medical Research involving Human Subjects. Each panelist gave written informed consent42 and we registered the trial at clinicaltrials.gov (NCT01407939).
Taste Stimuli
Stimuli, presented at room temperature when evaluated by panelists, included the currently available liquid formulation of Kaletra (Abbott Laboratories, USA) which contains the following excipients: ethanol, high fructose corn syrup, saccharin, sodium chloride, citric acid, menthol, peppermint oil, cotton candy, vanilla, and other flavors. Panelists also evaluated a battery of generally recognized as safe (GRAS) taste stimuli: common excipients (0.6 M sucrose, 0.3 M sodium chloride, 20% v/v ethanol) and bitter-tasting stimuli commonly used in taste research (but not contained in Kaletra): 0.008 M caffeine, 0.5 M urea, 4.92 × 10−7 M denatonium, 560 μM propylthiouracil (PTU), and 1.19 × 10−4 M quinine. We included PTU and quinine because they are medications used historically to treat thyroid disorders43,44 and malaria45, respectively. We also included PTU as a control stimulus since the association between its taste and TAS2R38 genotype is one of the most studied and robust traits in human genetics29,42,46–49 and is now regarded as a benchmark in genotype-taste phenotype and genome wide association studies50.
Psychophysical Procedures
Panelists rated the taste sensations (i.e., bitterness, sweetness, saltiness, sourness, savoriness), and irritation using the general Labeled Magnitude Scale (gLMS) and palatability using the hedonic gLMS. Both scales have been validated and shown to be superior to other rating scales (e.g., 9-point scales) when comparing taste sensations among individuals40,41,51,52. The gLMS53 rates taste sensation in terms of no sensation (0), barely detectable (1.4), weak (5.8), moderate (16), strong (35), very strong (53), and strongest imaginable (100). The hedonic gLMS scale40 rates affective experiences using the same anchors in terms of the strongest imaginable liking (100) to strongest imaginable disliking (−100) of any kind, with zero equal indicating no preference. Both scales allow for the rating of perceived intensity along a vertical axis lined with adjectives that are spaced quasi-logarithmically to yield ratio-quality data52,53.
After abstaining from eating for at least 1 hour, panelists tasted but did not swallow, in randomized order, 5 mL of each taste stimulus. After tasting each sample for 5 sec via a swish-and-spit protocol, panelists rated its taste and palatability on a computer with Compusense five Plus software (Compusense Inc., Guelph, Canada). One minute separated the presentation of each stimulus, during which panelists rinsed their mouths with water. Several months later, panelists were retested using identical methodologies to establish reliability of Kaletra ratings over time. While Kaletra was tasted without nose clips (to mimic the patient’s experience) during both test days, the other taste stimuli were tasted without nose clips on the second day only.
Genotype Procedures
Participants provided DNA samples extracted from saliva (BuccalAmp, Epicenter, Madison, WI, or Genotek, Kanata, Canada). Samples were diluted to a concentration of 5 ng μL−1, which was used as templates in Taqman assays and assayed in duplicate for single-nucleotide polymorphisms (Applied Biosystems, Foster City, CA) using previously established methods54. We selected candidate genes that encode for: 1) bitter taste receptors TAS2R38 Ala49Pro rs71359855; TAS2R60 rs459503556,57, TAS2R44 rs10845294; TAS2R4 rs2234002 because ritonavir is often described as bitter58; 2) the sweet taste receptor TAS1R3 rs3574481359 because high fructose corn syrup and saccharin are excipients; and 3) transient receptor potential [TRP] cation channel subfamily M member 5, TRPM5 rs230169, the final step in the sweet taste receptor transduction cascade that leads to receptor depolarization60; and 4) TRPM8 rs7593557, one of the main cold temperature sensors since menthol and peppermint oil are excipients61.
Statistical Analyses
We computed descriptive statistics, including tests for normality in the taste ratings of Kaletra, and Pearson correlations to assess test-retest reliability and relationships among psychophysical ratings of different taste stimuli. Analyses of variance were used to probe whether taste ratings of Kaletra differed by candidate genotype. Data were analyzed using Statistica (version 13.1, StatSoft, Tulsa, OK) and Stata/IC (version 14.2, College Station, TX) and data are expressed as mean ± standard error of the mean (SEM). For all analyses, the statistical significance level was set at p ≤ 0.05 (two tailed).
Results
Panelists
Panelists averaged 36.4 ± 0.9 (range, 23–58) years of age and represented the ethnic diversity of the city in which they reside, Philadelphia, PA, USA62 (67% African-American, 15% White, 2% Asian, 16% more than one race). Most panelists (n=73, 87%) returned several months later (10.7±0.3 months) for the second (retest) session.
Psychophysics
As expected, ratings of saltiness, sourness and savoriness were rarely given so the analyses focused on variation in ratings of bitterness and palatability (primary outcomes) and then sweetness and irritation, each of which was normally distributed (Shapiro-Wilk normality test: bitterness, W=0.90; irritation, W=0.93; sweetness, W=0.80; all p-values<0.001). Upon first presentation of Kaletra, bitterness ratings averaged 17 (± 2) and ranged from 0 (no sensation) to 57 (very strong), sweetness ratings averaged 13 (± 2) and ranged from 0 (no sensation) to 53 (very strong), whereas irritation ratings averaged 27 (± 2) and ranged from 0 (no sensation) to 96 (very strong to strongest imaginable). Likewise, hedonic ratings of its taste varied widely, ranging from +35 (moderate like) to −92 (very strong to strongest imaginable dislike). The average rating was −28 ± 2, in the range of “moderate to strong dislike” sensation. Hedonic ratings for the taste of Kaletra were negatively related to ratings of bitterness [r(82df)= −0.64, p<0.001] and irritation [r(82df)=−0.73, p<0.001] but not sweetness (p=0.42). The higher the ratings of bitterness, the higher the ratings of irritation [r(82df)=0.70, p<0.001]; multiple regression revealed both were significant predictors of hedonic ratings of Kaletra (p’s<0.001).
As shown in Figure 1, the bitterness [r(71df)=0.36, p=0.002], irritation [r(71df)=0.54, p<0.001], and hedonic [r(71df)=0.66; p=<0.001] ratings for Kaletra overall were reliable over time. Panelists who did not return (n=11) did not rate the taste of the drug as more unpleasant during the first session than those who came back for retesting (p=0.69). Palatability ratings for the taste of Kaletra were significantly related to palatability ratings for the excipient ethanol [r(71df)=0.31, p= 0.007] and for the bitter stimulus denatonium [r(71df)=0.23, p= 0.045] but not for quinine, urea, caffeine, PTU, sodium chloride, sucrose, or citric acid (all p-values>0.18).
Figure 1.
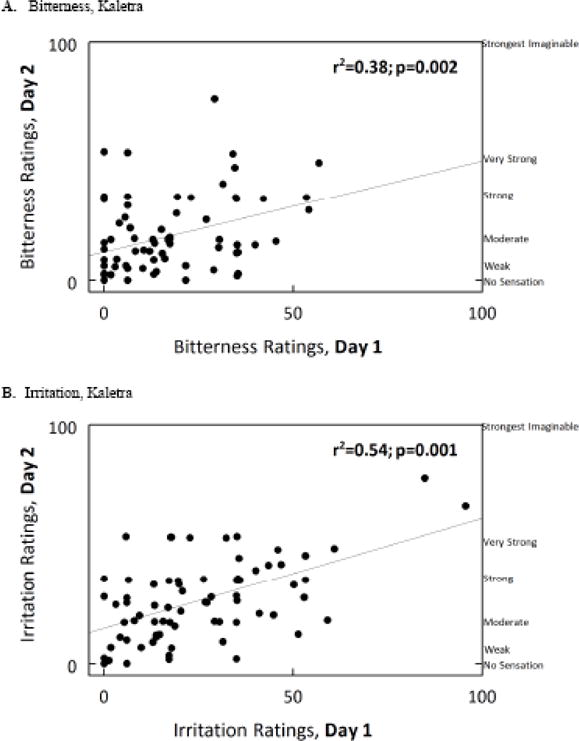
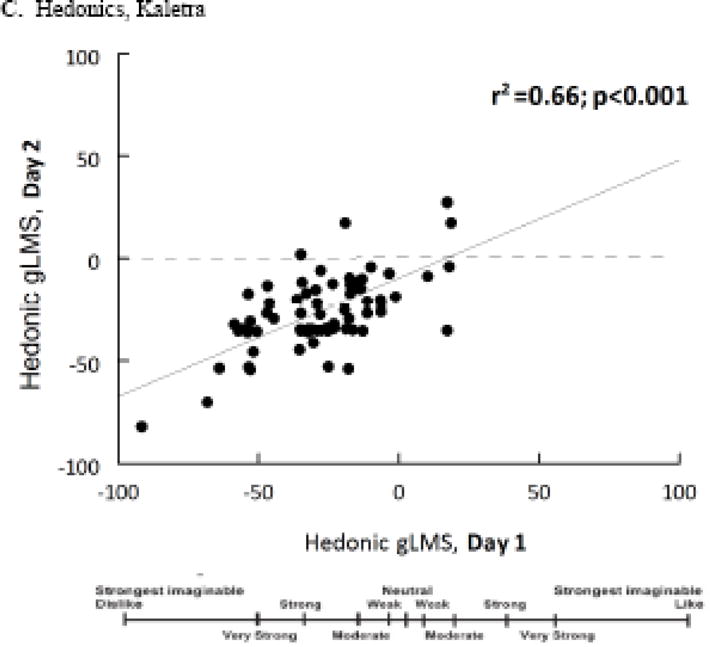
Genotyping
As shown in Table 1, the genotype call rates for the candidate genes were high (94–100%) and met Hardy-Weinberg equilibrium. Bitterness and irritation ratings for Kaletra varied by TAS2R60 genotype, with CC individuals rating the bitterness of Kaletra lower than CT or TT individuals (Figure 2). However, this genotype did not account for variation in the bitterness ratings for ethanol or denatonium (Table 1). For the TRPM8 genotype (but not TRPM5), individuals with an A allele perceived Kaletra to taste less sweet. The expected pattern was observed for PTU ratings and TAS2R38 genotype. The association displayed a heterozygote effect: the bitter-sensitive (P) form such that two copies of the bitter sensitive form (P) conferred higher bitterness and irritation and lower pleasantness ratings than did a single copy (AP), and results for both the PP and AP genotypes were different from those for the bitter-insensitive AA genotype (Figure 2). We tested for specificity of response by examining the effects of TAS2R60 on bitter response to PTU and TAS2R38 on response to Kaletra but found no relationships (TAS2R60 on PTU: p=0.22, TAS2R38 on Kaletra: p=0.71).
Table 1.
Genotype associations with taste ratings of Kaletra™ and PTU (control drug)
| Chromosome | Gene | Variant Name | Call Rate (%) | HWEa p-Value | Taste Quality, Drug | p-Value |
|---|---|---|---|---|---|---|
| 7 | TAS2R60 | rs4595035 | 94.0 | 0.8928 | Bitterness, Kaletra | p=0.017 |
| Irritation, Kaletra | p=0.054 | |||||
| Hedonics, Kaletra | p=0.073 | |||||
|
| ||||||
| 7 | TAS2R38 | rs713598 | 100.0 | 0.5436 | Bitterness, Kaletra | p=0.594 |
| Irritation, Kaletra | p=0.504 | |||||
| Hedonics, Kaletra | p=0.907 | |||||
|
| ||||||
| 12 | TAS2R44 | rs10845293 | 96.4 | 0.3247 | Bitterness, Kaletra | p=0.252 |
| Irritation, Kaletra | p=0.914 | |||||
| Hedonics, Kaletra | p=0.277 | |||||
|
| ||||||
| 7 | TAS2R4 | rs2234002 | 97.6 | 0.2202 | Bitterness, Kaletra | p=0.769 |
| Irritation, Kaletra | p=0.694 | |||||
| Hedonics, Kaletra | p=0.837 | |||||
|
| ||||||
| 1 | TAS1R3 | rs35744813 | 97.6 | 0.9057 | Bitterness, Kaletra | p=0.780 |
| Irritation, Kaletra | p=0.388 | |||||
| Sweetness, Kaletra | p=0.670 | |||||
| Hedonics, Kaletra | p=0.572 | |||||
|
| ||||||
| 2 | TRPM8 | rs7593557 | 96.4 | 0.3542 | Bitterness, Kaletra | p=0.344 |
| Irritation, Kaletra | p=0.424 | |||||
| Sweetness, Kaletra | p=0.008 | |||||
| Hedonics, Kaletra | p=0.893 | |||||
|
| ||||||
| 11 | TRPM5 | rs2301699 | 98.8 | 0.7780 | Bitterness, Kaletra | p=0.830 |
| Irritation, Kaletra | p=0.597 | |||||
| Sweetness, Kaletra | p=0.367 | |||||
| Hedonics, Kaletra | p=0.206 | |||||
|
| ||||||
| 7 | TAS2R60 | rs4595035 | 94.0 | 0.8928 | Bitterness, PTU | p=0.218 |
| Irritation, PTU | p=0.527 | |||||
| Hedonics, PTU | p=0.420 | |||||
|
| ||||||
| 7 | TAS2R38 | rs713598 | 100.0 | 0.5436 | Bitterness, PTU | p=0.00008 |
| Irritation, PTU | p=0.00068 | |||||
| Hedonics, PTU | p=0.00000 | |||||
|
| ||||||
| 12 | TAS2R44 | rs10845293 | 96.4 | 0.3247 | Bitterness, PTU | p=0.972 |
| Irritation, PTU | p=0.355 | |||||
| Hedonics, PTU | p=0.925 | |||||
|
| ||||||
| 7 | TAS2R4 | rs2234002 | 97.6 | 0.2202 | Bitterness, PTU | p=0.0164 |
| Irritation, PTU | p=0.0451 | |||||
| Hedonics, PTU | p=0.067 | |||||
|
| ||||||
| 1 | TAS1R3 | rs35744813 | 97.6 | 0.9057 | Bitterness, PTU | p=0.919 |
| Irritation, PTU | p=0.897 | |||||
| Sweetness, PTU | p=0.256 | |||||
| Hedonics, PTU | p=0.948 | |||||
|
| ||||||
| 2 | TRPM8 | rs7593557 | 96.4 | 0.3542 | Bitterness, PTU | p=0.353 |
| Irritation, PTU | p=0.430 | |||||
| Sweetness, PTU | p=0.682 | |||||
| Hedonics, PTU | p=0.743 | |||||
|
| ||||||
| 11 | TRPM5 | rs2301699 | 98.8 | 0.7780 | Bitterness, PTU | p=0.939 |
| Irritation, PTU | p=0.620 | |||||
| Sweetness, PTU | p=0.863 | |||||
| Hedonics, PTU | p=0.709 | |||||
HWE, Hardy-Weinburg equilibrium. Bold indicates significant at p<0.05.
Figure 2.

Discussion
Not all adult panelists had the same sensory experience when tasting the liquid formulation of the HIV treatment drug Kaletra (LPV/r), a finding consistent with clinical reports on pediatric HIV patients2,5,63. This individual variation was consistent over time and related to how bitter and irritating the liquid formulation tasted. These individual differences were genetic in origin. That the genotype-phenotype association between the TAS2R38 genotype and bitterness ratings of PTU was observed as expected29,42,46–49 provides internal validity for the following genotype-phenotype findings.
Those who perceived Kaletra as tasting less bitter possessed nonfunctional receptors of the TAS2R60 gene, albeit to date considered an orphan receptor57. While variation in perceived sweetness did not associate with sweet receptor gene TAS1R3, it did associate with gene related to variation in the TRPM8 gene, also known as the cold and menthol receptor 1. Indeed Kaletra contains the excipients saccharin and high fructose corn syrup and all panelists perceived sweetness, albeit at varying levels. Although taste stimuli were presented at room temperature, we hypothesized that individuals with functional TRPM8 genotypes perceived greater cooling sensation due to activation of receptor by menthol and in turn perceived less sweetness (or greater sweetness adaptation). We based this hypothesis on the following lines of evidence. First, the cold-receptor TRPM8 is activated by low levels of menthol61, an excipient of Kaletra. Second, while no research to date has determined whether menthol-containing solutions in the oral cavity changes how cool the solution tastes, we know that direct application of menthol to the skin leads to perceptions of coolness64. Third, actual cooling of the oral cavity leads to reductions in the perceived intensity of some sweeteners, including saccharin, an excipient in Kaletra65. Clearly more research is needed to determine the contribution of the taste-mixture interactions of the excipients66 to variation in how the patient perceives the formulation16.
Variation in the ratings of the taste of Kaletra was strongly tied to individual differences in the taste of ethanol, one of its excipients, and denatonium, a bitter agent once added to detergents, paints, and antifreeze to deter pediatric poisoning but children varied in how effective it was in deterrence67,68. That personal variation in either ethanol, as previously reported by Nolden and colleagues69, or denatonium ratings was not explained by TAS2R60 or TRPM8 genotypes suggests that some other ingredients in this liquid formulation, most likely the APIs, are driving the genotype-phenotype relationships.
Our experimental approach was to test not a liquid formulation in development but one currently used by millions of children in treatment. Studying the liquid formulation as a whole was chosen because our primary aim was to establish whether there was wide variation in adults, as has been reported clinically for children35,36. But by having the panelists taste the liquid formulation, we cannot determine whether the distaste was due solely to the active ingredients (LPV/r) or to the taste-mixture interaction among the wide range of excipients needed to dissolve the APIs (e.g., ethanol69) and/or to mask bitterness of the drug (e.g., sweeteners)66.
These data provide proof of principle that a genotyped adult sensory panel can be used to assess whether individual variation exists in the taste of pediatric formulations (see also ref32). Panelists used validated psychophysical tools that allow for the comparisons across individual adults53 but tools that are more cognitively demanding than that typically used to assess taste perception and hedonics in children70. We genotyped the panel for a few known candidate genes and related variation in genotype to variation in ratings since we were aware clinically of its importance in medication acceptance. However, we emphasize the preliminary nature of our findings and that the number of comparisons made increases the possibility of an alpha error highlighting the need and importance for replication in a larger cohort.
These findings provide the necessary tools and foundation for the next steps which in addition to replication will include evaluating the contribution of additional known genes regulating multiple points along the taste transduction pathway, in the periphery and along the central pathway71. That many bitter compounds do not act through known taste receptors72, suggests other receptor families and pathways exist7,72. This research also lays the foundation for objectively measuring individual differences—the hallmark of human perception—by not only panelists but also patients. Direct assessments of the variability in taste acceptance/palatability of LPV/r among pediatric patients is critical71, especially because such human variation cannot be measured by sensors such as the electronic tongue in its current form.71 The approach of converging information from genotyped adult panels and pediatric patients should not be limited to LPV/r but should extend to other drugs and therapeutics and their excipients including flavor volatiles.
Taste-testing standards for pediatric formulations are past due. To meet these goals, we need nonproprietary and validated psychophysical methodologies to assess personal variation in the taste of a medicine among children71. In developing such methods, we need to take the special needs of pediatric populations into consideration,2 including their sense of taste, which sometimes differs from that of adults73. These methods should determine initial responses to both the taste of the drug and tolerance after swallowing, to capture the full range of individual differences in sensory response, including the impact of the full range of receptors along the throat, gastrointestinal tract and other parts of the body7. Factors that impact taste perception may relate to side effects of the drug (e.g., vomiting, nausea, abdominal distress14). Of interest, side effects were evident in the initial phase of treatment of Kaletra, whether children were given either liquid or “sprinkles” formulations mixed with food, perhaps due in part to the dissolved API binds to these downstream “taste” receptors5. In some cases, these aversive side effects conditioned future responses—merely smelling the drug after prior exposures resulted in gagging and vomiting in some infants, making adherence challenging for some5,63.
Caregivers view improving the taste of a drug as the most needed intervention to increase antiretroviral therapy adherence in their children.35 This point is particularly important for children in sub-Saharan Africa, where more than 200,000 children are born each year infected with HIV, many of whom are prescribed LPV/r. Without treatment, more than 50% of HIV-infected infants die before their second birthday74. The time is ripe to focus on personal variation to the taste of their medicine75 since a drug, no matter how powerful, is ineffective when the child rejects it71. By taking the perspective of precision medicine that “nobody is average”, a greater understanding of the genetic and phenotypic variation underlying the flavor of a given medicine may inform the clinical care of the patient and lead to molecular targets to improve palatability.
Acknowledgments
We acknowledge the expert technical assistance of Kristi Roberts, Loma Inamdar, Ashley Reiter, and Jennifer Chapman; the valuable discussions with Drs. Alissa Nolden and Danielle Reed; and the genotyping by Dr. Danielle Reed. All authors participated in the research, data analyses, and JM and EL wrote the manuscript. All authors have approved the final version of the manuscript.
Funding
The research was supported by the National Institute of Deafness and Other Communication Disorders (NIDCD), National Institutes of Health (NIH), grant R01 DC011287. Genotyping was performed at the Genotyping and DNA/RNA Analysis Core (NIH-NIDCD core grant P30DC011735) under the supervision of Dr. Reed. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIDCD or NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical Trials Registration: Genetic variability in taste perception study: https://clinicaltrials.gov/ct2/show/NCT01841710; registry number NCT01841710, registered 24 October 2013.
Abbreviations: API, active pharmaceutical ingredient; gLMS, general Labeled Magnitude Scale; GPCRs, G protein-coupled receptors; GRAS, generally recognized as safe; HIV, human immunodeficiency virus; HWE, Hardy-Weinberg equilibrium; LPV/r, lopinavir/ritonavir; PTU, propylthiouracil; SEM, standard error of the mean; TRP, transient receptor potential
Conflicts of interest: none.
References
- 1.World Health Organization. Medicines: rational use of medicines, Fact sheet N°338. 2010 http://www.wiredhealthresources.net/resources/NA/WHO-FS_MedicinesRationalUse.pdf. Accessed April 19, 2017.
- 2.Ivanovska V, Rademaker CM, van Dijk L, Mantel-Teeuwisse AK. Pediatric drug formulations: a review of challenges and progress. Pediatrics. 2014;134:361–372. doi: 10.1542/peds.2013-3225. [DOI] [PubMed] [Google Scholar]
- 3.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59:674–676. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer R, Griffin F. Pharmacogenetic aspects of gustation. Arzneimittelforschung. 1964;14:673–686. [PubMed] [Google Scholar]
- 5.Musiime V, Fillekes Q, Kekitiinwa A, et al. The pharmacokinetics and acceptability of lopinavir/ritonavir minitab sprinkles, tablets, and syrups in african HIV-infected children. J Acquir Immune Defic Syndr. 2014;66:148–154. doi: 10.1097/QAI.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 6.Foster SR, Roura E, Thomas WG. Extrasensory perception: odorant and taste receptors beyond the nose and mouth. Pharmacol Ther. 2014;142:41–61. doi: 10.1016/j.pharmthera.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Finger TE, Kinnamon SC. Taste isn’t just for taste buds anymore. F1000 Biol Rep. 2011;3:20. doi: 10.3410/B3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee RJ, Cohen NA. Role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Curr Opin Allergy Clin Immunol. 2015;15:14–20. doi: 10.1097/ACI.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikut-Ligaj D, Trzcielinska-Lorych J. How taste works: cells, receptors and gustatory perception. Cell Mol Biol Lett. 2015;20:699–716. doi: 10.1515/cmble-2015-0042. [DOI] [PubMed] [Google Scholar]
- 10.An SS, Liggett SB. Taste and smell GPCRs in the lung: Evidence for a previously unrecognized widespread chemosensory system. Cell Signal. 2017 doi: 10.1016/j.cellsig.2017.02.002. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan JM, Margolskee RF. Taste cells of the gut and gastrointestinal chemosensation. Mol Interv. 2008;8:78–81. doi: 10.1124/mi.8.2.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reszka E, Nowakowska-Swirta E, Kupczyk M, et al. Expression of bitter taste receptors in the human skin in vitro. J Clinic Res Bioeth. 2015;6:218. [Google Scholar]
- 13.Miller LG, Daum RS, Creech CB, et al. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med. 2015;372:1093–1103. doi: 10.1056/NEJMoa1403789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyrot des Gachons C, Beauchamp GK, Stern RM, Koch KL, Breslin PA. Bitter taste induces nausea. Curr Biol. 2011;21:R247–248. doi: 10.1016/j.cub.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Giacoia GP, Taylor-Zapata P, Zajicek A. Eunice Kennedy Shriver National Institute of Child Health and Human Development Pediatrics Formulation Initiative: Proceedings from the Second Workshop on Pediatric Formulations. Clin Ther. 2012;34:S1–S10. doi: 10.1016/j.clinthera.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 16.Salunke S, Liu F, Batchelor H, et al. European Paediatric Formulation Initiative (EuPFI)-Formulating ideas for better medicines for children. AAPS PharmSciTech. 2017;18:257–262. doi: 10.1208/s12249-016-0584-1. [DOI] [PubMed] [Google Scholar]
- 17.Tuleu C, Breitkreutz J. Educational paper: formulation-related issues in pediatric clinical pharmacology. Eur J Pediatr. 2013;172:717–720. doi: 10.1007/s00431-012-1872-8. [DOI] [PubMed] [Google Scholar]
- 18.Salunke S, Tuleu C. Formulating better medicines for children-Still too far to walk. Int J Pharm. 2016;511:1124–1126. doi: 10.1016/j.ijpharm.2016.06.114. [DOI] [PubMed] [Google Scholar]
- 19.Giacoia GP, Taylor-Zapata P, Mattison D. Eunice Kennedy Shriver National Institute of Child Health and Human Development Pediatric Formulation Initiative: selected reports from working groups. Clin Ther. 2008;30:2097–2101. doi: 10.1016/j.clinthera.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Venables R, Stirling H, Batchelor H, Marriott J. Problems with oral formulations prescribed to children: a focus group study of healthcare professionals. Int J Clin Pharmacol Res. 2015;37:1057–1067. doi: 10.1007/s11096-015-0152-x. [DOI] [PubMed] [Google Scholar]
- 21.Venables R, Marriott J, Stirling H. FIND OUT: key problems with children’s medicines formulations … It’s a taste issue! Int J Pharm Pract. 2012;20:23. [Google Scholar]
- 22.Cram A, Breitkreutz J, Desset-Brethes S, Nunn T, Tuleu C. Challenges of developing palatable oral paediatric formulations. Int J Pharm. 2009;365:1–3. doi: 10.1016/j.ijpharm.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Matsui D. Assessing palatability of medicines in children. Paediatr Perinatal Drug Ther. 2007;8:55–60. [Google Scholar]
- 24.Liu F, Ranmal S, Batchelor HK, et al. Formulation factors affecting acceptability of oral medicines in children. Int J Pharm. 2015;492:341–343. doi: 10.1016/j.ijpharm.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Zajicek A, Fossler MJ, Barrett JS, et al. A report from the pediatric formulations task force: perspectives on the state of child-friendly oral dosage forms. AAPS J. 2013;15:1072–1081. doi: 10.1208/s12248-013-9511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Medicines Agency Committee for Medicinal Products for Human Use and Paedatric Committee. Guideline on pharmaceutical development of medicines for paediatric use (adopted) 2013 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf.
- 27.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maagdenberg H, Vijverberg SJ, Bierings MB, et al. Pharmacogenomics in pediatric patients: Towards personalized medicine. Paediatr Drugs. 2016;18:251–260. doi: 10.1007/s40272-016-0176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bufe B, Breslin PA, Kuhn C, et al. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed DR, Zhu G, Breslin PA, et al. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 2010;19:4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayes JE, Feeney EL, Nolden AA, McGeary JE. Quinine bitterness and grapefruit liking associate with allelic variants in TAS2R31. Chem Senses. 2015;40:437–443. doi: 10.1093/chemse/bjv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bennett SM, Zhou L, Hayes JE. Using milk fat to reduce the irritation and bitter taste of ibuprofen. Chemosens Percept. 2012;5:231–236. doi: 10.1007/s12078-012-9128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Antiretroviral therapy of HIV Infection in infants and children: towards universal access: recommendations for a public health approach-2010 revision. Geneva, Switzerland: WHO; 2010. [PubMed] [Google Scholar]
- 34.Martinez BL, Riordan FA. Novel strategies in the use of lopinavir/ritonavir for the treatment of HIV infection in children. HIV AIDS (Auckl) 2010;2:59–67. doi: 10.2147/hiv.s6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dyke RB, Lee S, Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 36.Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawless HT, Heymann H. Sensory evaluation of food - principles and practices. 2nd. New York: Springer; 2010. [Google Scholar]
- 38.Gilbert JL, Guthart MJ, Gezan SA, et al. Identifying breeding priorities for blueberry flavor using biochemical, sensory, and genotype by environment analyses. PLoS One. 2015;10:e0138494. doi: 10.1371/journal.pone.0138494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwieterman ML, Colquhoun TA, Jaworski EA, et al. Strawberry flavor: diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS One. 2014;9:e88446. doi: 10.1371/journal.pone.0088446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalva JJ, Sims CA, Puentes LA, Snyder DJ, Bartoshuk LM. Comparison of the hedonic general Labeled Magnitude Scale with the hedonic 9-point scale. J Food Sci. 2014;79:S238–245. doi: 10.1111/1750-3841.12342. [DOI] [PubMed] [Google Scholar]
- 41.Snyder DJ, Prescott J, Bartoshuk LM. Modern psychophysics and the assessment of human oral sensation. Adv Otorhinolaryngol. 2006;63:221–241. doi: 10.1159/000093762. [DOI] [PubMed] [Google Scholar]
- 42.Drayna D, Coon H, Kim UK, et al. Genetic analysis of a complex trait in the Utah Genetic Reference Project: a major locus for PTC taste ability on chromosome 7q and a secondary locus on chromosome 16p. Human Genetics. 2003;112:567–572. doi: 10.1007/s00439-003-0911-y. [DOI] [PubMed] [Google Scholar]
- 43.Rivkees SA. Controversies in the management of Graves’ disease in children. J Endocrinol Invest. 2016;39:1247–1257. doi: 10.1007/s40618-016-0477-x. [DOI] [PubMed] [Google Scholar]
- 44.Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 45.Nikolaeva ND, Tsibina VF. The treatment of malaria in children with omnoquinon. Pediatriia. 1945;65 [PubMed] [Google Scholar]
- 46.Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28:111–142. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duffy VB, Davidson AC, Kidd JR, et al. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–1637. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen AL, McGeary JE, Knopik VS, Hayes JE. Bitterness of the non-nutritive sweetener acesulfame potassium varies with polymorphisms in TAS2R9 and TAS2R31. Chem Senses. 2013;38:379–389. doi: 10.1093/chemse/bjt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mennella JA, Pepino MY, Duke FF, Reed DR. Psychophysical dissection of genotype effects on human bitter perception. Chem Senses. 2011;36:161–167. doi: 10.1093/chemse/bjq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Genick UK, Kutalik Z, Ledda M, et al. Sensitivity of genome-wide-association signals to phenotyping strategy: the PROP-TAS2R38 taste association as a benchmark. PLoS One. 2011;6:e27745. doi: 10.1371/journal.pone.0027745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartoshuk LM. Psychophysics: a journey from the laboratory to the clinic. Appetite. 2004;43:15–18. doi: 10.1016/j.appet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “Labeled Magnitude Scale’ for measuring sensations of taste and smell. Chem Senses. 1996;21:323–324. doi: 10.1093/chemse/21.3.323. [DOI] [PubMed] [Google Scholar]
- 53.Bartoshuk LM, Duffy VB, Green BG, et al. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 54.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–222. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mennella JA, Pepino MY, Duke FF, Reed DR. Age modifies the genotype-phenotype relationship for the bitter receptor TAS2R38. BMC Genet. 2010;11:60. doi: 10.1186/1471-2156-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knaapila A, Hwang LD, Lysenko A, et al. Genetic analysis of chemosensory traits in human twins. Chem Senses. 2012;37:869–881. doi: 10.1093/chemse/bjs070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thalmann S, Behrens M, Meyerhof W. Major haplotypes of the human bitter taste receptor TAS2R41 encode functional receptors for chloramphenicol. Biochem Biophys Res Commun. 2013;435:267–273. doi: 10.1016/j.bbrc.2013.04.066. [DOI] [PubMed] [Google Scholar]
- 58.O’Neil MJ, Heckelman PE, Dobbelaar PH, et al. The Merck index: an encylopedia of chemicals, drugs, and biological. 15th. Cambridge, UK: Royal Society of Chemistry; 2013. [Google Scholar]
- 59.Mennella JA, Reed DR, Mathew PS, Roberts KM, Mansfield CJ. A spoonful of sugar helps the medicine go down”: bitter masking by sucrose among children and adults. Chem Senses. 2015;40:17–25. doi: 10.1093/chemse/bju053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liman ER. TRPM5 and taste transduction. Handb Exp Pharmacol. 2007:287–298. doi: 10.1007/978-3-540-34891-7_17. [DOI] [PubMed] [Google Scholar]
- 61.Paschke M, Tkachenko A, Ackermann K, Hutzler C, Henkler F, Luch A. Activation of the cold-receptor TRPM8 by low levels of menthol in tobacco products. Toxicol Lett. 2017;271:50–57. doi: 10.1016/j.toxlet.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 62.Pew Charitable Trust. Philadelphia: The State of the City, A 2016 update. 2016 http://www.pewtrusts.org/en/research-and-analysis/reports/2016/03/philadelphia-the-state-of-the-city-a-2016-update. Accessed June 21, 2017.
- 63.Buchanan AL, Montepiedra G, Sirois PA, et al. Barriers to medication adherence in HIV-infected children and youth based on self- and caregiver report. Pediatrics. 2012;129:e1244–1251. doi: 10.1542/peds.2011-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campero M, Baumann TK, Bostock H, Ochoa JL. Human cutaneous C fibres activated by cooling, heating and menthol. J Physiol. 2009;587:5633–5652. doi: 10.1113/jphysiol.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Green BG, Nachtigal D. Temperature affects human sweet taste via at least two mechanisms. Chem Senses. 2015;40:391–399. doi: 10.1093/chemse/bjv021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Green BG, Lim J, Osterhoff F, Blacher K, Nachtigal D. Taste mixture interactions: suppression, additivity, and the predominance of sweetness. Physiol Behav. 2010;101:731–737. doi: 10.1016/j.physbeh.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.White NC, Litovitz T, Benson BE, Horowitz BZ, Marr-Lyon L, White MK. The impact of bittering agents on pediatric ingestions of antifreeze. Clin Pediatr (Phila) 2009;48:913–921. doi: 10.1177/0009922809339522. [DOI] [PubMed] [Google Scholar]
- 68.Silbert JE, Frude N. Bittering agents in the prevention of pediatric poisonings: Children’s reactions for denatonium benzoate (Bitrex) Arch Emerg Med. 1991;8:1–7. doi: 10.1136/emj.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nolden AA, McGeary JE, Hayes JE. Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol Behav. 2016;156:117–127. doi: 10.1016/j.physbeh.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Forestell CA, Mennella JA. The ontogeny of taste perception and preference throughout childhood. In: Doty RL, editor. Handbook of Olfaction and Gustation. 3rd. New York: Wiley-Liss; 2015. pp. 797–830. [Google Scholar]
- 71.Mennella JA, Spector AC, Reed DR, Coldwell SE. The bad taste of medicines: Overview of basic research on bitter taste. Clin Ther. 2013;35:1225–1246. doi: 10.1016/j.clinthera.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lossow K, Hubner S, Roudnitzky N, et al. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 2016;291:15358–15377. doi: 10.1074/jbc.M116.718544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mennella JA, Bobowski NK. The sweetness and bitterness of childhood: Insights from basic research on taste preferences. Physiol Behav. 2015;152:502–507. doi: 10.1016/j.physbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Penazzato M, Prendergast A, Tierney J, Cotton M, Gibb D. Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age. Cochrane Database Syst Rev. 2012;7:CD004772. doi: 10.1002/14651858.CD004772.pub3. [DOI] [PubMed] [Google Scholar]
- 75.Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat. 2016;2016:1654938. doi: 10.1155/2016/1654938. [DOI] [PMC free article] [PubMed] [Google Scholar]


