Abstract
Objective
Complex Crawford extent II thoracoabdominal aortic aneurysms (TAAA) can be treated in a hybrid manner with proximal thoracic endovascular aneurysm repair, followed by staged distal open thoracoabdominal repair. The purpose of this study was to evaluate the outcomes and healthcare associated value of this new method compared to traditional open repair over 10 years.
Methods
A prospectively collected database was used to identify all patients with extent II TAAA undergoing repair at a single institution between 2005 and 2015. Patient characteristics, post-operative outcomes, and incidence of major adverse events (MAE = renal failure, spinal cord ischemia, death) were compared. After adjusting for time since surgery, value was analyzed looking at quality (1/MAE) divided by cost (total health system cost). This is multiplied by a constant to set the value of open TAAA repair to 100.
Results
A total of 113 consecutive patients underwent extent II TAAA repairs, of which 25 (22.1%) had a staged hybrid approach with a median of 129 days between procedures. No baseline differences in demographic or comorbidity variables existed between groups (p>0.05). The hybrid group had shorter operative time (255 vs 306 minutes; p=0.01), shorter postoperative length of stay (LOS) (10.1 vs 13.3 days; p=0.02), as well as reduced blood loss (1300 vs 2600 mL; p=0.01) at the time of open operation. Despite higher rates of acute kidney injury in the hybrid group (76.0%vs 51.1%, p=0.03) there was no difference in renal failure (8.0% vs 4.5%, p=0.84) The incidence of MAE was lower in the staged hybrid group (20.0% vs 48.9%; p=0.01), without a difference in hospital mortality (4.0 vs 3.4%, p=0.89). Median total cost was higher in the hybrid group ($112,920 vs $72,037, p=0.003). Value was improved in the hybrid group by 56% using mean cost and 178% by median cost.
Conclusions
The 20% major adverse event rate associated with staged hybrid repair of extent II TAAA was significantly decreased compared to open repair, with a relative reduction of over 50%. Despite higher total hospital costs, staged hybrid repair had 56% to 178% higher healthcare related value compared to standard open repair. In an era of increasing focus on costs and quality, staged hybrid repair of extensive TAAAs is associated with fewer complications than open TAAA repair resulting in a good value investment from a resource utilization perspective.
Introduction
Thoracoabdominal aortic aneurysms (TAAA) have a dismal, progressive course with an inflection point at 7cm where over 40% will rupture.1-3 Since no effective medical therapy currently exists, standard treatment involves open surgical replacement of the aneurysmal aorta. Crawford classification categorizes TAAAs, where extent II involves the longest length of aorta from the left subclavian artery to the aortoiliac bifurcation. Typically repair of extent II aneurysms involves replacement of the descending and abdominal aorta to the bifurcation. As these repairs are the most extensive performed for aortic aneurysms, they are associated with the highest complication rates.3-8
Extent II aneurysm repair has incrementally evolved and protocols currently emphasize multimodal organ protection.3, 9-11 This includes permissive hypothermia, circulatory support with selective renal and visceral perfusion, cerebrospinal fluid drainage, and aggressive reimplantation of segmental arteries. Newer evidence suggests that staged hybrid repair can supplement these techniques to further reduce morbidity.12-18 The results suggest that thoracic endovascular repair followed by open distal repair is a safe and reasonable repair strategy. However, there is limited evidence regarding outcomes compared to open repair. Additionally, staged hybrid repair requires multiple procedures that are likely to attract critique in an era of value based care models. New technology is associated with high development costs and these charges will require justification with improved outcomes.
The concept of health care related value was most eloquently promoted by Porter and Teisberg where they describe restructuring our health system to maximize health outcomes per dollar spent.19 Critical to this idea is shifting from a provider and volume based analysis of outcomes to a patient centered analysis of efficiency.20, 21 This idea is separate from cost-effectiveness analyses, and instead encompasses quality, efficiency, costs and complications. The result is a simple metric, a ratio of outcomes to cost. This idea is being translated into alternative payment models that reward value, with CMS aiming to have 50% of alternative based payments related to value and quality by 2018.22 With such a focus on value, it is surprising to note the dearth of value related research in surgery.23 The purpose of the present study was to compare morbidity, mortality, cost and value for staged hybrid versus open repair of extent II thoracoabdominal aneurysms. We hypothesized that patients will have improved outcomes with the staged hybrid approach, but this new technology will be associated with increase healthcare costs resulting in similar value for the care of these complex patients.
Methods
Study Population
The records of 113 consecutive patients that underwent Crawford Extent II thoracoabdominal aneurysm repair between 2005 and 2015 were identified from a prospectively maintained database of aortic patients at our institution as well as an institutional Vascular Quality Initiative (VQI) database. Patients underwent either single stage open surgical repair or staged hybrid repair with TEVAR of the proximal aorta followed by open surgical repair of the distal aorta. The methodology has been previously described in detail with 18 of the 113 patients included in prior reports.14, 15 Briefly, TEVAR was performed with stent graft placement from the left subclavian artery to just above the celiac artery. Left carotid-subclavian artery bypass was performed at the discretion of the surgeon, but typically prior to any planned coverage of the left subclavian artery by the TEVAR. Distal repair was performed by thoracoretroperitoneal approach with a branched Dacron graft sewn directly to the proximal stent graft.
Inclusion criteria were Crawford extent II classification, while no exclusion criteria were applied. The database includes preoperative characteristics, operative details and short-term outcomes. Our institutional Clinical Data Repository (CDR) was queried to identify which Crawford Extent II thoracoabdominal aneurysm repair patients also underwent TEVAR. Detailed chart review was performed for all patients to capture complications and long-term outcomes. Detailed cost and charge information was obtained from the institutional CDR. The University of Virginia Institutional Review Board approved this study with exemption of consent due to the retrospective nature of the analysis (IRB Protocol # 17900).
Measures
Patients were stratified into open or hybrid repair defined as TEVAR performed on the proximal aspect of the TAAA followed by open repair of the remaining distal aneurysm. Acute kidney injury was defined as an increase in creatinine of 0.5 mg/dL or new dialysis. The primary outcome of composite major adverse event (MAE) was defined as renal failure, spinal cord ischemia or death. Secondary outcomes included in-hospital results, cost, and value. To account for inflation, cost data was adjusted to 2015 dollars using the market basket for the Center for Medicare and Medicaid Services (CMS) Inpatient Prospective Payment System. Value was calculated as inverted outcomes divided by cost.23, 24 This is multiplied by a constant to set the value of open TAAA repair to 100 where an increasing number indicates increasing value. This equates to:
Statistical Analysis
Continuous variables are represented as mean and standard deviation if normally distributed, otherwise presented as median and interquartile range (IQR), while categorical variables as number and percentage. Univariate analysis was used to evaluate baseline characteristics and short-term outcomes. Continuous variables were analyzed by Mann-Whitney U Test or Student's T-Test as appropriate, while categorical variables were assessed by Chi-Squared Test. All analyses were performed with SAS Version 9.4 (SAS Institute, Cary, NC) and significance was determined by an alpha < 0.05.
Results
Study Population
A total of 113 consecutive patients underwent Crawford extent II thoracoabdominal aneurysm repair during the study period, of whom 25 (22.1%) had a staged hybrid approach. The median year of intervention for the open cohort was 2009 and for the staged hybrid cohort was 2011. The baseline characteristics of the two groups are displayed in Table I, with no statistically significant differences identified. While the mean creatinine prior to open repair was higher for the staged hybrid cohort, this did not reach statistical significance (1.3 mg/dL vs 1.1 mg/dL, p=0.24). The median time between TEVAR and open repair in the staged hybrid group was 129 days (IQR 55-396).
Table I. Demographics and Comorbidities.
| Baseline Characteristics | Staged Hybrid (n=25) | Standard Open (n=88) | p-value |
|---|---|---|---|
| Age | 53 ± 17 | 58 ± 17 | 0.15 |
| Sex (male) | 16 (64.0%) | 52 (59.1%) | 0.66 |
| Coronary Artery Disease | 8 (32.0%) | 38 (33.6%) | 0.32 |
| Chronic Kidney Disease | 5 (20.0%) | 18 (20.5%) | 0.96 |
| Chronic Obstructive Pulmonary Disease | 5 (20.0%) | 30 (34.1%) | 0.18 |
| Diabetes Mellitus Type 2 | 4 (16.0%) | 13 (14.8%) | 0.88 |
| Hyperlipidemia | 12 (48.0%) | 42 (47.7%) | 0.98 |
| Hypertension | 17 (68.0%) | 60 (68.2%) | 0.99 |
| Tobacco Use | 15 (60.0%) | 55 (62.5%) | 0.82 |
| Prior Descending Aortic Dissection | 23 (92.0%) | 30 (34.1%) | <0.0001 |
Procedural Outcomes
The TEVAR procedure methods and results are displayed in Table II. Endoleaks were identified in 9 patients, 5 of whom required reintervention for type Ia endoleaks. One patient with a history of stage III chronic kidney disease developed renal failure. Almost 50% of patients required a carotid-subclavian bypass, with a median time from bypass to TEVAR of 2 days. Overall, the median length of stay was 6 days and median days before the staged open repair was 129.
Table II. Thoracic Endovascular Aortic Aneurysm Repair (TEVAR) Characteristics.
| TEVAR Characteristics | Incidence |
|---|---|
| Percutaneous Access | 11 (44.0%) |
| Carotid-Subclavian Bypass | 12 (48.0%) |
| Lumbar Drain | 21 (84.0%) |
| Endoleak | 9 (36.0%) |
| Reintervention | 5 (20.0%) |
| Spinal Cord Ischemia | 0 (0%) |
| Non-Fatal Myocardial Infarction | 0 (0%) |
| Length of Stay (days) ‡ | 6 [4,7] |
| Days Between Procedures‡ | 129 [62,163] |
presented as median and interquartile range due to skewedness
Operative characteristics for open TAAA repair (excluding TEVAR) did differ between hybrid and standard open patients, as demonstrated in Table III. The surgical component of hybrid cases involved more frequent use of cerebrospinal fluid drainage, left heart bypass, and selective visceral and renal perfusion. Despite the increased adjunctive techniques, mean operative time was lower (256 min vs 306 min, p=0.01).
Table III. Thoracoabdominal Aneurysm Repair (TAAA) Surgical Characteristics and Outcomes.
| Operative Characteristics | Staged Hybrid† (n=25) | Standard Open (n=88) | p-value |
|---|---|---|---|
| Lumbar Drain | 23 (92.0%) | 54 (61.4%) | 0.01 |
| Left Heart Bypass | 24 (96.0%) | 54 (61.4%) | 0.001 |
| Celiac Artery Perfusion | 12 (48.0%) | 17 (19.3%) | 0.01 |
| Superior Mesenteric Artery Perfusion | 13 (52.0%) | 19 (21.6%) | 0.01 |
| Renal Artery Perfusion | 12 (48.0%) | 18 (20.5%) | 0.01 |
| Operative Time (minutes) | 256 ± 97 | 306 ± 80 | 0.01 |
| Estimated Blood Loss (mL) | 1319 ± 1645 | 2601 ± 2859 | 0.01 |
|
| |||
| Short-Term Outcomes | |||
|
| |||
| In-hospital mortality | 1 (4.0%) | 3 (3.4%) | 0.89 |
| Spinal Cord Ischemia | 2 (8.0%) | 17 (19.3%) | 0.18 |
| Acute Kidney Injury | 19 (76.0%) | 45 (51.1%) | 0.03 |
| Renal Failure | 2 (8.0%) | 4 (4.5%) | 0.84 |
| Non-Fatal Myocardial Infarction | 1 (4.0%) | 3 (3.4%) | 0.99 |
| Reoperation | 9 (36.0%) | 27 (30.7%) | 0.61 |
| Length of Stay (days) | 10.1 ± 4.0 | 13.3 ± 12.3 | 0.04 |
| Major Adverse Event | 5 (20.0%) | 43 (48.9%) | 0.01 |
This excludes the TEVAR hospitalization.
There was no statistical difference in in-hospital mortality (4.0% vs 3.4%, p=0.89) also shown in Table III. While there was a higher rate of acute kidney injury in the hybrid cohort (76% vs 51%, p=0.03), AKI resolved in 89% of hybrid patients and 91% of standard open repair patients (p=0.84). Consequently, there was no significant difference in rate of renal failure requiring dialysis (8% vs 4.5%, p=0.84). The composite measure of death, spinal cord ischemia and renal failure was significantly lower in the hybrid repair cohort (20% vs 49%, p=0.01). Additionally, the median length of stay after open repair was significantly lower for the hybrid patients (10 days vs 13 days, p=0.04).
Cost and Value
Detailed cost and charge data are represented in Table IV. The median hospital cost for the open surgical component was significantly lower for the hybrid repair cohort ($54,518 vs $72,037, p=0.03). However, the median hospital cost for the TEVAR procedure was $51,352. Thus, the total median cost for hybrid TAAA repair was significantly higher than conventional open operation ($112,920 vs $72,037, p=0.003). It is important to note that the standard open repair cohort had significantly higher variation in cost, where the mean cost for hybrid repair was $144,714 versus $112,897 for the standard open group (p=0.15) (Figure 1).
Table IV. Median hospital costs and physician charges.
| Financial Outcomes | Staged Hybrid | Standard Open | p-value |
|---|---|---|---|
| TEVAR Hospital Cost | 51,352 (42,048-88,105) | - | |
| TEVAR Physician Charges | 13,724 (11,129-25,401) | - | |
| TAAA Repair Hospital Cost | 54,518 (44,738-77,619) | 72,037 (47,678-149,766) | 0.032 |
| TAAA Repair Physician Charges | 33,913 (20,801-64,816) | 23,570 (15,670-51,322) | 0.111 |
| Total Hospital Cost | 112,921 (100,511-154,416) | 72,037 (47,678-149,766) | 0.003 |
| Total Physician Charges | 72,021 (37,016-100,520) | 23,570 (15,670-51,322) | <0.0001 |
All costs were adjusted for medical inflation and presented as median 2015 dollars (interquartile range). TEVAR = Thoracic Endovascular Aortic Aneurysm Repair; TAAA = Thoracoabdominal Aneurysm
Figure 1. Total Hospital Cost.
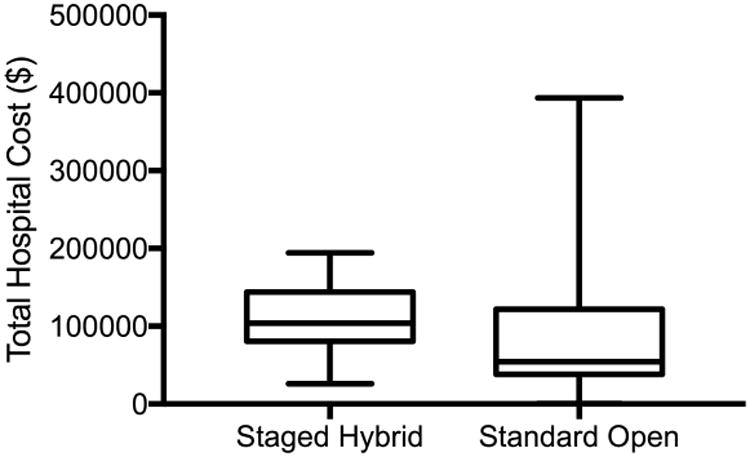
Box and Whiskers plot for the total hospital cost demonstrating the median, interquartile range, and range.
Total physician charges were significantly higher in the staged hybrid group ($72,021 vs 23,570, p<0.0001) due to additional charges during TEVAR (Table IV). However, the physician charges for the TAAA open repair were not significantly different ($33,913 vs $23,570, p=0.11). Using the standard open procedure as the benchmark for value, the staged hybrid procedure has increased value (Table V). The value is increased by 56% when using median hospital cost and by 78% when mean costs are utilized.
Table V. Value Comparison.
| Component Variables | Staged Hybrid (n=25) | Standard Open (n=88) | p-value |
|---|---|---|---|
| Major Adverse Event | 5 (20.0%) | 43 (48.9%) | 0.017 |
| Total Cost (median) | $112,921 | $72,037 | 0.003 |
| Value | 156 | 100 |
Total cost was adjusted for inflation and represents 2015 dollars.
Discussion
Repair of extent II TAAA is associated with a high rate of morbidity, with an overall 42% rate of composite major adverse events in this single center experience. However, the MAE rate for staged hybrid repair was significantly lower than standard open rate at 20% compared to 49%. Staged hybrid TAAA repair was associated with a significantly lower rate of spinal cord ischemia. While acute kidney injury rates were higher in the hybrid repair cohort, the high rate of recovery resulted in no difference in the rate of renal failure requiring dialysis. The median total cost was significantly higher in the staged hybrid cohort at $113,000 compared to $72,000 for the standard open repair. Considering both major adverse events and cost, staged hybrid repair represents better value by over 58% compared to the standard open repair technique.
Spinal cord ischemia can be a life altering complication that occurs with unfortunately high frequency after extent II TAAA repair ranging from 7% to over 30%.5, 7, 8, 25 The rates of spinal cord ischemia demonstrated in this analysis are consistent with the literature, and were significantly lower in the group undergoing staged hybrid repair at 8% compared to 19% in the standard open repair cohort. The hybrid repair methodology effectively converts an extent II TAAA into an extent III/IV TAAA. These less extensive aneurysm repairs are associated with much lower rates of spinal cord ischemia.7, 26 Additionally, it has been previously demonstrated that staged open procedures, first thoracic aneurysm repair followed by distal abdominal repair, was associated with a decreased rate of spinal cord injury from 17% to 0%.27 Our results demonstrate that this outcome can be replicated using TEVAR. An additional benefit may come from temporary aneurysm sac perfusion associated with type 1b endoleaks partially perfusing the spinal cord until the open surgery.28 While the reduction seen with staged hybrid repair is confounded by a higher rate of lumbar drain use, this likely does not account for the 50% reduction in MAE.
Renal failure after standard open repair of extent II TAAA is 16-26% in contemporary series.5, 7, 29 The rate of AKI after TEVAR varies with complexity of lesion and definition of kidney injury, but has been reported to be between 17-23%.30, 31 It is therefore not surprising to see increased AKI in our staged hybrid group since these patients are undergoing a previous procedure causing renal insult in one fifth of all patients. Despite a median interval of 129 days between TEVAR and open distal repair, they still experienced increased frequency of AKI. Other limited reports of staged hybrid repair indicate similar renal failure rates as reported in this single center study.18 Despite higher rates of AKI, detailed analysis reveals low incidence of progression to renal failure. These results suggest selective renal perfusion is effective and staged hybrid repair does not increase the risk for renal failure.
The resource utilization associated with the TEVAR component of the hybrid repair is not trivial, and is in fact higher than isolated TEVAR.32 This is not surprising considering almost 50% requiring a carotid-subclavian bypass. This not only impacted the cost, but also resulted in a 6 day median length of stay. Importantly, the open surgery component was significantly less costly for the hybrid cohort compared to the standard open group. The most likely explanation relates to the lower rate of major adverse events associated with hybrid repair. It has been demonstrated that complications are the most significant predictor of increased costs, and this effect is consistent across a wide variety of surgeries evaluated.33 This effect is even more predictive of cost than the Society of Thoracic Surgeons Predicted Risk of Mortality, the gold standard risk prediction model.34 These analyses demonstrate the importance of costs associated with treating complications, including supplementary supporting therapies/medications and longer stays with boarding and staffing costs.
The evaluation of surgical costs should not be completed in isolation. The important question is what outcomes are associated with the costs of care? This is becoming an increasing emphasis with changing reimbursement models. The rise of Accountable Care Organizations, pay-for-performance, bundled payments, and global billing is forcing consideration of value. This can be applied to surgery by comparing a ratio of cost to complications as previously demonstrated by Yount and colleagues.23, 24 This metric allows a reasonable assessment of short-term outcomes in relation to cost. The major complications associated with TAAA repair include renal failure, spinal cord ischemia and death. A composite of these measures is a reasonable assessment of major adverse events. Using this metric, we demonstrate that despite increased cost, staged hybrid repair represents reasonable value. The decreased rate of MAE more than compensates for increased hospital costs resulting in an increase in value of between 56% and 78%. This variability is due to high cost outliers associated with standard open repair as demonstrated in the box and whiskers plot. The median costs represent a conservative estimate of value at 156% for hybrid repair. However, to better account for the high-cost outliers, the mean costs demonstrate an even larger value increase at 178%. The outliers are likely due to open repair being associated with not only an increased complication rate but increased complication severity.
This study is limited by the single center, retrospective nature of the analysis with a potential for selection bias. While baseline characteristics were mostly similar, a higher percentage of hybrid repair patients had prior aortic dissection. Additionally, surgical practices changed over time and this is apparent and clearly explained in the operative characteristics. Additionally, there may be dropout bias from patients who underwent TEVAR but did not progress to open repair. A thorough review of records was performed and no patients that fit this description were identified. Additionally, others have reported no deaths or major adverse events associated with initial TEVAR prior to distal repair.18 Finally, value would ideally be assessed in a risk-adjusted manner such as has been previously done in the cardiothoracic surgery literature.24 Robust risk models are unfortunately unavailable although baseline assessment of patients demonstrated similar comorbidities and risk factors.
Although not available for analysis in this cohort, entirely endovascular TAAA repair represents another opportunity to assess the value of technological innovation. Current spinal cord ischemia rates are high, but as the technology advances and adjuncts such as temporary aneurysm sac perfusion help decrease this rate, its value should be assessed.28
Conclusion
The 20% major adverse event rate associated with staged hybrid repair of extent II TAAA was significantly decreased compared to open TAAA repair, with a relative reduction of over 50%. Despite higher total hospital costs, staged hybrid repair had 56% to 78% higher healthcare related value compared to standard open repair. In an era of increasing focus on costs as well as quality, staged hybrid repair of extensive aortic aneurysms is associated with fewer complications than open repair resulting in a good value investment from a resource utilization perspective.
Acknowledgments
Special thanks to Joshua Samudre and J. Michael Cullen MD for their hard work on data collection and dedication to the success of this project.
Funding: The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presentations: 41st Annual Meetings of the Southern Association for Vascular Surgery, Naples, FL from January 18-21, 2017 and Society for Clinical Vascular Surgery, Orlando, FL from March 10-13.
IRB: University of Virginia institutional review board protocol 17900
Conflicts of Interest: None to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crawford ES, DeNatale RW. Thoracoabdominal aortic aneurysm: observations regarding the natural course of the disease. J Vasc Surg. 1986;3(4):578–82. doi: 10.1067/mva.1986.avs0030578. [DOI] [PubMed] [Google Scholar]
- 2.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74(5):S1877–80. doi: 10.1016/s0003-4975(02)04147-4. discussion S92-8. [DOI] [PubMed] [Google Scholar]
- 3.Frederick JR, Woo YJ. Thoracoabdominal aortic aneurysm. Ann Cardiothorac Surg. 2012;1(3):277–85. doi: 10.3978/j.issn.2225-319X.2012.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coselli JS, Bozinovski J, LeMaire SA. Open surgical repair of 2286 thoracoabdominal aortic aneurysms. Ann Thorac Surg. 2007;83(2):S862–4. doi: 10.1016/j.athoracsur.2006.10.088. discussion S90-2. [DOI] [PubMed] [Google Scholar]
- 5.Coselli JS, LeMaire SA, Conklin LD, Koksoy C, Schmittling ZC. Morbidity and mortality after extent II thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 2002;73(4):1107–15. doi: 10.1016/s0003-4975(02)03370-2. discussion 15-6. [DOI] [PubMed] [Google Scholar]
- 6.LeMaire SA, Price MD, Green SY, Zarda S, Coselli JS. Results of open thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg. 2012;1(3):286–92. doi: 10.3978/j.issn.2225-319X.2012.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murana G, Castrovinci S, Kloppenburg G, Yousif A, Kelder H, Schepens M, et al. Open thoracoabdominal aortic aneurysm repair in the modern era: results from a 20-year single-centre experience. Eur J Cardiothorac Surg. 2016;49(5):1374–81. doi: 10.1093/ejcts/ezv415. [DOI] [PubMed] [Google Scholar]
- 8.Svensson LG, Crawford ES, Hess KR, Coselli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17(2):357–68. discussion 68-70. [PubMed] [Google Scholar]
- 9.Coselli JS, LeMaire SA. Left heart bypass reduces paraplegia rates after thoracoabdominal aortic aneurysm repair. Ann Thorac Surg. 1999;67(6):1931–4. doi: 10.1016/s0003-4975(99)00390-2. discussion 53-8. [DOI] [PubMed] [Google Scholar]
- 10.Coselli JS, LeMaire SA. Tips for successful outcomes for descending thoracic and thoracoabdominal aortic aneurysm procedures. Semin Vasc Surg. 2008;21(1):13–20. doi: 10.1053/j.semvascsurg.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Estrera AL, Sandhu HK, Charlton-Ouw KM, Afifi RO, Azizzadeh A, Miller CC, 3rd, et al. A Quarter Century of Organ Protection in Open Thoracoabdominal Repair. Ann Surg. 2015;262(4):660–8. doi: 10.1097/SLA.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 12.Bischoff MS, Brenner RM, Scheumann J, Zoli S, Di Luozzo G, Etz CD, et al. Staged approach for spinal cord protection in hybrid thoracoabdominal aortic aneurysm repair. Ann Cardiothorac Surg. 2012;1(3):325–8. doi: 10.3978/j.issn.2225-319X.2012.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canaud L, Karthikesalingam A, Jackson D, Cresswell L, Cliff M, Markar SS, et al. Clinical outcomes of single versus staged hybrid repair for thoracoabdominal aortic aneurysm. J Vasc Surg. 2013;58(5):1192–200. doi: 10.1016/j.jvs.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Flohr TF, Johnston WF, Tracci MC, Cherry KJ, Upchurch GR, Jr, et al. Staged hybrid repair of extensive thoracoabdominal aortic aneurysms secondary to chronic aortic dissection. J Vasc Surg. 2016;63(1):62–9. doi: 10.1016/j.jvs.2015.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Johnston WF, Upchurch GR, Jr, Tracci MC, Cherry KJ, Ailawadi G, Kern JA. Staged hybrid approach using proximal thoracic endovascular aneurysm repair and distal open repair for the treatment of extensive thoracoabdominal aortic aneurysms. J Vasc Surg. 2012;56(6):1495–502. doi: 10.1016/j.jvs.2012.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin PH, Kougias P, Bechara CF, Weakley SM, Bakaeen FG, Lemaire SA, et al. Clinical outcome of staged versus combined treatment approach of hybrid repair of thoracoabdominal aortic aneurysm with visceral vessel debranching and aortic endograft exclusion. Perspect Vasc Surg Endovasc Ther. 2012;24(1):5–13. doi: 10.1177/1531003511432768. [DOI] [PubMed] [Google Scholar]
- 17.Mangialardi N, Costa P, Bergeron P, Serrao E, Ronchey S. Staged hybrid repair of thoracoabdominal aortic aneurysm after chronic type B aortic dissection. Vascular. 2010;18(6):336–43. doi: 10.2310/6670.2010.00061. [DOI] [PubMed] [Google Scholar]
- 18.Vivacqua A, Idrees JJ, Johnston DR, Soltesz EG, Svensson LG, Roselli EE. Thoracic endovascular repair first for extensive aortic disease: the staged hybrid approachdagger. Eur J Cardiothorac Surg. 2016;49(3):764–9. doi: 10.1093/ejcts/ezv274. [DOI] [PubMed] [Google Scholar]
- 19.Porter ME, Teisberg EO. Redefining health care: creating value-based competition on results. Boston, Mass: Harvard Business School Press; 2006. [Google Scholar]
- 20.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 21.Porter ME, Lee TH. From Volume to Value in Health Care: The Work Begins. JAMA. 2016;316(10):1047–8. doi: 10.1001/jama.2016.11698. [DOI] [PubMed] [Google Scholar]
- 22.Burwell SM. Setting value-based payment goals--HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–9. doi: 10.1056/NEJMp1500445. [DOI] [PubMed] [Google Scholar]
- 23.Yount KW, Turrentine FE, Lau CL, Jones RS. Putting the value framework to work in surgery. J Am Coll Surg. 2015;220(4):596–604. doi: 10.1016/j.jamcollsurg.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 24.Yount KW, Rich JB, Lau CL, Ghanta RK, Yarboro LT, Kern JA, et al. The Costs of Our Traditional Yardsticks for Quality: Is Value Improving in Cardiac Surgery? 42nd Annual Western Thoracic Surgery Association Presentation. 2016 [Google Scholar]
- 25.Gilling-Smith GL, Worswick L, Knight PF, Wolfe JH, Mansfield AO. Surgical repair of thoracoabdominal aortic aneurysm: 10 years' experience. Br J Surg. 1995;82(5):624–9. doi: 10.1002/bjs.1800820517. [DOI] [PubMed] [Google Scholar]
- 26.Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg. 2002;236(4):471–9. doi: 10.1097/00000658-200210000-00010. discussion 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etz CD, Zoli S, Mueller CS, Bodian CA, Di Luozzo G, Lazala R, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg. 2010;139(6):1464–72. doi: 10.1016/j.jtcvs.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Kasprzak PM, Gallis K, Cucuruz B, Pfister K, Janotta M, Kopp R. Editor's choice--Temporary aneurysm sac perfusion as an adjunct for prevention of spinal cord ischemia after branched endovascular repair of thoracoabdominal aneurysms. Eur J Vasc Endovasc Surg. 2014;48(3):258–65. doi: 10.1016/j.ejvs.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Safi HJ, Harlin SA, Miller CC, Iliopoulos DC, Joshi A, Mohasci TG, et al. Predictive factors for acute renal failure in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg. 1996;24(3):338–44. doi: 10.1016/s0741-5214(96)70189-1. discussion 44-5. [DOI] [PubMed] [Google Scholar]
- 30.Jeon YH, Bae CH. The Risk Factors and Outcomes of Acute Kidney Injury after Thoracic Endovascular Aortic Repair. Korean J Thorac Cardiovasc Surg. 2016;49(1):15–21. doi: 10.5090/kjtcs.2016.49.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drews JD, Patel HJ, Williams DM, Dasika NL, Deeb GM. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann Thorac Surg. 2014;97(6):2027–33. doi: 10.1016/j.athoracsur.2014.02.045. discussion 33. [DOI] [PubMed] [Google Scholar]
- 32.Gillen JR, Schaheen BW, Yount KW, Cherry KJ, Kern JA, Kron IL, et al. Cost analysis of endovascular versus open repair in the treatment of thoracic aortic aneurysms. J Vasc Surg. 2015;61(3):596–603. doi: 10.1016/j.jvs.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D, et al. The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg. 2011;254(6):907–13. doi: 10.1097/SLA.0b013e31821d4a43. [DOI] [PubMed] [Google Scholar]
- 34.Osnabrugge RL, Speir AM, Head SJ, Fonner CE, Fonner E, Jr, Ailawadi G, et al. Costs for surgical aortic valve replacement according to preoperative risk categories. Ann Thorac Surg. 2013;96(2):500–6. doi: 10.1016/j.athoracsur.2013.04.038. [DOI] [PubMed] [Google Scholar]


