Abstract
External cricoid pressure is increasingly used to augment the upper esophageal sphincter (UES).
Objective
To determine the effect of: 1) pressures applied to cricoid, supra-cricoid and sub-cricoid regions on the length and amplitude of UES high-pressure zone (UESHPZ). 2) external cricoid pressure on LES tone.
Study Design
case-control study.
Methods
We studied 11 patients with supraesophageal reflux (58 ± 12 yr) and 10 healthy volunteers (47 ± 19 yr). We tested 20, 30, 40 mmHg pressures to cricoid, 1 cm proximal and 1 cm distal to cricoid. In an additional 15 healthy volunteers (46 ± 23 yr), we studied the effect of external cricoid pressure on LES tone. UES and LES pressures were determined using high-resolution manometry.
Results
There was significant increase of UESHPZ length with application of pressure at all sites. The increase of UESHPZ length was relatively symmetric, more orad and more caudad when the pressure was applied at the cricoid, supra-cricoid and sub-cricoid levels, respectively. The magnitude of pressure increase was greatest at the middle and orad part of the UESHPZ when the pressure was applied at the cricoid and supra-cricoid levels, respectively. The corresponding magnitude of increase in the caudad part of the UESHPZ was not observed with pressure at sub-cricoid level. There was no change of the LES pressure with application of cricoid pressure.
Conclusions
Effect of external pressure on UESHPZ is site dependent. Sub-cricoid pressure has the least effect on UESHPZ. External cricoid pressure at 20–40mmHg has no effect on the LES pressure.
Keywords: regurgitation, cricoid pressure, upper esophageal sphincter, lower esophageal sphincter, supraesophageal reflux disease
Introduction
The Upper Esophageal Sphincter high-pressure zone (UESHPZ) constitutes the main barrier against reflux of esophageal and gastric contents into the pharynx.1–4 Esophagopharyngeal reflux due to incompetence of the upper esophageal sphincter (UES) is fundamental to the development of reflux induced aerodigestive tract disorders. 5–9
Until recently, there has been no reliable method to remedy or compensate for UES incompetence. Recent studies in patients with supra-esophageal reflux disease (SERD) and healthy controls have shown that external pressures directed perpendicularly on the cricoid cartilage is proportionally transmitted onto the UESHPZ resulting in predictable luminal pressure augmentation. 10, 11 These studies have also shown that applying a device designed to exert external pressure on the cricoid cartilage inducing a sustained increase in intraluminal UES pressure between 20 and 30 mm Hg prevents pharyngeal entry of simulated esophageal reflux.10 These studies, however, did not quantify the effect of external pressure applied to areas orad and caudad to the cricoid. In addition to physiologic interest, this information may have practical implications in using UES pressure augmentation techniques in clinical practice.
The use of external cricoid pressure for medical purposes dates back to the 18th century. In 1771, Monro proposed external cricoid pressure to prevent gastric air insufflation during resuscitation of nearly drowned victims.12 In 1961, Sellick proposed external cricoid pressure to prevent aspiration of gastric content during anesthesia induction.13 Shaker et al in 2014 proposed sustained long duration external cricoid pressure for prevention of pharyngeal reflux during recumbency and sleep.10 Later, Silvers et al demonstrated the efficacy of this technique using a “UES Assist Device” based on the principle of controlled modest external cricoid pressure in improving the ENT/airway symptoms of patients with supra-esophageal reflux disease.14
Of interest, some studies in the anesthesiology field have reported relaxation of the lower esophageal sphincter (LES) due to external cricoid pressure.15–18 These studies, however, have used methods and instruments that have significant limitations in overcoming the physiological pressure variation within the LES high-pressure zone, the to-and-fro movement of the LES with respiration,19,20 as well as the effect of neck movement on sensor location within the LES. In these circumstances, the recording site could easily move from a higher to a lower pressure area within the sphincter and yield a spurious relaxation. To overcome these problems, the use of a sleeve device, which reliably and continuously measures the highest pressure within the LES, was introduced. 21, 22 In recent years, with the introduction of high-resolution manometry (HRM), the principle of sleeve recording has been adopted as an electronic sleeve (e-sleeve), which also continuously measures the highest pressure within the entire length of the LES. Systematic evaluation of the effect of external cricoid pressure on LES resting tone using state-of-the-art HRM technique is still awaited.
Therefore, our aims in this study were to: 1) Determine and compare the effect of pressures applied to cricoid, supra-cricoid and sub-cricoid regions on the length and amplitude of the UESHPZ. 2) Evaluate the effect of application of a range of external cricoid pressures on the LES tone.
Methods
Subjects
To study the effect of pressure site on UESHPZ, we studied 11 subjects (5 male, 58 ± 12 years) with SERD and 10 healthy volunteers (5 male, 47 ± 19 years). SERD patients had a variety of supraesophageal reflux symptoms including chronic cough, excess phlegm, hoarseness, and throat clearing. All subjects were followed by their primary gastroenterologist in a tertiary care referral center with established diagnosis of gastroesophageal reflux disease and were on long-term acid-suppressive therapy. Despite ongoing therapy, all patients complained of persistent troublesome regurgitation along with at least one of the above supra-esophageal manifestations. The age difference between the SERD group and normal subjects was not statistically different (p=0.1). To study the effect of external cricoid pressure on the LES tone, another cohort comprised of 15 healthy volunteers (10 male, 46 ± 23 years) were studied since the lower segment of the esophagus and LES were not included in the manometry studies of the initial two cohorts. The Medical College of Wisconsin institutional review board approved the studies and all participants signed written informed consent prior to their studies.
Study protocol
After 6 hours of fasting, participants underwent trans-nasal placement of the manometric catheter following topical 2% lidocaine application. The catheter was positioned to cover the entire pharynx and UES. The manometry catheter contained 36 circumferential solid-state pressure sensors spaced 1 cm apart measuring at a sampling rate of 50 Hz (Given imaging, Los Angeles, CA). After a 10-minute adaptation period, external pressure was applied to the center of the cricoid, 1 cm proximal (supra-cricoid), and 1 cm distal to the cricoid (sub-cricoid). To study the effect of external cricoid pressure on the LES, the manometry catheter was advanced to the stomach first then pulled back till both the UES and LES could be recorded in their entire pressure zones. The external pressure was only applied at the center of the cricoid. Participants were asked to refrain from swallowing as long as possible during the period of pressure application and 10 seconds after pressure release. Following the placement of the manometry catheter, the remainder of the study was performed in the recumbent position.
To apply external pressures to the cricoid, supra-cricoid, and sub-cricoid, we used a simple handmade laboratory device comprised of a small pressure cushion (0.8 cm × 0.5 cm × 2.5 cm) for targeting the pressure to a specific site (figure 1). Externally applied pressures were monitored in real time by using a small noncompliant air-filled bulb (10 ml), which was connected to a pressure sensor (SomnaTherapeutics, Glendale, WI) (figure 1). We tested 0, 20, 30, 40 mmHg pressures by pressing the cushion perpendicularly with the index finger and maintained for 10 seconds to the selected sites. Each pressure application was repeated 3 times with at least 10 seconds of external pressure free interval in between. UES, pharyngeal and proximal esophageal pressures were determined using HRM. We allowed 3-mmHg fluctuations around the target pressure with respiration of the participants.
Figure 1.
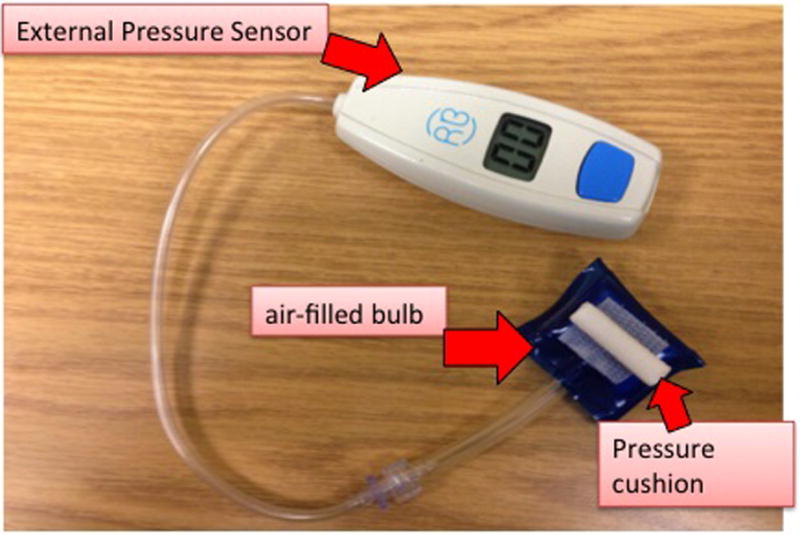
A simple handmade laboratory device comprised of a small pressure cushion (0.8 cm × 0.5 cm × 2.5 cm) was used for targeting the pressure to a specific site. The cushion was attached to a small noncompliant air-filled bulb (10 ml), which was connected to an external pressure sensor.
Manometric parameters
To measure the orad expansion of the UESHPZ, defined with 20 mmHg isobaric contour in the ManoView program, the difference of the upper border positions with and without application of external pressure was calculated. The similar method was applied to measure the caudad expansion of the UESHPZ. To map the UES pressure change with the application of external pressure at a given specific site, four pressure sensors 1 cm apart were placed to cover the orad to caudad part of the UES with the middle two sensors at the center of the UESHPZ. The mean pressure increase during the 10-second interval when the external pressure was applied was calculated at each pressure sensor. The length of the UESHPZ was measured at baseline and under different external pressure conditions. An example of the manometric UES pressure contour plot with and without application of external pressure is illustrated in figure 2.
Figure 2.

An example of UES pressure accentuation in response to application of 40 mmHg pressure to center of cricoid (2A), supra-cricoid (2B) and sub-cricoid (2C). The UESHPZ was outlined with 20 mmHg isobaric contour. As seen, the marginal expansion of UESHPZ for cricoid pressure is relatively symmetric (2A), expansion is more orad (2B) for supra-cricoid and more caudad (2C) for sub-cricoid pressure application.
To measure the change in the LES pressure during the application of graded external cricoid pressure, we first identified the upper and lower border of the LES and then used the e-sleeve function carried by the ManoView program. The mean LES pressure was the gradient above the gastric pressure and averaged during each 10-second interval commensurate with the on and off external cricoid pressure periods.
Statistical analysis
All statistical analyses were conducted using SAS 9.3 software. Comparisons were made using two sample t-test, paired t-test, linear trend tests and two-way repeated ANOVA as appropriate. P-values <0.05 were considered statistically significant. All data are expressed as mean ± SE.
Results
In all participants, all the applied external cricoid pressures were well tolerated. There was no untoward outcomes or complaints.
Length of UESHPZ
In healthy volunteers, the length of the UESHPZ increased from baseline of 2.8 ± 0.2 cm to 3.4 ± 0.2 cm, 3.6 ± 0.2 cm and 3.7 ± 0.2 cm in response to 20, 30 and 40 mmHg when applying external pressure at the center of cricoid, respectively (p<0.05).
In SERD patients, baseline UESHPZ length was shorter than healthy volunteers, though the difference was not statistically significant. With application of external pressure at cricoid level, the UESHPZ length increased significantly from baseline of 2.5 ± 0.2 cm to 2.9 ± 0.2 cm, 3.2 ± 0.2 cm and 3.4 ± 0.2 cm in response to 20, 30 and 40 mmHg, respectively (p<0.05). Similar degree of increase in UESHPZ length was observed when applying supra-cricoid and sub-cricoid pressure in all participants (figure 3). There was a significant linear trend between the length of the UESHPZ and the applied external cricoid pressure (p trend<0.05). There was no difference between healthy volunteers and SERD patients with regard to UESHPZ length under different external pressure conditions.
Figure 3.

UESHPZ length when applying external pressure at different locations in healthy volunteers (3A) and SERD patients (3B). The figure shows significant increase of UESHPZ length with application of external pressure at center of cricoid, supra-cricoid and sub-cricoid region compared to baseline, respectively.
*P<0.05 when compared to baseline; #P trend<0.05
Marginal expansion of UESHPZ
When applying external pressure to the middle of the cricoid, the expansion of the UESHPZ was relatively symmetric. However, the increase of the length of the UESHPZ was more orad when the pressure was applied at the supra-cricoid level. The caudad expansion was observed when the pressure was applied at the sub-cricoid level (figure 4). This pattern was similar in both healthy volunteers and SERD patients. The extent of the orad expansion and caudad expansion was positively associated with the magnitude of applied pressure, that is the higher the pressure, the greater the magnitude of marginal expansion (p trend <0.05).
Figure 4.

Increase of the UESHPZ length when applying external pressure at different locations in healthy volunteers (4A) and SERD patients (4B). The marginal expansion of UESHPZ is relatively symmetric, more orad and more caudad in response to external pressure at center of cricoid, supra-cricoid and sub-cricoid region, respectively. The extent of the orad expansion and caudad expansion was positively associated with the pressure applied.
* P trend<0.05
Mapping the pressure increase of UES
Table 1 (table 1) showed the mean pressure of the UES at each sensor level at baseline and with external cricoid pressure in SERD patients and healthy volunteers. The increase of UES pressure at each sensor level with applied external pressure is shown in figure 5. Irrespective of the specific site that the external pressure was applied, almost all of the sensors showed significant pressure increase compared to no pressure conditions. The magnitude of pressure increase was greatest at the middle two pressure sensors when the external pressure was applied at the center of cricoid level. When the pressure applied at the supra-cricoid level, the magnitude of pressure increase was greatest at the upper two pressure sensors, namely, orad part of the UES. However, the corresponding magnitude of increase in the caudad part of the UESHPZ was much less when the external pressure was applied at the sub-cricoid level (p<0.05, figure 5). The overall magnitude of UES pressure increase was not statistically different between supra-cricoid region and cricoid region, though the latter had greater pressure augmentation in the caudad part of the UES. When comparing healthy volunteers to SERD patients, healthy group demonstrated greater correspondent center augmentation of UES pressure in response to external pressure at center of cricoid (p<0.05). The difference was not evident between these two groups for supra-cricoid or sub-cricoid pressure application.
Table 1.
mean pressure of UES (mmHg) at each sensor level at baseline and with external pressure at center of cricoid level in SERD patients and healthy subjects (mean ± SE)
| Baseline (mmHg) | Cricoid pressure (mmHg) | |||
|---|---|---|---|---|
| 20mmHg | 30mmHg | 40mmHg | ||
| SERD patients | ||||
| UES_0 | 19.0 ± 3.5 | 27.0 ± 6.0 | 33.1 ± 10.2 | 28.4 ± 6.0 |
| UES_1 | 39.3 ± 7.1 | 52.6 ± 9.3 | 54.7 ± 5.5 | 57.3 ± 4.0 |
| UES_2 | 43.9 ± 7.0 | 53.7 ± 8.7 | 54.2 ± 5.3 | 58.0 ± 6.7 |
| UES_3 | 18.3 ± 4.4 | 20.5 ± 6.1 | 22.1 ± 3.9 | 24.9 ± 5.0 |
| Healthy subjects | ||||
| UES_0 | 13.6 ± 6.0 | 24.7 ± 6.0 | 25.3 ± 4.8 | 27.9 ± 6.2 |
| UES_1 | 46.4 ± 7.9 | 72.6 ± 13.0 | 74.6 ± 14.9 | 84.8 ± 15.6 |
| UES_2 | 43.7 ± 5.8 | 62.4 ± 8.2 | 68.5 ± 9.1 | 86.9 ± 9.0 |
| UES_3 | 17.7 ± 3.8 | 29.4 ± 4.3 | 26.2 ± 4.1 | 29.1 ± 5.3 |
UES_0: upper border of UES; UES_1: 1cm below UES_0; UES_2: 2cm below UES_0; UES_3: 3cm below UES_0 (lower border of UES)
Figure 5.
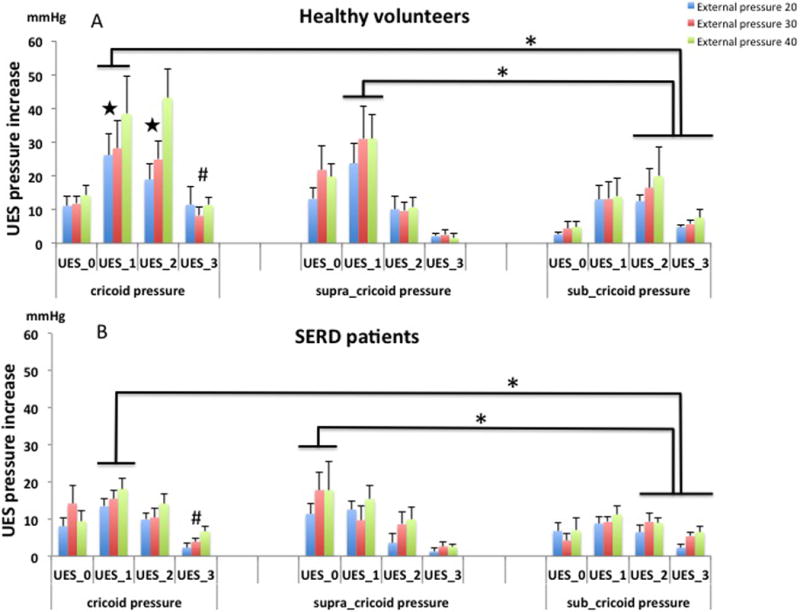
UES pressure increase in response to different locations of external pressure (mean pressure increase ± SE) in healthy volunteers (5A) and SERD patients (5B). The magnitude of pressure increase was greatest at the middle and orad part of the UESHPZ when the external pressure was applied at the cricoid and supra-cricoid levels, respectively. However, the corresponding magnitude of increase in the caudad part of the UESHPZ was much less when the external pressure was applied at the sub-cricoid level.
UES_0: upper border of UES; UES_1: 1cm below UES_0; UES_2: 2cm below UES_0; UES_3: 3cm below UES_0 (lower border of UES)
* P<0.05 when compared to caudal region of the UES
# P<0.05 when compared to UES_3 at supra-cricoid region
|P<0.05 when compared to SERD patients
The effect of external cricoid pressure on LES tone
For all tested external cricoid pressures, volunteers tolerated the maneuver well without gagging, retching, discomfort or swallowing. Comparison of the maximum LES pressure averaged over the ten second periods of external cricoid pressure with that of ten second periods without external cricoid pressure did not show any significant change. This finding was observed for all levels of tested pressures during all periods of external cricoid pressures of 20, 30 and 40 mmHg (18.8 ± 7.7 mmHg, 18.6 ± 8.2 mmHg and 19.3 ± 7.4 mmHg, respectively, compared to off pressure periods, 19.2 ±7.7 mmHg, figure 6 and figure 7).
Figure 6.

Effect of external cricoid pressure on the LES. There is no significant change of the mean LES pressure with applied cricoid pressures of 20, 30 and 40 mmHg when compared to baseline LES pressure.
Figure 7.

An example of a manometric tracing of the effect of cricoid pressure on LES tone. Figure 7A Color contour plot that displays UES and LES pressure profile before, during and after application of cricoid pressure at 40 mmHg. Figure 7B Corresponding line plot with the UES pressure (upper green line) and e-Sleeve recording of the LES pressure (bottom brown line). As seen, there is no discernible change in LES pressure with application of cricoid pressure.
Discussion
In this study, we determined the effect of external pressures applied perpendicularly to cricoid, supra-cricoid and sub-cricoid on the UESHPZ length and pressure amplitude. Study findings indicate that a.) Pressures applied to areas superior and inferior to cricoid cartilage generate UESHPZ pressure signatures different than that of pressures applied to the cricoid cartilage and b.) External pressures applied to cricoid and supra-cricoid regions augment the UES significantly more than the pressures applied caudad to cricoid area and c.) Application of external cricoid pressure at 20–40 mmHg does not affect the LES pressure.
UESHPZ is shaped by the pressures generated by its main component namely the cricopharyngeus muscle (CP) and the pressures generated by contiguous muscles bordering the superior aspect of the CP namely the inferior pharyngeal constrictor (IPC) and inferior aspect of the CP i.e. the most proximal portion of the striated esophagus.23–27 Studies utilizing concurrent high-resolution ultrasonography, manometry and fluoroscopy have defined the relative contribution of each of the three components to the UESHPZ.28 These studies confirm prior assertions that the majority of the length of the UESHPZ in the center as well as its peak pressure is contributed by the CP while inferior constrictor and proximal esophagus donate to the two proximal and distal tails of the high-pressure zone respectively.24, 26,27
The findings of the present study suggest that the orad extension in length and increase in pressure of the UESHPZ by application of pressure proximal to the cricoid cartilage is due to augmentation in the IPC area of the UESHPZ while caudad extension in length and increase in pressure of the UESHPZ by application of pressure distal to the cricoid cartilage is due to augmentation in the proximal esophageal area of the UESHPZ. These augmentations are most likely due to enhanced approximation of respective tissues in the anterior wall of the UESHPZ to the posterior wall of pharyngo-esophageal segment.
Findings of the present study also indicate that application of similar external pressures to areas distal to the cricoid cartilage induces significantly less pressure augmentation than pressures applied to areas proximal to the cricoid cartilage. The reason for this difference is likely due to the differences in anatomical arrangements. Applying external pressure at the laryngeal level and cricoid arch results in squeezing of the muscular pharyngo-esophageal tissue between the cartilaginous larynx/cricoid and the bony vertebral region, which are two hard surfaces, and thus increases pressure. In the sub-cricoid region, this is less effective as trachealis is a soft structure and thus the pressure is dispersed when tracheal rings are compressed. In addition, the spine drops away from the larynx below this level thus widening the space in an anterior-posterior direction.
Available data and clinical experience indicate that despite unprecedented success of acid suppressive therapy in treatment of esophagitis and control of heartburn, this chemical approach has limited effect in preventing regurgitation of gastric content into the pharynx and reflux attributed aerodigestive and airway disorders. Prior studies estimate that 51–90% of GERD patients also complain of regurgitation.29,30 Available data also indicate that compared to heartburn, regurgitation symptom is not as responsive to medical therapy aimed at acid suppression and a therapeutic gain of 5–35% using proton pump inhibitors for the control of regurgitation in patients who reported the symptom at baseline.29 More importantly, available studies indicate that supraesophageal and airway symptoms are reported by over half of the GERD patients.31,32 These findings are in addition to a large number of patients who present with similar complaints without overt heartburn or esophagitis.
A number of studies have implicated the pharyngeal reflux of gastric content in the development of supraesophageal symptoms.33–35 The UES constitutes the main barrier against the entry of refluxate into the pharynx.1–4 Recent studies have shown that intraesophageal pressure increase during reflux events, is generally <15 mmHg 36 and application of a relatively modest external cricoid pressure resulting in a 20 to 30 mm Hg intraluminal UES pressure increase would be adequate to prevent pharyngeal reflux under experimental condition10 and improve the supraesophageal symptoms.14 This physical approach to management of complications of reflux disease expands the existing non-pharmacologic approaches aimed at enhancement of sphincter barrier using surgical techniques.37,38 The findings of the present study provide additional information to inform most effective use of this UES enhancement technique. Further study is required to correlate pharyngeal reflux symptom and UES pressure augmentation with application of external pressure at different sites of UESHPZ (e.g. center, orad and caudad of UESHPZ).
The increase of the UESHPZ length may have clinical applicability. Our data showed that with application of external cricoid pressure, the length of UESHPZ increased by 22–32% in healthy subjects and 16–36% in SERD patients. Theoretically, increase in the length of UESHPZ may change the flow dynamics by increase of fluid resistance and pressure drop when refluxate transverses from the lower border to upper border of the UESHPZ. According to flow dynamics, if the length of UESHPZ increases by 20–30%, the pressure of refluxate will drop additional 20–30%. For example, if the pressure of refluxate is 15 mmHg when it enters the UES, with the increase of UESHPZ length, the pressure will drop to 10–12 mmHg when it approaches to the upper border of UESHPZ. Practically, the pressure change of refluxate caused by increase of UESHPZ length alone is small, however, combined with direct augmentation of UES pressure by applying external cricoid pressure, it may have additive effect in preventing the entry of refluxate into hypopharynx and larynx.
Previous anesthesiology literature suggested external cricoid pressure induces relaxation of the LES.15–18 Findings of the present study using state-of-the-art high-resolution manometry are in sharp contrast with these previous reports by documenting absence of any change in LES pressure during application of external cricoid pressures ranging 20–40 mmHg. This discrepancy can be explained by the differences in recording techniques and instruments, and the inability of older devices to overcome limitations imposed by axial asymmetry of the LES pressure zone and external cricoid pressure-induced catheter movement potentially yielding spurious relaxation recordings.
Conclusion
In summary, while external pressure increases both the length and pressure of the UESHPZ, its effects are site dependent. Sub-cricoid pressure has the least effect on the UESHPZ. In addition, external cricoid pressure at 20–40 mmHg has no effect on the LES pressure.
Acknowledgments
This work was supported in part by NIH grants P01DK068051, R01DK025731 and T32DK061923 grants
Abbreviations
- UES
Upper Esophageal Sphincter
- UESHPZ
Upper Esophageal Sphincter high-pressure zone
- LES
Lower Esophageal Sphincter
- SERD
Supraesophageal reflux disease
- CP
Cricopharyngeus muscle
Footnotes
Conflict of interest:
Dr. Reza Shaker is the inventor and patent holder of the Reza Band and has an interest in Somna Therapeutics.
Level of Evidence: 3b
References
- 1.Creamer B, Schlegel J. Motor responses of the esophagus to distention. J Appl Physiol. 1957;10:498–504. doi: 10.1152/jappl.1957.10.3.498. [DOI] [PubMed] [Google Scholar]
- 2.Gerhardt DC, Shuck TJ, Bordeaux RA, Winship DH. Human upper esophageal sphincter. Response to volume, osmotic, and acid stimuli. Gastroenterology. 1978;75:268–274. [PubMed] [Google Scholar]
- 3.Wallin L, Boesby S, Madsen T. The effect of HCl infusion in the lower part of the oesophagus on the pharyngo-oesophageal sphincter pressure in normal subjects. Scand J Gastroenterol. 1978;13:821–826. doi: 10.3109/00365527809182197. [DOI] [PubMed] [Google Scholar]
- 4.Enzmann DR, Harell GS, Zboralske FF. Upper esophageal responses to intraluminal distention in man. Gastroenterology. 1977;72:1292–1298. [PubMed] [Google Scholar]
- 5.Kamani T, Penney S, Mitra I, Pothula V. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol. 2012;269:2219–2225. doi: 10.1007/s00405-012-2028-1. [DOI] [PubMed] [Google Scholar]
- 6.Groome M, Cotton JP, Borland M, McLeod S, Johnston DA, Dillon JF. Prevalence of laryngopharyngeal reflux in a population with gastroesophageal reflux. Laryngoscope. 2007;117:1424–1428. doi: 10.1097/MLG.0b013e31806865cf. [DOI] [PubMed] [Google Scholar]
- 7.Lowden M, McGlashan JA, Steel A, Strugala V, Dettmar PW. Prevalence of symptoms suggestive of extra-oesophageal reflux in a general practice population in the UK. Logoped Phoniatr Vocol. 2009;34:32–35. doi: 10.1080/14015430902735847. [DOI] [PubMed] [Google Scholar]
- 8.Szczesniak MM, Williams RB, Cook IJ. Mechanisms of esophagopharyngeal acid regurgitation in human subjects. PLoS One. 2011;6(7):e22630. doi: 10.1371/journal.pone.0022630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szczesniak MM, Williams RB, Brake HM, Maclean JC, Cole IE, Cook IJ. Upregulation of the esophago-UES relaxation response: a possible pathophysiological mechanism in suspected reflux laryngitis. Neurogastroenterol Motil. 2010;22:381–386. e389. doi: 10.1111/j.1365-2982.2009.01452.x. [DOI] [PubMed] [Google Scholar]
- 10.Shaker R, Babaei A, Naini SR. Prevention of esophagopharyngeal reflux by augmenting the upper esophageal sphincter pressure barrier. Laryngoscope. 2014;124(10):2268–74. doi: 10.1002/lary.24735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babaei A, Jiao H, Mei L, Kern M, Shaker R. Correlation of Externally Applied Cricoid Pressure with Luminal Upper Esophageal Sphincter Pressure. Gastroenterology. 2014;146(S1):S–856. [Google Scholar]
- 12.Salem MR, Sellick BA, Elam JO. The historical background of cricoid pressure in anesthesia and resuscitation. Anesth Analg. 1974;53:230–232. [PubMed] [Google Scholar]
- 13.Sellick BA. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet. 1961;2:404–406. doi: 10.1016/s0140-6736(61)92485-0. [DOI] [PubMed] [Google Scholar]
- 14.Silvers SL, Vaezi MF, Vakil MB, et al. Prospective study of upper esophageal sphincter assist device for treating extraesophageal reflux. Otolaryngol Open J. 2016;2(1):31–38. [Google Scholar]
- 15.Garrard A, Campbell AE, Turley A, Hall JE. The effect of mechanically-induced cricoid force on lower oesophageal sphincter pressure in anaesthetised patients. Anaesthesia. 2004;59(5):435–9. doi: 10.1111/j.1365-2044.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- 16.Tournadre JP, Chassard D, Berrada KR, Bouletreau P. Cricoid cartilage pressure decreases lower esophageal sphincter tone. Anesthesiology. 1997;86(1):7–9. doi: 10.1097/00000542-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Salem MR, Bruninga KW, Dodlapatii J, Joseph NJ. Metoclopramide does not attenuate cricoid pressure-induced relaxation of the lower esophageal sphincter in awake volunteers. Anesthesiology. 2008;109(5):806–10. doi: 10.1097/ALN.0b013e31818a37dc. [DOI] [PubMed] [Google Scholar]
- 18.Thorn K, Thorn SE, Wattwil M. The effects of cricoid pressure, remifentanil, and propofol on esophageal motility and the lower esophageal sphincter. Anesth Analg. 2005;100(4):1200–3. doi: 10.1213/01.ANE.0000147508.31879.38. [DOI] [PubMed] [Google Scholar]
- 19.Liu J, Parashar VK, Mittal RK. Asymmetry of lower esophageal sphincter pressure: is it related to the muscle thickness or its shape? Am J Physiol. 1997;272:G1509–1517. doi: 10.1152/ajpgi.1997.272.6.G1509. [DOI] [PubMed] [Google Scholar]
- 20.Winans CS. Manometric asymmetry of the lower-esophageal high-pressure zone. Am J Dig Dis. 1977;22:348–354. doi: 10.1007/BF01072193. [DOI] [PubMed] [Google Scholar]
- 21.Dent J. new technique for continuous sphincter pressure measurement. Gastroenterology. 1976;71(2):263–7. [PubMed] [Google Scholar]
- 22.Linehan JH, Dent J, Dodds WJ, Hogan WJ. Sleeve device functions as a Starling resistor to record sphincter pressure. Am J Physiol. 1985;248:G251–G255. doi: 10.1152/ajpgi.1985.248.2.G251. [DOI] [PubMed] [Google Scholar]
- 23.Lang IM, Dantas RO, Cook IJ, Dodds WJ. Videoradiographic, manometric and electromyographic assessment of upper esophageal sphincter. Am J Physiol. 1991;260:G911–G919. doi: 10.1152/ajpgi.1991.260.6.G911. [DOI] [PubMed] [Google Scholar]
- 24.Sokol EM, Heitman P, Wolf BS, Cohen BR. Simultaneous cineradiographic and manometric study of the larynx, hypopharynx, and cervical esophagus. Gastroenterology. 1966;51:960–974. [PubMed] [Google Scholar]
- 25.Asoh R, Goyal RK. Manometry and electromyography of the upper esophageal sphincter in the opossum. Gastroenterology. 1978;74:514–520. [PubMed] [Google Scholar]
- 26.Cook IJ, Dodds WJ, Dantas RO, Massey B, Kern M, Lang IM, Brasseur JG, et al. Opening mechanisms of the upper esophageal sphincter. Am J Physiol. 1989;257:G748–G759. doi: 10.1152/ajpgi.1989.257.5.G748. [DOI] [PubMed] [Google Scholar]
- 27.Kahrilas PJ, Dodds WJ, Dent J, Logemann JA, Shaker R. Upper esophageal sphincter function during deglutition. Gastroenterology. 1988;95:52–62. doi: 10.1016/0016-5085(88)90290-9. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez LV, Dua KS, Surapaneni SN, Rittman T, Shaker R. Anatomic-manometric correlation of the upper esophageal sphincter: a concurrent US and manometry study. Gastrointest Endosc. 2010;72(3):587–92. doi: 10.1016/j.gie.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419–1425. doi: 10.1038/ajg.2011.146. [DOI] [PubMed] [Google Scholar]
- 30.Kahrilas PJ, Jonsson A, Denison H, Wernersson B, Hughes N, Howden CW. Regurgitation is less responsive to acid suppression than heartburn in patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:612–619. doi: 10.1016/j.cgh.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 31.Mearin F, Ponce J, Ponce M, Balboa A, Gonzalez MA, Zapardiel J. Frequency and clinical implications of supraesophageal and dyspeptic symptoms in gastroesophageal reflux disease. Eur J Gastroenterol Hepatol. 2012;24:665–674. doi: 10.1097/MEG.0b013e3283512139. [DOI] [PubMed] [Google Scholar]
- 32.Ylitalo R, Ramel S, Hammarlund B, Lindgren E. Prevalence of extraesophageal reflux in patients with symptoms of gastroesophageal reflux. Otolaryngol Head Neck Surg. 2004;131:29–33. doi: 10.1016/j.otohns.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Shang ZM, Huang WN, Hao JY. A study of esophageal function and reflux characteristics of gastroesophageal reflux disease in patients presenting with chronic cough [in Chinese] Zhonghua Nei Ke Za Zhi. 2011;50:931–934. [PubMed] [Google Scholar]
- 34.Satou Y, Oguro H, Murakami Y, Onoda K, Mitaki S, Hamada C, Mizuhara R, et al. Gastroesophageal reflux during enteral feeding in stroke patients: a 24-hour esophageal pH-monitoring study. J Stroke Cerebrovasc Dis. 2013;22:185–189. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope. 1991;101:1–78. doi: 10.1002/lary.1991.101.s53.1. [DOI] [PubMed] [Google Scholar]
- 36.Torrico S, Kern M, Aslam M, Narayanan S, Kannappan A, Ren J, Sui Z, et al. Upper esophageal sphincter function during gastroesophageal reflux events revisited. Am J Physiol Gastrointest Liver Physiol. 2000;279:G262–G267. doi: 10.1152/ajpgi.2000.279.2.G262. [DOI] [PubMed] [Google Scholar]
- 37.Galmiche J, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Langstrom G, et al. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969–1977. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 38.Ganz R, Peters J, Horgan S, Bemelman W, Dunst C, Edmundowicz S, Lipham JC, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:719–727. doi: 10.1056/NEJMoa1205544. [DOI] [PubMed] [Google Scholar]


