Abstract
Objective
To assess the feasibility of performing national, randomized trials of dietary interventions for localized prostate cancer.
Methods
The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) is a phase 3 clinical trial testing the efficacy of a high-vegetable diet to prevent progression in prostate cancer patients on active surveillance. Participants were randomized to a validated diet counseling intervention or a control condition. Chi-Square and Kruskal Wallis analyses were used to assess between-group differences at baseline.
Results
From 2011 to 2015, 478 (103%) of a targeted 464 patients were randomized at 91 study sites. At baseline, mean (SD) age was 64 (6) years and PSA 4.9 (2.1) ng/mL. Fifty-six (12%) participants were African-American, 17 (4%) Hispanic/Latino, and 16 (3%) Asian-American. There were no significant between-group differences for age (p-value = 0.98), race/ethnicity (p-value = 0.52), geographic region (p-value = 0.60), time since prostate cancer diagnosis (p-value = 0.85), PSA (p-value = 0.96), clinical stage (T1c or T2a, p-value = 0.27), or Gleason sum (Gleason 6 or 3+4 = 7, p-value = 0.76). In a pre-planned analysis, the baseline prostate biopsy samples of the first 50 patients underwent central pathology review to confirm eligibility, with an expectation that <10% would become ineligible. One (2%) of 50 patients became ineligible.
Conclusion
The MEAL Study demonstrates the feasibility of implementing national, multi-institutional phase 3 clinical trials of diet for prostate cancer and of testing interventions to prevent disease progression in active surveillance.
Introduction
Active surveillance (AS) provides a safe alternative to immediate treatment for men with low-risk and select men with low-volume, intermediate-risk prostate cancer. However, up to 40% of AS patients will undergo surgery, radiation, or androgen deprivation within 5 years, most for clinical progression. A minority will opt for curative therapy despite not meeting objective criteria for progression.1–5
Prevention of clinical progression is a potential strategy to reduce morbidity and health care costs in AS patients. But while numerous studies have identified clinical and pathological variables associated with AS progression, only one has tested an intervention designed to reduce its incidence: the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial.6,7 Further clinical trials are needed to develop prevention therapies and integrate them into AS treatment paradigms.
One potential prevention therapy is diet modification. Diet may influence the risks of prostate cancer incidence, progression, metastases, and death. Preliminary evidence suggests that prostate cancer patients who increase their vegetable and decrease their fat intakes experience increased progression-free, prostate cancer-specific, and overall survival.8–10 In addition, pre-clinical and epidemiological data indicate that components of cruciferous vegetables (isothiocyanates) and tomatoes (lycopene and other carotenoids) induce apoptosis of prostate cancer cells, inhibit carcinogenesis, induce the expression of cytoprotective enzymes, promote genomic stability, and decrease the risk of lethal prostate cancer.11–15
Using social cognitive theory, we designed and successfully pilot tested an intervention that promotes vegetable intake in prostate cancer patients. Among AS patients, our intervention significantly increased total daily vegetable intake and blood carotenoid levels.16,17
The Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) is a phase 3 trial testing the efficacy of this dietary intervention to prevent clinical progression in AS patients.18 MEAL is being conducted by the Alliance for Clinical Trials in Oncology (formerly the Cancer and Leukemia Group B), a National Cancer Institute (NCI)-sponsored cooperative group that conducts cancer control and treatment trials within the NCI National Clinical Trial Network (NCTN). Members of the NCTN, which include academic institutions, community hospitals and cancer centers across the United States, have been able to participate.
As MEAL is the first national, multi-center, randomized clinical trial measuring the impact of a dietary intervention on prostate cancer, we assessed the effectiveness of the MEAL study to enroll and randomize AS patients.
Materials and Methods
The MEAL Study protocol has been described in detail previously.18 Briefly, MEAL is a randomized, phase 3 trial that utilizes a validated, telephone-based counseling program to increase vegetable consumption among AS patients.17,19 From December 2010 to September 2015, AS patients were recruited from participating urology and medical oncology clinics. Financial incentives were not utilized. Eligible patients were randomized 1:1 to either the telephone-based counseling intervention or a control condition in which they received printed materials from the Prostate Cancer Foundation (PCF) recommending consumption of a healthy, vegetable-rich diet (www.pcf.org). Dynamic allocation (minimization method) was used for stratified randomization. Randomization was stratified by age (< 70 years vs. ≥ 70 years), race (African American vs. Other) and time since diagnostic prostate biopsy (0–12 months prior to registration vs. > 12 and ≤ 24 months prior to registration).
Major inclusion and exclusion criteria
Eligible patients were 50 to 80 years of age with biopsy-proven adenocarcinoma of the prostate, clinical stage T1c or T2a and a serum prostate-specific antigen (PSA) < 10 ng/mL, diagnosed within 24 months of baseline with an extended pattern (≥10 cores) biopsy with < 25% of total cores and ≤ 50% of any single core involving cancer. Grade criteria were Gleason 6 for men ≤ 70 years and Gleason ≤ 3 + 4 = 7 for men > 70 years. A single pathologist performed centralized pathology review to confirm eligibility. Participants were not required to have a confirmatory biopsy prior to study entry. The initial biopsy showing diagnosis of prostate cancer was used for the purposes of determining eligibility. However, if a subsequent biopsy performed before patient enrollment showed that the patient was ineligible, he was not enrolled to the study.
Exclusion criteria included prior surgery, radiation, minimally-invasive ablation, or androgen deprivation for prostate cancer and consumption of ≥ 6 servings per day of fruits and vegetables at baseline. Consumption of dietary supplements was permitted. Patients taking 5-alpha reductase inhibitors (5ARIs) were eligible after discontinuation of drug followed by a 90-day washout period.
Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines.
Intervention
The telephone counseling protocol, performed centrally at the Moores University of California San Diego Comprehensive Cancer Center, followed a step-wise, phased approach that employed strategies adopted from social cognitive theory and motivational interviewing techniques to effect and maintain positive behavior change.18 After randomization, each intervention participant was assigned to a personal counselor who encouraged consumption of at least 7 daily vegetable-fruit servings (defined as a half-cup cut up raw or cooked vegetables, fruit, or 100% vegetable juice), including at least 2 servings each of cruciferous vegetables and tomatoes.
The intervention was divided into 4 phases over a 24-month period.18 To ensure intervention fidelity, counselors completed an intensive 80-hour training program. A registered dietitian supervised the intervention team and conducted regular performance reviews. To standardize the intervention and minimize the potential for bias, a detailed, relational database provided counselors with a computer-assisted coaching protocol for each participant contact.18
Primary outcome
The primary outcome is clinical progression defined as PSA >10 ng/nL; PSA doubling time (PSADT) <3 years; or any one of the following findings on repeat prostate biopsy: >25% of cores positive for cancer, >50% of any one core positive for cancer, Gleason sum ≥ (3 + 4) = 7 for men < 70 years, or Gleason sum ≥ (4 + 3) = 7 for men ≥ 70 years.
Secondary outcomes
Secondary outcomes include incidence of prostate cancer treatment in patients who did not meet progression criteria, prostate-cancer specific anxiety as measured by the Memorial Anxiety Scale for Prostate Cancer (Max-PC), urinary symptoms as measured by the International Prostate Symptom Score (I-PSS), and quality of life (QoL) as measured by the Functional Assessment of Cancer Therapy Scale-Prostate (FACT-P), and Expanded Prostate Cancer Index Composite 26 (EPIC-26).18
Outcome evaluation
Follow-up duration is 24 months. PSA is evaluated every 3 months starting from baseline. Beginning 6 months after baseline, PSADT is calculated at the Alliance Statistics and Data Center as log2 divided by the slope (the least squares estimator) of log (PSA) observations over time using the latest 3 PSA measurements.
Participants who do not receive definitive treatment with surgery or radiation undergo an end-of-study biopsy 24 months after baseline. Additional for-cause biopsies are performed as clinically indicated at the discretion of the treating physician. An independent team of telephone assessors evaluates the diets of participants at baseline, 12 months, and 24 months by telephone interview using the Nutrition Data Systems for Research (NDS-R, current version 2010, University of Minnesota Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN) software and nutrient database.
Fasting blood samples are collected at baseline, 12 months, and 24 months and analyzed for plasma carotenoids (total carotenoids, α-carotene, β-carotene, lutein, lycopene, and cryptoxanthin) using high-performance liquid chromatography methodology.16
Statistics
Patients were randomized 1:1 to receive the dietary intervention (experimental arm) or dietary information (control arm). The log-rank test with a two-sided α = 5% and a sample size of 418 has 80% power to detect a difference in progression rate of 20% in the control versus 10% in the experimental arm during the 24-month follow-up period.
Under the exponential distribution assumption for the time to progression, the 2-year progression rate of 20% versus 10% corresponds to a hazard ratio (HR) of 2.1. Assuming a 10% dropout rate, targeted enrollment was 464 patients. We based the expected 10% dropout rate on our pilot study, in which the observed dropout rate was 2%; we conservatively assigned an expected 5-fold increase in the dropout rate compared to the pilot.17 Randomization was stratified by age (≤ 70 years versus > 70 years), race (African American versus Other) and time since original diagnostic prostate biopsy (≤12 months prior to registration versus > 12 to 24 months prior to registration).
We are comparing time to clinical progression between the two arms using the log-rank test for univariate analysis and Cox’s proportional hazards regression for multivariate analysis adjusting for stratification and other prognostic factors; comparing the probability to proceed to treatment within 2 years using the chi-squared test; and estimating the time trajectory of QoL using the generalized estimating equation method.18,20
We are comparing the changes from baseline in mean daily intakes of total vegetables, crucifers, tomato products, beans/legumes and fat between the two study arms with two-sample t-tests at 12 and 24 months and the changes in plasma carotenoid concentrations from baseline between the two arms using a two-sample t-test.
This phase 3 trial was monitored at least twice annually by the Alliance Data and Safety Monitoring Committee, a standing committee composed of individuals from within and outside of the Alliance. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Statistical analyses were conducted by the Alliance Statistics and Data Center.
Results
Randomization
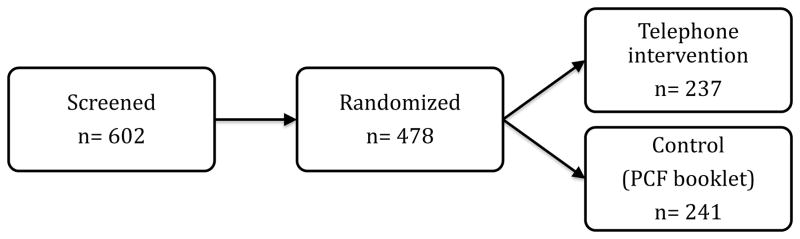
During enrollment, 478 of 464 patients (103%) were randomized from 91 sites. Of these, 237 were randomized to the telephone intervention arm and 241 were randomized to the control arm of PCF printed materials. The screen failure rate was 21% (Figure 1).
Figure 1.
Screening and randomization of men enrolled in the Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]). PCF—Prostate Cancer Foundation.
Baseline characteristics
Mean (SD) age was 63.5 (6.4) years and PSA 4.9 (2.1) ng/mL. Fifty-six (12%) participants were African-American, 17 (4%) Hispanic or Latino, and 16 (3%) Asian-American (Table 1). There were no significant differences between groups with respect to age (p-value = 0.98), race/ethnicity (p-value = 0.52), geographic region (p-value = 0.60), time since prostate cancer diagnosis (p-value = 0.85), PSA (p-value = 0.96), clinical stage (p-value = 0.27), or Gleason sum (p-value = 0.76) (Table 1).
Table 1.
Baseline characteristics of men enrolled in the Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance])
| MEAL Intervention (N=237) | PCF Booklet (N=241) | Total (N=478) | P-value | |
|---|---|---|---|---|
| Age (years) | 0.97802 | |||
| N | 237 | 241 | 478 | |
| Mean (SD) | 63.6 (6.4) | 63.5 (6.5) | 63.5 (6.4) | |
| Median | 64.0 | 64.0 | 64.0 | |
| Q1, Q3 | 59.0, 67.0 | 59.0, 68.0 | 59.0, 68.0 | |
| Range | (50.0–80.0) | (47.0–77.0) | (47.0–80.0) | |
| Race/ethnicity | 0.52272 | |||
| Unknown | 1 (0.4%) | 1 (0.4%) | 2 (0.4%) | |
| Non-Hispanic White | 193 (50.8%) | 187 (49.2%) | 380 (78.0%) | |
| Black or African American | 25 (10.5%) | 31 (12.9%) | 56 (11.7%) | |
| Hispanic or Latino | 10 (4.2%) | 7 (2.9%) | 17 (3.6%) | |
| Asian | 6 (2.5%) | 10 (4.1%) | 16 (3.3%) | |
| Native Hawaiian or Pacific Islander | 0 (0.0%) | 1 (0.4%) | 1 (0.2%) | |
| American Indian or Alaska Native | 1 (0.4%) | 0 (0.0%) | 1 (0.2%) | |
| Not Reported | 1 (0.4%) | 2 (0.8%) | 3 (0.6%) | |
| More than one race | 0 (0.0%) | 2 (0.8%) | 2 (0.4%) | |
| Region | 0.60051 | |||
| Midwest | 42 (17.7%) | 54 (22.4%) | 96 (20.1%) | |
| North East | 61 (25.7%) | 56 (23.2%) | 117 (24.5%) | |
| South | 43 (18.1%) | 45 (18.7%) | 88 (18.4%) | |
| West | 91 (38.4%) | 86 (35.7%) | 177 (37.0%) | |
| Time since cancer diagnosis | 0.85481 | |||
| ≤ 12 months | 203 (98.7%) | 205 (98.3%) | 408 (85.4%) | |
| > 12 and ≤ 24 months | 34 (14.3%) | 36 (14.9%) | 70 (14.6%) | |
| PSA (ng/mL) | 0.95872 | |||
| N | 235 | 238 | 473 | |
| Mean (SD) | 4.9 (2.1) | 4.9 (2.2) | 4.9 (2.1) | |
| Median | 4.8 | 4.7 | 4.7 | |
| Q1, Q3 | 3.6, 6.1 | 3.4, 6.0 | 3.5, 6.0 | |
| Range | (0.0–13.5) | (0.8–11.0) | (0.0–13.5) | |
| Pending verification3 | 5 | |||
| Clinical T stage | 0.27101 | |||
| T1c | 194 (81.8%) | 196 (81.3%) | 390 (81.6%) | |
| T2a | 23 (9.7%) | 32 (13.3%) | 55 (11.5%) | |
| Pending verification3 | 20 (8.5%) | 13 (5.4%) | 33 (6.9%) | |
| Gleason sum | 0.75621 | |||
| 6 | 225 (94.9%) | 228 (94.6%) | 453 (94.8%) | |
| 3+4=7 | 4 (1.7%) | 5 (2.1%) | 9 (1.9%) | |
| Pending verification3 | 8 (3.4%) | 8 (3.3%) | 16 (3.3%) |
Chi-Square
Kruskal Wallis
By The Alliance for Clinical Trials in Oncology central review office
Pathology central review
In a pre-planned analysis, the baseline prostate tissue samples of the first 50 patients underwent central pathology review to confirm eligibility. The expectation was that <10% of the patients would become ineligible after central review, and only 1 (2%) of 50 patients became ineligible.
Discussion
The MEAL Study is the first phase 3 clinical trial of a dietary intervention for prostate cancer. These initial results demonstrate that implementation of a large-scale clinical trial of diet for prostate cancer—with appropriately balanced study arms, a racially diverse patient sample, and broad national representation from both academic and community facilities—is feasible. These results also confirm the viability of performing large randomized trials of interventions to prevent clinical progression in AS patients.
Accrual for MEAL exceeded targets. The low screen failure rate (21%) suggests robust generalizability. Study groups were comparable with respect to age, race/ethnicity, geographic region, PSA, clinical stage, and Gleason sum. Over 21% of MEAL participants represent racial/ethnic minorities, slightly less than the enrollment goal of 29%. Nevertheless, a large proportion—12%—is African American (Table 1). Clinical progression data for African Americans on AS has been inconsistent: some studies have suggested increased progression risk; others have not.21,22 The final results of MEAL, projected to be available in 2018, should thus substantially inform care of African American men on AS.
Prior studies have observed beneficial prostate cancer-specific effects of vegetable-intense diets in AS patients. In a cohort study of 93 AS patients, adoption of lifestyle changes—including a low-fat, plant-based diet—was associated with diminished serum PSA concentrations and decreased rates of progression to curative treatment for up to 2 years.9,23 Gene expression profiling of prostate biopsies in a subset of these men (n=30) identified significant post-lifestyle intervention changes in cellular processes related to carcinogenesis,9 and analyses of peripheral blood mononuclear cells showed significantly increased telomerase activity and longer telomeres,24 intimating that nutritional and other lifestyle changes may potentially promote chromosome stability.
In contrast to prior diet interventions, which required intensive in-person counseling sessions,23 the MEAL intervention produces robust diet changes through a centralized phone-based counseling system.16–18 Compared to in-person counseling, a phone-based system for dietary intervention is advantageous for several reasons. First, for patients, it minimizes economic burdens and removes practical barriers to care. Second, it promotes intervention fidelity through standardized treatment protocols. Finally, it provides substantial economies of scale and efficient delivery of care to relatively large patient populations.
Our results validate the feasibility of implementing trials to prevent clinical progression in AS patients. The overwhelming majority of AS studies have focused on quantifying risks of clinical progression rather than identifying and testing therapies to prevent it.1–5 MEAL is the first phase 3 prevention study undertaken in AS patients since the REDEEM trial. REDEEM assessed the efficacy of dutasteride to prevent progression—defined as Gleason sum ≥ 7 on follow-up biopsy or incident curative therapy—in 340 AS patients over 3 years. Compared to placebo, dutasteride significantly reduced progression incidence by 38%.6
Patients with low-risk prostate cancer are entering AS with increasing frequency.25,26 Reductions in the number of AS patients who undergo surgery or radiation would minimize treatment-associated morbidity, improve patient quality of life, and contain health care costs. However, interventions to prevent progression, like dutasteride, remain underutilized and understudied: consequently, even as the prevalence of patients on AS increases, the incidence of AS progression has not changed appreciably over the past decade. Still, at least one additional prevention trial is currently enrolling: a randomized phase 2 study of a prostate cancer vaccine, PROSTVACÒ (PSA-TRICOM), in 150 AS patients (clinicaltrials.gov). Further AS prevention trials, based on the successful MEAL and REDEEM models, could substantively inform care of the AS population.
Conclusion
The MEAL Study demonstrates the feasibility of implementing randomized trials of diet for prostate cancer and of testing interventions to prevent clinical progression in AS patients.
Acknowledgments
Support: Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under the Award Number UG1CA189823 (Alliance for Clinical Trials in Oncology NCORP Grant), U10CA037447, U10CA011789, U10CA041287, U10CA059518, U10CA077651, U10CA077658, U10CA138561, U10CA180791, U10CA180850, U10CA180866, National Cancer Institute 1R01 CA132951-01A1, Department of Defense PC073412, and The Prostate Cancer Foundation. The study is also supported, in part, by grants from the National Cancer Institute (CA31946) to the Alliance for Clinical Trials in Oncology (Monica M. Bertagnolli, M.D., Chair) and to the Alliance Statistics and Data Center (Daniel J. Sargent, Ph.D., CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the Department of Defense.
Footnotes
Clinicaltrials.gov Id: NCT01238172
Conflicts of Interest
None
References
- 1.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–277. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 3.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 4.Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol. 2015;193(3):807–811. doi: 10.1016/j.juro.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 5.Newcomb LF, Thompson IM, Jr, Boyer HD, et al. Outcomes of Active Surveillance for Clinically Localized Prostate Cancer in the Prospective, Multi-Institutional Canary PASS Cohort. J Urol. 2016;195(2):313–320. doi: 10.1016/j.juro.2015.08.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleshner NE, Lucia MS, Egerdie B, et al. Dutasteride in localised prostate cancer management: the REDEEM randomised, double-blind, placebo-controlled trial. Lancet. 2012;379(9821):1103–1111. doi: 10.1016/S0140-6736(11)61619-X. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton Z, Parsons JK. Prostate Cancer Prevention: Concepts and Clinical Trials. Curr Urol Rep. 2016;17(4):35. doi: 10.1007/s11934-016-0587-1. [DOI] [PubMed] [Google Scholar]
- 8.Richman EL, Kenfield SA, Chavarro JE, et al. Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA Internal Medicine. 2013;173(14):1318–1326. doi: 10.1001/jamainternmed.2013.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frattaroli J, Weidner G, Dnistrian AM, et al. Clinical events in prostate cancer lifestyle trial: results from two years of follow-up. Urology. 2008;72(6):1319–1323. doi: 10.1016/j.urology.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 10.Richman EL, Carroll PR, Chan JM. Vegetable and fruit intake after diagnosis and risk of prostate cancer progression. Int J Cancer. 2012;131(1):201–210. doi: 10.1002/ijc.26348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber NJ, Zhang X, Zhu G, et al. Lycopene inhibits DNA synthesis in primary prostate epithelial cells in vitro and its administration is associated with a reduced prostate-specific antigen velocity in a phase II clinical study. Prostate Cancer Prostatic Dis. 2006;9(4):407–413. doi: 10.1038/sj.pcan.4500895. [DOI] [PubMed] [Google Scholar]
- 12.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10(9):949–954. [PubMed] [Google Scholar]
- 13.Nordstrom T, Van Blarigan EL, Ngo V, et al. Associations between circulating carotenoids, genomic instability and the risk of high-grade prostate cancer. Prostate. 2016;76(4):339–348. doi: 10.1002/pros.23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol. 2012;19(2):134–141. doi: 10.1111/j.1442-2042.2011.02906.x. [DOI] [PubMed] [Google Scholar]
- 15.Zu K, Mucci L, Rosner BA, et al. Dietary lycopene, angiogenesis, and prostate cancer: a prospective study in the prostate-specific antigen era. J Natl Cancer Inst. 2014;106(2):djt430. doi: 10.1093/jnci/djt430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsons JK, Newman V, Mohler JL, et al. The Men’s Eating and Living (MEAL) study: a Cancer and Leukemia Group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology. 2008;72(3):633–637. doi: 10.1016/j.urology.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 17.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Marshall J. Dietary modification in patients with prostate cancer on active surveillance: a randomized, multicentre feasibility study. BJU Int. 2008;101(10):1227–1231. doi: 10.1111/j.1464-410X.2007.07365.x. [DOI] [PubMed] [Google Scholar]
- 18.Parsons JK, Pierce JP, Mohler J, et al. A randomized trial of diet in men with early stage prostate cancer on active surveillance: rationale and design of the Men’s Eating and Living (MEAL) Study (CALGB 70807 [Alliance]) Contemporary Clinical Trials. 2014;38(2):198–203. doi: 10.1016/j.cct.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parsons JK, Bennett JL. Outcomes of retropubic, laparoscopic, and robotic-assisted prostatectomy. Urology. 2008;72(2):412–416. doi: 10.1016/j.urology.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 20.Bang H, Jung SH, George SL. Sample size calculation for simulation-based multiple-testing procedures. J Biopharm Stat. 2005;15(6):957–967. doi: 10.1080/10543400500265710. [DOI] [PubMed] [Google Scholar]
- 21.Leapman MS, Freedland SJ, Aronson WJ, et al. Pathologic and Biochemical Outcomes among African-American and Caucasian Men with Low-Risk Prostate Cancer in the SEARCH Database: Implications for Active Surveillance Candidacy. J Urol. 2016 doi: 10.1016/j.juro.2016.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundi D, Faisal FA, Trock BJ, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85(1):155–160. doi: 10.1016/j.urology.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ornish D, Weidner G, Fair WR, et al. Intensive lifestyle changes may affect the progression of prostate cancer. J Urol. 2005;174(3):1065–1069. doi: 10.1097/01.ju.0000169487.49018.73. discussion 1069–1070. [DOI] [PubMed] [Google Scholar]
- 24.Ornish D, Lin J, Chan JM, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. The Lancet Oncology. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 25.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314(1):80–82. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 26.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67(1):44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]