Abstract
For thousands of years, plants and their products have been used as the mainstay of medicinal therapy. In recent years, besides attempts to isolate the active ingredients of medicinal plants, other new applications of plant products, such as their use to prepare drug delivery vehicles have been discovered. Nanobiotechnology is a branch of pharmacology that can provide new approaches for drug delivery by the preparation of biocompatible carrier nanoparticles (NPs). In this article we review recent studies on four important plant proteins that have been used as carriers for targeted delivery of drugs and genes. Zein is a water-insoluble protein from maize; Gliadin is a 70% alcohol-soluble protein from wheat and corn; Legumin is a casein-like protein from leguminous seeds such as peas; Lectins are glycoproteins naturally occurring in many plants that recognize specific carbohydrate residues. NPs formed from these proteins show good biocompatibility, possess the ability to enhance solubility, and provide sustained release of drugs and reduce their toxicity and side effects. The effects of preparation methods on the size and loading capacity of these NPs are also described in this review.
Keywords: Plant proteins, Zein, Gliadin, Legumin, Lectin, Drug delivery, Nanocarrier, Sustained release
1-Introduction
In recent years, biotechnology and biomedical science have played a major role in devising drug delivery systems and especially, progress in incorporation of nanotechnology for biomedical investigations has offered innovative materials and approaches for drug delivery applications, which provide many advantages over traditional routes of administering pharmaceutical products (1–8). Many macromolecules (proteins, polymers, carbohydrates, etc) have been used to prepare NPs and other forms of nanocarriers for drug delivery. The term “nanoparticles” describes solid, colloidal particles consisting of macromolecular substances that vary in size from 10 nm to 1000 nm, however, particles >200 nm are less often studied and nanomedicine usually refers to particles <200 nm (9–11).
The application of proteins derived from animal/insect sources such as gelatin(12), collagen(13), albumin (14), casein (15) and silk (16) have been widely investigated for drug delivery purposes as nanocarriers. However, some of these carriers may pose a risk of infectious pathogens due to contamination with animal tissues. On the other hand, there are significant numbers of individuals who avoid consuming animal-based proteins as food due to religious beliefs or personal preferences. Plant based-proteins derived from abundant natural plant resources have been employed as an alternative source of material to overcome the above-mentioned limitations. Also, plant proteins are often less expensive than animal proteins and possess functional groups which can be easily employed either to adsorb or to covalently couple molecules capable of modifying the targeting properties of NPs (17). Meanwhile, in recent years, some other biologically-derived elements, such as viruses and bacteria have been adapted to act as targeted drug delivery vehicles (18–20). These biological elements include viral vectors, virus-like particles, virosomes, recombinant bacteria, “microbots”, bacterial ghosts (21), as well as various kinds of living cells (22) that have been employed to either to transport a therapeutic cargo, or to produce it in situ. However, despite promising potential for efficient in-vivo targeted delivery, there remain worrisome biosafety issues related to immunogenicity caused by human viruses, virus-like particles and virosomes etc. (23). Additionally, well-documented proof will be needed to convince health authorities and dispel concerns of the biomedical community about the safety of employing bacteria for medical treatments (24).
Several plant-derived polysaccharides, such as cellulose, hemicelluloses, pectin, inulin, alginates, carageenans, rosin, gums and musilages in various forms have been used to prepare implants, films, beads, microparticles, and NPs, and these formulations have also been investigated for drug delivery purposes (25).
Reddy et al.(26) published a review paper describing the potential of plant proteins for medical applications, and recently some review papers have been published concerned with general classes of protein-based nanocarriers, and these have also included some plant-protein derived nanocarriers (17, 27–29). In this review, the drug delivery applications of plant-derived proteins with particulate sizes that fall into the range of 10 to 100nm (ultrafine) and 100 to 1000nm (fine) as drug carriers along with recent studies and advances are summarized.
Zein is a water-insoluble plant protein derived from maize and owing to its ability to maintain humidity levels in prepared goods, has found many applications in the packaging and food industries. It contains many nonpolar amino acids such as alanine, phenylalanine, leucine and proline, but because of its protein structure and its hydrophobic properties, can be phagocytosed by macrophages and can therefore stimulate the immune system (30–32). Currently, commercial zein, is provided in two forms, yellow and white zein (33). According to its solubility and molecular weight, zein is divided into three types: “alpha” 21–25 kDa; “beta” 17–18 kDa; and “gamma” 27 kDa. These types comprise 75–87%, 15%, and 5–10% of total natural zein protein respectively. Alpha zein has an isoelectric pH point at 6.8 but the surface charge of zein can vary according to the environmental conditions. Due to the special structure of zein proteins, they can form globules with a diameter of 150 to 550 nm having somewhat different shapes in vivo (17). Zein therefore has the potential to be loaded with other cargo molecules and can avoid interaction with other hydrophilic polymer systems in the body (34). Gamma zein is able to deliver drugs through the cell membrane due to the interaction of its N-terminus with the cell membrane (35, 36). The N-terminus sequence of zein can be described as a member of the proline-rich class of cell penetrating peptides (CPPs), and is therefore used in intracellular drug delivery systems (37). In recent years, there have been intensive ongoing investigations aimed at developing zein nanoparticles for drug delivery applications along with in vivo pharmacokinetic preclinical studies on animal models for evaluating oral bioavailability and toxicology of zein-based drug delivery, which have produced considerably enhanced oral bioavailability of drugs encapsulated by these nanocarriers (38–42).
Another common plant protein is gluten which is the main storage protein in the seeds of wheat and corn, and is obtained from the industrial processing of starch (31). Based on its solubility, gluten is divided into two constituents; monomeric gliadin that is soluble in 70% alcohol with a molecular weight of 25–100 kDa, and polymeric glutenin that is rather insoluble due to disulfide bond cross-linking with a molecular weight of 106 kDa (43, 44). Glutenin nanoparticles have demonstrated excellent stability, which eliminates the need for usage of potentially cytotoxic crosslinking agents. Additionally, recent in-vivo preclinical and in-vitro studies have revealed the high potential of glutenin nanoparticles for therapeutic drug delivery (45).
The amino acid composition analysis shows the presence of large amounts of non-polar neutral amino acids in gliadin and only small amounts of charged polar amino acids are present in its structure (46, 47). The high proline content in gliadin causes it to interact with dermal and epidermal keratin protein and allows it to mediate controlled drug release (48).
Legumin is an albumin-like protein which plays a storage role in pea seeds (49). The main storage proteins in pea seeds are called globulins (50), and the two most abundant fractions are legumin and vicilin. These proteins are water-soluble depending on the pH and ionic strength. Legumin NPs have been utilized in pharmaceutical applications such as cutaneous or transdermal administration of drugs (51). These particles have been used as interesting pharmaceutical carriers for the delivery of antitumor drugs in local cancer treatment for an improved cytostatic effect (52).
Lectins are another group of naturally occurring proteins or glycoprotein’s, which were first discovered in plants. However, they are also present in other living organisms. They are distinguished by their ability to bind non-covalently and specifically to carbohydrate residues attached to biomolecules such as proteins and lipids (53–55). The first study on lectin proteins was conducted by Hermann Stillmark in 1888 who reported that ricin protein extracted and partially purified from castor beans, possessed an agglutinating property (56). In the 1950s, lectins were named for preparations derived from plants, which could recognize and distinguish blood groups via the different sugar residues expressed (57). Later in 1972, Sharon et al. listed a large number of different lectin proteins which had up to then been purified (58). In recent decades, there has been a substantial body of knowledge accumulated on plant lectins, including their biochemical properties, biological functions and specific carbohydrate binding affinities (59–61). A variety of lectin-NP conjugates have been prepared in the last decade and proposed for drug delivery applications.
2- Zein
2-1 Protein structure and properties
The tertiary structure of zein is ribbon-like, with dimensions of 16 nm long, 4.6 nm wide and 1.2 nm thick with distinct hydrophobic and hydrophilic segments (62). Based on small-angle X-ray scattering, the structure of zein consists of 10 successive helical sequences, arranged in an anti-parallel fashion and stabilized by hydrogen bonds between the turns. At each end there is a final glutamine-rich helix (63) (figure 1). The specific structure of zein makes it behave as an amphiphilic polymer, and when it interacts with molecules such as drugs, this interaction can influence its tertiary structure. Hydrophilic drugs open the coiled structure of zein and allow formation of smooth films, whereas lipophilic compounds enhance the curvature of the zein particles and form smaller spheres. Amphiphilic compounds allow the formation of interconnecting channels, thereby decreasing the curvature and forming a sponge (64). As compared to animal proteins such as silk and collagen, zein has more negative charge that makes it suitable for carrying the positive charged drug into the body (26). Although zein has an overall amphiphilic structure, it can have a better interaction with hydrophobic drugs in sustained release delivery systems, followed by hydrophilic and amphiphilic compounds respectively (65). Zein, having a high proportion of apolar amino acid residue (>50%), is an alcohol soluble protein. The variation in concentration of ethanol or isopropanol in the aqueous solution along with thermal treatment (at 60°C for 10 min) has been studied to evaluate the change in physical properties of the suspension of zein particles. It was found that the particle size was increased by raising the ethanol concentration in the ethanol/water solvent mixture initially from 70% (13.4nm) to 85% (29 nm) and the isopropanol concentration of isopropanol/water solution from 65 to 95%. This occurred due to unfolding occurring at elevated temperature, and exposure of internal hydrophobic leading to hydrophobic interactions which caused aggregation of zein molecules (66).
Figure 1.
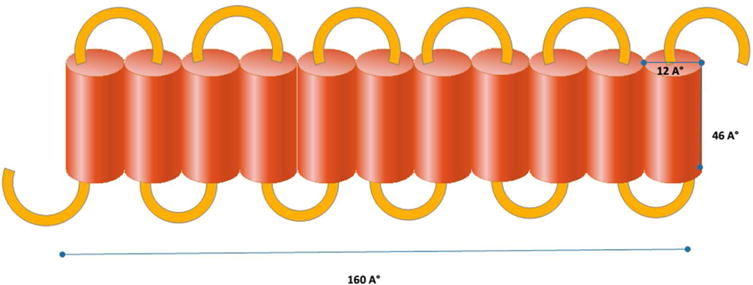
Structure of a single unit of zein
In table 1 various protein NP preparation methods, generated particle size along with encapsulation efficiency of some therapeutics with zein NPs are listed. In the following section, the latest studies and applications of zein in drug delivery systems are covered.
Table 1.
Comparison between different zein NPs prepared by different methods
| Zein NPs (ZN) | Size nm | Loading and encapsulati on efficiency | Method of preparation | Description/Results | Ref. |
|---|---|---|---|---|---|
| Sodium caseinate/ZN loaded with Thymol | 300 | 65,8–97.1 % | Direct pouring SC into Zein solution | Thymol loaded core-shell nanodelivery exhibited antibacterial properties against food pathogens and offred sustained release. | (73) |
| Thymol & carvacral/ZN | 800 | 50% | Liquid-liquid dispersion | The thymol and carvacrol encapsulated in zein NPS demonstrated antimicrobial properties and their antioxidant capabilities along with release kinetics were studies. | (75) |
| Cranberry procyanidin/ZN | 392–447 | 48–79% | Liquid-liquid dispersion | The cytotoxicity of ZN encapsulated CP was evaluated toward cancerous lukemia HL-60cells, which demonstrated to be less than administrating individual CPs. | (76) |
| Catalase & superoxide dismutase/ZN | 256 | 30–50% | Phase separation method | Oral delivery of active antioxidant agents, catalase or superoxide dismutase (SOD), which were used to treat pathogenesis of rheumatoid arthritis (RA) was evaluated. | (69) |
| Tangeretin/lactoglubulin/ZN | 245–253 | 73% | Anti-solvent precipitation | lactoglubulin/ZN coreshell particles with hydrophilic shell were used to encapsulate tangeretin, additive in food and beverages, to improve its solubility and dispersion in aqueous medium. | (77) |
| Curcumin/ZN | 175–900 | 85–90% | Electro-hydrodynamic atomization | curcumin-loaded ZN NPs were evaluated for the storage stability, morphology and the potential of curcumin to be employed as natural colorant in food products. | (92) |
| Aceclofenac-pantoprazole-zein-eudragit nanofibers (NF) | 50–200 | 36% for aceclofenac 66% for pantaperazol |
Single nozzle electrospinning | The dual drug delivery system, aceclofenac and pantoprazole-loaded zein NPs provided a combination that can be employed to avoid the toxicity caused by non-steroidal anti inflammatory Drugs. | (85) |
| KET/zein-PVP-core-sheath NF | 730±1 90 | ------- | Coaxial electrospinning | Ketoprofen-loaded core-sheath nanocarrier released the entrapped drug in two phases. The hydrophilic sheath structure released the drug immediately whereas the core part performed sustained release. | (86) |
| pDNA/zein nanospheres | 158–397 | 65% | Coacervation technique | The encapsulated DNA was protected from degradation and the pDNA/zein nanospheres exhibited sustained release over 7 days. | (36) |
| TOC/Chitosan-ZN | 200–800 | 76.5–86.5% | ------- | chitosan-ZN improved the sustained release and protected the encapsulated α-tocopherol (TOC) from gastrointestinal enzymes. Also provided a suitable carrier for delivering hydrophobic materials, such as TOC. | (68) |
| Daidzin/TPGS-ZN | 210–221 | 63% | Antisolvent method | TPGS improved the encapsulation efficiency of drug in ZN NPs and also hindered its ifflux. ZN provided biocompatible medium and TZN(emulsified ZN) offered sustained releasE. | (41) |
2-2 Application of zein NPs in drug delivery systems
2-2-1 Zein NPs generated by various phase separation methods
Zein NPs are the most intensively investigated group of plant proteins for drug delivery applications either individually or in the form of hybrids with other materials to improve stability as well as other properties. Mesoporous silica SBA-15 and recombinant human bone morphogenetic protein-2 (rhBMP-2) was loaded onto a zein-based scaffold and hydroxypropyltrimethyl ammonium chloride derivatized chitosan (HACC) was incorporated into it as an anti-infective agent. The developed zein-HACC-S20 displayed good antibacterial activity against E. coli and S. aureus lasting for 5 days and was also able to release rhBMP-2 at a low controlled rate for up to 27 days (67). Luo et al. (68) proposed a nanohybrid formed by a complex between chitosan and zein for encapsulation of alpha-tocopherol (TOC, the active compound in vitamin E) to protect it from destruction in the stomach. The average particle size after drug loading varied from 200 to 800 nm and there was a burst release of the drug followed by a slower sustained release.
To control reactive oxygen species (ROS), one of the important pathogenic elements in rheumatoid arthritis (RA), the oral administration of antioxidant enzymes are desirable. Zein NPs were generated using a phase separation method and folate-conjugated enzymes, superoxide dismutase (SOD) and catalase, were loaded onto zein NPs to protect them from gastrointestinal degradation after oral administration and also to target them to activated macrophages via folate receptors. The particle size in this system was 255.21 nm with 30–50 % loading efficiency. The use of zein increased the therapeutic activity of the proteins from 5–10% with free catalase and SOD, to 40–60% with the zein-loaded enzymes, and also protected the enzymes from pepsin as well as the acidic conditions of the stomach (69).
Coacervation or desolvation (sometimes referred to as anti-solvent precipitation, drawing-out precipitation or solvent displacement) is based on the variable solubility of proteins in different solvents which is a function of pH, ionic strength and electrolytes (70, 71). Desolvation is a technology applied for preparing NPs from a wide range of polymeric macromolecules, and is based on desolvation by changes in charge and pH, or by addition of a desolvating agent (ethanol or concentrated inorganic salt solutions). The main advantage is that this process does not require an increase in temperature and, therefore is applicable to heat-sensitive drugs (48).
Zou et al. (41) generated zein nanoparticles (ZN) and emulsified these particles with TPGS1000 (TPGS) to form TZN for oral administration of daidzin. The ZN and TZN were prepared by a modified antisolvent method. The results indicated that the average size was around 200 nm, and the presence of TPGS (a food additive) enhanced drug encapsulation from 53% in ZN to 63% in TZN, and moreover the cellular uptake and drug release was significantly higher in intestinal Caco-2 cells. These authors carried out in vivo investigations on bioavailability and pharmacokinetics performed by analyzing mice plasma for daidzin metabolites after oral delivery of daidzin loaded TZN. These studies confirmed improved oral bioavailability of diadzin by the TZN drug delivery system.
In a separate study, Luo et al. (72) used caseinate (CAS) to stabilize ZN to enhance the cellular uptake and transportation in Caco-2 cells. The best uptake occurred with a 2:1 ratio of CAS:zein at 1 mg/ml where 40% was taken up by the cells after 4h. The size of these particles was 120–140 nm and the uptake occurred through an energy-dependent endocytosis mechanism. Moreover, Li et al. (73) reported a two-phase sodium caseinate (SC)/ZN preparation by a simple antisolvent procedure via direct pouring of SC into a ZN solution. The average size of the NPs was around 300 nm and after freeze drying, they were able to be re-dispersed in deionized water. The carrier showed good antimicrobial activity against E. coli and Salmonella spp and could be a possible preservative against microbial contamination in the food industry.
The liquid-liquid dispersions method has been widely utilized both for generation of protein NPs and encapsulation of some compounds within zein NPs. The process generally involves, preparation of a zein solution in 60–90% aqueous ethanol, shearing the stock solution by pouring into bulk deionized water which causes emulsification of the solution into small droplets, separation of alcohol from the droplets and partition into the water solution due to its immiscibility with water, precipitation of the undissolved zein NPs and encapsulation of the co-dissolved nonpolar compound (74). Wu et al. (75) used a liquid-liquid dispersion method to encapsulate two antimicrobial essential oils, thymol and carvacrol, into ZN with an average size of <800nm. The antioxidant compounds extracted from cranberries known as procyanidins (CPs) were also able to be loaded into ZN by employing liquid-liquid dispersion owing to the formation of both hydrogen bonds and hydrophobic interactions between zein and the CPs. The average particle size was 392 to 447 nm depending on the CPs/zein ratio. This CPs zein reduced the cytotoxicity of procyanidins in pro-myelocytic leukemia L-60 cells in comparison with CPs-solution (76). The combination of ZN and beta-lactoglobulin is proposed to be a good carrier of lipophilic drugs. The tangeretin-encapsulated lactoglobulin-coated ZN NPs were produced via liquid-liquid dispersion and used for delivery of water-insoluble tangeretin (a polymethoxyflavone). First, zein and tangeretin were dissolved in ethanol solution (90%, v/v), then this organic phase was introduced to aqueous solution of beta-lactoglobulin in saline to provide an amphiphilic surface coating of ZN. The yielded core-shell colloidal NPs were stable at low salt concentrations and pH values outside the isoelectric point, but aggregated at the isoelectric pH and at high salt concentrations (77). In a similar fashion, ZN coated with an alginate shell was proposed as nano-delivery system. Zein alginate core/shell NPs were generated via an antisolvent precipitation to form the zein core and, electrostatic deposition to form the alginate shell. The core diameter was 80 nm, surrounded by a 40 nm thick shell and having an overall diameter of 160 nm. The ZN showed good thermal stability at pH 7, without change in size after heating at 90° C for 2 h (78). In another study, ZNs were generated with food-grade nonionic surfactant, Tween 80 coating as a food stabilizer via antisolvent precipitation. The developed NPs had a 76 nm diameter zein core and a Tween 80 sheath with 6 nm thickness and the obtained coreshell nanostructures could be employed as food grade delivery systems for encapsulating, protecting, and releasing bioactive molecules (79). Regier et al. (36) applied the coacervation technique to prepare ZN, then loaded hydrophilic plasmid DNA (pDNA) into the nanospheres with a loading efficiency that depended on the zein-pDNA ratio, with a maximum of 65.3 ± 1.9% and a density of 6.1 ± 0.2 mg DNA/g zein. The encapsulation protected the pDNA from DNAase I degradation and could provide sustained release for gene therapy.
Luo et al. (80). encapsulated two drugs (indol-3-carbinol (I3C) and 3,3′ diindolylmethane (DIM)), into zein/carboxymethyl chitosan (CMCS) by combining liquid-liquid phase separation and ionic gelation methods. These nanospheres could provide good controlled release of both I3C and DIM. In another study, Lai et al. (81) encapsulated 5-fluorouracil (5-FU) into ZN using a phase separation procedure for liver targeting. The results showed higher accumulation of drug in the liver after intra-vascular administration when the zein:5-FU ratio was 3:1 v/v. It could remain in the bloodstream for 24h. The average size of these particles was 11.4 nm, the encapsulation efficiency was 60.7%, and 4°C was the best storage temperature.
Another technology, which closely resembles anti-solvent precipitation, is modification of the (anti-)solvent system by employing supercritical fluids, such as CO2 as the anti-solvent. This system has advantages of the absence of residual solvent, mild operating temperatures, and a narrow particle size distribution and has been used as a tool for encapsulation (71). Hu et al. (82) used the “solution-enhanced dispersion by supercritical fluids” (SEDS) technique (figure 2) to load the poorly water-soluble compound, lutein, into ZN. The study showed that the application of the SEDS technique could influence the morphology, drug loading, and size of the NPs. A ratio of lutein/zein of 1:18 w/w, and a pressure of 10 Mpa coupled with a solution flow rate of 10ml/min at 45°C could produce lutein-loaded ZN in a narrow size-range with controlled drug release.
Figure 2.
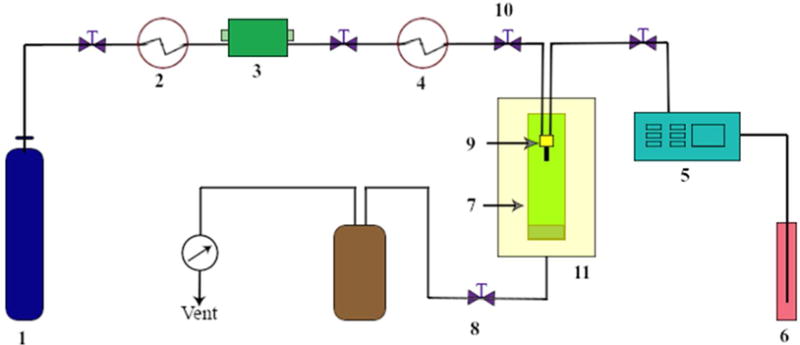
Schematic diagram for the apparatus and process of the solution enhanced dispersion by supercritical fluids (SEDS) technique; the apparatus consists of three major systems: a CO2 supply system, an organic solution delivery system, and a high pressure vessel. The components are: 1) CO2 cylinder, 2) cooling system, 3) piston pump, 4) heat exchanger, 5) HPLC pump, 6) solution, 7) high pressure Wessel, 8) back pressure regulator, 9) coaxial nozzle, 10) pressure meter, 11) gas bath.
2-2-2 Zein nanofibers generated by electrospinning
The incorporation of various therapeutics, growth factors, vitamins etc into polymeric nanofibers prepared from natural materials has been investigated for numerous delivery applications via oral, pulmonary, transdermal and ocular administration routes (83). The electrospinning method is the most popular method employed for preparation of nanofibers from natural or synthetic materials for drug delivery purposes. The method is generally based on applying high voltage (1 to 30Kv) to polymer solutions or melts which generates highly electrified droplets with charge evenly distributed over the surface. When subjected to the higher potential, the droplet acquires a deformed shape named as “Taylor cone” and by further increasing the voltage beyond the threshold value, the electric field overcomes the surface tension and the jetting of charged solution and solvent evaporation occurs leading to the transition between liquid and solid and formation of a nonwoven fabric mat (84).
Aceclofenac and pantoprazole (members of two different classes of drugs) were simultaneously transported by composite nano-fibers made by electrospinning zein/eudragit mixtures. Aceclofenac could be loaded onto zein, whereas pantoprazole, an acid labile proton pump inhibitor, was carried by eudragit S 100 to inhibit gastric acid secretion. The nanocarrier was prepared via single nozzle electrospinning process (figure 3A) then the drug was loaded in nanofibers. The sustained release of the drug was demonstrated for up to 8h both in vitro and in vivo. This study showed less gastrointestinal toxicity by controlled release of NSAIDs (85). Jian et al. used coaxial electrospining to provide a biphasic drug release (BDR) system (fig 3B). They loaded ketoprofen (KET), a poorly-water soluble NSAID, onto core-sheath nano-fibers. The sheath part consisted of hydrophilic polyvinylpyrrolidone (PVP) as a filament-forming matrix covering a core matrix of zein. The average size of the nano-fibers was 730 ± 190 nm and they had good compatibility with KET. In this system 42.3% of loaded drug was released immediately and the remaining drug was released over 10h (86). In a similar study Yang et al. used acetic acid as the sheath liquid in a coaxial electrospining process to coat zein and ferulic acid (FA) as the core fluid. The results obtained from The proposed modified coaxial electrospinning process indicated that the generated nanofibers possessed higher quality and improved functional performance, such as sustained-release profiles (87). Another modified coaxial electrospinnig technique was used to prepare ibuprofen (IBU) loaded zein nanofibers using dimethylformamide as the sheath fluid. Zein exhibited good compatibility with IBU due to the formation of hydrogen bonds and the drug sustained release over 10 h in vitro was observed (88). Streptomycin sulfate was incorporated into electrospun nanofibers made from a mixture of polyurethane (PU) with added zein and cellulose acetate (CA). The presence of zein in the polymer mixture, improved the overall hydrophobicity and the ability to mediate cell attachment (89). Curcumin is a natural occurring compound with various therapeutic applications and desirable features of free radical scavenging and stimulating cell proliferation. The electrospinning method has been adapted to prepare a zein-curcumin fibrous scaffold. The in vitro curcumin release from electrospun fibers was studied and the initial burst release of curcumin from the scaffold was observed, followed by sustained release without any toxicity towards mouse fibroblasts (90).
Figure 3.
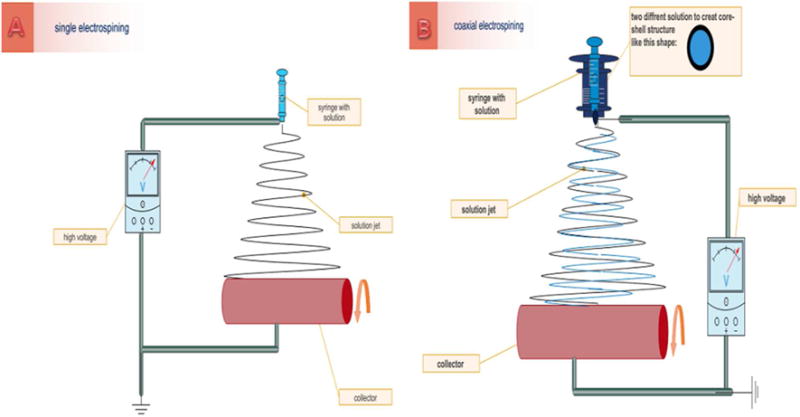
Electrospinning setup consists of a spinneret (typically a hypodermic syringe needle) connectedto a high-voltage, direct current power supply, a syringe pump, and a grounded collector. A polymer solution, is loaded into the syringe and this liquid is extruded from the needle tip at a constant rate by a syringe pump A) single electrospinning B) coaxial electrospinning (A coaxial setup uses a multiple solution feed system which allows for the injection of one solution into another at the tip of the) spinneret.
Other encapsulation techniques have also been investigated for efficient delivery of curcumin. To prevent the possibility of macrophage uptake and consequent immunogenicity which can be due to the protein origin and hydrophobicity of zein nano/microparticles, PEGylation of zein has been proposed. The hydrophobic anticancer drug, curcumin was encapsulated into a nanocarrier consisted of ZN conjugated with methoxy poly(ethylene glycol) (mPEG) to form micelle structures with an external hydrophilic shell. The results displayed higher drug uptake in drug resistant NCI/ADR-RES cancer cells in vitro (91). In another study, Gomez-Estaca et al. (92) encapsulated curcumin into ZN by an electrohydrodynamic atomization technique with an encapsulation efficiency of around 85–90%. It was observed that the ZN size could be increased from 175 to 900 nm by increasing the zein concentration from 2.5 to 15% (w/w).
Zein nanocarriers are also promising for gene therapy applications. Karthikeyan et al. (93) developed siRNA loaded zein nanofibers for the first time and the potential of electrospun zein nanofibers for safe delivery of siRNA and sustained release was demonstrated. In vitro study revealed gene silencing efficiency of 26%, which was obtained by total 35% released siRNA.
These studies demonstrate the potential of zein protein as a promising nanocarrier for clinical applications which should be further investigated for sustained and biphasic drug release and gene delivery systems.
3- Gliadin
3-1 Protein structure and properties
The gliadin structure consists of two main parts: a hydrophobic central domain (CD) containing highly repetitive amino acid sequences such as glutamine and proline, surrounded by hydrophobic terminal domains (TD). These two domains give amphiphilic properties to this molecule (94). According to the size of the TD, three type of gliadin are recognized; α/β gliadin, γ gliadin, and ω gliadin in which α/β and γ gliadins have a long carboxy-TD chain and a short amino-TD chain with high amphiphilic properties, whereas in ω gliadin, both carboxyl and amino TD chains are short and the CD comprises 90–95% of the protein structure (95). However, use of gliadin may cause immune response in celiac disease. In this disease, glutamine and proline containing proteins get ingested in the gut stimulate Th1 and Th17 cells, leading to malabsorption and villous atrophy (96). Antibodies against gliadin have been detected in patients who suffer from celiac disease, therefore it would be important to take this into account if considering administration of gliadin nano-carriers in those patients (97).
3-2 Applications of gliadin NPs in drug delivery systems
The liquid anti-solvent precipitation (desolvation) is the most widely employed strategy for preparation of gliadin NPs and encapsulation, which is based on the lowering of the solvent power during dissolution of bioactive compounds. As mentioned before, a mixture of the bio-component and the excipient are dissolved in a mixture of a suitable organic solvent and water, then an anti-solvent is added to this mixture (71). The size of the generated gliadin NPs, (which is a determinant characteristic for medical purposes), depends on the organic medium and can be optimized by varying the solubility parameter δ of the protein solvent phase, so that, when δ is closer to the protein solubility parameter, the size of the NPs is smaller (150nm when δMixture corresponds to the gliadin solubility parameter δG). Besides considering the solubility parameter for achieving the desired particle size, the environmental acceptability issue should also be taken into account before choosing a suitable organic solvent: generally an ethanol and water mixture is always preferred (98),(99).
The large surface area of the GNP provides the ability for good interaction with biological surfaces (100). Since gliadin is rich in neutral and hydrophobic amino acids, hydrogen bonding and hydrophobic interaction can link it to the mucosal surface and lipid-rich tissue. Carbazole-incorporated gliadin NPs have been prepared via a desolvation procedure and utilized for delivery of lipophilic drug carbazole for oral administration (101). Thereby, gliadin is a suitable material to produce various mucoadhesive NPs that can stick to mucosal surfaces, and be used for oral drug delivery of lipophilic molecules (102). Some unique properties of GNPs such as its affinity for the upper gastrointestinal tract, the small size of GNPs that increase penetration into the gastric mucosa and its ability to protect antibiotics, make it suitable in treatment of Helicobacter pylori infection (103). Taking advantage of the bioadhesive ability of gliadin, it was applied for delivery of clarithromycin and omeprazole to the stomach (the site of H. pylori infection). The drug loaded NPs were prepared via desolvation and the estimated size of these GNPs were 400 to 650 nm with around 75% loading efficiency and with sustained release behavior at pH 1.2 and 37°C (104). Similarly, a long-lasting mucoadhesive preparation of amoxicillin-loaded GNPs was more effective against H. pylori infection than free amoxicillin, due to its extended gastrointestinal residence time (105). Moreover, Ramteke et al. (106) produced a triple antibiotic combination therapy consisting of amoxicillin, clarithromycin and omeprazole entrapped in GNP to increase the effectiveness of H. pylori therapy.
In another study, owing to the mucoadhesive properties of GNPs, the oral administration of gliadin-encapsulated superoxide dismutase was studied to treat feline immunodeficiency virus (FIV) infection (107).
In a separate study, Fajardo et al. (108) used cinnamaldehyde as cross-linker to bind gliadin to lysozyme, a naturally occurring antimicrobial agent that is used against Gram-positive microorganisms. In this research, the effect of the cinnamaldehyde on the release of the lysozyme from the gliadin was studied against Listeria innocua at pH 6.2. As expected, the results showed that increasing the cross-linker led to a decrease in lysozyme release and lower antimicrobial activity.
Kim et al. (109) utilized the amine groups of the gliadin protein as a polymerization initiator to produce an amphiphilic combination of gliadin-ethylene cyanoacrylate (ECA) and increase the water solubility of GNP. Gliadin plays a hydrophilic role whereas poly ECA provides the hydrophobic part on the surface. Duclairoir et al. (110) studied the interaction of GNPs with three different types of drug; vitamin E (VE) as a hydrophobic compound, linalool/linalyl acetate mixture (LLA) as a slightly polar compound mixture, and benzalkonium chloride (BZC) as an amphiphilic cationic drug. The results of this study showed the average size of these NPs was around 450–475 nm and the entrapment of VE and LLA was higher than BZC because of the non-polarity of the carrier, whereas the sustained release of the VE was more than LLA and BZC. LLA-GNPs and BZC-GNPs exhibited burst release of the drugs that confirmed the better interaction of gliadin with non-polar compounds in comparison with either polar or amphiphilic bioactive molecules.
The encapsulation of lipophilic retinoic acid into GNPs was reported by Duclairoir et al. (111). Gliadin NPs were prepared by a desolvation method and they exhibited satisfying carrier characteristics for all-trans-retinoic acid (RA) with good entrapment efficiency: about 75% of added drug at 60 μg• (mg gliadin) −1 and a payload of 76.4 μg• (mg gliadin)−1 NPs. A rapid release of 20% of the drug after 15 min in sink conditions was observed followed by a slower diffusion process as a second step.
In a different strategy, Gulfam et al. used electrospray deposition to generate gliadin and gliadin-gelatin composite NPs for delivery of anticancer drugs, such as cyclophosphamide, against breast cancer cells. The average size of the GNPs varied from 220–450 nm according to the percentage of gliadin. The non-hybrid gliadin NPs gradually released cyclophosphamide over 48 h while gliadin-gelatin hybrid NPs provided a more rapid drug release. Moreover, GNPs containing 7% loading of drug induced apoptosis in vitro within 24 h (112).
4- Legume proteins
4-1 Legumin and Vicilin
4-1-1 Protein structure and properties
Legumin and vicilin are known as storage proteins in leguminous seeds, since they are laid down at an early stage of the life cycle of seed development for future use at a metabolically more active stage. Many different proteins exist in seeds and the storage proteins should be distinguished from those proteins which have metabolic or structural roles (113).
Generally, pea proteins comprise 20–30% by weight of pea seeds, mainly consisting of globulins (65–80%), glutelins and albumin. Globulins contain three different components including legumin, vicilin and convicilin. The 11S globulin components represented by legumin have molecular weights of 350 and 400 kDa whereas the 7S globulin component has a molecular weight of 150 kDa consisting of vicilin and convicilin (50). According to work by Bailey et al. (114) who created a model for legumin with ten polypeptide chains, a molecular weight of 320kDa is preferred. There are some differences and also similarities between legumin and vicilin. Their shape and the size of the subunit domains in an aqueous solution are similar, but the molecular weight of legumin (360 kDa) is higher than that of vicilin (200kDa) (115). Moreover legumin is resistant to coagulation at high temperatures (116). In both proteins, the carboxy-terminus of the subunits exhibits a conserved core region that is predominantly hydrophobic with most of the residues in a beta-sheet conformation. The amino-terminus regions of the subunits from the two different proteins cannot be aligned, although they typically contain a high proportion of residues in common (117, 118), predicted to be in a helical conformation i.e. leucine, glycine, and threonine (114). There is an inserted polypeptide span in the legumin proteins with a high proportion of acidic amino acids (between the central and carboxy-terminus) (117). These residues are predicted to be in a helical conformation (118).
4-1-2 Application of legumin and vicilin in drug delivery
Legumin and vicilin NPs can be prepared by a simple controlled desolvation (pH-coacervation) method (119, 120). These coacervates are not stable enough and must be made rigid with physical or chemical cross linking to prevent the formation of aggregates. Glutaraldehyde is used as the most common cross-linking agent (121). A limitation in particle growth, and hence a decrease in the NP size results by increasing the anti-solvent/solvent ratio (122). However agglomeration can result from the use of high salt concentration due to its neutralizing effect on surface charge (123). Because legumin is hydrophobic, when solvent ionic strength is increased, the protein solubility would be increased, resulting in smaller NPs (124). The cross-linking agent, glutaraldehyde is known as a toxic material, so it is necessary to remove it as completely as possible (125). Legumin proteins need the presence of a surfactant such as Poloxamer in their fabrication process to stabilize them against aggregation. It was found that an increase in the product yield could result from an increase in the surfactant concentration even with no detectable change in the particle size (29). The legumin NP preparation process is shown below in Figure 4 with the first chromatographic procedure done in order to purify legumin, and followed by the production of legumin coacervates by stirring with sodium phosphate citrate buffer. The coacervation method is the most frequently applied method for the synthesis of legumin NPs. By varying the surfactant content, pH, and ionic strength, the percentage yield of legumin coacervates can be optimized. The optimum pH to obtain coacervates in submicron size was near to neutrality and the results showed an opalescent appearance.
Figure 4.
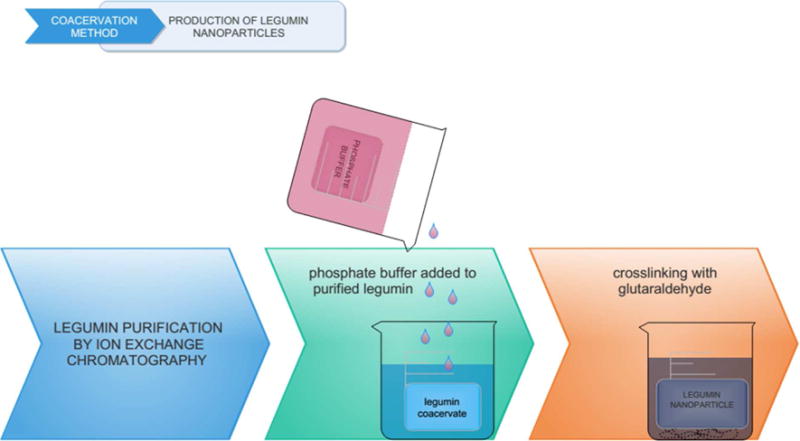
Production of Legumin NPs by the coacervation method: First, the extracted plant legumin is purified by ion exchange chromatography; then, phosphate buffer as desolvating agent is added to the purified protein and finally, the generated protein NP coacervates are stabilized through crosslinking by glutaraldehyde.
Irache et al. (124) synthesized legumin NPs with a size around 250 nm by crosslinking with glutaraldehyde using a pH-coacervation method (near neutral pH). The NPs at neutral pH were more stable, whereas in an acidic environment the NPs suffered rapid degradation.
Surface adsorption or drug entrapment inside the particles are the most common methods of drug loading with legumin (126).
Vicilin NPs have been prepared and used as drug carriers in a complex system in which these particles were conjugated with UIex europaeus lectin molecules due to their sugar-binding property via a two-stage carbodiimide method. The NPs had an average diameter of approximately 660 nm and the proposed delivery system was used in-vitro studies which revealed high potential of the prepared conjugate toward bovine submaxillary gland mucin (BSM) glycoprotein as a biological model (127).
4-2 Soy protein Isolate
It is known that soybeans which are classified as legumes contain proteins consisting of albumins and globulins, and as stated previously, legumes also include legumin and vicilin (113). The protein known as soybean isolate (SPI) consists of four prominent protein sub types, 2S, 7S, 11S and 15S, among which glycinin (11S protein) and β-conglycinin (7S protein) comprise more than 80% (128). Soybeans are an abundant natural protein source, which offer satisfactory water solubility, and SPI has been employed to develop different kinds of nano-sized drug delivery vehicles, owing to its good encapsulation ability. Since SPI is degraded in the fluid of the gastrointestinal tract, this limits its routes of delivery to injection-based delivery (parenteral) methods. Many recent studies have therefore been SPI for oral delivery, by fabricating hybrid and complex nanocarriers in conducted to adapt combination with other natural products (129–131). In a study conducted by Teng et al. (132), SPI nanoparticles were developed based on a desolvation approach with ethanol as desolvating agent, and curcumin was used as a model drug. The preparation process included different steps of incorporation, cross-linking, dispersion, evaporation and desolvation. The average size of the nanoparticles was found to range from 220.1 to 286.7 nm. The efficiency of encapsulation and loading of the drug were 97.2% and 2.7%, respectively. Basak et al. (133) prepared hybrid composite particles from S PI and sodium alginate with addition of the clay mineral, cloisite 30B in an aqueous solution. Different amounts of vincristine sulfate were added to the SPI-cloisite 30 B solution followed by stirring for 1h, and then the polymer-drug complexes were dried at room temperature. Drug release kinetics were analyzed by plotting the cumulative release data versus time, that was fitted to an exponential equation which indicated a non-Fickian type of kinetics. The release of the drug was studied under va rying pH conditions and it was found that the drug release depended on the pH and the composition of the matrix.
5- Lectin
5-1 Protein structure and properties
The lectins are another class of glycoproteins isolated from legumes consisting of two or four identical, or almost identical, subunits (or protomers) of 25–30 kDa, and each contains a single carbohydrate binding site with the same sugar specificity. The subunits of the legume lectins all consist of single polypeptide chains of about 250 amino acids which may carry one or two N-linked oligosaccharides. In some lectins (e.g., those from pea and lentil) the polypeptides are divided into a light (α) and heavy (β) chain. Legume lectins exhibit significant sequence homologies, along with about 20% of invariant amino acids, and close to 20% of similar ones (134).
Plant lectins are divided into several groups according to the molecular structure including merolectins (possessing one carbohydrate-recognition/binding domain e.g. havein), hololectins (having two or more carbohydrate- recognition/binding domains with homological structure which bind to structurally similar sugars e.g. concanavalin A, ConA), chimerolectins (fusion proteins consisting of additional non-related domains and/or tandemly arrayed-binding domains e.g. ricin) and superlectins (lectins with at least two different carbohydrate binding domains e.g. TXCL-1)(54, 135).
Plant lectins based on their carbohydrate binding specificities are classified into polyspecific or monospecific. Lectins reversibly bind through their non-catalytic domain to specific sugar residues such as monosaccharides or oligosaccharides including galactose, glucose and glucose derivatives, mannose and mannose-containing glycans (55, 136, 137). These carbohydrate residues are involved in triggering various cell recognition and adhesion processes. Many vesicular transport systems such as endocytosis in cells are accomplished through recognition of and binding to carbohydrate residues on the cell surface (138–142).
Various studies have investigated the stability of lectins exposed to different conditions. For example, it is known that Solanum tuberosum lectin (STL), a glycoprotein composed of two identical subunits, remained stable throughout a pH range of 4–10 and its thermal stability did not change below 50°C. In addition, the endocytic capacity of lectins is also a critical characteristic. In this regard, STL shows a high endocytic capacity in rabbit conjunctival epithelial cells (143, 144).
5-2 The function of lectin-conjugates in drug delivery systems
For many years, lectins have captured the intense interest of many researchers with diverse applications in the medical, biological and agricultural fields (145, 146). Some of the most studied areas are anti-insect activity (147, 148), antimicrobial activity (149–151), antiviral activity (152–155), and use as biomarkers for disease diagnosis (156–158). They have been widely studied in anti-cancer research (154, 156, 159 161), and anti-cancer activities have been found for several plant lectins including wheat germ agglutinin (WGA) (162) and ricin (163), and autophagic effects caused by P. cyrtonema lectin (PCL) and ConA in tumor cells (164).
On the other hand, lectins have long shown great potential for drug targeting applications (142, 165). Therefore, lectin-mediated drug delivery systems have become a major field of study. Lectin-mediated drug delivery systems were first proposed in the 1980s (166). In recent years, drug delivery applications of lectins have also attracted a large number of studies (143, 167–170). These drug delivery applications of lectins include epithelial delivery (171–174), pulmonary delivery (173), ocular delivery (155), mucosal delivery (141, 148, 169, 175, 176), oral antigen delivery (154, 177), and protein and peptide drug delivery systems (173, 177).
The targeting ability of lectins can be explained by a mechanism in which lectins bind to carbohydrates on the exterior surface of specific cells followed by a cellular uptake process, and when internalized lectins show a particularly strong binding to nuclear pore membranes (54, 171). The majority of cell surface proteins and lipids in cell membranes are glycosylated by forming glycan arrays, which offer suitable binding sites for lectins. Different combinations of various sugars allow the existence of the immense range of glycan arrays in nature and the subsequent enormous variety in the chemical signatures of different cells. Therefore, the lectins attached to the drug and the glycosylated surface of cells can interact with each other (141, 174). In this manner, a specific lectin protein can target different cells and tissues. Therefore, the great potential of lectins has been exploited in targeted drug delivery systems (142, 171). Two major mechanisms are employed for preparing lectin mediated drug delivery systems: 1) direct lectin based targeting in which the carbohydrate groups bound to the delivery system are recognized by endogenous lectins on the cell surface. These endogenous lectins have been found in many mammalian cells and, 2) reverse lectin based targeting wherein the exogenous lectins attached to the delivery system recognize the carbohydrate groups located on glycoproteins and glycolipids on the cell surface (178). However, some drawbacks have been observed for lectin-based drug delivery systems. Owing to the plant origin of lectins, they have a considerable potential for immune-stimulatory effects (179). This recognition by the immune system could have adverse effects on the immune function and could cause cytotoxicity which can cause harmful side-effects (180, 181).
In table 2, some recent researches based on lectin-polymeric NP conjugates as drug delivery systems are summarized.
Table 2.
Recent researches about lectin-NP conjugates in drug delivery systems
| Carrier NP/size | Conjugation efficiency (amount or percentage) | Particle preparation method | Utilized Drug | Drug loading and entrapment (amount or efficiency) | Functio nalizing Lectin species | Description/ Results | Ref |
|---|---|---|---|---|---|---|---|
| PEG-PLGA NPs/<135 nm | 94% | emulsion/solvent evaporation technique | Haloperidol | Loading: 0.96% Encapsulation: 73.2% |
Solanum tubeersu m lectin (STL) | Used for treatment of schizophrenia Haloperidol concentration in brain tissue increased by 1.5–3 fold Release efficiency: 6–8% |
(182) |
| PLGA NPs/PLGA NP: 436 nm, PNA-PLGA-NP: 525nm, WGA-PLGA-NP: 474nm | PNAconj ugates:52.2% WGA conjugates:22% |
oil-in-water solvent evaporation method (simple oil/water emulsification technique) | Glucocorticoid | Encapsulatio n efficiency: PNA conjugate: 72%, WGA conjugate:63 % | Peanut (PNA) and Wheat gernm agglutinin (WGA) | For treatment of inflammatory bowel disease Murine colitis models used for analysis Higher bioadhesion and selectivity to inflamed tissue |
(183) |
| Nanogel carrier consisted of concanavalin A interpeneterated in poly(NIPAM). ConA@poly(NIPAM): 26 to 65 nm And 650nm for uncrosslinked nanogels |
92% | Free radical precipitation copolymerization of NIPAM and MBAAm using APS/TEMED as an initiating system in the presence of ConA. | Insulin | Yield of insulin loading was 39.8 wt% | Concanavalin A from Jack bean | The porous nanogel could provide high insulin loading capacity the reversible The rapid volume change of the nanogels as a function of glucose concentration could regulate the insulin release |
(184) |
|
Concanavalin A-conjugated poly(ethylene glycol)– poly(lactic acid) NPs NP :149.4±2.2 ConA-NP: 158.6± 1.8 nm |
92.6± 2.1% | NPs were prepared by the emulsion/solvent evaporation method with biodegradable and biocompatible PEGated PLA | coumarin 6 | Encapsulation efficiency: 69.4±2.9%, | Concanavalin A (the Jack bean) | ConA-conjugated PEG-PLA NPs for intranasal drug delivery to the cervical lymph nodes were developedd. Conjugation of ConA did not increase the cytotoxicity or nasal ciliotoxicity of NPs but significantly improved their cellular uptake by Calu-3 cells and targeting efficiency to the DCLNs |
(185) |
| Concanavalin-A (Con-A)-conjugated gastroretentive poly (lactic-co-glycolic acid) (PLGA) NPs | – | The PLGA NPs were prepared by solvent evaporation Method and The coupling of lectin with polymeric carriers was carried out using carbodiimide technique | acetohydroxamic acid (AHA) and clarithromycin (CLR) | The values for entrapment efficiency were ranged between 40.06±1.21 to 55.02 ±0.91 and 80.16±0.23 to 90.12±0.14 % for AHA and CLR NPs, resDectivelv. | Concana valin A | The controlled release of drug, i.e., AHA and CLR, from these NPs occured along with mucoadhesive property that could be advantageous against H. pylori infection in gastric environment. The Con-A-conjugated PLGA NPs could be suitable for the incorDoration of other antibiotics with urease inhibitor for the -effective treatment of H. Dvlori infection. |
(186) |
Mucosal targeted drug delivery systems have been the goal of several of these studies. In recent decades, lectins have been exploited to enhance targeting to lymphoid tissue (187). Lectins have been proved to increase the adhesion of NPs to the intestinal epithelium resulting in enhanced drug penetration (54, 171). According to these studies, lectins including WGA, odorranalectin, and UEA have been conjugated with NPs resulting in improved bioadhesive delivery systems (139, 141, 174–176, 188).
It has been shown that targeting ligands can enhance particle uptake via binding to specific receptors on the cell surfaces. A common approach is to coat the NPs by the lectin-ligands (143, 167, 168, 171, 174). Lectin proteins can bind with high specificity to carbohydrate moieties of the glycocalyx of intestinal enterocytes and also to the mucus layer. In this case, lectin conjugated NPs can be exploited as a mucoadhesive system to target mucosal surfaces (149, 189).
The thin layer of mucus adhering to epithelial cells contains highly glycosylated proteins named mucins which provide a great potential for lectin-mediated drug delivery systems (167). Furthermore, it is proposed that plant lectins are ideal reagents for direct delivery of drugs to the gut epithelium since they are stable at low pH conditions and they have specificity for binding to epithelial cells (155, 167). Figure 5 shows the process consisting of three steps of binding, internalization and intracellular transport of lectins or lectin conjugates into a specific cell in an epithelial barrier.
Figure 5.
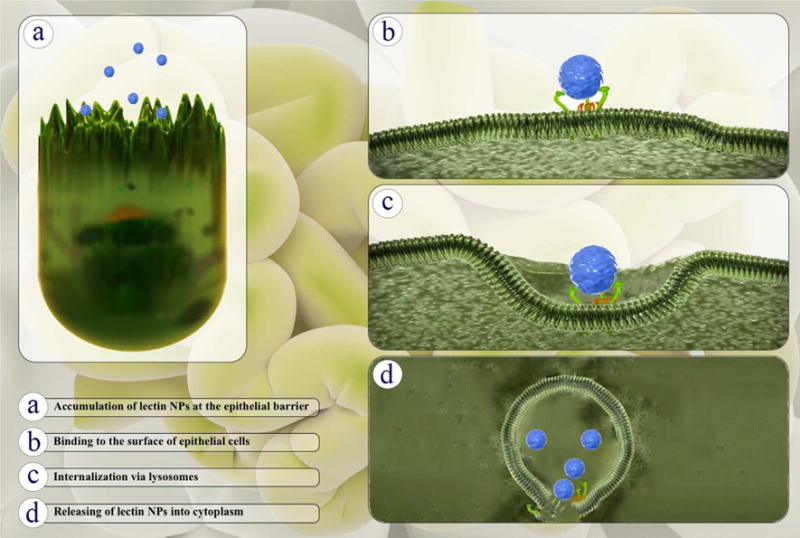
Three main steps to target specific cells at epithelial barriers by lectins including: Binding, Internalization and Intracellular transport of lectins or lectin conjugates
Uptake of the Lectin-polymeric NP conjugates can be improved via the increased interactions with mucus (143, 170, 190, 191). Ying Liu et al. (141) suggested a mechanism for intestinal transport of wheat germ agglutinin (WGA)-grafted lipid NPs for oral delivery of bufalin to target intestinal mucosal tissue. Fig. 6 shows the transport mechanism of the drugs from WGA-grafted lipid NPs to intestinal mucosal cells. According to this mechanism, glycosylated structures are recognized by WGA and causing bioadhesion. This is followed by the triggering of endocytosis via cell signaling cascades.
Figure 6.
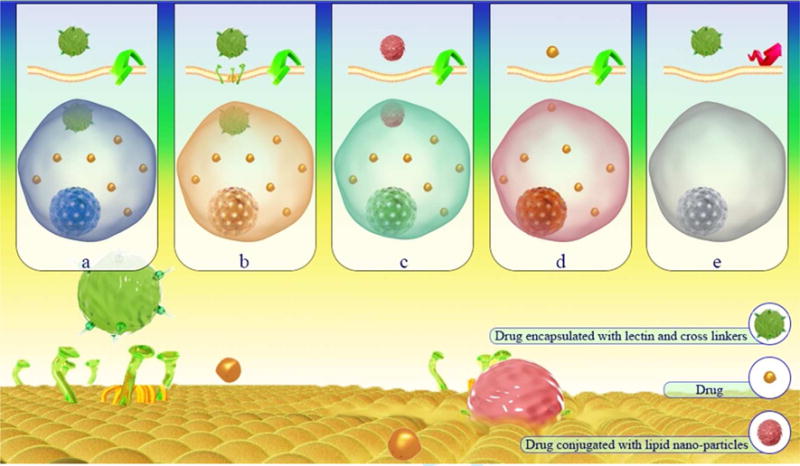
Schematic for intestinal drug transport using WGA-conjugated lipid NPs targeted to intestinal mucosal cells: (a,c) fluid phase endocytosis, (b) binding of WGA to glycoproteins followed by receptor-mediated endocytosis, (d) transcellular transport, (e) endocytosis might be blocked by absence of receptors or unsuitable conditions
Recently, several efforts based on novel drug delivery systems using lectins have been conducted for treatment of inflammatory bowel disease (IBD). Moulari et al. (183) exploited a lectin-mediated drug delivery system loaded with glucocorticoids for active targeting and selective bioadhesion to the inflamed tissue in an experimental murine colitis model. Peanut agglutinin (PNA) and WGA lectins were attached to the surface of the NPs via covalent binding. The results of quantitative adhesion analysis confirmed a higher binding and selective bioadhesion for lectin mediated NPs compared to unconjugated NPs which led to an enhanced anti-inflammatory therapeutic efficacy for the glucocorticoid-loaded lectin-conjugated delivery systems. WGA lectin conjugates strongly bound to healthy intestinal mucosa. However, PNA lectin conjugated NPs revealed much-improved bioadhesion and subsequent therapeutic effects compared to those of WGA. Moreover, the resistance of lectin moieties to degradation by digestive processes was evaluated in simulated gastro-intestinal fluids suggesting a high stability of lectin-conjugates.
Many studies for treatment of central nervous system (CNS) disorders and diseases have been investigated using lectins (182, 192). Some studies have investigated whether therapeutic drugs can be delivered to the CNS via intranasal administration (193–195). Chen et al. (169) studied the in vitro and in vivo brain delivery after nasal adsorption of NPs using Solanum tuberosum lectin (STL) conjugated biodegradable MePEG-PLGA NPs. STL was exploited as a surface biorecognition ligand through covalent coupling. STL-conjugated NPs showed good mucoadhesion, followed by enhanced internalization of NPs in Calu 3 cells. Brain delivery of the STL-mediated conjugates via nasal administration was improved. The safety and biocompatibility of STL-mediated delivery was also confirmed by low cytotoxicity and only minor irritation of the cilia. Among CNS disorders, schizophrenia has been also been studied (193, 195). In a study conducted by Piazza et al. (182) intranasal administration of haloperidol-loaded therapeutic NPs to increase transport to the brain was investigated. STL lectin-functionalized, polyethylene glycol-block-poly (D,L)-lactic-co-glycolic acid (PEG-PLGA) NPs were loaded with haloperidol. The in vitro release of haloperidol demonstrated showed minimal drug leakage, and they found efficient drug transport through the nasal epithelium to target brain tissue. Furthermore, this study revealed that a lower amount of drug dose was needed in order to cause a therapeutic effect for treatment of schizophrenia.
Bladder cancer is considered a challenging neoplastic malignancy in oncology around the world (196). Many efforts have been conducted to improve intravesical delivery using anti cancer drugs. Neutsch et al. (197) studied the potential of lectin conjugates as delivery systems to urothelial cells. Multivalent protein bioconjugates were formed via covalent coupling of WGA as a targeting ligand, to fluorescent-labeled bovine serum albumin (fBSA). Their study showed that fBSA payload protein conjugated to WGA resulted in improved binding to cancer cells. This approach could be used for preferential internalization of macromolecules to bladder cancer as well as triggering efficient endogenous uptake via lectin-mediated endocytosis. Terminal accumulation in degradative lysosomal compartments was seen using WGA-induced internalization. Therefore, lectin bioconjugates are promising for delivery of small molecule drugs and also complex biomolecules in intravesical therapy for bladder cancer.
6- Conclusion
The use of plant proteins as drug carriers can provide controlled release possibilities and can also enhance the distribution and compatibility of the drugs in the biological environment. Plant proteins such as zein, gliadin, legumin and lectins possess favorable properties such as low toxicity, ready availability, easily modifiable structures with free functional groups, and good stability. These plant proteins are basically part of natural foodstuffs and their biological compatibility has been established by millennia of evolution. Different methods of preparation to produce various types of NPs can be utilized. The binding of cargo to the carrier can be achieved either by covalent conjugation using a cleavable linker or by non-covalent encapsulation. Zein has probably been used the most to prepare drug-carrying protein NPs due to its low cost, easy availability, “generally regarded as safe” status, together with its high drug-loading capacity. There have been a few recent attempts toward developing novel stimuli-responsive (smart) nanocarriers based on zein NPs that incorporate fluorescent markers for cell imaging, and also dual-responsive drug delivery systems for targeted and controlled delivery of therapeutics (190, 191). These can be regarded as only the first steps toward developing smart plant-derived drug delivery vehicles. Apart from these reports, several recent preclinical in-vivo studies, have been performed to evaluate the possibility of employing plant protein-based nanocarriers in living animals. These studies have important implications for the future possible widespread use of these natural proteins for medical applications.
The studies using gliadin and legumin as drug carriers are less common and only a relatively few reports have been published, but since these efforts show acceptable results, these two proteins could be good candidates for further researches. Lectins show great potential for drug delivery via interaction with carbohydrates on mucosal surfaces. However, rather than individual lectin NPs, there have been a large number of lectin-NP conjugates reported so far as drug carriers. Meanwhile, their ability to be recognized by the immune system means that this possibility should be taken into account when designing in vivo studies. Further progress in the application of plant proteins as drug delivery systems requires deeper investigation into the nature of protein-drug interactions, studying the binding kinetics of protein nanocarriers to the cell receptors for targeted therapy and optimizing several related parameters.
Acknowledgments
Michael R Hamblin was supported by US NIH grant R01AI050875.
References
- 1.Karimi M, Eslami M, Sahandi-Zangabad P, Mirab F, Farajisafiloo N, Shafaei Z, et al. pH- Sensitive stimulus-responsive nanocarriers for targeted delivery of therapeutic agents. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2016;8(5):696–716. doi: 10.1002/wnan.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karimi M, Avci P, Ahi M, Gazori T, Hamblin MR, Naderi-Manesh H. Evaluation of Chitosan-Tripolyphosphate Nanoparticles as a p-shRNA Delivery Vector: Formulation, Optimization and Cellular Uptake Study. Journal of Nanopharmaceutics and Drug Delivery. 2013;1(3):266–78. doi: 10.1166/jnd.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mofazzal Jahromi MA, Karimi M, Azadmanesh K, Naderi Manesh H, Hassan ZM, Moazzeni SM. The effect of chitosan-tripolyphosphate nanoparticles on maturation and function of dendritic cells. Comparative Clinical Pathology. 2014;23(5):1421–7. [Google Scholar]
- 4.Karimi M, Sahandi Zangabad P, Ghasemi A, Amiri M, Bahrami M, Malekzad H, et al. Temperature-Responsive Smart Nanocarriers for Delivery Of Therapeutic Agents: Applications and Recent Advances. ACS Applied Materials & Interfaces. 2016;8(33):21107–33. doi: 10.1021/acsami.6b00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart Internal Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. IOP Concise Physics. 2015 [Google Scholar]
- 6.Karimi M, Zare H, Bakhshian Nik A, Yazdani N, Hamrang M, Mohamed E, et al. Nanotechnology in diagnosis and treatment of coronary artery disease. Nanomedicine. 2016;11(5):513–30. doi: 10.2217/nnm.16.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimi M, Ghasemi A, Zangabad PS, Rahighi R, Basri SMM, Mirshekari H, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016 doi: 10.1039/c5cs00798d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karimi M, Zangabad PS, Ghasemi A, Hamblin MR. Smart External Stimulus-Responsive Nanocarriers for Drug and Gene Delivery. Morgan & Claypool Publishers; 2015. Light-sensitive nanocarriers. [Google Scholar]
- 9.Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Experimental and molecular pathology. 2009;86(3):215–23. doi: 10.1016/j.yexmp.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karimi M, Bahrami S, Ravari SB, Zangabad PS, Mirshekari H, Bozorgomid M, et al. Albumin nanostructures as advanced drug delivery systems. Expert opinion on drug delivery. 2016 doi: 10.1080/17425247.2016.1193149. (just-accepted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karimi M, Mirshekari H, Aliakbari M, Sahandi-Zangabad P, Hamblin MR. Smart mesoporous silica nanoparticles for controlled-release drug delivery. Nanotechnology Reviews [Google Scholar]
- 12.Khan H, Shukla RN, Bajpai AK. Genipin-modified gelatin nanocarriers as swelling controlled drug delivery system for in vitro release of cytarabine. Materials Science and Engineering: C. 2016;61:457–65. doi: 10.1016/j.msec.2015.12.085. [DOI] [PubMed] [Google Scholar]
- 13.El-Samaligy MS, Rohdewald P. Reconstituted collagen nanoparticles, a novel drug carrier delivery system. Journal of Pharmacy and Pharmacology. 1983;35(8):537–9. doi: 10.1111/j.2042-7158.1983.tb04831.x. [DOI] [PubMed] [Google Scholar]
- 14.Elzoghby AO, Samy WM, Elgindy NA. Albumin-based nanoparticles as potential controlled release drug delivery systems. Journal of Controlled Release. 2012;157(2):168–82. doi: 10.1016/j.jconrel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Elzoghby AO, Abo El-Fotoh WS, Elgindy NA. Casein-based formulations as promising controlled release drug delivery systems. Journal of Controlled Release. 2011;153(3):206–16. doi: 10.1016/j.jconrel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Mottaghitalab F, Farokhi M, Shokrgozar MA, Atyabi F, Hosseinkhani H. Silk fibroin nanoparticle as a novel drug delivery system. Journal of Controlled Release. 2015;206:161–76. doi: 10.1016/j.jconrel.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Elzoghby AO, Samy WM, Elgindy NA. Protein-based nanocarriers as promising drug and gene delivery systems. Journal of controlled release. 2012;161(1):38–49. doi: 10.1016/j.jconrel.2012.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Gujrati V, Kim S, Kim S-H, Min JJ, Choy HE, Kim SC, et al. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS nano. 2014;8(2):1525–37. doi: 10.1021/nn405724x. [DOI] [PubMed] [Google Scholar]
- 19.Din MO, Danino T, Prindle A, Skalak M, Selimkhanov J, Allen K, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 2016;536(7614):81–5. doi: 10.1038/nature18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y, Nolte RJM, Cornelissen JJLM. Virus-based nanocarriers for drug delivery. Advanced Drug Delivery Reviews. 2012;64(9):811–25. doi: 10.1016/j.addr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nature reviews Drug discovery. 2011;10(7):521–35. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 22.Fliervoet LAL, Mastrobattista E. Drug delivery with living cells. Advanced Drug Delivery Reviews. 2016 doi: 10.1016/j.addr.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 23.Slomovic S, Pardee K, Collins JJ. Synthetic biology devices for in vitro and in vivo diagnostics. Proceedings of the National Academy of Sciences. 2015;112(47):14429–35. doi: 10.1073/pnas.1508521112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claesen J, Fischbach MA. Synthetic microbes as drug delivery systems. ACS synthetic biology. 2014;4(4):358–64. doi: 10.1021/sb500258b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beneke CE, Viljoen AM, Hamman JH. Polymeric plant-derived excipients in drug delivery. Molecules. 2009;14(7):2602–20. doi: 10.3390/molecules14072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy N, Yang Y. Potential of plant proteins for medical applications. Trends in biotechnology. 2011;29(10):490–8. doi: 10.1016/j.tibtech.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Nitta SK, Numata K. Biopolymer-based nanoparticles for drug/gene delivery and tissue engineering. International journal of molecular sciences. 2013;14(1):1629–54. doi: 10.3390/ijms14011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salatin S, Jelvehgari M, Maleki-Dizaj S, Adibkia K. A sight on protein-based nanoparticles as drug/gene delivery systems. Therapeutic Delivery. 2015;6(8):1017–29. doi: 10.4155/tde.15.28. [DOI] [PubMed] [Google Scholar]
- 29.Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y. Protein Nanoparticles as Drug Delivery Carriers for Cancer Therapy. BioMed Research International. 2014;2014:12. doi: 10.1155/2014/180549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podaralla S, Averineni R, Alqahtani M, Perumal O. Synthesis of Novel Biodegradable Methoxy Poly (ethylene glycol)‚ ÄìZein Micelles for Effective Delivery of Curcumin. Molecular pharmaceutics. 2012;9(9):2778–86. doi: 10.1021/mp2006455. [DOI] [PubMed] [Google Scholar]
- 31.Joye IJ, Nelis VA, McClements DJ. Gliadin-based nanoparticles: fabrication and stability of food-grade colloidal delivery systems. Food Hydrocolloids. 2015;44:86–93. [Google Scholar]
- 32.Patel AR, Velikov KP. Zein as a source of functional colloidal nano-and microstructures. Current Opinion in Colloid & Interface Science. 2014;19(5):450–8. [Google Scholar]
- 33.Podaralla S, Perumal O. Influence of Formulation Factors on the Preparation of Zein Nanoparticles. Aaps Pharmscitech. 2012;13(3):919–27. doi: 10.1208/s12249-012-9816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Sun Q, Wang H, Zhang L, Wang J-Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2005;26(1):109–15. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Zhong Q, Jin M. Zein nanoparticles produced by liquid–liquid dispersion. Food Hydrocolloids. 2009;23(8):2380–7. [Google Scholar]
- 36.Regier MC, Taylor JD, Borcyk T, Yang Y, Pannier AK. Fabrication and characterization of DNA-loaded zein nanospheres. Journal of nanobiotechnology. 2012;10(1):44. doi: 10.1186/1477-3155-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Wang Y, Zhang X, Zhang W, Guo S, Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. Journal of Controlled Release. 2014;174:126–36. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 38.Penalva R, González-Navarro CJ, Gamazo C, Esparza I, Irache JM. Zein nanoparticles for oral delivery of quercetin: Pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomedicine: Nanotechnology, Biology and Medicine. doi: 10.1016/j.nano.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 39.Hädrich G, Vaz GR, Maidana M, Kratz JM, Loch-Neckel G, Favarin DC, et al. Anti-inflammatory effect and toxicology analysis of oral delivery quercetin nanosized emulsion in rats. Pharmaceutical research. 2016;33(4):983–93. doi: 10.1007/s11095-015-1844-6. [DOI] [PubMed] [Google Scholar]
- 40.Penalva R, Esparza I, Larraneta E, González-Navarro CJ, Gamazo C, Irache JM. Zein-based nanoparticles improve the oral bioavailability of resveratrol and its anti-inflammatory effects in a mouse model of endotoxic shock. Journal of agricultural and food chemistry. 2015;63(23):5603–11. doi: 10.1021/jf505694e. [DOI] [PubMed] [Google Scholar]
- 41.Zou T, Gu L. TPGS emulsified zein nanoparticles enhanced oral bioavailability of daidzin: in vitro characteristics and in vivo performance. Molecular pharmaceutics. 2013;10(5):2062–70. doi: 10.1021/mp400086n. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Shen L, Xu L, Yang Y. Controlled delivery of hollow corn protein nanoparticles via non- toxic crosslinking: in vivo and drug loading study. Biomedical microdevices. 2015;17(1):1–8. doi: 10.1007/s10544-014-9926-5. [DOI] [PubMed] [Google Scholar]
- 43.Nesterenko A, Alric I, Silvestre Fß, Durrieu V. Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Industrial crops and products. 2013;42:469–79. [Google Scholar]
- 44.Bietz J, Rothfus J. Comparison of peptides from wheat gliadin and glutenin. Cereal chemistry. 1970;47(4):381–92. [Google Scholar]
- 45.Reddy N, Shi Z, Xu H, Yang Y. Development of wheat glutenin nanoparticles and their biodistribution in mice. Journal of Biomedical Materials Research Part A. 2015;103(5):1653–8. doi: 10.1002/jbm.a.35302. [DOI] [PubMed] [Google Scholar]
- 46.Byers M, Miflin BJ, Smith SJ. A quantitative comparison of the extraction of protein fractions from wheat grain by different solvents, and of the polypeptide and amino acid composition of the alcohol-soluble proteins. Journal of the Science of Food and Agriculture. 1983;34(5):447–62. doi: 10.1002/jsfa.2740340506. [DOI] [PubMed] [Google Scholar]
- 47.He H, Roach R, Hoseney R. Effect of nonchaotropic salts on flour bread-making properties. Cereal chemistry. 1992;69(4):366–71. [Google Scholar]
- 48.Pinto Reis C, Neufeld RJ, Ribeiro AJ, Veiga F. Nanoencapsulation I. Methods for preparation of drug-loaded polymeric nanoparticles. Nanomedicine: Nanotechnology, Biology and Medicine. 2006;2(1):8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Sailaja A, Amareshwar P, Chakravarty P. Different techniques used for the preparation of nanoparticles using natural polymers and their application. Int J Pharm Pharm Sci. 2011;3(Suppl 2):45–50. [Google Scholar]
- 50.Nehete JY, Bhambar RS, Narkhede MR, Gawali SR. Natural proteins: Sources, isolation, characterization and applications. Pharmacognosy Reviews. 2013;7(14):107–16. doi: 10.4103/0973-7847.120508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sripriyalakshmi S, Jose P, Ravindran A, Anjali C. Recent trends in drug delivery system using protein nanoparticles. Cell biochemistry and biophysics. 2014;70(1):17–26. doi: 10.1007/s12013-014-9896-5. [DOI] [PubMed] [Google Scholar]
- 52.Mirshahi T, Irache J, Nicolas C, Mirshahi M, Faure J, Gueguen J, et al. Adaptive immune responses of legumin nanoparticles. Journal of drug targeting. 2002;10(8):625–31. doi: 10.1080/1061186021000066237. [DOI] [PubMed] [Google Scholar]
- 53.Peumans WJ, van Damme EJ, Barre A, Roug√© P. The Molecular Immunology of Complex Carbohydrates‚ Äî. Vol. 2. Springer; 2001. ClassificaSon of plant lecSns in families of structurally and evolutionary related proteins; pp. 27–54. [DOI] [PubMed] [Google Scholar]
- 54.Lehr C-M, Haas J. Developments in the area of bioadhesive drug delivery systems. Expert opinion on biological therapy. 2002;2(3):287–98. doi: 10.1517/14712598.2.3.287. [DOI] [PubMed] [Google Scholar]
- 55.Peumans WJ, Van Damme EJ. Lectins as plant defense proteins. Plant Physiology. 1995;109(2):347–52. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peumans WJ, Damme EJV. Plant lectins: versatile proteins with important perspectives in biotechnology. Biotechnology and Genetic Engineering Reviews. 1998;15(1):199–228. [Google Scholar]
- 57.Boyd WC, Shapleigh E. Specific Precipitating Activity of Plant Agglutinins (Lectins) Science (New York, NY) 1954;119(3091):419–-. doi: 10.1126/science.119.3091.419. [DOI] [PubMed] [Google Scholar]
- 58.Sharon N, Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972;177(4053):949–59. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- 59.Loris R, Hamelryck T, Bouckaert J, Wyns L. Legume lectin structure. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1998;1383(1):9–36. doi: 10.1016/s0167-4838(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 60.Damme EJ, Kaku H, Perini F, Goldstein IJ, Peeters B, Yagi F, et al. Biosynthesis, primary structure and molecular cloning of snowdrop (Galanthus nivalis L.) lectin. European Journal of Biochemistry. 1991;202(1):23–30. doi: 10.1111/j.1432-1033.1991.tb16339.x. [DOI] [PubMed] [Google Scholar]
- 61.Meyer A, Rypniewski W, Szyma≈Ñski M, Voelter W, Barciszewski J, Betzel C. Structure of mistletoe lectin I from Viscum album in complex with the phytohormone zeatin. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics. 2008;1784(11):1590–5. doi: 10.1016/j.bbapap.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Matsushima N, Danno G-i, Takezawa H, Izumi Y. Three-dimensional structure of maize< i>α</i>-zein proteins studied by small-angle X-ray scattering. Biochimica et Biophysica Acta (BBA)-Protein Structure and Molecular Enzymology. 1997;1339(1):14–22. doi: 10.1016/s0167-4838(96)00212-9. [DOI] [PubMed] [Google Scholar]
- 63.Sousa F, Luzardo-Álvarez A, Blanco-Méndez J, Martín-Pastor M. NMR techniques in drug delivery: Application to zein protein complexes. International journal of pharmaceutics. 2012;439(1):41–8. doi: 10.1016/j.ijpharm.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 64.Wang Q, Yin L, Padua GW. Effect of hydrophilic and lipophilic compounds on zein microstructures. Food Biophysics. 2008;3(2):174–81. [Google Scholar]
- 65.Karthikeyan K, Vijayalakshmi E, Korrapati PS. Selective Interactions of Zein Microspheres with Different Class of Drugs: An In Vitro and In Silico Analysis. AAPS PharmSciTech. 2014:1–9. doi: 10.1208/s12249-014-0151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Ye R, Liu J. Understanding of dispersion and aggregation of suspensions of zein nanoparticles in aqueous alcohol solutions after thermal treatment. Industrial Crops and Products. 2013;50:764–70. [Google Scholar]
- 67.Zhou P, Xia Y, Cheng X, Wang P, Xie Y, Xu S. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials. 2014;35(38):10033–45. doi: 10.1016/j.biomaterials.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y, Zhang B, Whent M, Yu LL, Wang Q. Preparation and characterization of zein/chitosan complex for encapsulation of α-tocopherol, and its in vitro controlled release study. Colloids and Surfaces B: Biointerfaces. 2011;85(2):145–52. doi: 10.1016/j.colsurfb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Alwahab NSA, Moazzam ZM. Zein-based oral drug delivery system targeting activated macrophages. International journal of pharmaceutics. 2013;454(1):388–93. doi: 10.1016/j.ijpharm.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 70.Podaralla SK, Perumal OP, Kaushik RS. Design and Formulation of Protein-Based NPDDS. Drug Delivery Nanoparticles Formulation and Characterization. 2009;191:69. [Google Scholar]
- 71.Joye IJ, McClements DJ. Production of nanoparticles by anti-solvent precipitation for use in food systems. Trends in Food Science & Technology. 2013;34(2):109–23. [Google Scholar]
- 72.Luo Y, Teng Z, Wang TT, Wang Q. Cellular uptake and transport of zein nanoparticles: Effects of sodium caseinate. Journal of agricultural and food chemistry. 2013;61(31):7621–9. doi: 10.1021/jf402198r. [DOI] [PubMed] [Google Scholar]
- 73.Li K-K, Yin S-W, Yin Y-C, Tang C-H, Yang X-Q, Wen S-H. Preparation of water-soluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization. Journal of Food Engineering. 2013;119(2):343–52. [Google Scholar]
- 74.Zhong Q, Tian H, Zivanovic S. ENCAPSULATION OF FISH OIL IN SOLID ZEIN PARTICLES BY LIQUID-LIQUID DISPERSION. Journal of Food Processing and Preservation. 2009;33(2):255–70. [Google Scholar]
- 75.Wu Y, Luo Y, Wang Q. Antioxidant and antimicrobial properties of essential oils encapsulated in zein nanoparticles prepared by liquid–liquid dispersion method. LWT-Food Science and Technology. 2012;48(2):283–90. [Google Scholar]
- 76.Zou T, Li Z, Percival SS, Bonard S, Gu L. Fabrication, characterization, and cytotoxicity evaluation of cranberry procyanidins-zein nanoparticles. Food hydrocolloids. 2012;27(2):293–300. [Google Scholar]
- 77.Chen J, Zheng J, McClements DJ, Xiao H. Tangeretin-loaded protein nanoparticles fabricated from zein/β-lactoglobulin: Preparation, characterization, and functional performance. Food chemistry. 2014;158:466–72. doi: 10.1016/j.foodchem.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 78.Hu K, McClements DJ. Fabrication of biopolymer nanoparticles by antisolvent precipitation and electrostatic deposition: Zein-alginate core/shell nanoparticles. Food Hydrocolloids. 2015;44:101–8. [Google Scholar]
- 79.Hu K, McClements DJ. Fabrication of surfactant-stabilized zein nanoparticles: A pH modulated antisolvent precipitation method. Food Research International. 2014;64:329–35. doi: 10.1016/j.foodres.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 80.Luo Y, Wang TT, Teng Z, Chen P, Sun J, Wang Q. Encapsulation of indole-3-carbinol and 3, 3′- diindolylmethane in zein/carboxymethyl chitosan nanoparticles with controlled release property and improved stability. Food chemistry. 2013;139(1):224–30. doi: 10.1016/j.foodchem.2013.01.113. [DOI] [PubMed] [Google Scholar]
- 81.Lai L, Guo H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. International journal of pharmaceutics. 2011;404(1):317–23. doi: 10.1016/j.ijpharm.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 82.Hu D, Lin C, Liu L, Li S, Zhao Y. Preparation, characterization, and in vitro release investigation of lutein/zein nanoparticles via solution enhanced dispersion by supercritical fluids. Journal of Food Engineering. 2012;109(3):545–52. [Google Scholar]
- 83.Sridhar R, Lakshminarayanan R, Madhaiyan K, Amutha Barathi V, Lim KHC, Ramakrishna S. Electrosprayed nanoparticles and electrospun nanofibers based on natural materials: applications in tissue regeneration, drug delivery and pharmaceuticals. Chemical Society Reviews. 2015;44(3):790–814. doi: 10.1039/c4cs00226a. [DOI] [PubMed] [Google Scholar]
- 84.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. Journal of Controlled Release. 2014;185:12–21. doi: 10.1016/j.jconrel.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 85.Karthikeyan K, Guhathakarta S, Rajaram R, Korrapati PS. Electrospun zein/eudragit nanofibers based dual drug delivery system for the simultaneous delivery of aceclofenac and pantoprazole. International journal of pharmaceutics. 2012;438(1):117–22. doi: 10.1016/j.ijpharm.2012.07.075. [DOI] [PubMed] [Google Scholar]
- 86.Jiang Y-N, Mo H-Y, Yu D-G. Electrospun drug-loaded core–sheath PVP/zein nanofibers for biphasic drug release. International journal of pharmaceutics. 2012;438(1):232–9. doi: 10.1016/j.ijpharm.2012.08.053. [DOI] [PubMed] [Google Scholar]
- 87.Yang J-M, Zha L-s, Yu D-G, Liu J. Coaxial electrospinning with acetic acid for preparing ferulic acid/zein composite fibers with improved drug release profiles. Colloids and Surfaces B: Biointerfaces. 2013;102:737–43. doi: 10.1016/j.colsurfb.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 88.Huang W, Zou T, Li S, Jing J, Xia X, Liu X. Drug-loaded zein nanofibers prepared using a modified coaxial electrospinning process. AAPS PharmSciTech. 2013;14(2):675–81. doi: 10.1208/s12249-013-9953-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Unnithan AR, Gnanasekaran G, Sathishkumar Y, Lee YS, Kim CS. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydrate Polymers. 2014;102:884–92. doi: 10.1016/j.carbpol.2013.10.070. [DOI] [PubMed] [Google Scholar]
- 90.Dhandayuthapani B, Anila M, Ravindran Girija A, Yutaka N, Venugopal K, Yasuhiko Y, et al. Hybrid fluorescent curcumin loaded zein electrospun nanofibrous scaffold for biomedical applications. Biomedical Materials. 2012;7(4):045001. doi: 10.1088/1748-6041/7/4/045001. [DOI] [PubMed] [Google Scholar]
- 91.Podaralla S, Averineni R, Alqahtani M, Perumal O. Synthesis of Novel Biodegradable Methoxy Poly (ethylene glycol)–Zein Micelles for Effective Delivery of Curcumin. Molecular pharmaceutics. 2012;9(9):2778–86. doi: 10.1021/mp2006455. [DOI] [PubMed] [Google Scholar]
- 92.Gomez-Estaca J, Balaguer M, Gavara R, Hernandez-Munoz P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocolloids. 2012;28(1):82–91. [Google Scholar]
- 93.Karthikeyan K, Krishnaswamy VR, Lakra R, Kiran MS, Korrapati PS. Fabrication of electrospun zein nanofibers for the sustained delivery of siRNA. Journal of Materials Science: Materials in Medicine. 2015;26(2):1–8. doi: 10.1007/s10856-015-5439-x. [DOI] [PubMed] [Google Scholar]
- 94.Wang P, Tao H, Wu F, Yang N, Chen F, Jin Z, et al. Effect of frozen storage on the foaming properties of wheat gliadin. Food Chemistry. 2014;164:44–9. doi: 10.1016/j.foodchem.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 95.Thewissen BG, Celus I, Brijs K, Delcour JA. Foaming properties of wheat gliadin. Journal of agricultural and food chemistry. 2011;59(4):1370–5. doi: 10.1021/jf103473d. [DOI] [PubMed] [Google Scholar]
- 96.Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine & growth factor reviews. 2014;25(1):45–55. doi: 10.1016/j.cytogfr.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 97.Neves MM, González-García MB, Santos-Silva A, Costa-García A. Voltammetric immunosensor for the diagnosis of celiac disease based on the quantification of anti-gliadin antibodies. Sensors and Actuators B: Chemical. 2012;163(1):253–9. [Google Scholar]
- 98.Duclairoir C, Irache JM, Nakache E, Orecchioni AM, Chabenat C, Popineau Y. Gliadin nanoparticles: formation, all-trans-retinoic acid entrapment and release, size optimization. Polymer international. 1999;48(4):327–33. [Google Scholar]
- 99.Orecchioni A-M, Duclairoir C, Renard D, Nakache E. Gliadin characterization by sans and gliadin nanoparticle growth modelization. Journal of nanoscience and nanotechnology. 2006;6(9–10):9–10. doi: 10.1166/jnn.2006.455. [DOI] [PubMed] [Google Scholar]
- 100.Jahanshahi M, Babaei Z. Protein nanoparticle: a unique system as drug delivery vehicles. African Journal of Biotechnology. 2008;7(25) [Google Scholar]
- 101.Arangoa M, Ponchel G, Orecchioni A, Renedo M, Duchene D, Irache J. Bioadhesive potential of gliadin nanoparticulate systems. European journal of pharmaceutical sciences. 2000;11(4):333–41. doi: 10.1016/s0928-0987(00)00121-4. [DOI] [PubMed] [Google Scholar]
- 102.Arangoa MA, Campanero MA, Renedo MJ, Ponchel G, Irache JM. Gliadin nanoparticles as carriers for the oral administration of lipophilic drugs. Relationships between bioadhesion and pharmacokinetics. Pharmaceutical research. 2001;18(11):1521–7. doi: 10.1023/a:1013018111829. [DOI] [PubMed] [Google Scholar]
- 103.Lopes D, Nunes C, Martins MCL, Sarmento B, Reis S. Eradication of< i> Helicobacter pylori</i>: Past, present and future. Journal of Controlled Release. 2014;189:169–86. doi: 10.1016/j.jconrel.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 104.Ramteke S, Jain NK. Clarithromycin-and omeprazole-containing gliadin nanoparticles for the treatment of Helicobacter pylori. Journal of drug targeting. 2008;16(1):65–72. doi: 10.1080/10611860701733278. [DOI] [PubMed] [Google Scholar]
- 105.Umamaheshwari R, Ramteke S, Jain NK. Anti-Helicobacter pylori effect of mucoadhesive nanoparticles bearing amoxicillin in experimental gerbils model. Aaps Pharmscitech. 2004;5(2):60–8. doi: 10.1208/pt050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ramteke S, Ganesh N, Bhattacharya S, Jain NK. Triple therapy-based targeted nanoparticles for the treatment of Helicobacter pylori. Journal of drug targeting. 2008;16(9):694–705. doi: 10.1080/10611860802295839. [DOI] [PubMed] [Google Scholar]
- 107.Carillon J, Rouanet J-M, Cristol J-P, Brion R. Superoxide dismutase administration, a potential therapy against oxidative stress related diseases: several routes of supplementation and proposal of an original mechanism of action. Pharmaceutical research. 2013;30(11):2718–28. doi: 10.1007/s11095-013-1113-5. [DOI] [PubMed] [Google Scholar]
- 108.Fajardo P, Balaguer MP, Gomez-Estaca J, Gavara R, Hernandez-Munoz P. Chemically modified gliadins as sustained release systems for lysozyme. Food Hydrocolloids. 2014;41:53–9. [Google Scholar]
- 109.Kim S, Kim YS. Production of gliadin-poly (ethyl cyanoacrylate) nanoparticles for hydrophilic coating. Journal of nanoparticle research. 2014;16(2):1–10. [Google Scholar]
- 110.Duclairoir C, Orecchioni A-M, Depraetere P, Osterstock F, Nakache E. Evaluation of gliadins nanoparticles as drug delivery systems: a study of three different drugs. International journal of pharmaceutics. 2003;253(1):133–44. doi: 10.1016/s0378-5173(02)00701-9. [DOI] [PubMed] [Google Scholar]
- 111.Loveday SM, Singh H. Recent advances in technologies for vitamin A protection in foods. Trends in food science & technology. 2008;19(12):657–68. [Google Scholar]
- 112.Gulfam M, Kim J-e, Lee JM, Ku B, Chung BH, Chung BG. Anticancer drug-loaded gliadin nanoparticles induce apoptosis in breast cancer cells. Langmuir. 2012;28(21):8216–23. doi: 10.1021/la300691n. [DOI] [PubMed] [Google Scholar]
- 113.Derbyshire E, Wright D, Boulter D. Legumin and vicilin, storage proteins of legume seeds. Phytochemistry. 1976;15(1):3–24. [Google Scholar]
- 114.Bailey CJ, Boulter D. The structure of legumin, a storage protein of broad bean (Vicia faba) seed. European Journal of Biochemistry. 1970;17(3):460–6. doi: 10.1111/j.1432-1033.1970.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 115.Popello IA, Suchkov VV, Grinberg VY, Tolstoguzov VB. Liquid/liquid phase equilibrium in globulin/salt/water systems: legumin. Journal of the Science of Food and Agriculture. 1990;51(3):345–53. [Google Scholar]
- 116.Schwenke KD, Zirwer D, Gast K, GÖRnitz E, Linow K-J, Gueguen J. Changes of the oligomeric structure of legumin from pea (Pisum sativum L.) after succinylation. European Journal of Biochemistry. 1990;194(2):621–7. doi: 10.1111/j.1432-1033.1990.tb15661.x. [DOI] [PubMed] [Google Scholar]
- 117.Argos P, Narayana S, Nielsen N. Structural similarity between legumin and vicilin storage proteins from legumes. The EMBO journal. 1985;4(5):1111. doi: 10.1002/j.1460-2075.1985.tb03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Properties Patel P, Panchal H. In silico Structure Modeling and Comparative Analysis of Characterization of Protein Polymers Useful for Protein-Based Nano Particulate Drug Delivery Systems (NPDDS): A Bioinformatics Approach. International Journal of Peptide Research and Therapeutics. 2014;20(1):103–8. [Google Scholar]
- 119.Ezpeleta I, Irache JM, Stainmesse S, Gueguen J, Orecchioni A-M. Preparation of small-sized particles from vicilin (vegetal protein from Pisum sativum L.) by coacervation. European journal of pharmaceutics and biopharmaceutics. 1996;42(1):36–41. [Google Scholar]
- 120.Mirshahi T, Irache J, Gueguen J, Orecchioni A. Development of drug delivery systems from vegetal proteins: legumin nanoparticles. Drug development and industrial pharmacy. 1996;22(8):841–6. [Google Scholar]
- 121.Ezpeleta I, Rache J, Gueguen J, Orecchioni A. Properties of glutaraldehyde cross-linked vicilin nano-and microparticles. Journal of microencapsulation. 1997;14(5):557–65. doi: 10.3109/02652049709006809. [DOI] [PubMed] [Google Scholar]
- 122.Wang G, Siggers K, Zhang S, Jiang H, Xu Z, Zernicke RF, et al. Preparation of BMP-2 containing bovine serum albumin (BSA) nanoparticles stabilized by polymer coating. Pharmaceutical research. 2008;25(12):2896–909. doi: 10.1007/s11095-008-9692-2. [DOI] [PubMed] [Google Scholar]
- 123.Langer K, Balthasar S, Vogel V, Dinauer N, Von Briesen H, Schubert D. Optimization of the preparation process for human serum albumin (HSA) nanoparticles. International Journal of Pharmaceutics. 2003;257(1):169–80. doi: 10.1016/s0378-5173(03)00134-0. [DOI] [PubMed] [Google Scholar]
- 124.Irache JM, Bergougnoux L, Ezpeleta I, Gueguen J, Orecchioni A-M. Optimization and in vitro stability of legumin nanoparticles obtained by a coacervation method. International Journal of Pharmaceutics. 1995;126(1):103–9. [Google Scholar]
- 125.Wang G, Uludag H. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opinion on Drug Delivery. 2008;5(5):499–515. doi: 10.1517/17425247.5.5.499. [DOI] [PubMed] [Google Scholar]
- 126.Merodio M, Arnedo A, Renedo MJ, Irache JM. Ganciclovir-loaded albumin nanoparticles: characterization and in vitro release properties. European journal of pharmaceutical sciences. 2001;12(3):251–9. doi: 10.1016/s0928-0987(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 127.Ezpeleta I, Irache JM, Stainmesse S, Chabenat C, Gueguen J, Orecchioni A-M. Preparation of lectin-vicilin nanoparticle conjugates using the carbodiimide coupling technique. International Journal of Pharmaceutics. 1996;142(2):227–33. [Google Scholar]
- 128.Nishinari K, Fang Y, Guo S, Phillips G. Soy proteins: A review on composition, aggregation and emulsification. Food Hydrocolloids. 2014;39:301–18. [Google Scholar]
- 129.Teng Z, Luo Y, Wang Q. Carboxymethyl chitosan–soy protein complex nanoparticles for the encapsulation and controlled release of vitamin D3. Food Chemistry. 2013;141(1):524–32. doi: 10.1016/j.foodchem.2013.03.043. [DOI] [PubMed] [Google Scholar]
- 130.Deng X-X, Zhang N, Tang CH. Soy Protein Isolate as a Nano Carrier for Enhanced Water Dispersibility, Stability and Bioaccessibility of β-Carotene. Journal of the Science of Food and Agriculture. 2016 doi: 10.1002/jsfa.8033. [DOI] [PubMed] [Google Scholar]
- 131.Chen F-P, Ou S-Y, Tang C-H. Core–Shell Soy Protein–Soy Polysaccharide Complex (Nano)particles as Carriers for Improved Stability and Sustained Release of Curcumin. Journal of Agricultural and Food Chemistry. 2016;64(24):5053–9. doi: 10.1021/acs.jafc.6b01176. [DOI] [PubMed] [Google Scholar]
- 132.Teng Z, Luo Y, Wang Q. Nanoparticles synthesized from soy protein: preparation, characterization, and application for nutraceutical encapsulation. Journal of agricultural and food chemistry. 2012;60(10):2712–20. doi: 10.1021/jf205238x. [DOI] [PubMed] [Google Scholar]
- 133.Basak A, Chahatray R, Nayak P. Soy Protein Isolate Blended with Cloisite 30B for Controlled Release of Anticancer Drug Vincristine. World. 2014;3(1):39–44. [Google Scholar]
- 134.Lis H, Sharon N. Lectins: carbohydrate-specific proteins that mediate cellular recognition. Chemical reviews. 1998;98(2):637–74. doi: 10.1021/cr940413g. [DOI] [PubMed] [Google Scholar]
- 135.Damme EJV, Peumans WJ, Barre A, Rougé P. Plant lectins: a composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Critical Reviews in Plant Sciences. 1998;17(6):575–692. [Google Scholar]
- 136.Barre A, Bourne Y, Van Damme EJ, Peumans WJ, Roug√© P. Mannose-binding plant lectins: different structural scaffolds for a common sugar-recognition process. Biochimie. 2001;83(7):645–51. doi: 10.1016/s0300-9084(01)01315-3. [DOI] [PubMed] [Google Scholar]
- 137.Teuschl AH, Neutsch L, Monforte X, R√°nzler D, van Griensven M, Gabor F, et al. Enhanced cell adhesion on silk fibroin via lectin surface modification. Acta biomaterialia. 2014;10(6):2506–17. doi: 10.1016/j.actbio.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 138.Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. Journal of Biological Chemistry. 2007;282(5):2753–64. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- 139.Teuschl AH, Neutsch L, Monforte X, Rünzler D, van Griensven M, Gabor F, et al. Enhanced cell adhesion on silk fibroin via lectin surface modification. Acta biomaterialia. 2014;10(6):2506–17. doi: 10.1016/j.actbio.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 140.Han JW, Yoon KS, Klochkova TA, Hwang M-S, Kim GH. Purification and characterization of a lectin, BPL-3, from the marine green alga Bryopsis plumosa. Journal of Applied Phycology. 2011;23(4):745–53. [Google Scholar]
- 141.Liu Y, Wang P, Sun C, Zhao J, Du Y, Shi F, et al. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. International journal of pharmaceutics. 2011;419(1):260–5. doi: 10.1016/j.ijpharm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 142.Li H, Dong W-f, Zhou J-y, Xu X-m, Li F-q. Triggering effect of N-acetylglucosamine on retarded drug release from a lectin-anchored chitosan nanoparticles-in-microparticles system. International journal of pharmaceutics. 2013;449(1):37–43. doi: 10.1016/j.ijpharm.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 143.Chirra HD, Desai TA. Emerging microtechnologies for the development of oral drug delivery devices. Advanced drug delivery reviews. 2012;64(14):1569–78. doi: 10.1016/j.addr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qaddoumi M, Lee VH. Lectins as endocytic ligands: an assessment of lectin binding and uptake to rabbit conjunctival epithelial cells. Pharmaceutical research. 2004;21(7):1160–6. doi: 10.1023/b:pham.0000033002.93967.5f. [DOI] [PubMed] [Google Scholar]
- 145.Dan X, Liu W, Ng TB. Development and Applications of Lectins as Biological Tools in Biomedical Research. Medicinal Research Reviews. 2016;36(2):221–47. doi: 10.1002/med.21363. [DOI] [PubMed] [Google Scholar]
- 146.Iordache F, Ionita M, Mitrea LI, Fafaneata C, Pop A. Antimicrobial and Antiparasitic Activity of Lectins. Current Pharmaceutical Biotechnology. 2015;16(2):152–61. doi: 10.2174/138920101602150112151907. [DOI] [PubMed] [Google Scholar]
- 147.Hakim RS, Baldwin K, Smagghe G. Regulation of midgut growth, development, and metamorphosis. Annual review of entomology. 2010;55:593–608. doi: 10.1146/annurev-ento-112408-085450. [DOI] [PubMed] [Google Scholar]
- 148.Thakur K, Kaur M, Kaur S, Kaur A, Kamboj SS, Singh J. Purification of Colocasia esculenta lectin and determination of its anti-insect potential towards Bactrocera cucurbitae. Journal of Environmental Biology. 2013;34(1):31–6. [PubMed] [Google Scholar]
- 149.Magalhães A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osório H, et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology. 2009;19(12):1525–36. doi: 10.1093/glycob/cwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mukhopadhyay B, Martins MB, Karamanska R, Russell DA, Field RA. Bacterial detection using carbohydrate-functionalised CdS quantum dots: a model study exploiting< i> E. coli</i> recognition of mannosides. Tetrahedron Letters. 2009;50(8):886–9. [Google Scholar]
- 151.Sá RA, Gomes FS, Napoleão TH, Santos ND, Melo CM, Gusmão NB, et al. Antibacterial and antifungal activities of Myracrodruon urundeuva heartwood. Wood Science and Technology. 2009;43(1–2):85–95. [Google Scholar]
- 152.Swanson MD, Winter HC, Goldstein IJ, Markovitz DM. A lectin isolated from bananas is a potent inhibitor of HIV replication. Journal of Biological Chemistry. 2010;285(12):8646–55. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Granell A, Fernández-del-Carmen A, Orzáez D. In planta production of plant-derived and non-plant-derived adjuvants. Expert review of vaccines. 2010;9(8):843–58. doi: 10.1586/erv.10.80. [DOI] [PubMed] [Google Scholar]
- 154.Hamid R, Masood A, Wani IH, Rafiq S. Lectins: proteins with diverse applications. Journal of Applied Pharmaceutical Science. 2013;3(4 Suppl 1):S93–S103. [Google Scholar]
- 155.Gavrovic-Jankulovic M, Prodanovic R. Drug delivery: plant lectins as bioadhesive drug delivery systems. Journal of Biomaterials and Nanobiotechnology. 2011;2(05):614–21. [Google Scholar]
- 156.Lu Q, Li N, Luo J, Yu M, Huang Y, Wu X, et al. Pinellia pedatisecta agglutinin interacts with the methylosome and induces cancer cell death. Oncogenesis. 2012;1(10):e29. doi: 10.1038/oncsis.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bergström M, Åström E, Påhlsson P, Ohlson S. Elucidating the selectivity of recombinant forms of Aleuria aurantia lectin using weak affinity chromatography. Journal of Chromatography B. 2012;885:66–72. doi: 10.1016/j.jchromb.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 158.Basuki JS, Esser L, Duong HT, Zhang Q, Wilson P, Whittaker MR, et al. Magnetic nanoparticles with diblock glycopolymer shells give lectin concentration-dependent MRI signals and selective cell uptake. Chemical Science. 2014;5(2):715–26. [Google Scholar]
- 159.Zhang G, Sun J, Wang H, Ng T. First isolation and characterization of a novel lectin with potent antitumor activity from a< i> Russula</i> mushroom. Phytomedicine. 2010;17(10):775–81. doi: 10.1016/j.phymed.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 160.Zuo Z, Fan H, Wang X, Zhou W, Li L. Purification and characterization of a novel plant lectin from Pinellia ternata with antineoplastic activity. SpringerPlus. 2012;1(1):1–9. doi: 10.1186/2193-1801-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pernot M, Frochot C, Vanderesse R, Barberi-Heyob M. Targeting strategies in photodynamic therapy for cancer treatment. In: Michael H, Ying-Ying H, editors. Handbook of Photomedecine. Taylor & Francis-CRC Press; 2013. pp. 341–52. [Google Scholar]
- 162.Narayanan S, Surendranath K, Bora N, Surolia A, Karande AA. Ribosome inactivating proteins and apoptosis. FEBS letters. 2005;579(6):1324–31. doi: 10.1016/j.febslet.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 163.Plattner VE, Wagner M, Ratzinger G, Gabor F, Wirth M. Targeted drug delivery: binding and uptake of plant lectins using human 5637 bladder cancer cells. European Journal of Pharmaceutics and Biopharmaceutics. 2008;70(2):572–6. doi: 10.1016/j.ejpb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 164.Liu B, Cheng Y, Bian H-j, Bao J-k. Molecular mechanisms of Polygonatum cyrtonema lectin- induced apoptosis and autophagy in cancer cells. Autophagy. 2009;5(2):253–5. doi: 10.4161/auto.5.2.7561. [DOI] [PubMed] [Google Scholar]
- 165.Jung MG, Lee KP, Choi H-G, Kang S-H, Klochkova TA, Han JW, et al. Characterization of carbohydrate combining sites of Bryohealin, an algal lectin from Bryopsis plumosa. Journal of applied phycology. 2010;22(6):793–802. [Google Scholar]
- 166.Bies C, Lehr C-M, Woodley JF. Lectin-mediated drug targeting: history and applications. Advanced drug delivery reviews. 2004;56(4):425–35. doi: 10.1016/j.addr.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 167.Gabor F, Bogner E, Weissenboeck A, Wirth M. The lectin–cell interaction and its implications to intestinal lectin-mediated drug delivery. Advanced drug delivery reviews. 2004;56(4):459–80. doi: 10.1016/j.addr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 168.Neutsch L, Wirth E-M, Spijker S, Pichl C, Kählig H, Gabor F, et al. Synergistic targeting/prodrug strategies for intravesical drug delivery—Lectin-modified PLGA microparticles enhance cytotoxicity of stearoyl gemcitabine by contact-dependent transfer. Journal of Controlled Release. 2013;169(1):62–72. doi: 10.1016/j.jconrel.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 169.Chen J, Zhang C, Liu Q, Shao X, Feng C, Shen Y, et al. Solanum tuberosum lectin-conjugated PLGA nanoparticles for nose-to-brain delivery: in vivo and in vitro evaluations. Journal of drug targeting. 2012;20(2):174–84. doi: 10.3109/1061186X.2011.622396. [DOI] [PubMed] [Google Scholar]
- 170.Ensign LM, Cone R, Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Advanced drug delivery reviews. 2012;64(6):557–70. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang X-Y, Koller R, Wirth M, Gabor F. Lectin-coated PLGA microparticles: thermoresponsive release and in vitro evidence for enhanced cell interaction. International journal of pharmaceutics. 2012;436(1):738–43. doi: 10.1016/j.ijpharm.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 172.Jain SK, Gupta M, Sahoo AK, Pandey AN, Jain AK. Lectin conjugated gastro-retentive microspheres of amoxicillin for effective treatment of Helicobacter pylori. CURRENT SCIENCE. 2014;106(2):267–76. [Google Scholar]
- 173.Gupta A, Gupta RK, Gupta G. Targeting cells for drug and gene delivery: emerging applications of mannans and mannan binding lectins. Journal of Scientific and Industrial Research. 2009;68(6):465–83. [Google Scholar]
- 174.Wang X-Y, Koller R, Wirth M, Gabor F. Lectin-Grafted PLGA Microcarriers Loaded with Fluorescent Model Drugs: Characteristics, Release Profiles, and Cytoadhesion Studies. Scientia pharmaceutica. 2014;82(1):193–205. doi: 10.3797/scipharm.1312-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wen Z, Yan Z, Hu K, Pang Z, Cheng X, Guo L, et al. Odorranalectin-conjugated nanoparticles: preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. Journal of Controlled Release. 2011;151(2):131–8. doi: 10.1016/j.jconrel.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 176.da Cruz Cabral L, Fernández Pinto V, Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. International journal of food microbiology. 2013;166(1):1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 177.Diesner SC, Wang X-Y, Jensen-Jarolim E, Untersmayr E, Gabor F. Use of lectin-functionalized particles for oral immunotherapy. Therapeutic delivery. 2012;3(2):277–90. doi: 10.4155/tde.11.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Minko T. Drug targeting to the colon with lectins and neoglycoconjugates. Advanced Drug Delivery Reviews. 2004;56(4):491–509. doi: 10.1016/j.addr.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 179.des Rieux A, Fievez V, Garinot M, Schneider Y-J, Préat V. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. Journal of controlled release. 2006;116(1):1–27. doi: 10.1016/j.jconrel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 180.Liu B, Bian H-j, Bao J-k. Plant lectins: potential antineoplastic drugs from bench to clinic. Cancer Letters. 2010;287(1):1–12. doi: 10.1016/j.canlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 181.Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: carbohydrates, lectins and implications in cancer therapeutics. Acta histochemica. 2011;113(3):236–47. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Piazza J, Hoare T, Molinaro L, Terpstra K, Bhandari J, Selvaganapathy PR, et al. Haloperidol- loaded intranasally administered lectin functionalized poly (ethylene glycol)–block-poly (d, l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(1):30–9. doi: 10.1016/j.ejpb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 183.Moulari B, Béduneau A, Pellequer Y, Lamprecht A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. Journal of Controlled Release. 2014;188:9–17. doi: 10.1016/j.jconrel.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 184.Ye T, Yan S, Hu Y, Ding L, Wu W. Synthesis and volume phase transition of concanavalin A- based glucose-responsive nanogels. Polymer Chemistry. 2014;5(1):186–94. [Google Scholar]
- 185.Shao X, Liu Q, Zhang C, Zheng X, Chen J, Zha Y, et al. Concanavalin A-conjugated poly (ethylene glycol)–poly (lactic acid) nanoparticles for intranasal drug delivery to the cervical lymph nodes. Journal of microencapsulation. 2013;30(8):780–6. doi: 10.3109/02652048.2013.788086. [DOI] [PubMed] [Google Scholar]
- 186.Jain SK, Haider T, Kumar A, Jain A. Lectin-Conjugated Clarithromycin and Acetohydroxamic Acid-Loaded PLGA Nanoparticles: a Novel Approach for Effective Treatment of H. pylori. AAPS PharmSciTech. 2016;17(5):1131–40. doi: 10.1208/s12249-015-0443-5. [DOI] [PubMed] [Google Scholar]
- 187.Harokopakis E, Hajishengallis G, Michalek SM. Effectiveness of liposomes possessing surface- linked recombinant B subunit of cholera toxin as an oral antigen delivery system. Infection and immunity. 1998;66(9):4299–304. doi: 10.1128/iai.66.9.4299-4304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gao X, Chen J, Tao W, Zhu J, Zhang Q, Chen H, et al. UEA I-bearing nanoparticles for brain delivery following intranasal administration. International journal of pharmaceutics. 2007;340(1):207–15. doi: 10.1016/j.ijpharm.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 189.Bae YH, Lee ES, Kim D, Youn YS, Oh KT. inventors; Google Patents, assignee. Drug delivery vehicle that mimics viral properties. US2013. [Google Scholar]
- 190.Clark M, Hirst BH, Jepson MA. Lectin-mediated mucosal delivery of drugs and microparticles. Advanced drug delivery reviews. 2000;43(2):207–23. doi: 10.1016/s0169-409x(00)00070-3. [DOI] [PubMed] [Google Scholar]
- 191.Irache JM, Durrer C, Duch√™ne D, Ponchel G. Bioadhesion of lecSn-latex conjugates to rat intestinal mucosa. Pharmaceutical research. 1996;13(11):1716–9. doi: 10.1023/a:1016405126656. [DOI] [PubMed] [Google Scholar]
- 192.Pardridge WM. Blood–brain barrier delivery. Drug discovery today. 2007;12(1):54–61. doi: 10.1016/j.drudis.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 193.Piazza J, Hoare T, Molinaro L, Terpstra K, Bhandari J, Selvaganapathy PR, et al. Haloperidol- loaded intranasally administered lectin functionalized poly (ethylene glycol)‚ Äìblock-poly (d, l)-lactic-co-glycolic acid (PEG‚ ÄìPLGA) nanoparticles for the treatment of schizophrenia. European Journal of Pharmaceutics and Biopharmaceutics. 2014;87(1):30–9. doi: 10.1016/j.ejpb.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 194.Ma Y-P, Ma M-M, Cheng S-M, Ma H-H, Yi X-M, Xu G-L, et al. Intranasal bFGF-induced progenitor cell proliferation and neuroprotection after transient focal cerebral ischemia. Neuroscience letters. 2008;437(2):93–7. doi: 10.1016/j.neulet.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 195.Luppi B, Bigucci F, Abruzzo A, Corace G, Cerchiara T, Zecchi V. Freeze-dried chitosan/pectin nasal inserts for antipsychotic drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2010;75(3):381–7. doi: 10.1016/j.ejpb.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 196.Kamel MH, Moore PC, Bissada NK, Heshmat SM. Potential years of life lost due to urogenital cancer in the United States: trends from 1972 to 2006 based on data from the SEER database. The Journal of urology. 2012;187(3):868–71. doi: 10.1016/j.juro.2011.10.142. [DOI] [PubMed] [Google Scholar]
- 197.Neutsch L, Eggenreich B, Herwig E, Marchetti-Deschmann M, Allmaier G, Gabor F, et al. Lectin bioconjugates trigger urothelial cytoinvasion – A glycotargeted approach for improved intravesical drug delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2012;82(2):367–75. doi: 10.1016/j.ejpb.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 198.Liang H, Li J, He Y, Xu W, Liu S, Li Y, et al. Engineering Multifunctional Films Based on Metal-Phenolic Networks for Rational pH-Responsive Delivery and Cell Imaging. ACS Biomaterials Science & Engineering. 2016;2(3):317–25. doi: 10.1021/acsbiomaterials.5b00363. [DOI] [PubMed] [Google Scholar]
- 199.Qi H, Cao J, Xin Y, Mao X, Xie D, Luo J, et al. Dual responsive zein hydrogel membrane with selective protein adsorption and sustained release property. Materials Science and Engineering: C. 2017;70(Part 1):347–56. doi: 10.1016/j.msec.2016.09.010. [DOI] [PubMed] [Google Scholar]


