Abstract
Optical trapping is a powerful and widely used laboratory technique in the biological and materials sciences that enables rapid manipulation and measurement at the nanometer scale. However, expanding the analytical throughput of this technique beyond the serial capabilities of established single-trap microscope-based optical tweezers remains a current goal in the field. In recent years, advances in nanotechnology have been leveraged to create innovative optical trapping methods that increase the number of available optical traps and permit parallel manipulation and measurement of arrays of optically trapped targets. In particular, nanophotonic trapping holds significant promise for integration with other lab-on-a-chip technologies to yield compact, robust analytical devices. In this review, we highlight progress in nanophotonic manipulation and measurement, as well as the potential for implementing these on-chip functionalities in biological research and biomedical applications.
Graphical Abstract
The combination of high-throughput optical manipulation of biomolecular arrays with lab-on-a-chip technology offers the potential to revolutionize biological and biomedical research. Our review covers progress in nanophotonic optical trapping technologies with a focus on high-throughput applications in the biosciences.
1. INTRODUCTION
Since Arthur Ashkin’s pioneering work in the 1980s1, 2, optical trapping technologies have revolutionized the scientific investigation of interactions ranging from the millimeter to the nanometer scale. The ability to apply forces of tens of piconewtons and manipulate trapped targets with sub-nanometer precision has firmly established optical trapping as a versatile and powerful technique for studying biological processes like motor protein kinetics, protein and RNA folding, DNA and chromatin dynamics, and active matter micro-rheology3–10. Optical traps have also been used to manipulate cells and viruses11–13, and emerging nanophotonic trapping systems (described below) have shown promise for use in cell and pathogen processing tasks like sensing, sorting, transporting, and counting.
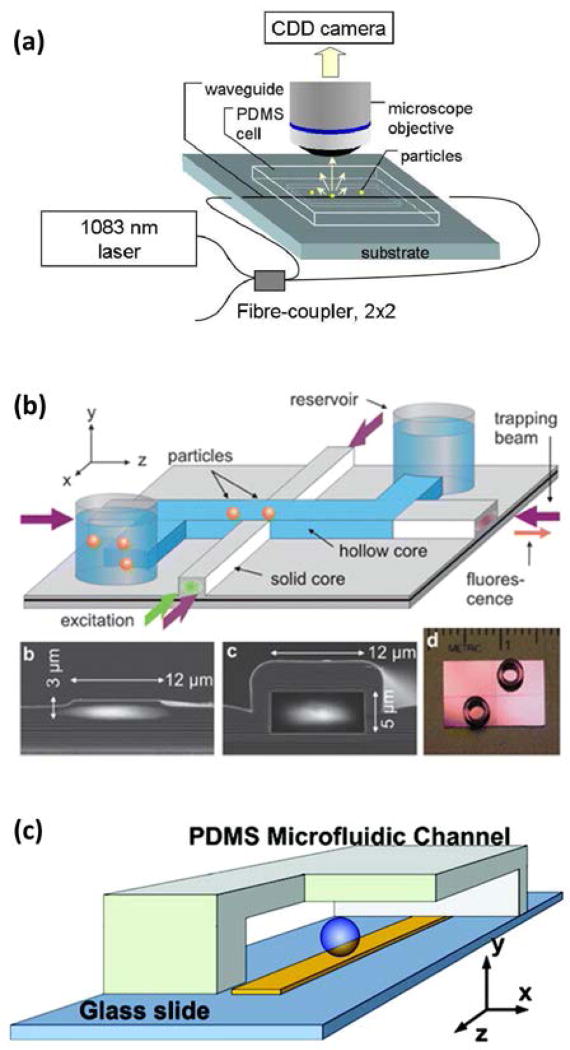
Optical trapping occurs when material in a non-uniform electric field experiences a force in the direction of increasing field intensity. In a traditional “optical tweezers” apparatus, a laser beam is focused with a microscope objective to a diffraction-limited trapping region of high field intensity (Figure 1a). While the capabilities of a traditional microscope-based optical trapping apparatus enable manipulation and measurement with sub-nanometer precision, the single optical trap at the beam focus restricts throughput to one sample at a time. However, most cellular and biochemical processes are stochastic, and require a number of measurements to fully characterize the dynamics of a biomolecular process. The desire to advance beyond serial measurement and controllably manipulate multiple targets has therefore motivated the development of a variety of array-based optical trapping techniques.
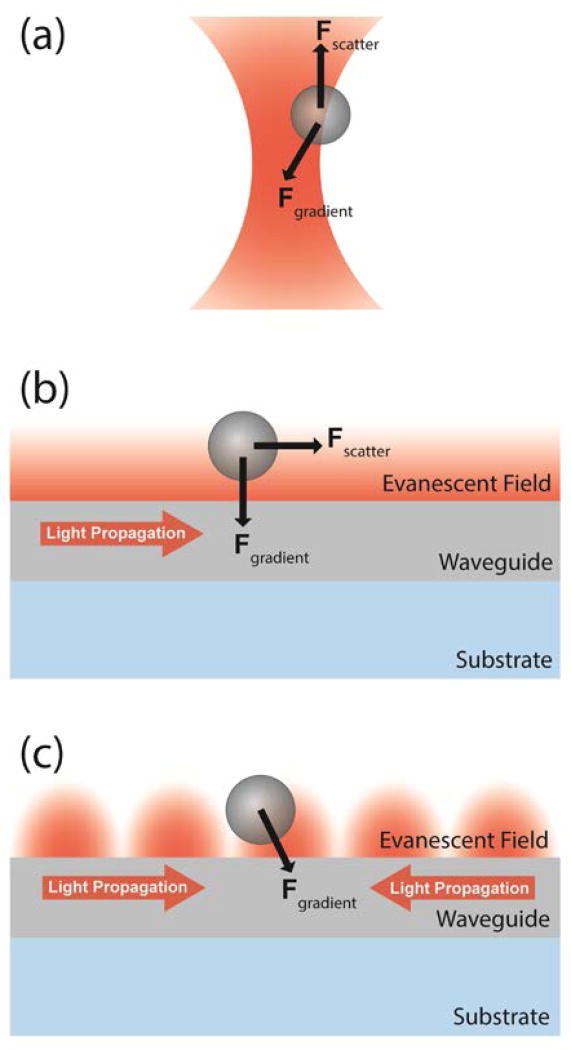
Figure 1.
Optical trapping geometries. (a) A dielectric bead in a tightly focused beam near the focus of a microscope objective experiences an optical gradient force toward the focal point, and a scattering force that displaces the bead from the trap center. (b) Near the surface of a nanophotonic waveguide, a dielectric bead experiences an optical gradient force toward the surface due to the evanescent field decay. Light scattered by the trapped bead imparts a scattering force that can propel the bead along the waveguide. (c) The scattering force is eliminated by establishing a stationary standing wave in the waveguide.
In this review, we highlight the progress that has been made toward parallel manipulation and measurement using on-chip optical trapping systems, with specific attention to their applications in biomolecular and cellular research and diagnostics. Although traditional microscope-based optical trapping systems have been adapted to achieve parallel manipulation, the future of array-based optical trap manipulation and measurement is likely to be defined by nanophotonic optical trapping systems. Rather than using bulky optical elements (e.g. lenses, mirrors, wave plates, beam splitter cubes, modulators) to prepare and focus a free-space laser beam, nanophotonic devices confine field propagation along chip-based light-guiding surface structures. Doing so produces an evanescent field – a region of non-uniform optical field intensity that supports optical trapping and manipulation (Figure 1b). Importantly, nanophotonic devices are compatible with “lab-on-a-chip” (LOC) functionalities like microfluidic transport systems. Broadly speaking, LOC miniaturization offers a variety of unique advantages such as reduced reagent requirements, integrated sample preparation and delivery, short diffusion lengths, parallel measurement for increased throughput and/or multiplexing capabilities, as well as a number of practical advantages (e.g. system portability, enhanced stability, decreased production costs)14–16. A number of nanophotonic structures and functionalities have been developed in recent years that can serve as building blocks to create compact, LOC optical trapping systems for integrated, high-throughput measurement and manipulation. While the full implementation of these functionalities within nanophotonic platforms is still under development, existing technology already has the potential to revolutionize biological and biomedical research with the projected availability of robust, compact, low cost, and user-friendly devices that are capable of high-throughput, high precision single-target analysis.
2. BACKGROUND
The traditional microscope-based optical trap, formed at the focal point of a microscope objective, was the first optical trap to be interfaced with biological materials11, 12 and now has a rich history of applications in biophysical and biomedical research. It was initially implemented as a single three-dimensional trapping center to manipulate a single cell, virus, or microsphere molecular handle, but a host of novel adaptations have since been developed. Specifically, to expand observation and control capabilities, microscope-based optical traps have been adapted to rotate birefringent dielectric targets17–19, and have also been interfaced with other single-molecule tools including fluorescent reporters, micropipettes, and magnetic tweezers20.
Another avenue of development has been the continued effort to trap and/or manipulate multiple dielectric targets in parallel, either to increase measurement throughput or to enable more complex manipulation of molecular structures. In particular, two complimentary microscope-based strategies have been implemented to distribute a single laser beam over multiple traps: time-sharing optical traps21 (temporal distribution) and holographic optical traps22 (spatial distribution). Although independent manipulation of tens of optical traps has been demonstrated using the time-sharing approach23, and arrays of hundreds of traps have been achieved with holographic techniques24, increasing the number of traps when using these methods requires a proportional increase in laser power to maintain a given trap stiffness. Alternatively, nanophotonic trapping methods offer an opportunity to increase the number of traps without increasing laser power or degrading individual trap strength, while also shedding the need for bulky beam preparation configurations and expensive beam control elements (e.g., acousto-optic modulators or spatial light modulators).
Unlike microscope-based optical traps, which confine trapped targets to a beam focus, most nanophotonic traps confine targets near surface structures that guide light with total internal reflection (TIR). Notably, TIR produces an evanescent field (i.e., near-field) that propagates along the surface boundary, but for which the field strength decays exponentially with distance from the boundary surface (Figure 1b). This exponential decay creates a strong field gradient, and thus an optical force toward the surface that acts on nearby dielectric target material. As a result, the motion of material in a nanophotonic trap is essentially restricted to a translational plane within about a hundred nanometers from a surface. For the same waveguide dimension design, waveguides with a larger contrast in refractive index (n) between the core and the cladding layer exhibit evanescent fields with shorter decay lengths, and therefore produce stronger optical gradient forces at a given laser power. The field gradients needed to optically trap micron-sized targets, like cells, with pN-scale forces can be achieved at low optical powers (~mW) using common waveguide materials (e.g., ion-doped glass, n≈1.48; SU-8 polymer, n≈1.55; Si3N4, n≈1.98; Ta2O5, n≈2.15; and Si, n≈3.48; note: indices are reported at operational wavelengths of 1550 nm for Si, and 1064 nm for the other materials)25–29. While the large refractive index of Si provides exceptional light confinement, the increased absorption of light by water at 1550 nm, compared with 1064 nm (where Si is opaque), generates local, power-dependent heating near nanophotonic structures. This heating can significantly influence the motion of trapping targets via thermophoretic effects30, and also potentially alter or damage biological samples.
For traveling waves propagating within the waveguide, momentum transferred to the target by scattered and absorbed photons can produce an additional optical force in the direction of field propagation. This scattering force can be eliminated by establishing a stationary standing wave in the trapping waveguide (Figure 1c). As highlighted below, this approach permits precise and controllable positioning of an array of near-field optical traps31–33.
Compared with traditional microscope-based optical traps, near-field nanophotonic traps offer specific practical advantages related to the number and minimum size of targets that can be optically manipulated. Because the majority of the field energy is confined within a high-refractive index guiding structure (e.g., a waveguide), rather than in the aqueous solution where trapping occurs, trapping any single dielectric target has little influence on the phase or intensity of the trapping field. This property allows arrays of 10s-100s of targets to be trapped on one nanophotonic structure using a single light source without significant loss of stiffness per additional trapped particle. Confining field energy within the waveguides also helps to reduce sample heating, because the materials used for waveguides and their underlying substrates tend to have lower optical absorption and greater thermal conductivity than water or biological samples34. Constrained by thermal heating and the diffraction limit of a tightly focused beam, microscope-based optical traps are generally limited to dielectric targets with diameters (d) of 0.5 µm or larger for practical applications34, 35. Notable exceptions have been achieved with smaller biological targets, including optical trapping of individual HIV-1 virions36. Strong gradient forces achieved by tight field confinement within nanophotonic structures however, permit routine trapping and manipulation of sub-100 nm dielectric particles, as well as biological targets like viruses, DNA, and proteins, as discussed below.
While the implementation of nanophotonic manipulation techniques in biological and biomedical studies is still emerging, their promise for incorporation into compact, user-friendly LOC devices has motivated the development of a number of nanophotonic trapping and manipulation components. Together, these comprise a tool-box of building blocks from which future end-user devices can be constructed for precise, high-throughput manipulation and measurement of biomolecular arrays. Like many other LOC systems, nanophotonic devices are typically fabricated using standard CMOS-compatible nanofabrication techniques. This shared property enables straightforward integration of nanophotonic trapping devices with high-speed microelectronic control systems and versatile microfluidic sample handling capabilities. Within this framework of on-chip manipulation, we present developments made by the nanophotonic trapping community organized by the type of manipulation achieved: (1) fixed-velocity traps, (2) fixed-position traps, (3) variable-position traps with micron precision, and (4) variable-position traps with nanometer precision. In each case, we will highlight the reported or proposed application of the technology for biomolecular research and diagnostics.
3. FIXED-VELOCITY TRAPS
Fixed-velocity nanophotonic traps are characterized by a continuous optical scattering force that propels a trapped dielectric along the surface of a nanophotonic waveguide. The fundamental groundwork for all future devices in near-field particle manipulation was developed in the seminal studies by Kawata and Sugiura in the early-to-mid 1990s37. In the first demonstration of near-field optical propulsion, polystyrene and glass microparticles were propelled through water in an evanescent field produced by TIR of a laser beam at a sapphire prism surface37. Kawata and Tani later extended that work using polystyrene microparticles that were trapped above waveguides, and then propelled along their length over distances exceeding 1 cm38. In both cases, an optical gradient force pulled nearby microparticles toward the surface due to the rapid decay of the evanescent optical field. Trapped particles were also longitudinally translated along the surface due to the scattering force in both cases (Figure 1b). These two optical forces, in combination with a fluid drag force from the aqueous solution, caused optically trapped beads to migrate along the surface with a constant velocity that varied with the diameter and material composition of the microparticles, as well as the power of the coupled laser.
These studies demonstrated that particle localization and transport is limited by both the particle properties, and the patterning and geometry of light-guiding structures engineered on the chamber surface. Perhaps even more notably, multiple particles were simultaneously trapped and manipulated in parallel in both of the seminal studies, indicating feasibility of large-array manipulation in future applications. Recognizing that near-field trapping and scattering forces depend on the geometric and dielectric properties of the trapped material, the authors forecasted future applications of their design in cell sorting and mixing according to material parameters of the trapped target such as size, chemical composition, and shape.
3.1 Waveguide Propulsion
In the decades following these pioneering studies, improvements in micro- and nanofabrication techniques and resources, along with increasingly straightforward integration with microfluidic delivery systems16, led to expanded exploration of LOC-compatible fixed-velocity trapping and transport systems. Manually controlled particle sorting was the first application to be demonstrated using this technology. In that report by Grujic et al., polystyrene microspheres that were trapped and transported along a waveguide were sorted at a y-branch junction by shifting the optical power between the split waveguide branches39. The ion-exchange doped glass waveguides used in that study (and the previous by Kawata) offered a modest refractive index contrast relative to water, but little refractive index contrast relative to the glass substrate. By transitioning to silicon nitride (Si3N4) as a higher refractive index waveguiding material to increase field confinement, Gaugiran et al. increased both the gradient and radiation forces by more than an order of magnitude25. This permitted, for the first time, the trapping and transport of biological cells (Figure 2a). Red blood cells and yeast cells were transported along the Si3N4 waveguides at approximately 1 µm/s with guiding powers of approximately 60 mW and 40 mW at the trapping region, respectively. Using Ta2O5, an even higher refractive index waveguide material, Ahluwalia et al. later demonstrated greater transport velocities for trapped red blood cells27, as well as the use of variable-force trapping to analyze red blood cell deformability during storage28. These studies, performed in static aqueous sample chambers, confirmed the potential of waveguide-based nanomanipulation techniques for use in trapping, transport, and analysis applications with biological cells.
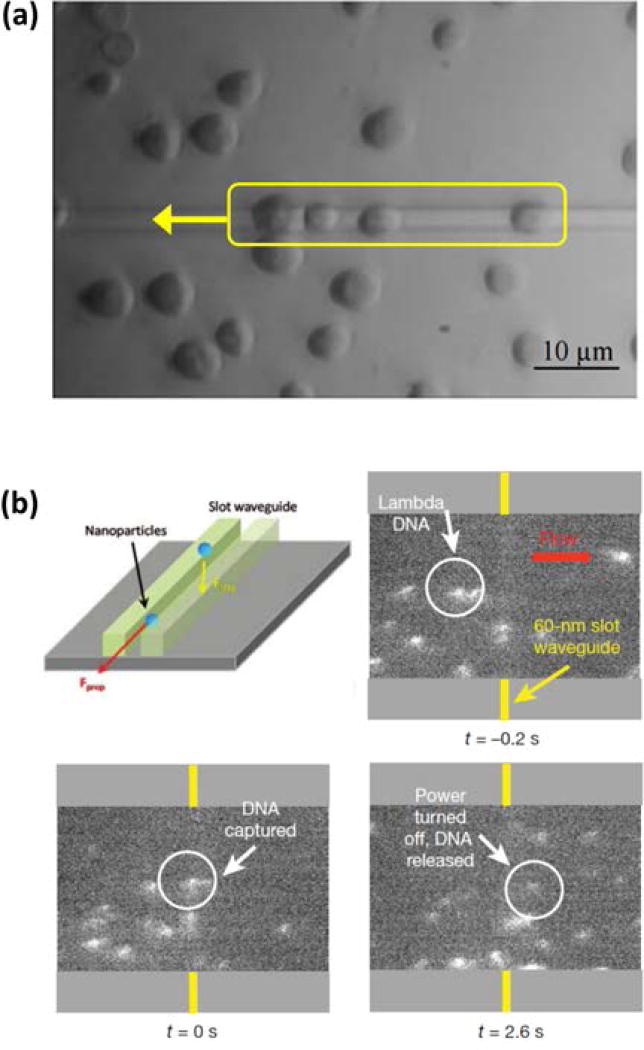
Figure 2.
Examples of fixed-velocity nanophotonic traps. (a) The simplest fixed-velocity nanophotonic trap design is a basic waveguide. Yeast cells were trapped and transported to the left along the waveguide25. (b) Two parallel waveguides in close vicinity to each other generate a high strength fixed-velocity “slot waveguide” trap. Controlled trapping and release of stained λ-DNA was demonstrated in the presence of microfluidic flow29, 45. (Adapted with permission from: Ref 25, Copyright Optical Society; Ref29, Copyright 2009 American Chemical Society.; Ref45, Copyright 2009 Nature Publishing Group).
As previously mentioned, microfluidic sample handling offers a number of advantages, including increased sample throughput and the ability to dynamically exchange solution buffer contents. Moving beyond static fluid chambers toward a more versatile sample delivery system, Schmidt et al. integrated waveguide-based fixed-velocity optical traps with a microfluidic flow channel26. Using waveguides formed from SU-8 polymer, they directly captured passing particles from a pressure-driven fluid flow and demonstrated stable particle transport in directions both perpendicular and anti-parallel to the direction of fluid flow. They also characterized the chromatographic and fractionation properties of their system by introducing pairs of polystyrene microparticles with differing diameters. In that size regime, both the gradient and scattering optical forces are greater for larger particles, supporting the possibility of using such a system for sorting cells or biofunctionalized microparticles. In related work, Khan et al. used Si3N4 waveguides to sort a heterogeneous mixture of glass microparticles, achieving successful fractionation despite directly observed particle collisions on the waveguide40. In the most advanced analytical application demonstrated so far with a simple waveguide propulsion design, initially reported by Schein et al., the size and surface interactions of nanoparticles trapped on a waveguide were precisely characterized by monitoring scattered light41, 42. These waveguide propulsion demonstrations show that the manipulation technique is versatile with respect to the waveguiding material and the size and composition of transported targets and that they are compatible with microfluidic delivery systems that will be needed for biological studies in end-user devices.
3.2 Slot Waveguide Propulsion
It is well documented that both optical gradient and scattering forces decrease with particle radius43, 44, making optical trapping and transport of nanoscale dielectric particles difficult to achieve on conventional rectangular waveguides. To overcome this challenge and further increase both the gradient and scattering forces on nanoparticles (d ≤ 100 nm), Yang et al. designed and fabricated a slot waveguide in a silicon-on-insulator (SOI) substrate45. Si has a high refractive index, which reduces the decay length of the evanescent field and increases the optical field gradient. Furthermore, with the slot waveguide design, a significant portion of the guided optical mode resides in the exposed slot region between two closely spaced (<150 nm) rectangular silicon ridges (Figure 2b). Dielectric material trapped in the slot region is exposed to greater optical intensity and thus experiences a greater scattering force than material trapped above a single waveguide. In that report, the slot waveguide approach was applied to the trapping and transport of 75 nm polystyrene nanospheres, as well as trapping of 48 kbp long λ-DNA molecules for the first time within a nanophotonic trap. Considering that many viruses and cell organelles have dimensions on this same scale, the ability to trap and transport dielectric targets in this size regime holds significant potential for use in future LOC-compatible biomolecular studies.
3.3 Whispering Gallery Mode Resonators
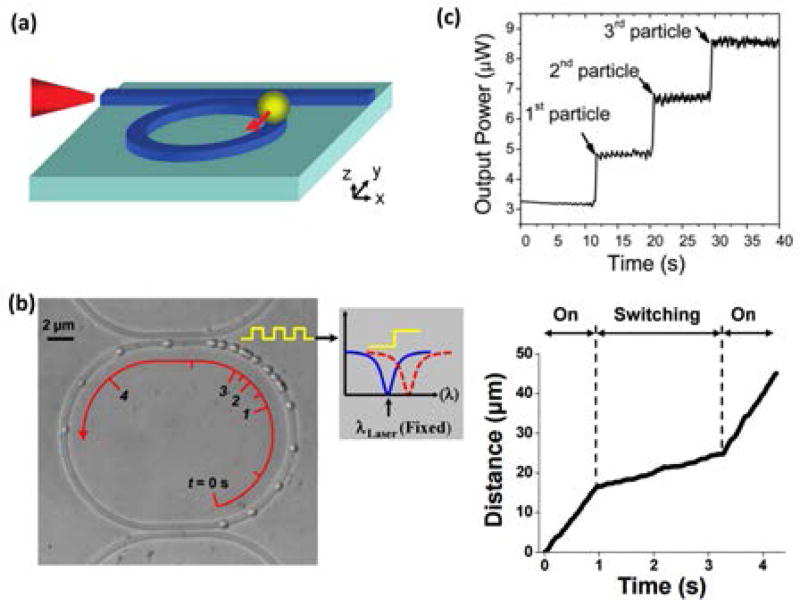
Using an alternative method to increase both the optical gradient and scattering forces, multiple research groups have amplified the guided optical field intensity in a waveguide structure by adopting the geometry of a closed-ring “whispering gallery mode” (WGM) resonator (Figure 3)46–50. Photons that are coupled to the waveguide resonators from an adjacent bus waveguide can circulate through the structure many times before being absorbed or scattered. If the optical excitation wavelength meets the resonance condition, coupled photons constructively interfere with each other and amplify the field intensity in the resonator. In addition, particles that are optically transported along the bus waveguide can be transferred to a particular ring resonator by tuning the excitation wavelength in the bus waveguide to match the resonance wavelength of a particular ring. Similar controllable transfer can also be achieved by using the thermo-optic effect to modulate the phase of the field in a waveguide that is coupled to the resonator (Figure 3b)49. By integrating an on-chip microheater with a waveguide resonator, Soltani et al. demonstrated rapid control of the field intensity in the resonator, allowing dynamic modulation of the velocity of trapped particles on the resonator. Additionally, Lin et al. notably demonstrated that the number, and velocity, of beads trapped on a ring could be determined by monitoring the output power from the bus waveguide, a technology which could be directly translated to counting cells48 (Figure 3c). The ability to transport selectively trapped dielectric targets over long distances before storing, counting, or characterizing them marks major progress in the development of LOC-style microanalysis systems for use in biomolecular assays.
Figure 3.
Fixed-velocity trapping has been localized on whispering gallery mode resonators. (a) Schematic representation47. (b) Overlaid images of a single microparticle that was transported around a WGM resonator at 2 user-controlled velocities (taken at 0.24 s time intervals)49. (c) The number of optically trapped targets on a WGM resonator was determined by changes in the output power transmitted through the bus waveguide48. (Reprinted with permission from: Ref.47, Copyright 2010 American Chemical Society; Ref48, Copyright 2011 Royal Chemistry Society).
While advances in fixed-velocity nanophotonic trapping and transport techniques hold promise for particle and cell sorting and counting assays, practical limitations remain. Namely, there is a limited ability to precisely control the location of trapped targets, and therefore a restricted capacity to extract information about forces that may be acting on them – a critical measurement in many standard optical trapping experiments. In our next section, we examine fixed-position optical traps that may be integrated with fixed-velocity waveguide traps in a manner similar to the previously discussed WGM resonators, but with more precise position localization capacities that can accommodate force measurements.
4. FIXED-POSITION TRAPS
Although the ability to optically control transport of trapped target material remains central in the development of future on-chip analytical systems, some measurement approaches, like optical and force spectroscopy, require the optically trapped target to occupy a trapping center at a precisely localized position. This is most directly achieved by engineering trapping features at fixed positions on a substrate, and three dominant approaches to fixed-position trapping have emerged within the nanophotonic field: divergent-field traps, photonic crystal resonators, and plasmonic tweezers. Nanophotonic trapping and manipulation aside, the application of photonic crystals and plasmonic nanostructures in analytical and biomolecular studies is well documented in literature, particularly for sensing and optical spectroscopy51–57. Given the focus of this review, we will highlight examples in which these technologies have either been integrated with optically controlled transport systems or developed for force spectroscopy applications.
4.1 Divergent-field Traps
Optical trapping in divergent-field traps relies on the abrupt variation in the field intensity that results from a termination or change in the width of a waveguide. The rapid decrease in field strength at such a junction produces a gradient force that is capable of stably trapping particles. A proof-of-concept example by Cai and Poon used single-mode Si3N4 waveguides to transport particles to tapered waveguide junctions where, depending on the junction geometry, one or more polystyrene particles (d = 1 µm) became trapped58. They also arranged for successive substitution, where an incoming particle replaced an existing trapped particle by pushing it from the trap center. The authors identified applications in pharmaceutical synthesis and droplet-based DNA computation for their junction trap technology. In an alternate design used by Helleso et al., Boerkamp et al., and Helle et al., a single source waveguide was split into two waveguides that looped toward each other, terminating microns apart in opposing directions (Figure 4a)59–61. Dielectric material was trapped in the space between the waveguide termini where counter propagating beams crossed. Using this design, Boerkamp et al. engineered a microfluidic channel through the region between the opposing waveguide facets, and performed Raman spectroscopy on optically trapped polystyrene microspheres60. Helleso et al. trapped both polystyrene microspheres and red blood cells59. In follow-up work by the same group, Helle et al. demonstrated that their trap center was vertically displaced from the surface61, a feature that may prove useful in preventing surface interactions or adhesion forces when analyzing biological samples. Expanding the number of targets trapped by a single divergent-field waveguide, Leake et al. placed the terminal facet of a multimode interference waveguide at the edge of a microfluidic channel62. Up to six microspheres were simultaneously trapped, and by changing the source wavelength, the trap arrays could be toggled between two unique interference patterns. This demonstration exhibited both array trapping and a discrete form of manipulation in a compact on-chip system.
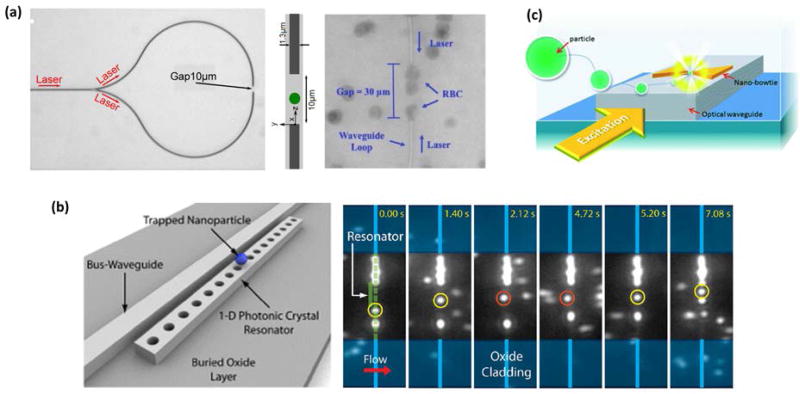
Figure 4.
Examples of fixed-position nanophotonic traps. (a) Divergence-based junction traps balance the optical gradient and scattering forces to trap targets like red blood cells (RBC)59, 61. (b) Stronger fixed-position traps are achieved with photonic crystal (PhC) resonators. A nanoparticle that was transported along a bus waveguide (yellow circles) became trapped at a side-coupled PhC resonator (orange circles) before being released and transported along the bus waveguide again63. (c) Plasmonic nanostructures also allow fixed-position trap arrays with strong trapping forces78. Each of these fixed-position traps were waveguide coupled and combined with fixed-velocity target transport. (Adapted with permission from: Ref61, Copyright 2015 Optical Society; Ref59, Copyright 2012 Royal Chemistry Society; Ref63, Copyright 2010 American Chemical Society; Ref78, Copyright 2014 Royal Chemistry Society).
4.2 Photonic Crystal Traps
Generally associated with a broad array of on-chip based applications, photonic crystals consist of two or more dielectric materials of differing refractive indices (e.g., Si and water) arranged in a periodic pattern. Such devices can be engineered to exhibit localized resonant modes where the optical field energy is strongly concentrated, resulting in strong optical gradient forces. Several studies from the lab of D. Erickson have investigated the use of 1D photonic crystal waveguide structures in near-field optical trapping and analysis30, 63–65. Using silicon devices, they demonstrated trapping of 48 nm and 62 nm polystyrene nanospheres, as well as the ability to optically transport a 500 nm polystyrene particle along a bus waveguide, transfer the particle to the photonic crystal trap, and then release the particle for further optical transport on the bus waveguide (Figure 4b)63. They later showed that switching from Si to Si3N4 devices and use of laser wavelength from 1550 nm to 1064 nm greatly reduced the local heating at the resonator that resulted from optical absorption by water30, 64. This permitted trapping of even smaller dielectric targets, including single molecules of Wilson disease proteins, quantum dots, and 22 nm polymer particles64. Interestingly, by analyzing the intensity of light scattered by a trapped particle, they quantified the interaction force between the resonator surface and a 100 nm trapped particle with sub-pN resolution – a technique they named nanophotonic force microscopy (NFM)65. Two-dimensional photonic crystal optical traps have also been reported for precisely measuring the size distribution of sub-100 nm gold nanoparticles66, and trapping and analyzing larger dielectric particles (d > 0.25 µm) and bacteria at sub-mW powers67–72. These latter devices hold potential for applications in bacteria sensing or analysis. Additionally, Renaut et al. demonstrated rotational orientation control for a pair of adjacent 1 µm microspheres trapped on a PhC73, indicating short-range nanomanipulation may be also achieved with PhC optical traps.
4.3 Plasmonic Traps
Like photonic crystal cavities, slot waveguides, and other sub-wavelength waveguiding structures, nanoplasmonic devices offer another approach to concentrate light at dimensions well below the diffraction limit. Nanoplasmonic devices consist of nanoscale metallic features in which resonant surface plasmons, i.e., collective oscillations of mobile valence electrons in the metal, can be excited by incident light within a particular frequency range. Plasmons produce strong electric fields at the metal-dielectric surface boundary that decay very quickly, giving rise to strong optical gradient forces. Nanoplamsonic devices can be patterned in large arrays using standard nanofabrication techniques, and consequently, have been used to demonstrate optical trapping of arrays of particles, bacteria, and yeast74, 75. Because plasmonic field enhancement tends to be strongest at sharp features and gap junctions, translational manipulation of an array of trapped targets is generally difficult to achieve with plasmonic devices. However, rotation of trapped targets was achieved by illuminating particular trap geometries with circularly polarized light76, 77. Near-field plasmonic traps have also been combined with traditional dielectric waveguides for on-chip optical excitation. Lin et al. fabricated plasmonic tweezers on top of a Si3N4 waveguide to trap a 1 µm polystyrene particle after it was optically propelled along the length of the waveguide78 (Figure 4c). In a different geometry, Cheng et al. embedded a silver nanowire in a poly(methyl methacrylate) (PMMA) nanofiber and demonstrated optical trapping of a sub-µm polystyrene particle that was optically transported along the fiber79. As a practical note, plasmonic absorption of light can induce significant local heating near the metallic nanostructures80, so care must be taken to control such heating when trapping biological samples.
Although fixed-position nanophotonic optical traps offer limited opportunity for manipulation of trapped targets, they serve a useful role in sensing when used in combination with other near-field nanomanipulation techniques. As described in this section, combining fixed-position trapping structures with fixed-velocity transport structures enables a number of procedural steps (e.g. collection, transport, localization, and analysis) to be implemented on-chip for spatial or temporal arrays of analytical targets. Aside from position localization, other specific advantages of fixed-position traps include the ability to pattern many traps over large areas during fabrication and the ability to stably trap targets at low optical powers (µW-mW). These properties make fixed-position optical traps particularly suited for multiplexed detection and sensing applications in complex analytical samples.
5. MICRON-PRECISION VARIABLE-POSITION TRAPS
As discussed in the previous section, when fixed-position and fixed-velocity traps are combined, they provide significant capacity for on-chip, parallelized manipulation and analysis of biomolecular or nanoscale materials. However, some biomolecular applications require the ability to controllably reposition optically trapped targets (e.g., moving trapped targets back and forth between multiple chemical microenvironments within a microfluidic channel). By tuning opposing scattering forces that are exerted by counter-propagating optical beams in light-guiding structures, position localization and reversible velocity control has been achieved with micron precision in multiple on-chip optical trapping platforms.
The earliest demonstration of on-chip optical trap positioning was reported in 2007 by Grujic et al., using sub-µm polystyrene spheres on a Cs+ ion-exchange waveguide in soda lime glass81. Light from a single laser source was split with a 2 × 2 fiber coupler, and then coupled into opposite ends of a single waveguide via direct fiber butting (Figure 5a). Interference effects were not observed, and particle positioning was tuned by changing the strength of opposing scattering forces. This was achieved by repositioning the input coupling fibers to modulate the ratio of input optical power at each end of the waveguide. Additionally, long chains of trapped particles formed over time, and they were able to be translated back and forth along the waveguide in parallel. Although velocity and position control was inconsistent along the length of the waveguide (due to scattering loss), this initial demonstration showed significant promise both for trap position tuning and parallel array manipulation.
Figure 5.
Variable-position nanophotonic traps that balance optical scattering forces to control the position of trapped targets. (a) The simplest configuration uses (incoherent) counter-propagating beams in a basic waveguide to trap and transport arrays of particles along the waveguide81. (b) The ARROW tunable loss-based trapping design allows for micron-scale spatial resolution in positioning of nanoparticles, and integrates well with on-chip fluorescence excitation and signal collection82. (c) Counter-propagating plasmons on a gold strip permitted tunable positioning of a bead over a ~30 µm region83. (Reprinted with permission from: Ref81, Copyright 2007 Optical Society; Ref82, Copyright 2009 Royal Chemistry Society; Ref83, Copyright 2010 American Chemical Society).
Intentionally taking advantage of optical propagation loss, Kuhn et al. demonstrated tunable optical trap positioning using a liquid-core AntiResonant Reflecting Optical Waveguide (ARROW)82. In this unique waveguide geometry, the sample analyte solution was contained within the core of the waveguide, and target material was trapped in the region where counter-propagating beams had equal intensity (Figure 5b). Limited by a large optical trapping volume (spanning several microns along the waveguide), tunable trapping of fluorescent silica beads and E. coli bacteria was nonetheless demonstrated. Interestingly, a perpendicular waveguide was used for on-chip fluorescence excitation of particles trapped at the appropriate position, and fluorescence signal was collected in the ARROW waveguide itself.
The approach of counter-propagating beams was also applied by Wang et al. to a prism beneath a plasmonic gold stripe to trap and control the position of polystyrene microspheres83. Localization along the stripe was achieved by balancing scattering forces of two counter-propagating surface plasmons (Figure 5c), and the position was tunable over an approximate range of 30 µm. Just as in the loss-based ARROW trap, the balanced-scattering approach used for position tuning in this device does not easily scale to support manipulation of arrays of targets on a single light-guiding feature, but a geometric array of closely patterned plasmonic stripe segments on a single surface may provide array manipulation opportunities.
6. NANOMETER-PRECISION VARIABLE-POSITION TRAPS
Experiments analogous to those implemented on traditional microscope-based optical tweezer instruments (e.g., force spectroscopy with sub-pN force resolution and sub-nm position resolution) require precise control over both the center position of an optical trap and the repositioning speed. While early work toward this goal yielded tunable position control with micronscale precision, described in the previous section, the ability to transport and reposition an array of nanophotonic optical traps with nanometer precision has only been achieved by establishing a standing wave within the trapping waveguide.
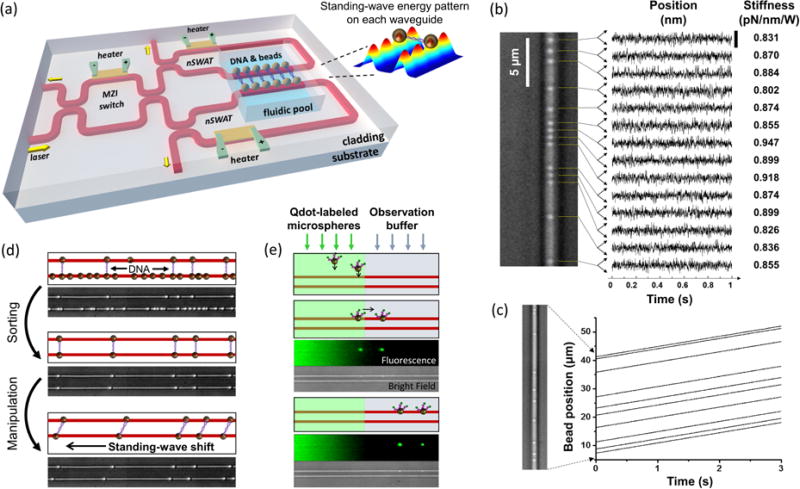
In 2014, Soltani et al. demonstrated the ability to precisely control and measure arrays of trapped particles using a novel near-field optical trapping device referred to as a nanophotonic standing wave array trap (nSWAT)31. The nSWAT device simultaneously integrates nanophotonic manipulation, microelectronics, and microfluidics. Initially fabricated with Si waveguides using an electron-beam lithography process31, second-generation nSWAT waveguides were fabricated in Si3N4 through photolithographic patterning32, 33. Transitioning from electron-beam lithography to photolithography reduced fabrication costs and processing time. Switching to Si3N4 from Si permitted operation at a shorter laser wavelength (1064 nm vs. 1550 nm), thus reducing sample heating and nonlinear optical absorption by the waveguide. In both cases, the nSWAT devices employ counter-propagating waves in a semiconductor waveguide (Si or Si3N4) to produce a standing wave, thus forming an array of three-dimensional optical traps at the interference antinodes of the evanescent field (Figure 6a). The translational position of the trap array along the nSWAT waveguide is controlled with sub-nm precision by adjusting the phase difference between the opposing beams (Figure 6b)32. The phase is modulated via the thermo-optic effect at high speed (30 kHz) using onchip microheaters49, enabling precisely controlled particle velocities (Fig. 6c)32. The nSWAT demonstrated optical trap stiffnesses on the order of 1 pN/nm per Watt of laser power at the trapping region, and thus forces are expected to be on the order of tens of piconewtons33. Inconsistent reporting of trap stiffness and power requirements in literature for nanophotonic trapping devices prevents direct comparison, but the performance characteristics of the nSWAT are comparable to traditional microscope-based optical tweezers32. Arrays of 10s of particles have been trapped and transported in parallel, with the force and position of each particle measured in time. Furthermore, the nSWAT platform is capable of applying both the constant velocity and constant force conditions that are commonly used in single-biomolecule studies, and, relative to standard microscope-based optical tweezers setups, it is exceptionally resistant to environmental noise and vibrations.
Figure 6.
Nanophotonic standing wave array trap (nSWAT). (a) The nSWAT integrates nanophotonics, microfluidics, and microelectronics for precision optical trapping applications in one on-chip device31. Input laser light is divided across two adjacent nSWAT waveguides using a Mach-Zehnder interferometer (MZI), allowing parallel manipulation of an array of DNA dumbells trapped on adjacent waveguides. Microheaters are used to tune the MZI power splitting ratio and to adjust the position of trap arrays on each trapping waveguide. (b) Holding an array of optical traps at a fixed position yielded particle trapping with sub-nm precision, and allowed the trap stiffness to be calculated32. (c) Constant-velocity transport of an array of trapped nanoparticles32. (d) DNA dumbbells were sorted using a combination of trapping and fluid flow forces, and the resulting dumbbell array was manipulated in parallel31. (e) Fluorescently labeled beads that were introduced in the left laminar flow were trapped and transported to the right laminar flow and held for observation31. The bottom waveguide was off in this demonstration.
To demonstrate the nSWAT’s potential for use in single-molecule biological studies, DNA dumbbell tethers, i.e., single molecules of DNA with a bead attached at each end, were sorted from a mixture of other free beads and DNA molecules, and then subsequently manipulated in parallel (Figure 6d)31. Molecular tethers are commonly stretched in single-molecule experiments to measure mechanical properties of biological polymers like nucleic acids, and also to characterize their interactions with other molecules like enzymes and molecular motors. When working with molecular tethers, sorting is an important step for removing incomplete tether complexes and ensuring a uniform sample population. By alternately turning the standing wave trap array on and off in adjacent nSWAT waveguides and using appropriately directed microfluidic flow, they showed that only fully intact DNA dumbbells were left bridging the two waveguides. Translating the optical trap array of one nSWAT relative to the other directly enabled stretching of multiple molecular dumbbells, and translating the trap arrays on both nSWAT waveguides at the same velocity enabled dumbbell tethers to be transported to other regions of microfluidic flow. This showed that stretching experiments commonly performed on traditional optical tweezers setups can be translated to the nSWAT platform with increased measurement throughput, augmented by the capacity to move tethers to different regions for chemical or biomolecular interactions.
In another proof-of-concept demonstration, fluorescently labeled beads were trapped from a flow stream that also contained free labels, and were then transported to an adjacent label-free buffer for observation (Figure 6e)31. This highlighted the nSWAT’s compatibility with fluorescence imaging, and established the potential of the device for applications in sequentially controlled reactions that require different microenvironments, as well as sorting, sampling, and monitoring of biomolecular interactions. Together, these demonstrations exhibit the versatility of the nSWAT platform for conducting high-throughput measurements in multistep reactions using a robust, compact on-chip platform.
While early demonstrations of variable-position nanophotonic traps with micron-precision showed promise for controlled, parallel manipulation of an array of dielectric targets, a number of practical considerations limited their broad implementation in array-based biomolecular studies. The nSWAT platform’s achievements in high-precision position localization and velocity control, resulting from the employed standing wave mechanism and thermo-optic phase modulators, respectively, match the performance of traditional microscope-based optical traps and are expected to enable onchip implementation of single-molecule experiments on parallel arrays of molecules.
6. CONCLUSION
We have presented a broad overview of the use of nanophotonic optical traps as they relate to parallelized manipulation and measurement of biomolecular target arrays. Over the past two decades, substantial progress has been made in transporting arrays of trapped targets over millimeter distances, confining them with nanometer precision, and improving single-target detection and characterization measurements. Recently demonstrated abilities to tunably manipulate arrays of analytical targets (including DNA dumbbells by nSWAT32) and quantify sub-pN forces (NFM65) using nanophotonic traps already meet or surpass specifications of traditional labscale high-precision measurement tools like optical tweezers and atomic force microscopes. Much of the current research applying nanophotonic manipulation to biological and biomedical applications is still at a proof-of-concept stage, but despite commercialization challenges84, devices employing nanophotonic manipulation have begun to emerge on the market85.
The next decade will likely bring substantial development in devices that integrate the basic trapping and manipulation functionalities summarized here with other cutting-edge LOC microfluidic designs, micromagnetic array manipulation systems86, on-chip detectors87, and on-chip light sources to power the trapping waveguides88. Low-power, on-chip light sources for fluorescence illumination (i.e., silicon LEDs or dye lasers) can already be incorporated for inclusive on-chip design89. Such near-field photonic devices may also soon be engineered to perform sensitive optical spectroscopy measurements using the trapping waveguide itself90–92. It is clear from the wealth of achievements presented in this review that near-field trapping and manipulation has emerged as an important sub-field in its own right, and we look forward to a future where high-throughput, high-precision measurements in science and medicine are made accessible on compact, low-cost, and user-friendly devices.
Acknowledgments
We would like to express gratitude to members of the Wang lab for critical reading of the manuscript, especially Dr. Shanna L. Moore. We also wish to thank Dr. Fan Ye for help in the initial planning stages of the project. Finally, we wish to acknowledge support from the National Institutes of Health under Ruth L. Kirschstein National Research Service Awards (2T32GM008267) to M.D.W. and (F32GM122167) J.E.B., National Science Foundation grant (MCB-1517764) to M.D.W., as well as the National Science Foundation Graduate Fellowship DGE1144153 to R.P.B.
Footnotes
James E. Baker Conflicts of interest: NA.
Ryan P. Badman Conflicts of interest: NA.
Michelle D. Wang Conflicts of interest: NA.
Author Contributions: The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. J.E.B and R.P.B., and M.D.W. researched for and planned the manuscript, and drafted the manuscript. All authors edited the manuscript.
The authors declare no competing financial interest.
Contributor Information
James E. Baker, Howard Hughes Medical Institute, Department of Physics – LASSP, Cornell University, Ithaca, NY 14853
Ryan P. Badman, Department of Physics, Cornell University, Ithaca, NY 14853
Michelle D. Wang, Howard Hughes Medical Institute, Department of Physics – LASSP, Cornell University, Ithaca, NY 14853
References
- 1.Ashkin A. Acceleration and Trapping of Particles by Radiation Pressure. Physical Review Letters. 1970;24:156. [Google Scholar]
- 2.Ashkin A, Dziedzic JM, Bjorkholm JE, Chu S. Observation of a Single-Beam Gradient Force Optical Trap for Dielectric Particles. Optics Letters. 1986;11:288–290. doi: 10.1364/ol.11.000288. [DOI] [PubMed] [Google Scholar]
- 3.Svoboda K, Schmidt CF, Schnapp BJ, Block SM. Direct Observation of Kinesin Stepping by Optical Trapping Interferometry. Nature. 1993;365:721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- 4.Finer JT, Simmons RM, Spudich JA. Single Myosin Molecule Mechanics - Piconewton Forces and Nanometer Steps. Nature. 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 5.Wang MD, Schnitzer MJ, Yin H, Landick R, Gelles J, Block SM. Force and velocity measured for single molecules of RNA polymerase. Science. 1998;282:902–907. doi: 10.1126/science.282.5390.902. [DOI] [PubMed] [Google Scholar]
- 6.Cecconi C, Shank EA, Bustamante C, Marqusee S. Direct observation of the three-state folding of a single protein molecule. Science. 2005;309:2057–2060. doi: 10.1126/science.1116702. [DOI] [PubMed] [Google Scholar]
- 7.Liphardt J, Onoa B, Smith SB, Tinoco I, Bustamante C. Reversible unfolding of single RNA molecules by mechanical force. Science. 2001;292:733–737. doi: 10.1126/science.1058498. [DOI] [PubMed] [Google Scholar]
- 8.Wang MD, Yin H, Landick R, Gelles J, Block SM. Stretching DNA with optical tweezers. Biophys J. 1997;72:1335–1346. doi: 10.1016/S0006-3495(97)78780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brower-Toland BD, Smith CL, Yeh RC, Lis JT, Peterson CL, Wang MD. Mechanical disruption of individual nucleosomes reveals a reversible multistage release of DNA. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1960–1965. doi: 10.1073/pnas.022638399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizuno D, Tardin C, Schmidt CF, MacKintosh FC. Nonequilibrium mechanics of active cytoskeletal networks. Science. 2007;315:370–373. doi: 10.1126/science.1134404. [DOI] [PubMed] [Google Scholar]
- 11.Ashkin A, Dziedzic JM, Yamane T. Optical Trapping and Manipulation of Single Cells Using Infrared-Laser Beams. Nature. 1987;330:769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- 12.Ashkin A, Dziedzic JM. Optical Trapping and Manipulation of Viruses and Bacteria. Science. 1987;235:1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Rahman MA, Ohta AT. Microtechnology for Cell Manipulation and Sorting. Springer; 2017. Optical Manipulation of Cells; pp. 93–128. [Google Scholar]
- 14.Squires TM, Quake SR. Microfluidics: Fluid physics at the nanoliter scale. Reviews of Modern Physics. 2005;77:977–1026. [Google Scholar]
- 15.Whitesides GM. The origins and the future of microfluidics. Nature. 2006;442:368–373. doi: 10.1038/nature05058. [DOI] [PubMed] [Google Scholar]
- 16.Abgrall P, Gue AM. Lab-on-chip technologies: making a microfluidic network and coupling it into a complete microsystem - a review. Journal of Micromechanics and Microengineering. 2007;17:R15–R49. [Google Scholar]
- 17.Friese MEJ, Nieminen TA, Heckenberg NR, Rubinsztein-Dunlop H. Optical alignment and spinning of laser-trapped microscopic particles. Nature. 1998;394:348–350. [Google Scholar]
- 18.La Porta A, Wang MD. Optical torque wrench: Angular trapping, rotation, and torque detection of quartz microparticles. Physical Review Letters. 2004;92 doi: 10.1103/PhysRevLett.92.190801. [DOI] [PubMed] [Google Scholar]
- 19.Deufel C, Forth S, Simmons CR, Dejgosha S, Wang MD. Nanofabricated quartz cylinders for angular trapping: DNA supercoiling torque detection. Nature Methods. 2007;4:223–225. doi: 10.1038/nmeth1013. [DOI] [PubMed] [Google Scholar]
- 20.Moffitt JR, Chemla YR, Smith SB, Bustamante C. Recent advances in optical tweezers. Annual Review of Biochemistry. 2008;77:205–228. doi: 10.1146/annurev.biochem.77.043007.090225. [DOI] [PubMed] [Google Scholar]
- 21.Sasaki K, Koshioka M, Misawa H, Kitamura N, Masuhara H. Pattern-Formation and Flow-Control of Fine Particles by Laser-Scanning Micromanipulation. Optics Letters. 1991;16:1463–1465. doi: 10.1364/ol.16.001463. [DOI] [PubMed] [Google Scholar]
- 22.Grier DG, Roichman Y. Holographic optical trapping. Applied Optics. 2006;45:880–887. doi: 10.1364/ao.45.000880. [DOI] [PubMed] [Google Scholar]
- 23.Mirsaidov U, Timp W, Timp K, Mir M, Matsudaira P, Timp G. Optimal optical trap for bacterial viability. Physical Review E. 2008;78 doi: 10.1103/PhysRevE.78.021910. [DOI] [PubMed] [Google Scholar]
- 24.Curtis JE, Koss BA, Grier DG. Dynamic holographic optical tweezers. Optics Communications. 2002;207:169–175. [Google Scholar]
- 25.Gaugiran S, Getin S, Fedeli JM, Colas G, Fuchs A, Chatelain F, Derouard J. Optical manipulation of microparticles and cells on silicon nitride waveguides. Optics Express. 2005;13:6956–6963. doi: 10.1364/opex.13.006956. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt BS, Yang AHJ, Erickson D, Lipson M. Optofluidic trapping and transport on solid core waveguides within a microfluidic device. Optics Express. 2007;15:14322–14334. doi: 10.1364/oe.15.014322. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia BS, Helleso OG, Subramanian AZ, Wilkinson JS, Chen J, Chen XY. Integrated platform based on high refractive index contrast waveguide for optical guiding and sorting. Complex Light and Optical Forces Iv. 2010;7613 [Google Scholar]
- 28.Ahluwalia BS, McCourt P, Oteiza A, Wilkinson JS, Huser TR, Helleso OG. Squeezing red blood cells on an optical waveguide to monitor cell deformability during blood storage. Analyst. 2015;140:223–229. doi: 10.1039/c4an01181c. [DOI] [PubMed] [Google Scholar]
- 29.Yang AHJ, Lerdsuchatawanich T, Erickson D. Forces and Transport Velocities for a Particle in a Slot Waveguide. Nano Letters. 2009;9:1182–1188. doi: 10.1021/nl803832q. [DOI] [PubMed] [Google Scholar]
- 30.Serey X, Mandal S, Chen YF, Erickson D. DNA Transport and Delivery in Thermal Gradients near Optofluidic Resonators. Physical Review Letters. 2012;108 doi: 10.1103/PhysRevLett.108.048102. [DOI] [PubMed] [Google Scholar]
- 31.Soltani M, Lin J, Forties RA, Inman JT, Saraf SN, Fulbright RM, Lipson M, Wang MD. Nanophotonic trapping for precise manipulation of biomolecular arrays. Nature Nanotechnology. 2014;9:448–452. doi: 10.1038/nnano.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye F, Badman RP, Inman JT, Soltani M, Killian JL, Wang MD. Biocompatible and High Stiffness Nanophotonic Trap Array for Precise and Versatile Manipulation. Nano Letters. 2016;16:6661–6667. doi: 10.1021/acs.nanolett.6b03470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye F, Soltani M, Inman J, Wang MD. Tunable Nanophotonic Array Traps with Enhanced Force and Stability. Optics Express. 2017 doi: 10.1364/OE.25.007907. (in press) [DOI] [PubMed] [Google Scholar]
- 34.Erickson D, Serey X, Chen YF, Mandal S. Nanomanipulation using near field photonics. Lab on a Chip. 2011;11:995–1009. doi: 10.1039/c0lc00482k. [DOI] [PubMed] [Google Scholar]
- 35.Neuman KC, Block SM. Optical trapping. Review of Scientific Instruments. 2004;75:2787–2809. doi: 10.1063/1.1785844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pang YJ, Song HN, Kim JH, Hout XM, Cheng W. Optical trapping of individual human immunodeficiency viruses in culture fluid reveals heterogeneity with single-molecule resolution. Nature Nanotechnology. 2014;9:624–630. doi: 10.1038/nnano.2014.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawata S, Sugiura T. Movement of Micrometer-Sized Particles in the Evanescent Field of a Laser-Beam. Optics Letters. 1992;17:772–774. doi: 10.1364/ol.17.000772. [DOI] [PubMed] [Google Scholar]
- 38.Kawata S, Tani T. Optically driven Mie particles in an evanescent field along a channeled waveguide. Optics Letters. 1996;21:1768–1770. doi: 10.1364/ol.21.001768. [DOI] [PubMed] [Google Scholar]
- 39.Grujic K, Helleso OG, Hole JP, Wilkinson JS. Sorting of polystyrene microspheres using a Y-branched optical waveguide. Optics Express. 2005;13:1–7. doi: 10.1364/opex.13.000001. [DOI] [PubMed] [Google Scholar]
- 40.Khan SA, Shi Y, Chang CM, Jan C, Fan SH, Ellerbee AK, Solgaard O. Optical separation of heterogeneous size distributions of microparticles on silicon nitride strip waveguides. Optics Express. 2015;23:8855–8866. doi: 10.1364/OE.23.008855. [DOI] [PubMed] [Google Scholar]
- 41.Schein P, Ashcroft CK, O'Dell D, Adam IS, DiPaolo B, Sabharwal M, Shi C, Hart R, Earhart C, Erickson D. Near-Field Light Scattering Techniques for Measuring Nanoparticle-Surface Interaction Energies and Forces. Journal of Lightwave Technology. 2015;33:3494–3502. doi: 10.1109/JLT.2015.2440216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Dell D, Schein P, Erickson D. Simultaneous Characterization of Nanoparticle Size and Particle-Surface Interactions with Three-Dimensional Nanophotonic Force Microscopy. Physical Review Applied. 2016;6 doi: 10.1103/PhysRevApplied.6.034010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harada Y, Asakura T. Radiation forces on a dielectric sphere in the Rayleigh scattering regime. Optics Communications. 1996;124:529–541. [Google Scholar]
- 44.Ng LN, Luff BJ, Zervas MN, Wilkinson JS. Forces on a Rayleigh particle in the cover region of a planar waveguide. Journal of Lightwave Technology. 2000;18:388–400. [Google Scholar]
- 45.Yang AHJ, Moore SD, Schmidt BS, Klug M, Lipson M, Erickson D. Optical manipulation of nanoparticles and biomolecules in sub-wavelength slot waveguides. Nature. 2009;457:71–75. doi: 10.1038/nature07593. [DOI] [PubMed] [Google Scholar]
- 46.Yang AHJ, Erickson D. Optofluidic ring resonator switch for optical particle transport. Lab on a Chip. 2010;10:769–774. doi: 10.1039/b920006a. [DOI] [PubMed] [Google Scholar]
- 47.Lin SY, Schonbrun E, Crozier K. Optical Manipulation with Planar Silicon Microring Resonators. Nano Letters. 2010;10:2408–2411. doi: 10.1021/nl100501d. [DOI] [PubMed] [Google Scholar]
- 48.Lin SY, Crozier KB. Planar silicon microrings as wavelength-multiplexed optical traps for storing and sensing particles. Lab on a Chip. 2011;11:4047–4051. doi: 10.1039/c1lc20574a. [DOI] [PubMed] [Google Scholar]
- 49.Soltani M, Inman JT, Lipson M, Wang MD. Electro-optofluidics: achieving dynamic control on-chip. Optics Express. 2012;20:22314–22326. doi: 10.1364/OE.20.022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang JW, Poon AW. Unfolding a design rule for microparticle buffering and dropping in microring-resonator-based add-drop devices. Lab on a Chip. 2014;14:1426–1436. doi: 10.1039/c3lc51186c. [DOI] [PubMed] [Google Scholar]
- 51.Threm D, Nazirizadeh Y, Gerken M. Photonic crystal biosensors towards on-chip integration. Journal of Biophotonics. 2012;5:601–616. doi: 10.1002/jbio.201200039. [DOI] [PubMed] [Google Scholar]
- 52.Baker JE, Sriram R, Miller BL. Two-dimensional photonic crystals for sensitive microscale chemical and biochemical sensing. Lab on a Chip. 2015;15:971–990. doi: 10.1039/c4lc01208a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with plasmonic nanosensors. Nature Materials. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 54.Petryayeva E, Krull UJ. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing-A review. Analytica Chimica Acta. 2011;706:8–24. doi: 10.1016/j.aca.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 55.Juan ML, Righini M, Quidant R. Plasmon nano-optical tweezers. Nature Photonics. 2011;5:349–356. [Google Scholar]
- 56.Kotnala A, Gordon R. Double nanohole optical tweezers visualize protein p53 suppressing unzipping of single DNA-hairpins. Biomedical Optics Express. 2014;5:1886–1894. doi: 10.1364/BOE.5.001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al Balushi AA, Kotnala A, Wheaton S, Gelfand RM, Rajashekara Y, Gordon R. Label-free free-solution nanoaperture optical tweezers for single molecule protein studies. Analyst. 2015;140:4760–4778. doi: 10.1039/c4an02213k. [DOI] [PubMed] [Google Scholar]
- 58.Cai H, Poon AW. Optical trapping of microparticles using silicon nitride waveguide junctions and tapered-waveguide junctions on an optofluidic chip. Lab on a Chip. 2012;12:3803–3809. doi: 10.1039/c2lc40636e. [DOI] [PubMed] [Google Scholar]
- 59.Helleso OG, Lovhaugen P, Subramanian AZ, Wilkinson JS, Ahluwalia BS. Surface transport and stable trapping of particles and cells by an optical waveguide loop. Lab on a Chip. 2012;12:3436–3440. doi: 10.1039/c2lc40375g. [DOI] [PubMed] [Google Scholar]
- 60.Boerkamp M, van Leest T, Heldens J, Leinse A, Hoekman M, Heideman R, Caro J. On-chip optical trapping and Raman spectroscopy using a TripleX dual-waveguide trap. Optics Express. 2014;22:30528–30537. doi: 10.1364/OE.22.030528. [DOI] [PubMed] [Google Scholar]
- 61.Helle OI, Ahluwalia BS, Helleso OG. Optical transport, lifting and trapping of micro-particles by planar waveguides. Optics Express. 2015;23:6601–6612. doi: 10.1364/OE.23.006601. [DOI] [PubMed] [Google Scholar]
- 62.Leake KD, Olson MAB, Ozcelik D, Hawkins AR, Schmidt H. Spectrally reconfigurable integrated multi-spot particle trap. Optics Letters. 2015;40:5435–5438. doi: 10.1364/OL.40.005435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandal S, Serey X, Erickson D. Nanomanipulation Using Silicon Photonic Crystal Resonators. Nano Letters. 2010;10:99–104. doi: 10.1021/nl9029225. [DOI] [PubMed] [Google Scholar]
- 64.Chen YF, Serey X, Sarkar R, Chen P, Erickson D. Controlled Photonic Manipulation of Proteins and Other Nanomaterials. Nano Letters. 2012;12:1633–1637. doi: 10.1021/nl204561r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schein P, Kang P, O'Dell D, Erickson D. Nanophotonic Force Microscopy: Characterizing Particle-Surface Interactions Using Near-Field Photonics. Nano Letters. 2015;15:1414–1420. doi: 10.1021/nl504840b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mirsadeghi SH, Young JF. Ultrasensitive Diagnostic Analysis of Au Nanoparticles Optically Trapped in Silicon Photonic Circuits at Sub-Milliwatt Powers. Nano Letters. 2014;14:5004–5009. doi: 10.1021/nl501424d. [DOI] [PubMed] [Google Scholar]
- 67.Renaut C, Dellinger J, Cluzel B, Honegger T, Peyrade D, Picard E, de Fornel F, Hadji E. Assembly of microparticles by optical trapping with a photonic crystal nanocavity. Applied Physics Letters. 2012;100 [Google Scholar]
- 68.Descharmes N, Dharanipathy UP, Diao Z, Tonin M, Houdre R. Single particle detection, manipulation and analysis with resonant optical trapping in photonic crystals. Lab on a Chip. 2013;13:3268–3274. doi: 10.1039/c3lc50447f. [DOI] [PubMed] [Google Scholar]
- 69.Descharmes N, Dharanipathy UP, Diao ZL, Tonin M, Houdre R. Observation of Backaction and Self-Induced Trapping in a Planar Hollow Photonic Crystal Cavity. Physical Review Letters. 2013;110 doi: 10.1103/PhysRevLett.110.123601. [DOI] [PubMed] [Google Scholar]
- 70.van Leest T, Caro J. Cavity-enhanced optical trapping of bacteria using a silicon photonic crystal. Lab on a Chip. 2013;13:4358–4365. doi: 10.1039/c3lc50879j. [DOI] [PubMed] [Google Scholar]
- 71.Tardif M, Jager JB, Marcoux PR, Uchiyamada K, Picard E, Hadji E, Peyrade D. Single-cell bacterium identification with a SOI optical microcavity. Applied Physics Letters. 2016;109 [Google Scholar]
- 72.Tonin M, Mor FM, Forró L, Jeney S, Houdré R. Thermal fluctuation analysis of singly optically trapped spheres in hollow photonic crystal cavities. Applied Physics Letters. 2016;109:241107. [Google Scholar]
- 73.Renaut C, Cluzel B, Dellinger J, Lalouat L, Picard E, Peyrade D, Hadji E, de Fornel F. On chip shapeable optical tweezers. Scientific Reports. 2013;3 doi: 10.1038/srep02290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Righini M, Ghenuche P, Cherukulappurath S, Myroshnychenko V, de Abajo FJG, Quidant R. Nano-optical Trapping of Rayleigh Particles and Escherichia coli Bacteria with Resonant Optical Antennas. Nano Letters. 2009;9:3387–3391. doi: 10.1021/nl803677x. [DOI] [PubMed] [Google Scholar]
- 75.Wong HMK, Righini M, Gates JC, Smith PGR, Pruneri V, Quidant R. On-a-chip surface plasmon tweezers. Applied Physics Letters. 2011;99 [Google Scholar]
- 76.Wang K, Schonbrun E, Steinvurzel P, Crozier KB. Trapping and rotating nanoparticles using a plasmonic nano-tweezer with an integrated heat sink. Nature Communications. 2011;2 doi: 10.1038/ncomms1480. [DOI] [PubMed] [Google Scholar]
- 77.Tsai WY, Huang JS, Huang CB. Selective Trapping or Rotation of Isotropic Dielectric Microparticles by Optical Near Field in a Plasmonic Archimedes Spiral. Nano Letters. 2014;14:547–552. doi: 10.1021/nl403608a. [DOI] [PubMed] [Google Scholar]
- 78.Lin PT, Chu HY, Lu TW, Lee PT. Trapping particles using waveguide-coupled gold bowtie plasmonic tweezers. Lab on a Chip. 2014;14:4647–4652. doi: 10.1039/c4lc00731j. [DOI] [PubMed] [Google Scholar]
- 79.Cheng C, Xu XH, Lei HX, Li BJ. Plasmon-assisted trapping of nanoparticles using a silver-nanowire-embedded PMMA nanofiber. Scientific Reports. 2016;6 doi: 10.1038/srep20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herzog JB, Knight MW, Natelson D. Thermoplasmonics: Quantifying Plasmonic Heating in Single Nanowires. Nano Letters. 2014;14:499–503. doi: 10.1021/nl403510u. [DOI] [PubMed] [Google Scholar]
- 81.Grujic K, Helleso OG. Dielectric microsphere manipulation and chain assembly by counter-propagating waves in a channel waveguide. Optics Express. 2007;15:6470–6477. doi: 10.1364/oe.15.006470. [DOI] [PubMed] [Google Scholar]
- 82.Kuhn S, Measor P, Lunt EJ, Phillips BS, Deamer DW, Hawkins AR, Schmidt H. Loss-based optical trap for on-chip particle analysis. Lab on a Chip. 2009;9:2212–2216. doi: 10.1039/b900555b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang K, Schonbrun E, Steinvurzel P, Crozier KB. Scannable Plasmonic Trapping Using a Gold Stripe. Nano Letters. 2010;10:3506–3511. doi: 10.1021/nl101653n. [DOI] [PubMed] [Google Scholar]
- 84.Hochberg M, Baehr-Jones T. Towards fabless silicon photonics. Nature Photonics. 2010;4:492–494. [Google Scholar]
- 85.Optofluidics. [Accessed March 1]; Available at: http://opfluid.com/
- 86.Rampini S, Li P, Lee GU. Micromagnet arrays enable precise manipulation of individual biological analyte-superparamagnetic bead complexes for separation and sensing. Lab on a Chip. 2016;16:3645–3663. doi: 10.1039/c6lc00707d. [DOI] [PubMed] [Google Scholar]
- 87.Dhakal A, Subramanian AZ, Wuytens P, Peyskens F, Le Thomas N, Baets R. Evanescent excitation and collection of spontaneous Raman spectra using silicon nitride nanophotonic waveguides. Optics Letters. 2014;39:4025–4028. doi: 10.1364/OL.39.004025. [DOI] [PubMed] [Google Scholar]
- 88.Chen SM, Li W, Wu J, Jiang Q, Tang MC, Shutts S, Elliott SN, Sobiesierski A, Seeds AJ, Ross I, et al. Electrically pumped continuous-wave III-V quantum dot lasers on silicon. Nature Photonics. 2016;10:307. [Google Scholar]
- 89.Tang SKY, Li ZY, Abate AR, Agresti JJ, Weitz DA, Psaltis D, Whitesides GM. A multi-color fast-switching microfluidic droplet dye laser. Lab on a Chip. 2009;9:2767–2771. doi: 10.1039/b914066b. [DOI] [PubMed] [Google Scholar]
- 90.Measor P, Seballos L, Yin DL, Zhang JZ, Lunt EJ, Hawkins AR, Schmidt H. On-chip surface-enhanced Raman scattering detection using integrated liquid-core waveguides. Applied Physics Letters. 2007;90 [Google Scholar]
- 91.Evans CC, Liu CY, Suntivich J. TiO2 Nanophotonic Sensors for Efficient Integrated Evanescent Raman Spectroscopy. Acs Photonics. 2016;3:1662–1669. [Google Scholar]
- 92.Dhakal A, Peyskens F, Clemmen S, Raza A, Wuytens P, Zhao HL, Le Thomas N, Baets R. Single mode waveguide platform for spontaneous and surface-enhanced on-chip Raman spectroscopy. Interface Focus. 2016;6 doi: 10.1098/rsfs.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]