Abstract
Dopamine signals mainly through D1 receptors (D1Rs) and D2Rs, and D1R- or D2R-expressing neurons contribute to distinct reward and addictive behaviors. Traditionally, transgenic mice expressing green fluorescent protein (GFP) under D1R or D2R promoters are used for fluorescent verification in electrophysiology studies, whereas Cre mice are employed for behavioral research. However, it is unknown whether the same neuronal populations are targeted in GFP and Cre mice. Additionally, while D1Rs and D2Rs are known to be expressed in different striatal neurons, their expression patterns outside the striatum remain unclear. The present study addressed these two questions by using several transgenic mouse lines expressing fluorescent proteins (GFP or tdTomato) or Cre under the control of D1R or D2R promoters. We found a high degree of overlap between GFP- and Cre-positive neurons in the striatum and hippocampus. Additionally, we discovered that D1Rs and D2Rs were highly segregated in the orbitofrontal cortex, prefrontal cortex, dorsal and ventral hippocampus, and amygdala: ~ 4–34% of neurons co-expressed these receptors. Importantly, slice electrophysiological studies demonstrated that D1R-positive and D1R-negative hippocampal neurons were functionally distinct in a mouse line generated by crossing Drd1a-Cre mice with a Cre reporter Ai14 line. Lastly, we discovered that chronic alcohol intake differentially altered D1R-positive and D2R-positive neuron excitability in the ventral CA1. These data suggest that GFP and Cre mice target the same populations of striatal neurons, D1R- or D2R-expressing neurons are highly segregated outside the striatum, and these neurons in the ventral hippocampal may exert distinct roles in alcohol addiction.
Keywords: Dopamine D1 receptor, dopamine D2 receptor, striatum, cortex, hippocampus, alcohol
INTRODUCTION
Dopaminergic signaling in the central nervous system plays critical roles in regulating several aspects of brain function, including motor behavior, motivation, learning and memory (Schultz, 2007). Altered dopaminergic transmission is the common mechanism underlying drug and alcohol abuse (Hyman et al., 2006; Koob and Volkow, 2010; Luscher and Malenka, 2011). Dopamine effects are determined by their receptors presented on the target neuron. The brain contains two major groups of dopamine receptors, which exert opposing actions: the excitatory D1 receptor (D1R) and the inhibitory D2 receptor (D2R) (Beaulieu and Gainetdinov, 2011). D1Rs and D2Rs are highly expressed by medium spiny neurons (MSNs) of the striatum (Beaulieu and Gainetdinov, 2011; Calabresi et al., 2014; Gerfen and Surmeier, 2011; Kreitzer and Malenka, 2008). The D1R-expressing MSNs (dMSNs) that project directly to the substantia nigra pars reticulata (SNr) form the direct-pathway; D2R-expressing MSNs (iMSNs) give rise to the indirect pathway, which projects to the external part of the globus pallidus (Gerfen and Surmeier, 2011; Kreitzer and Malenka, 2008). Until recently, the functions and roles of dMSNs and iMSNs in drug and alcohol addiction had remained elusive. However, novel techniques have enabled cell type-specific access to these neurons and have thus advanced the field by providing a more comprehensive understanding of these two pathways. These techniques include the use of fluorescent reporter mice expressing green fluorescent protein (GFP) and of transgenic mice expressing Cre-recombinase, under the control of the D1R or D2R promoter. Previous electrophysiological studies predominantly used Drd1a-eGFP (D1-GFP) and Drd2a-eGFP (D2-GFP) mice to selectively visualize dMSNs and iMSNs, respectively; these found enhanced glutamatergic input onto dMSNs following cocaine or alcohol exposure (Gong et al., 2003; Kim et al., 2011; Pascoli et al., 2012; Valjent et al., 2009; Wang et al., 2015). Meanwhile, behavioral studies often selectively manipulated MSN activities using Drd1a-Cre (D1-Cre) and Drd2-Cre (D2-Cre) mice, and showed that chemogenetic or optogenetic suppression of dMSNs in D1-Cre mice reduced cocaine or alcohol-related behaviors (Gong et al., 2007; Pascoli et al., 2012; Wang et al., 2015). To link these electrophysiological and behavioral results, it is important to clarify whether both studies targeted the same neuronal populations.
In addition to the striatum, which is the major area involved in drug addiction (Everitt and Robbins, 2013; Yager et al., 2015), dopamine receptors are also expressed in other addiction-related brain areas such as the cortex, hippocampus, and basolateral amygdala (BLA) (Koob and Volkow, 2010; Volkow and Morales, 2015). Although dopamine receptors are widely distributed in the brain, the receptor subtype densities vary between different areas. An understanding of these expression patterns provides novel insights into neural circuits involved in addiction.
To examine D1R and D2R expression patterns both in the striatum and other brain regions, we generated a range of double and triple transgenic mice where D1Rs and/or D2Rs were labelled using a fluorescent protein and/or Cre. We found that D1-GFP- and D2-Cre (tdTomato)-positive neurons overlapped in the striatum, as did D2-GFP- and D2-Cre (tdTomato)-positive neurons. Outside the striatum, triple transgenic models showed distinct patterns, which clear segregation of D1R-positive (D1R+) and D2R-positive (D2R+) neurons in the cortex, hippocampus, and BLA. We found that spike firing in hippocampal D1R+ and D2R+ neurons are differentially regulated by chronic alcohol consumption. The findings of this study provide a solid foundation for the study of D1R- and D2R-expressing neurons in the central nervous system and in drug addition.
MATERIALS AND METHODS
See Supporting Information for futher details
Reagents
Reagents were obtained from Sigma or Life technologies.
Animal breeding and genotyping
D1-tdTomato, D1-Cre, D2-eGFP, and D2-Cre mice were obtained from Mutant Mouse Regional Resource Centers. Ai6, Ai14, Snap25, and C57BL/6 mice were purchased from The Jackson Laboratory. Male and female 2–3-month-old mice were used in this study. All animal care and experimental procedures were approved by the Texas A&M University Institutional Animal Care and Use Committee.
Histology and cell counting
Fluorescent images were acquired using a confocal microscope (Fluorview-1200, Olympus) and analyzed using Imaris 8.3.1 (Bitplane, Switzerland), as previously reported (Cheng et al., 2016). Cell counting was performed in 11 triple transgenic mice (D1-tdTomato;D2-Cre;Snap25).
Behavioral test and the intermittent-access 20% alcohol 2-bottle-choice drinking procedure
D1-GFP mice and D1-Cre;Ai14 mice were tested for locomotor activity in an open field box, as previously described (Cheng et al., 2016). Male D1-Cre;Ai14 and D2-Cre;Ai14 mice were trained to consume 20% alcohol for 8 weeks using the intermittent access 2-bottle-choice drinking process as previously described (Cheng et al., 2016; Wang et al., 2015).
Slice electrophysiology
Slice preparation and electrophysiological recordings have been described previously (Cheng et al., 2016; Wang et al., 2015). Slices were maintained in external solution containing (in mM): 125 NaCl, 4.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1.25 NaH2PO4, 25 NaHCO3, 15 sucrose and 15 glucose. The electrodes containing (in mM): 123 potassium gluconate, 10 HEPES, 0.2 EGTA, 8 NaCl, 2 MgATP, 0.3 NaGTP, pH 7.2–7.3, corresponding to an osmolarity of ~280 mOsm.
Statistical analysis
Data were analyzed using a t test or two-way ANOVA with repeated measurement (Two-Way RM ANOVA).
RESULTS
Co-localization of GFP-positive and Cre-positive neurons in triple transgenic mice
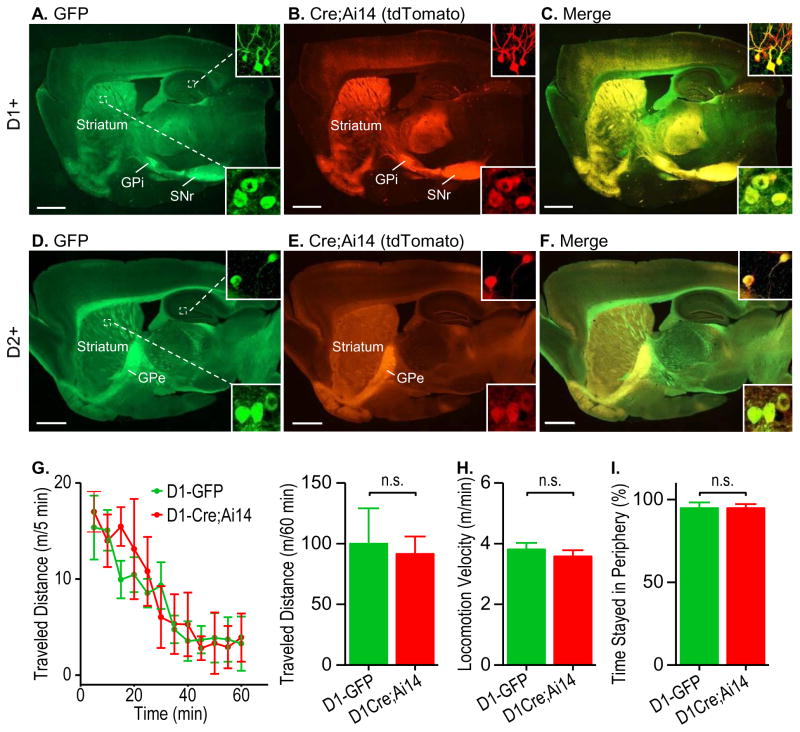
To compare the dMSNs identified in GFP and Cre mice, we studied D1-Cre;Ai14;D1-GFP mice. The Ai14 Cre reporter line expresses the red fluorescent protein, tdTomato, in the presence of Cre (Madisen et al., 2010). Two-month-old transgenic mice were perfused, sagittal sections were prepared, and fluorescent images were obtained. GFP-positive green fluorescent neurons were mainly located in the striatum and projected to the internal segment of the globus pallidus (GPi) and the SNr (Fig. 1A); the same Cre-positive neuron pattern was observed, as indicated by the red fluorescence (Fig. 1B). These findings were consistent with those of previous studies (Cheng et al., 2016; Gong et al., 2003). We also observed GFP and Cre expression in the hippocampus (Figs. 1A and 1B). Importantly, we found a close overlap of the GFP-positive and Cre-positive neurons in the striatum and hippocampus (Fig. 1C). These results suggest that the fluorescent neurons in D1-GFP mice and the Cre-positive neurons in D1-Cre mice represent the same neuronal population.
Figure 1. GFP-positive and Cre-positive mice showed highly similar histology and behavior.
(A–C) Representative dual-channel fluorescent images of a sagittal brain section of a D1-GFP;D1-Cre;Ai14 mouse showing D1R-driven GFP (A, green), Cre (B, red, tdTomato), and their co-localization (C, yellow). Note that both the green and red fluorescence was detected predominantly in the striatum and the target areas of D1R+ neurons: the internal part of globus pallidus (GPi) and substantia nigra pars reticulata (SNr). Insets: enlarged images of striatal (bottom right) and hippocampal (top right) neurons. Images are representative of results from three mice. (D–F) GFP- and Cre-positive neurons also showed significant co-localization in the D2-GFP;D2-Cre;Ai14 mouse brain. Note that both the green and red fluorescence was mainly detected in the striatum and the target area of D2R+ neurons: the external segment of the globus pallidus (GPe). (G) Locomotor activity did not differ between D1-GFP mice and D1-Cre;Ai14 mice. Left, The time course of distance traveled by the indicated mice within 60 min. Right, Bar graphs summarizing the total distance travelled in 60 min. n.s., not significant, p > 0.05. n = 5 mice/group. (H–I) Locomotion velocity (H) and time spent at periphery (I) were identical for D1-GFP and D1-Cre;Ai14 mice. n.s., p > 0.05. n = 5 mice/group. n = 5 mice/group. Scale: 1 mm (A–F).
To compare the iMSNs identified in GFP and Cre mice, we studied D2-GFP;D2-Cre;Ai14 mice. We found that GFP-positive green fluorescent neurons were located in the striatum and projected to the external segment of the globus pallidus (Fig. 1D), as did the Cre-positive red fluorescent neurons (Fig. 1E). Both green and red fluorescent neurons were also found in the hippocampus (Figs. 1D and 1E). Furthermore, we discovered a high degree of overlap of GFP- and Cre-positive neurons in the striatum and hippocampus (Fig. 1F). These results suggest that the GFP-positive neurons in D2-GFP mice and the Cre-positive neurons in D2-Cre mice represent the same neuronal population.
Next, we compared the behaviors of these two types of transgenic mice. Open field tests found no differences in the locomotor activities of D1-GFP mice and D1-Cre;Ai14 mice (Fig. 1G). They travelled the same distance during a 60-min testing session (Fig. 1H; t(8) = 0.26, p > 0.05); the travel speed was around 4 m/min for both groups (Fig. 1I; t(8) = 0.80, p > 0.05); and they spent the same time at the periphery (Fig. 1J; t(8) = 0.01, p > 0.05). These results suggest that these two lines of transgenic mice targeting dMSNs showed similar locomotor activities.
Distinct distribution patterns of D1R+ and D2R+ neurons in the cortex
In addition to regulating striatal activity, dopamine signaling also regulates cortical function (e.g., Chandler et al., 2014; Gao et al., 2001; Li et al., 2011; Tritsch and Sabatini, 2012), and the cortex has relatively high expression levels of dopamine receptors (Beaulieu and Gainetdinov, 2011; Tritsch and Sabatini, 2012). However, the detailed cortical expression patterns of D1Rs and D2Rs are not clear. Cortical GFP expression in D1-GFP or D2-GFP mice is weaker than that observed in the striatum (Gong et al., 2003; Valjent et al., 2009) and this has limited studies of D1R- and D2R-expressing cortical neurons. The development of D1- and D2-Cre mice (Gong et al., 2007) and strong Cre reporter lines (Madisen et al., 2010) provides an opportunity to elucidate cortical D1R- and D2R-expressing neurons more clearly.
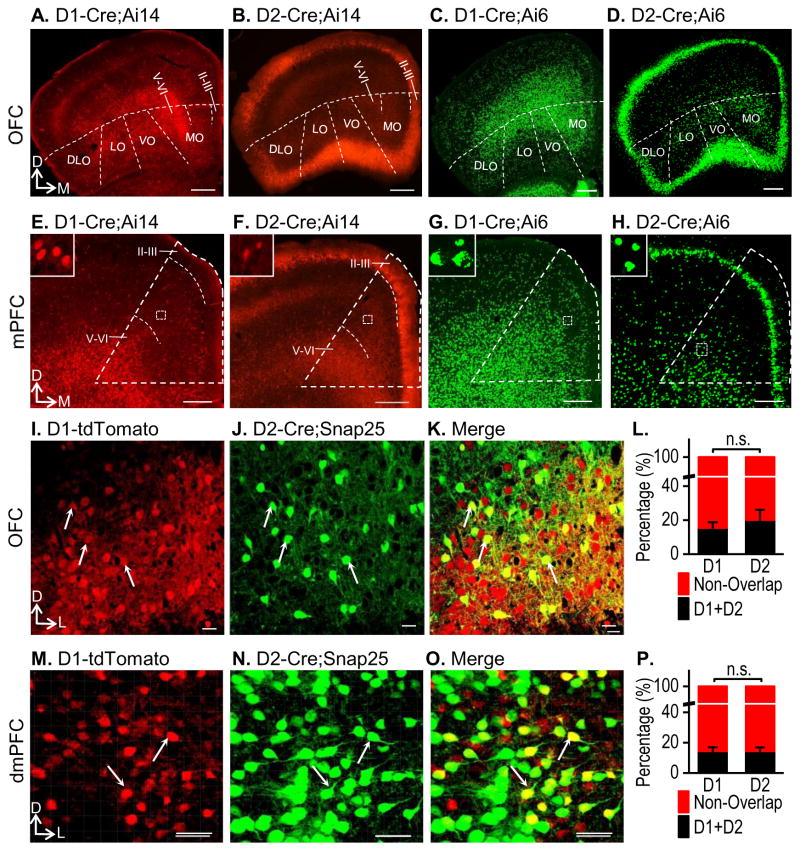
First, we examined the cortical distribution patterns of D1Rs or D2Rs in D1- or D2-Cre mice that were crossed with either Ai14 or Ai6 mice. Ai6 is a Cre reporter line that expresses the green fluorescent protein, ZsGreen, in the presence of Cre (Madisen et al., 2010). We used this line because it shows very strong fluorescence, mainly in the soma (Madisen et al., 2010), which facilitates sensitive neuronal detection. We targeted two addiction-related cortical areas: the orbital frontal cortex (OFC) and the medial prefrontal cortex (mPFC) (Everitt and Robbins, 2013; Koob and Volkow, 2010; Luscher and Malenka, 2011). We found that D1R+ neurons were mainly present in the deep layers (V–VI) of the OFC (Fig. 2A), while D2R expression was detected in the more superficial cortical layers (Fig. 2B). These different expression patterns were clear in D1-Cre;Ai6 (Fig. 2C) and D2-Cre;Ai6 (Fig. 2D) mice. Examination of D1R and D2R expression in the mPFC using the same reporters showed similar patterns (Figs. 2E–2H). D1R+ neurons were scattered mainly in the deep interior cortex (Figs. 2E and 2G), while D2R expression remained highly constrained to the superficial layers II–III of the mPFC (Figs. 2F and 2H). These results suggest that a considerable number of D1R+ and D2R+ neurons are present in diverse areas of the cortex, where D1Rs are primarily expressed in the deep layers, and D2Rs are predominantly found in the superficial layers.
Figure 2. Distinct expression patterns of D1Rs and D2Rs in the cortex.
(A–D) Fluorescent images of the OFC in D1- or D2-Cre mice that were crossed with Ai14 (tdTomato) or Ai6 (ZsGreen), as indicated, are shown. D1R+ neurons were located primarily in the deeper layers (V–VI) (A, C), while D2R+ neurons were in more superficial layers of the OFC (II–III) (B, D). OFC subregions are indicated: DLO, dorsolateral orbital cortex; LO, lateral orbital cortex; VO, ventral orbital cortex; MO, medial orbital cortex. Images show representative results from three mice. (E–H) mPFC images of the same animals used to analyze the OFC are shown, with the mPFC outlined by a dashed triangle. D1R+ neurons were located primarily in the deeper layers of the mPFC (E, G), whereas D2R+ neurons were mostly in the superficial layers of the mPFC, with some scattered in the deeper layers (F, H). Enlarged images of the dashed boxes are shown at the top left. (I–L) Co-localization of D1Rs and D2Rs in the OFC of D1-tdTomato;D2-Cre;Snap25 mice. Representative images show the D1R+ neurons (I, red), the D2R+ neurons (J, green), and some co-localization (K, yellow), as indicated by the arrows. Note that the percentage of overlapped D1R+ and D2R+ neurons did not differ (L). n.s., p > 0.05, n = 12 sections from 3 mice per group. (M–P) Co-localization of D1R- and D2R-expressing neurons in the dorsal mPFC (dmPFC) of D1-tdTomato;D2-Cre;Snap25 mice. The percentages of co-localization of D1R- and D2R-expressing neurons were identical (P). n.s., p > 0.05. n = 23 sections from 6 mice per group. D, dorsal; M, medial; L, lateral. Scale: 300 μm (A–D), 200 μm (E–H), 10 μm (I–K), 30 μm (M–O).
Co-localization of D1Rs and D2Rs has been reported in the nucleus accumbens (Bertran-Gonzalez et al., 2008; Kreitzer, 2009). It is unclear whether cortical D1Rs and D2Rs also co-localize, although electrophysiological recording has shown that some cortical neurons potentially respond to both D1R or D2R agonists (Li et al., 2011; Li et al., 2012). We thus examined whether there was any overlap of these neuronal types in the OFC or mPFC. To test this, we crossed D1-tdTomato mice, D2-Cre mice, and Snap25 mice to obtain the triple transgenic line, D1-tdTomato;D2-Cre;Snap25. In this line, tdTomato expression was driven by D1Rs and all red neurons were therefore D1R+ (Fig. 2I). Snap25 is a Cre reporter line that expresses GFP after Cre recombination (Madisen et al., 2015); D2R+ neurons were thus green due to their D2R-driven Cre expression (Fig. 2J). We selected the Snap25 line instead of the Ai6 line because the GFP was evenly expressed in the soma and dendrites in Snap25 mice after recombination; this helps to verify co-localization (Fig. 2K). We found that in layers III–IV of the OFC, 15.42% of the D1R+ neurons also expressed D2Rs, whereas 20.16% of the D2R+ neurons also expressed D1Rs (Figs. 2I–2K). These overlap rates were not significantly different (Fig. 2L; t(22) = −0.69, p > 0.05). In addition, we found that in male animals nearly 14.35% of D1R+ neurons in the transition area of layers II and V/VI of the dorsal mPFC (dmPFC) also expressed D2Rs, while 14.47% of D2R+ neurons expressed D1Rs in this region (Figs. 2M–2O and Table S1). These overlap rates were not significantly different (Fig. 2P; t(44) = −0.03, p > 0.05). Furthermore, we counted neurons in the superficial and deep layers and found that while no difference was detected in layer V/VI, the overlapping rate of D2R+ neurons was significantly lower than that of D1R+ neurons in layer II (Table S2; t(20) = −1.31, p > 0.05 for layer V/VI; t(20) = 2.79, p < 0.05 for layer II). Additionally, the overlapping rate of D1R+ neurons was identical in both layers II and V/VI, whereas the overlapping rate of D2R+ neurons was significantly lower in layer II than layer V/VI (Table S2; t(20) = −0.27, p > 0.05 for D1R+ neurons; t(20) = −4.07, p < 0.001 for D2R+ neurons). We also examined D1R+ and D2R+ neurons in the ventral mPFC (vmPFC) and found that the overlapping rates of both neuronal types were idential (Fig. S1; t(28) = 1.71, p > 0.05). Interestingly, the overlapping rates of both D1R+ and D2R+ neurons were significantly higher in the vmPFC than in the dmPFC (D1: 33.47 ± 3.30% vs. 14.35 ± 2.62%, t(36) = 4.55, p < 0.001; D2: 26.02 ± 2.82% vs. 14.47 ± 2.41, t(36) = 3.08, p < 0.01). Lastly, we did not observed sex difference in the dmPFC (Table S1). These findings suggest that although some mPFC and OFC neurons express both D1Rs and D2Rs, D1R+ and D2R+ neurons are generally segregated in the cortex.
Distinct expression patterns of D1Rs and D2Rs in the dorsal hippocampus
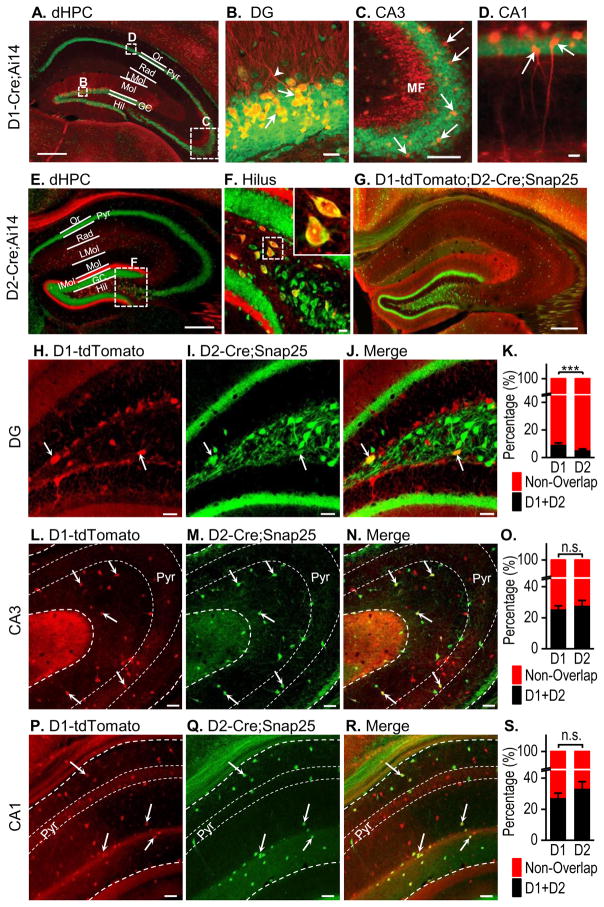
Dopaminergic inputs have been identified in the hippocampus (e.g., Du et al., 2016). Hippocampal expression of D1Rs and D2Rs has also been discovered in D1- and D2-GFP mice using immunohistochemical methods (Gangarossa et al., 2012). We re-examined D1R and D2R expression in the dorsal hippocampus using the D1- or D2-Cre;Ai14 mice because these show strong tdTomato expression; this removes the necessity for immunohistochemical enhancement of the fluorescent protein, which may not be possible in studies requiring live tissue imaging and identification (e.g., electrophysiological studies). As reported previously (Gangarossa et al., 2012), D1Rs were highly expressed in dentate granule cells (Figs. 3A and 3B), which axons formed mossy fibers that projected to the CA3 area (Fig. 3C). A few pyramidal neurons in the CA3 (Fig. 3C) and CA1 (Fig. 3D) were also D1R+. In contrast, D2Rs were mainly expressed in hilar mossy cells (Figs. 3E and 3F), which axons projected to the inner third molecular layer (Figs. 3E and 3F). We also examined the overlap between D1R and D2R expression in the sub-regions of the dorsal hippocampus using the D1-tdTomato;D2-Cre;Snap25 mice (Fig. 3G). In the dentate gyrus, neurons containing both D1Rs and D2Rs were observed most frequently in the hilus (Figs. 3H–3J). Interestingly, 9.76 ± 0.90% of D1R+ neurons expressed D2Rs and this rate was significantly higher than the 5.79 ± 0.52% of D2R+ neurons that expressed D1Rs (Fig. 3K; t(44) = 3.81, p < 0.001). The different overlap rates reflected the relative levels of positive neurons found in this area; 38.15 ± 2.53% of the total counted neuronal population were D1R+, significantly fewer than the 61.85 ± 2.53% that were D2R+ (t(44) = −6.62, p < 0.001). In the CA3, neurons expressing both D1Rs and D2Rs were mainly found in non-pyramidal cell layers, suggesting that they were probably interneurons (Figs. 3L–3N). Co-localization was fairly common, with 25.98% of D1R+ neurons expressing D2Rs and 28.28% of D2R+ neurons expressing D1Rs. These two percentages were not significantly different (Fig. 3O; t(22) = −0.69, p > 0.05). In the CA1, neurons expressing both D1Rs and D2Rs were also located outside the pyramidal layers (Figs. 3P–3R), and co-localization was common, with 27.71% of D1R+ neurons expressing D2Rs and 33.71% of D2R+ cells expressing D1Rs. These ratios were not significantly different (Fig. 3S; t(26) = −1.26, p > 0.05). We also examined the overlapping rates of D1R+ and D2R+ neurons in female dorsal dentate gyrus (dDG) and did not observe any difference between these two neuronal types (Table S1). There was no sex difference in overlapping rates of D1R+ or D2R+ neurons within the dDG. Together, these results suggest that D1R- and D2R-expressing neurons in the dorsal hippocampus are highly segregated, with some overlap in the hilus and non-pyramidal cell layers of the CA3 and CA1 regions.
Figure 3. Separation of D1R- and D2R-expressing neurons in the dorsal hippocampus.
D1-Cre;Ai14 (A–D), D2-Cre;Ai14 (E–F), and D1-tdTomato;D2-Cre;Snap 25 mice (G–R) were used. (A) Representative fluorescent image of a dorsal hippocampal (dHPC) section from a D1-Cre;Ai14 mouse, counter-stained with NeuroTrace green. The dHPC layers are labeled as Or (stratum oriens), Pyr (pyramidal cell layer), Rad (stratum radiatum), LMol (stratum lacunocum-molecularis), Mol (stratum molecularis), GC (granule cell layer), and Hil (hilus). Boxes indicate the zones shown in B–D at a higher magnification, where arrows indicate D1R+ granule cells (yellow) and dendrites (red) in the dentate gyrus (B), in the CA3 region (C), where the pyramidal cell bodies (green) and the mossy fibers (MF, red) are visible, and in the CA1 region (D), showing expression of D1Rs in some pyramidal neurons (arrows). (E) Representative fluorescent image of a dHPC section from a D2-Cre;Ai14 mouse, with the boxed sub-region of the dentate gyrus shown in panel F. (F) Enlarged image of the D2-Cre;Ai14 hilus with an inset showing two D2-Cre;Ai14-expressing neurons. (G) Representative fluorescent image of the dHPC from a D1-tdTomato;D2-Cre;Snap25 mouse. (H–J) Representative dual-channel fluorescent images of the D1-tdTomato;D2-Cre;Snap25 dentate gyrus with arrows indicating D1R+ neurons expressing tdTomato (H), D2R+ neurons expressing GFP (I), and some cells expressing both D1Rs and D2Rs (J). (K) Bar graph summarizing the overlap of D1R+ and D2R+ neurons in the dentate gyrus. **p < 0.01. n = 23 sections from 7 mice per group. (L–N) Representative images of the D1-tdTomato;D2-Cre;Snap25 CA3 area. Arrows indicate several neurons showing co-localization and these data are summarized in (O). n.s., p > 0.05. n = 12 sections from 5 mice per group. (P–R) Representative fluorescent images of the D1-tdTomato;D2-Cre;Snap25 CA1 area, with arrows indicating the neurons showing co-localization, as summarized in (S). n.s., p > 0.05. n = 14 sections from 6 mice per group. Scale: 300 μm (A, E, and G), 20 μm (B–D and F), 30 μm (H–J, L–N, and P–R).
Different distribution patterns of D1Rs and D2Rs in the ventral hippocampus
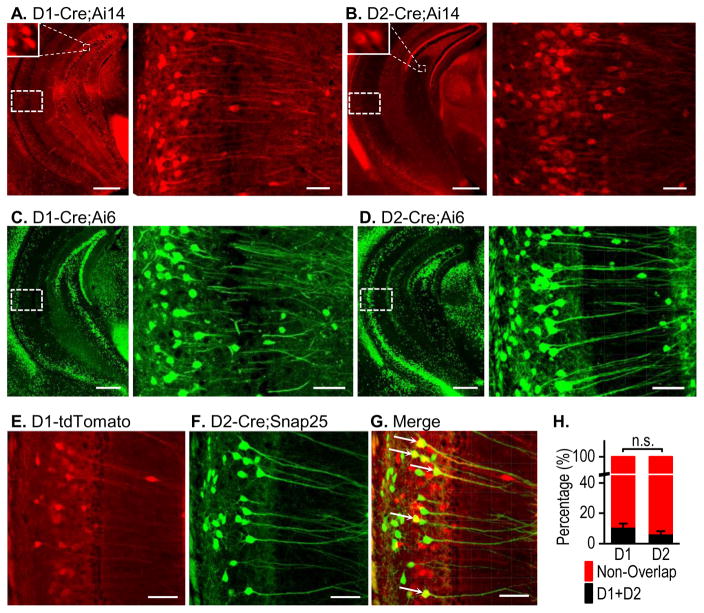
The ventral hippocampus plays a very important role in addiction and other psychiatric disorders that involve dopamine signaling (Bagot et al., 2015; Gunaydin and Kreitzer, 2016; Sesack and Grace, 2009). Therefore, we examined D1R and D2R distribution in this region. Consistent with their distribution patterns in the dorsal hippocampus, D1Rs were located in dentate granule cells (Fig. 4A, left), and D2Rs were found in the hilus (Fig. 4B, left). Interestingly, we observed high expression levels of both receptors in pyramidal cells of the CA1 and subiculum areas (Figs. 4A, right and 4B, right). To confirm these results, we also examined expression using D1- or D2-Cre;Ai6 mice. This produced very similar results as the D1-/D2-Cre;Ai14 study (Figs. 4C and 4D), where both D1Rs and D2Rs were clearly expressed in the pyramidal cell dendrites. Since both receptor types were highly expressed in the ventral hippocampus, we explored their co-localization using triple transgenic D1-tdTomato;D2Cre;Snap25 mice. Surprisingly, we did not observe very high co-expression of D1Rs (Figs. 4E and 4G) and D2Rs (Figs. 4F and 4G) in the CA1 or subiculum. The co-localization rates were 11.10 ± 2.08% for D1Rs and 6.77 ± 1.44% for D2Rs, and these were not significantly different (Fig. 4H; t(54) = 1.71, p > 0.05). We also examined the overlapping rates of D1R+ and D2R+ neurons in female ventral CA1 and observed a greater overlapping rate of D1 neurons than that of D2R+ neurons (Table S1; t(34) = 3.35, p < 0.01). However, there is no gender difference in D1R+ or D2R+ neurons (Table S1). These results indicate that although both D1Rs and D2Rs show some co-localization in the ventral hippocampus, they are highly segregated in the CA1 and subiculum areas.
Figure 4. Distinct expression patterns of D1Rs and D2Rs in the ventral hippocampus.
Representative fluorescent images of the ventral hippocampus of the indicated mice, showing D1R expression (A and C) and D2R expression (B and D) in the indicated mice. Top left insets show enlarged images of positive granule cells. The right-hand panels show an enlarged image of the CA1 region indicated by the dashed box on the left panel. Note the strong expression in the pyramidal neuron soma and dendrites. (E–G) Dual-channel fluorescent images of the ventral hippocampal CA1 area from a D1-tdTomato;D2-Cre;Snap25 mouse showing D1R expression in red (E), D2R expression in green (F), and co-localization in yellow (G). (H) Bar graph summarizing the co-localization of D1Rs and D2Rs in the ventral hippocampus. n,s., p > 0.05. n = 28 sections from 7 mice per group. Scale bars: 500 μm (A–D, left), 50 μm (A–D, right), and 70 μm (E–G).
Distinct expression patterns of D1Rs and D2Rs in the amygdala
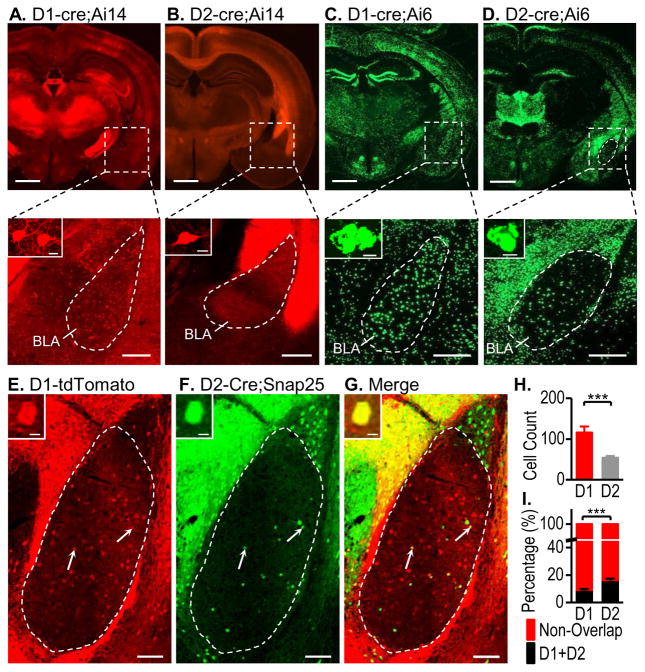
The amygdala receives dopaminergic inputs (Belujon and Grace, 2011; Koob and Volkow, 2010), but its detailed D1R and D2R expression patterns remain unclear. Thus, we investigated D1- or D2-Cre;Ai14 mice and detected a relatively high expression of D1Rs in the BLA (Fig. 5A), but weak D2R expression in this region (Fig. 5B). To further verify this finding, we compared the BLA of D1- and D2-Cre;Ai6 mice, where ZsGreen produced a much stronger signal than the tdTomato present in Ai14 mice (Madisen et al., 2010). This approach also revealed strong expression of D1Rs in the BLA (Figs. 5C), and few D2R+ neurons (Fig. 5D). These results indicate that D1R+ neurons predominate in the BLA and that D2R+ neurons are less common.
Figure 5. Distinct expression patterns of D1Rs and D2Rs in the amygdala.
Relatively high levels of D1R expression (A and C) and low levels of D2R expression (B and D) were observed in coronal sections showing the amygdala of the indicated mice (top panels). The BLA (boxed area) is enlarged in the lower panel, where the top left inset shows positive neurons. Note that red fluorescence (tdTomato) was visualized in the soma and dendrites of the D1-Cre;Ai14 mouse, while green fluorescence (ZsGreen) was only found in the soma of the D1- and D2-Cre;Ai5 mice. (E–G) Representative images of a coronal section of the amygdala from a D1-tdTomato;D2-Cre;Snap25 mouse with arrows showing abundant tdTomato-expressing D1R+ neurons (E), a few GFP-expressing D2R+ neurons (F), and some co-localization (yellow, G) within the outlined BLA area. The top left panels show an enlarged image of one co-expressing neuron. (H) Bar graphs depicting a higher average number of D1R+ neurons than of D2R+ neurons in the BLA area. **p < 0.01. n = 28 sections from 8 mice per group. (I) Bar graph showing that a significantly higher percentage of D1R+ neurons expressed D2Rs, as compared with the percentage of D2R+ neurons that expressed D1Rs in D1-tdTomato;D2-Cre;SNAP25 transgenic mice. ***p < 0.001. n = 28 sections from 8 mice per group. Scale bars: 2 mm (A–D top), 300 μm (A–D bottom), 10 μm (A–G insets), and 100 μm (E–G).
In addition, we used male D1-tdTomato;D2-Cre;Snap25 transgenic mice to count neurons expressing D1Rs and/or D2Rs (Figs. 5E–5G). We examined 28 BLA sections from 8 mice, and a total of 3248 D1R+ neurons and 1515 D2R+ neurons were counted. This corresponded to an average of 116 D1R+ neurons in the BLA, which was significantly higher than the average of 54 D2R+ neurons (Fig. 5L; t(54) = 4.05, p < 0.001). Co-localization analysis showed that 8.95 ± 1.03% of D1R-expressing neurons were D2R+; this was significantly lower than the 16.12 ± 1.31% of D2R+ neurons that expressed D1Rs (Fig. 5M; t(54) = −4.32, p < 0.001). We also counted BLA neurons in female mice and found that the overlapping rates of D1R+ and D2R+ neurons was significantly lower in females than in males (Table S1; t(44) = 3.63, p <0.001 in D1; t(44) = 2.52 p < 0.05 in D2). Together, these results indicate that the BLA expresses significantly more D1Rs than D2Rs, with some overlap between D1R+ and D2R+ neurons.
Electrophysiology of D1R+ and D2R+ neurons in the hippocampus
D1- and D2-GFP mice have been widely used in electrophysiological studies of striatal neurons (Valjent et al., 2009; Wang et al., 2015), but are used much less frequently for studies outside the striatum because of the low expression levels in these brain regions (Gong et al., 2003; Valjent et al., 2009). As stated above, our investigation of D1- and D2-Cre;Ai14 animals identified strong fluorescent signals in the cortex, hippocampus, and BLA, indicating that these mice had the potential to support electrophysiological studies of neurons outside the striatum.
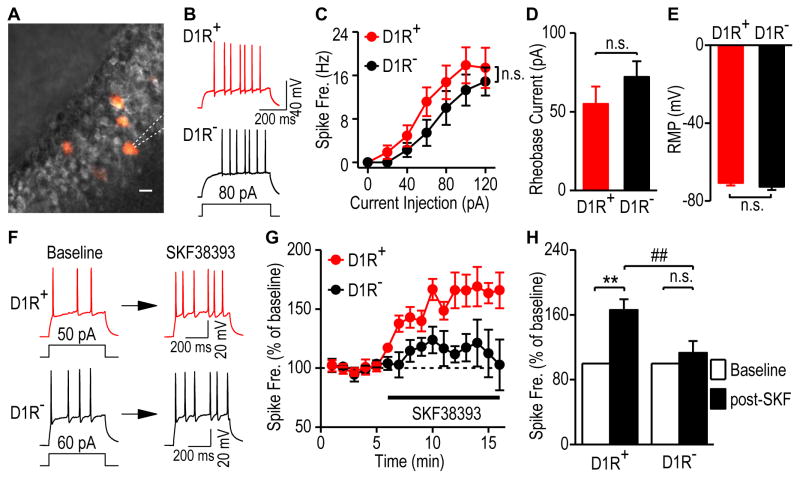
To investigate the activity of D1R+ hippocampal neurons, we measured evoked firing in D1R+ and D1R-negative (D1R−) granular cells (Fig. 6A). We found no difference in excitability (Figs. 6B and 6C; F(1,155) = 1.85, p > 0.05), rheobase currents (Fig. 6D; t(26) = −1.16, p > 0.05), or resting membrane potentials (Fig. 6E; t(26) = 0.93, p > 0.05) between these neuronal types. Bath application of the D1R agonist, SKF38393 (20 μM), produced a significant increase in the firing frequency of D1R+ neurons (165.94 ± 13.36% of the baseline, q = 6.63, p < 0.01), as shown in Figures 6F–6H. In contrast, SKF38393 only caused a small increase in the firing frequency of D1R− neurons (113.05 ± 14.75% of the baseline), which was not statistically significant (Figs. 6F–6H; q = 1.31, p > 0.05). These results showed that D1Cre;Ai14 mice facilitated accurate visualization of D1R+ neurons in the hippocampus and suggested that they could allow electrophysiological investigations of these neurons in other non-striatal brain regions.
Figure 6. Functional verification of D1R+ neurons in the dorsal hippocampus.
Whole-cell current-clamp recordings were conducted in fluorescent D1R+ and non-fluorescent D1R− granule cells of the dorsal hippocampus in D1-Cre;Ai14 mice. (A) Representative differential interference contrast (DIC, grey) and fluorescent (red) images of granule cells in a dorsal hippocampal slice. The dashed lines indicate a patch pipette. Scale bar: 10 μm. (B) Sample traces of membrane potentials in response to a step current injection in D1R+ and D1R− granule cells in the dorsal hippocampus. (C) Spike-firing frequency in response to a series of increasing current injections in these neurons. n.s., not significant, p > 0.05, Two-Way RM ANOVA. n = 14 neurons from 4 mice per group. (D–E) The rheobase current (D) and resting membrane potentials (RMP, E) did not differ between D1R+ and D1R− granule cells. n.s., p > 0.05, t test. n = 14 neurons from 4 mice per group. (F) Sample traces of membrane potentials in D1R+ (top) and D1R− (bottom) granule cells in response to a step current injection before (left, baseline) and after (right) bath application of a D1R agonist, SKF38393 (20 μM). (G) Time-course of averaged responses in evoked firing frequency before and during SKF38393 application, as indicated by the horizontal bar, in D1R+ (red) and D1R− (black) granule cells. (H) Bar graphs comparing SKF38393-induced changes in firing frequency of D1R+ and D1R− neurons. Baseline firing was calculated from 1–5 min and post-SKF38393 firing was averaged from 12–16 min. **p < 0.01, ##p < 0.01, two-way ANOVA with repeated measure, followed by SNK test. n = 4 neurons per group.
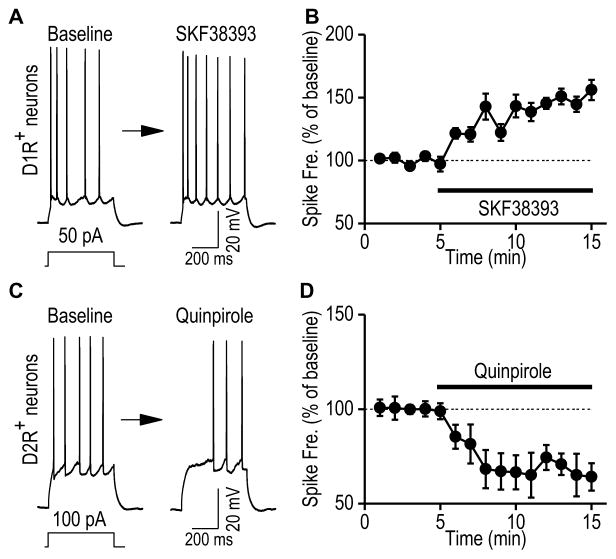
Next, we examined whether D1R and D2R activation differentially modulated hippocampal neuronal excitability. We selected ventral CA1 neurons to test this possibility because both receptors were found in CA1 pyramidal cells (Fig. 4). This is unlike the dDG where D1Rs and D2Rs are found in granule cells and hilar cells, repectively. We discovered that the D1R agonist SKF38393 significantly increased the spiking firing frequency (Figs. 7A,7B; 147.09 ± 5.39 % of the baseline at 5–10 min post-SKF38393 application, t(5) = −8.73, p < 0.001). In contrast, the D2R agonist quinpirole (20 μM) reduced the firing frequency (Figs. 7C, 7D; 67.87 ± 7.86 % of the baseline, t(6) = 4.09, p < 0.01). Together, these results indicate that dopamine receptor activation distinctly regulates excitability of ventral CA1 D1R+ or D2R+ neurons.
Figure 7. Dopamine receptor activation distinctly regulates excitability of D1R+ and D2R+ neurons in the ventral hippocampal CA1 area.
Whole-cell current-clamp recordings were conducted in fluorescent D1R+ and D2R+ neurons in the ventral CA1 area of the D1-Cre;Ai14 and D2-Cre;Ai14 mice, respectively. The evoked firing in response to a step current injection were measured before and after bath application of a D1R agonist (SKF38393, 20 μM) to D1R+ neurons, or a D2R agonist (quinpirole, 20 μM) to D2R+ neurons. (A) Sample traces showing evoked firing of D1R+ neurons before (left) and after (right) bath application of SKF38393. (B) Time-course of averaged responses in evoked firing frequency before and during SKF38393 application, as indicated by the horizontal bar in D1R+ neurons. n = 6 neurons from 4 mice. (C) Sample trace showing evoked firing of D2R+ neurons before and after bath application of quinpirole. (D) Time-course of averaged responses in evoked firing frequency before and during quinpirole application in D2R+ neurons. n = 7 neurons from 3 mice.
Chronic alcohol consumption distinctly regulates excitability of ventral CA1 neurons
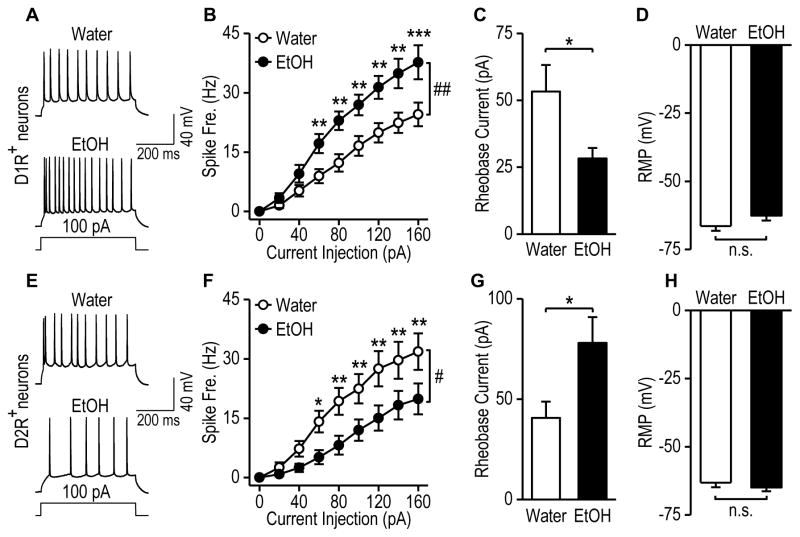
Alcohol is known to increase dopaminergic firing (Barak et al., 2014; Brodie et al., 1990). We thus examined whether chronic alcohol intake altered excitability of D1R+ and D2R+ ventral CA1 neurons. D1-Cre;Ai14 and D2-Cre;Ai14 mice were trained to consume 20% alcohol using the two-bottle choice drinking procedure (Cheng et al., 2016; Wang et al., 2015) and ventral CA1 neurons were recorded 24-h after the last drinking session. We found that chronic alcohol intake increased spike firing frequency (Figs. 8A and 8B; F(1, 274) = 11.07, p < 0.01) and reduced rheobase currents (Fig. 8C; t(36) = 2.17, p < 0.05) in D1R+ neurons withouth changing their resting membrane potentials (Fig. 8D; t(36) = −1.60, p > 0.05). In contrast, chronic alcohol consumption decreased firing frequency (Figs. 8E, 8F; F(1,255) = 6.49, p < 0.05) and increased rheobase currents (Fig. 8G; t(33) = −2.19, p < 0.05) in D2R+ ventral CA1 neurons. The resting membrane potential also remained unchanged in this neuronal type (Fig. 8H; t(33) = 0.79, p > 0.05). Lastly, we compared D1R+ and D2R+ neurons in water groups and found no difference in excitability (F(1,458) = 1.72, p > 0.05), rheobase currents (t(33) = 0.91, p > 0.05), or resting membrane potentials (t(33) = −1.34, p > 0.05). Collectively, these data suggests that chronic alcohol intake upregulates excitability of D1R+ neuron and downregulates excitibilty of D2R+ neurons in the ventral CA1.
Figure 8. Chronic alcohol consumption distinctly modulates D1R+ and D2R− neuron excitability in the ventral hippocampal CA1 area.
D1-cre;Ai14 and D2-cre;Ai14 mice were trained to consume alcohol for 8 weeks using the intermittent-access 2-bottle choice procedure. Twenty-four h after the last drinking session, ventral hippocampal slices were prepared for electrophysiologic recordings. (A–B) Alcohol consumption caused an increase in spike firing frequency. (A) Sample traces of evoked firing of fluorencent D1R+ neurons in the ventral CA1 of alcohol-drinking mice (EtOH) and water controls (Water). (B) Spike frequency in response to increasing current injections in D1R+ neurons from water and alcohol groups. ##p < 0.01 by Two-Way RM ANOVA. **p < 0.01, ***p < 0.001 versus the water group at the same stimulating intensities, post-hoc SNK test. (C) Alcohol consumption produced a reduction in rheobase current in D1R+ neurons. *p < 0.05 by t test. (D) Alcohol consumption did not alter resting membrane potential in D1R+ neurons. n.s., p > 0.05 by t test. (E–H) Chronic alcohol consumption led to reduced excitability of D2R+ neurons in the ventral hippocampal CA1 area. (E) Sample traces of evoked firing of D2R+ neurons in water and alcohol groups. (F) Spike frequency in response to increasing current injections in D2R+ neurons from alcohol and water groups of D2-cre;Ai14 mice. #p < 0.05 by Two-Way RM ANOVA. *p < 0.05, **p < 0.01, versus the EtOH group at the same stimulating intensities, post-hoc SNK test. (G) Alcohol consumption produced an increase in rheobase current. *p < 0.05 by t test. (H) Alcohol consumption did not alter resting membrane potentials in D2R+ neurons from the ventral hippocampal CA1 area. n.s., p > 0.05 by t test. A–D: n = 21 neurons from 4 mice (Water) and 17 neurons from 3 mice (EtOH). E–H: n = 14 neurons from 3 mice (Water) and n = 21 neurons from 3 mice (EtOH).
DISCUSSION
The present study of a range of transgenic mouse lines demonstrated that neurons expressing D1- or D2-eGFP, and D1- or D2-Cre, were highly co-localized in the striatum and hippocampus. These results indicate that fluorescent neurons in D1- or D2-GFP mice and Cre-positive neurons in D1- or D2-Cre mice represented the same neuronal populations. Importantly, by crossing D1- or D2-Cre mouse lines with Cre reporter lines, we found that D1R+ and D2R+ neurons were highly segregated in the OFC, mPFC, hippocampus (dorsal and ventral), and BLA. In addition, neuronal subpopulations were identified that expressed both D1Rs and D2Rs; the proportion of these ranged from 4% in the female OFC to 34% in the dorsal CA1 area. Lastly, electrophysiological recording demonstrated that the D1R+ and D1R− neurons were functionally distinct in the hippocampus and alcohol consumption distinctly regulates excitability of ventral hippocampal neurons.
Accessing the same populations of neurons with D1- or D2-GFP mice and D1- or D2-Cre mice
D1- or D2-GFP mice were initially created to visualize specific D1R- or D2R-expressing neurons (Gong et al., 2003; Valjent et al., 2009; Wang et al., 2015), while D1- or D2-Cre mice were generated to facilitate optogenetic and chemogenetic manipulation of specific neurons in behavioral studies (Cheng et al., 2016; Gong et al., 2007; Kravitz et al., 2012; Pascoli et al., 2012). Important cellular and behavioral discoveries have been made using these two types of transgenic mice (Cheng et al., 2016; Kravitz et al., 2012; Pascoli et al., 2012; Valjent et al., 2009; Wang et al., 2015). Our analysis of GFP- and Cre-expressing D1R+ or D2R+ neurons in triple transgenic mice found a high degree of overlap between GFP- and Cre-expressing striatal and hippocampal neurons, suggesting that these did in fact identify the same neuronal populations, at least in these two brain regions. These results provide a solid basis for correlating the cellular results obtained using D1- or D2-GFP mice with the behavioral results generated using D1- or D2-Cre mice. For instance, electrophysiological studies have shown that drug and alcohol exposure increased glutamatergic inputs to dMSNs of D1-GFP mice (Pascoli et al., 2012; Wang et al., 2015), while some behavioral studies have indicated that optogenetic or chemogenetic inhibition of dMSNs in D1-Cre mice reduced alcohol intake or cocaine reinforcement (Cheng et al., 2016; Kravitz et al., 2012). Since the GFP-positive and Cre-positive neurons are the same population, we may safely draw the conclusion that drug and alcohol-mediated changes in dMSNs may contribute to additive behaviors. In addition, previous studies have argued that different strains of mice exhibit behavioral differences, which limit comparisons between studies targeting the same population of striatal neurons (Kramer et al., 2011; Nelson et al., 2012). Our present comparison of the locomotor activities of D1-GFP and D1-Cre;Ai14 mice found that this behavior did not differ significantly, although these two mouse lines may exhibit differences in other behaviors. Thus, in order to produce cellular data that can be correlated with behavioral results, which are often based on D1- or D2-Cre mice, it may be best to use D1- or D2-Cre mice crossed with a Cre reporter line, such as Ai14 (Cheng et al., 2016).
Distinct expression of D1Rs and D2Rs outside the striatum
The present study examined the expression patterns of D1R+ and D2R+ neurons outside the striatum using Cre-mice crossed with Ai14 or Ai6 reporters. In the cortex, we found a considerable number of D1R+ and D2R+ neurons, which supported the importance of the frontal cortex dopaminergic reward system as a key determinant of decision-making behavior and behavior modification (Koob and Volkow, 2010; Luscher and Malenka, 2011). Furthermore, D1Rs and D2Rs were expressed in layers V–VI and II–III, respectively. As neurons in layers V–VI are more likely to have axonal projections to the basal ganglia and the spinal cord, dopamine-mediated regulation of D1R-expressing neurons in these layers may thus directly contribute to the modulation of voluntary movement. The high expression of D2Rs in layers II–III, but not layers V–VI, suggests that D2R signaling may indirectly regulate projecting cortical neurons.
The D1R+ and D2R+ neurons were also segregated in the hippocampus and BLA. Interestingly, we found abundant expression of D1Rs and D2Rs in the dorsal and ventral hippocampus. The high expression of both D1Rs and D2Rs in the ventral hippocampus is particularly interesting; both receptors are found in the CA1 and subiculum but are highly segregated. This suggests a cell type-specific, rather a region-specific, expression of D1Rs and D2Rs. Given that excitability of D1R+ and D2R+ ventral CA1 neurons are distinctly altred by chronic alcohol intake, it will be of great interest to determine whether these neurons exhibit opposite roles in addiction or other psychiatric disorders. Unexpectedly, D1R+ neurons predominated in the BLA, while D2R+ neurons were barely observable. This finding was somewhat consistent with the observation that amygdala neurons project to dMSNs, but not iMSNs, in the striatum (Wall et al., 2013). Interestingly, female mice have lower overlapping rates of BLA D1R+ and D2R+ neurons than male mice. It is not clear how dominant D1 signaling in the amygdala contributes to its role in emotion regulation in males and females (Janak and Tye, 2015; Neugebauer et al., 2004; Tye et al., 2011).
Overlap of D1R and D2R expression
Although neurons expressing D1Rs and D2Rs were generally highly segregated, some co-localization was observed. Overlap of D1R+ and D2R+ neurons was reported previously in the striatum, where the highest overlap rate of 17% was found in the nucleus accumbens shell (Bertran-Gonzalez et al., 2008). Outside the striatum, we found overlap rates ranging from ~4% to 34%. Relatively high rates of co-localization were present in the dorsal hippocampal CA1 and CA3 regions. Most of these neurons were outside the pyramidal layers, and may be interneurons. Some striatal interneurons were reported to express both D2Rs and another D1-type receptor, the D5R (Kreitzer and Malenka, 2008). D1Rs and D2Rs expressed in the same neurons can form heterodimers, which contribute to addiction and schizophrenia (Perreault et al., 2014).
Implications for the use of D1- or D2-Cre;Ai14 mice for electrophysiological or morphological studies
Unlike the striatum, where over 90% of neurons express either D1Rs or D2Rs, the majority of neurons in other brain regions do not express D1Rs or D2Rs. Previous studies of cortical or hippocampal dopamine signaling usually recorded neurons in slices without identifying the receptor expression. In this study, we showed that D1R+ hippocampal neurons in brain slices could be identified by their fluorescent signal and then recorded using whole-cell patch-clamp approaches; the D1R+ and D1R− neurons showed distinct responses to D1R activation. The critical advance that facilitates this approach is the use of double transgenic mice in which the fluorescent protein expression level is enhanced by Cre-Loxp recombination. In summary, we found that the GFP-positive neurons in D1- or D2-GFP mice and the Cre-positive neurons in D1- or D2-Cre mice represented the same neuronal populations and that neurons expressing D1Rs and D2Rs were highly segregated in the cortex, hippocampus, and BLA, although a minority of neurons did co-express these receptors. Importantly, we showed that double transgenic mice expressing Cre and Cre-reporter fluorescent proteins provide an ideal tool for the selective investigation of D1R and D2R function outside the striatum. Lastly, we found excessive alcohol consumption distinctly regulates excitability of these neurons, suggesting that they may differentially contribute to alcohol use disorder.
Supplementary Material
Acknowledgments
The authors thank Mr. Jonathan Artz, Ms. Britton Barbee, Mr. Eric Williams, Ms. Caroline Matlock, Mr. Nathaniel Teplitiskiy, Mr. Sebastian Melo, Mr. Jared Jerger, and Ms. Rachel Bonasera for technical support. This research was supported by NIAAA R01AA021505 (JW). Xiaoyan Wei was supported by a scholarship from the China Scholarship Council (No.20153170480).
Footnotes
Disclosure/Conflict of interest
The authors have no conflicts of interest to disclose.
Authors’ contributions
JW was responsible for the concept and design of the study. XYW, YC, and JL were responsible for the acquisition of all the images. JW, XYW, and TM were both responsible for the drafting, critical review, and final approval of the manuscript. TM and CH was responsible for the electrophysiological results. XYW was responsible for the mouse breeding. All authors critically reviewed the content and approved the final version for publication.
References
- Bagot RC, Parise EM, Pena CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolanos-Guzman CA, Cheer J, Deisseroth K, Han MH, Nestler EJ. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nature communications. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barak S, Wang J, Ahmadiantehrani S, Ben Hamida S, Kells AP, Forsayeth J, Bankiewicz KS, Ron D. Glial cell line-derived neurotrophic factor (GDNF) is an endogenous protector in the mesolimbic system against excessive alcohol consumption and relapse. Addiction biology. 2014 doi: 10.1111/adb.12152. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Annals of the New York Academy of Sciences. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci. 2008;28:5671–5685. doi: 10.1523/JNEUROSCI.1039-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain research. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M. Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci. 2014;17:1022–1030. doi: 10.1038/nn.3743. [DOI] [PubMed] [Google Scholar]
- Chandler DJ, Waterhouse BD, Gao WJ. New perspectives on catecholaminergic regulation of executive circuits: evidence for independent modulation of prefrontal functions by midbrain dopaminergic and noradrenergic neurons. Frontiers in neural circuits. 2014;8:53. doi: 10.3389/fncir.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Huang CC, Ma T, Wei X, Wang X, Lu J, Wang J. Distinct Synaptic Strengthening of the Striatal Direct and Indirect Pathways Drives Alcohol Consumption. Biological psychiatry. 2016 doi: 10.1016/j.biopsych.2016.05.016. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Deng W, Aimone JB, Ge M, Parylak S, Walch K, Zhang W, Cook J, Song H, Wang L, Gage FH, Mu Y. Dopaminergic inputs in the dentate gyrus direct the choice of memory encoding. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5501–5510. doi: 10.1073/pnas.1606951113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neuroscience and biobehavioral reviews. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Longueville S, De Bundel D, Perroy J, Herve D, Girault JA, Valjent E. Characterization of dopamine D1 and D2 receptor-expressing neurons in the mouse hippocampus. Hippocampus. 2012;22:2199–2207. doi: 10.1002/hipo.22044. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annual review of neuroscience. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Kreitzer AC. Cortico-Basal Ganglia Circuit Function in Psychiatric Disease. Annual review of physiology. 2016;78:327–350. doi: 10.1146/annurev-physiol-021115-105355. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual review of neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biological psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer PF, Christensen CH, Hazelwood LA, Dobi A, Bock R, Sibley DR, Mateo Y, Alvarez VA. Dopamine D2 receptor overexpression alters behavior and physiology in Drd2-EGFP mice. J Neurosci. 2011;31:126–132. doi: 10.1523/JNEUROSCI.4287-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annual review of neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60:543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Kellendonk C, Simpson EH, Kandel ER, Gao WJ. D2 receptor overexpression in the striatum leads to a deficit in inhibitory transmission and dopamine sensitivity in mouse prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12107–12112. doi: 10.1073/pnas.1109718108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Wang MJ, Gao WJ. Hyperdopaminergic modulation of inhibitory transmission is dependent on GSK-3beta signaling-mediated trafficking of GABAA receptors. Journal of neurochemistry. 2012;122:308–320. doi: 10.1111/j.1471-4159.2012.07790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knopfel T, Boyden ES, Reid RC, Carandini M, Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–958. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AB, Hang GB, Grueter BA, Pascoli V, Luscher C, Malenka RC, Kreitzer AC. A comparison of striatal-dependent behaviors in wild-type and hemizygous Drd1a and Drd2 BAC transgenic mice. J Neurosci. 2012;32:9119–9123. doi: 10.1523/JNEUROSCI.0224-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2004;10:221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Luscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39:156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annual review of neuroscience. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia Reward Network: Microcircuitry. Neuropsychopharmacology. 2009 doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76:33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends in neurosciences. 2009;32:538–547. doi: 10.1016/j.tins.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- Wall NR, De La Parra M, Callaway EM, Kreitzer AC. Differential innervation of direct- and indirect-pathway striatal projection neurons. Neuron. 2013;79:347–360. doi: 10.1016/j.neuron.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cheng Y, Wang X, Roltsch Hellard E, Ma T, Gil H, Ben Hamida S, Ron D. Alcohol Elicits Functional and Structural Plasticity Selectively in Dopamine D1 Receptor-Expressing Neurons of the Dorsomedial Striatum. J Neurosci. 2015;35:11634–11643. doi: 10.1523/JNEUROSCI.0003-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.