Abstract
Background
Data derived from prospective randomized clinical trials suggest differential comparative benefit between carotid angioplasty and stent (CAS) placement and carotid endarterectomy (CEA) in various age strata. We sought to investigate the impact of age on outcomes of CAS and CEA in general practice.
Methods
We analyzed the data from the Nationwide Inpatient Sample (NIS), which is representative of all admissions in the United States from 2005 to 2008. The primary end point was occurrence of stroke, cardiac complications, or death during the postprocedural period. Outcomes of interest were compared between patients aged ≥70 years and ≤70 years, undergoing CEA and CAS. Multivariate logistic regression was performed to determine the effect of age on occurrence of postoperative stroke, cardiac complications, or death. Covariates included in the logistic regression were patient’s age, gender, comorbid conditions, including hypertension, diabetes mellitus (DM), chronic lung disease, coronary artery disease (CAD), congestive heart failure (CHF), and renal failure; symptom status (symptomatic vs asymptomatic status), and hospital characteristics.
Results
Of the total 495,331 estimated patients who received treatment for CAD during the study period, 88% underwent CEA and the remaining 12% underwent CAS. Of the total procedures, 41% of the procedures were performed in patients aged <70 years compared to the remaining 59% that were performed among patients aged ≥70 years. For patients undergoing CAS, age >70 years was an important predictor of postoperative stroke (P = .0025; odds ratio [OR], 1.7; 95% confidence interval [CI], 1.2–2.5) and cardiac complications postprocedure (P = .045; OR, 1.3; 95% CI, 1.0–1.6). For patients undergoing CEA, age ≥70 years was associated with higher cardiac complications (P < .001; OR, 1.5; 95% CI, 1.3–1.7) and higher postoperative mortality risk (P = .0008; OR, 1.4; 95% CI, 1.1–1.8) compared to patients aged <70 years. The increased risk of composite end point (postoperative stroke/cardiac complications/ mortality) among patients aged >70 years was a significant factor for patients undergoing either CAS or CEA (OR of 1.3 for both procedures).
Conclusion
Our analysis suggests that most CAS and CEAs are performed in patients aged ≥70 years in general practice, and higher rates of postoperative complications are observed among these patients regardless of procedure choice.
Extracranial atherosclerotic carotid disease accounts for up to 15% to 20% of all ischemic strokes.1,2 According to the most recent American Heart Association/American Stroke Association guidelines about management of patients with extracranial carotid stenosis, both carotid angioplasty and stent (CAS) placement and carotid endarterectomy (CEA) are reasonable options for carotid disease revascularization. These recommendations also include a statement emphasizing the importance of age in patient selection. According to these guidelines, it is reasonable to choose CEA over CAS when revascularization is indicated in older patients; particularly when vascular anatomy is unfavorable for the endovascular approach.1 The age cutoff, however, is not mentioned in these guidelines.
In the recently published Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) trial, a crossover was observed at an age of approximately 70 years where CAS tended to show greater efficacy at younger ages, and CEA at older ages.3 However, the results of carotid revascularization in general practice setting vary considerably and are suboptimal in several settings.4,5 The differential outcomes in general practice have been attributed to the difference in patient population, variable experience of operators, failure to measure and diagnose quality/performance gaps, and implement quality improvement interventions in a timely fashion. Therefore, the age-related difference in outcomes identified in clinical trials may be augmented or blunted in the general practice setting.
Our objective was to identify if such suggested differential results in age strata are also observed in general practice at the national level among patients undergoing CEA and CAS.
METHODS
Study sample
In order to evaluate the characteristics and outcomes for patients undergoing CEA or CAS in the United States, we used the Nationwide Inpatient Sample (NIS) for the calendar year, from 2005 to 2008. The NIS is the largest database of its kind and includes all-payer discharge information from a national survey of nonfederal hospitals in the United States. The NIS provides a weighting strategy in order to draw estimates at the national level based on a 20% annual survey of hospitals. The statistical analyses are performed based on these weighted numbers and, therefore, the data provided in the Results section are national estimates. A complete overview and description of the NIS is available at http://www.hcup-us.ahrq.gov.
Identification of patients and procedures
We used International Classification of Disease, 9th Revision, Clinical Modification (ICD-9-CM) codes similar to previously published reports on carotid revascularization comparing CEA and CAS at the national level using NIS data for the year 2005.6 Diagnostic code fields were screened for ICD- 9-CM code for either CAS (00.63) or CEA (38.12) with the corresponding diagnostic code for carotid artery stenosis with (433.11) or without (433.10) stroke. We used the criterion which has been used previously6 to differentiate the symptomatic from asymptomatic patients with carotid stenosis. If a patient’s discharge diagnosis (diagnostic fields 1–15) was “carotid artery stenosis without mention of stroke” with no accompanying secondary diagnoses for transient ischemic attack (TIA), they were classified as “asymptomatic.” If a patient’s discharge diagnosis was either “carotid artery stenosis with stroke” or, if there was no mention of stroke, but a secondary diagnosis code included that for TIA, patients were classified as “symptomatic.” A patient was excluded from the final dataset if he or she had procedural codes listed for both CAS and CEA (<1% of total) during the index admission in the interest of keeping the cohorts as homogenous as possible to facilitate comparison between the two procedure types.
The variables abstracted were patients’ age, gender, race/ethnicity, comorbidities (congestive heart failure [CHF], coronary artery disease [CAD], diabetes mellitus [DM], hypertension, peripheral vascular disease, renal failure, obesity, and chronic lung disease), procedures performed (CEA or CAS), and hospital characteristics in which they were treated (rural, urban nonteaching, urban teaching hospitals) and discharge disposition. The NIS defines an urban hospital as one located in a metropolitan statistical area and a teaching hospital as one with American Medical Association-approved residency program and either membership in the Council of Teaching Hospitals or a ratio of full-time equivalent interns and residents to beds of 0.25 or higher. The NIS does not divide rural hospitals into teaching or nonteaching because of the small number of teaching hospitals in rural areas.
The primary outcome measures for this analysis were procedure-related complications, including postoperative neurological complications, cerebral infarction or hemorrhage (ICD-9-CM codes 997.00–997.09) and postoperative cardiac complications (ICD-9-CM code 997.1). A patient undergoing CAS or CEA that had any of these codes under one of their secondary ICD-9-CM diagnostic codes (up to 15), was classified as having had an iatrogenic stroke or cardiac complications. Secondary outcome measures included discharge disposition. Discharge disposition for the purpose of analysis was condensed to a dichotomous variable, home vs other destinations (rehabilitation facility, skilled nursing facility, nursing home, and death).
Statistical analysis
We sought to investigate the impact of age on outcomes associated with CAS and CEA in NIS. The primary end point was comparison of postoperative stroke, cardiac complications, and inhospital death during the postprocedural period between patients aged <70 years and those aged >70 years for CAS and CEA separately. We used a multivariate model, adjusting for demographic and hospital characteristics, and comorbid conditions between <70 years and those aged >70 years. In addition, we also compared discharge destination of home as a surrogate of good outcome. To ascertain the outcome variables in different age groups, we also evaluated the age factor by every decade increase from the age of 50 years. We also compared CEA and CAS in univariate analysis in both age groups, <70 years and those aged ≥70 years.
We used the SAS 9.1 software (SAS Institute, Cary, NC) to convert raw counts generated from the NIS database into weighted counts that we used to generate national estimates. The statistical analysis was performed based on these weighted numbers and incorporated the complex sampling of NIS, following Healthcare Cost and Utilization Project (HCUP) recommendations.7 We used the χ2 test for categorical data and analysis of variance for continuous data with a P < .05 considered statistically significant. Outcomes of interest were compared between CEA and CAS in patients aged ≥70 years and patients aged <70 years. Multivariate logistic regression was performed to determine the effect of age on postoperative stroke, cardiac complications, or death after adjusting for potential confounders. Potential confounders included in the logistic regression were gender, comorbid conditions (hypertension, DM, chronic lung disease, CAD, CHF, and renal failure), carotid stenosis symptom status (symptomatic vs asymptomatic), and hospital characteristics (teaching status and bed size).
RESULTS
Of the total 495,331 estimated patients who underwent CEA or CAS during the study period from 2005 to 2008 in the United States; 88% underwent CEA and the remaining 12% underwent CAS. The vast majority of patients (approximately 93% in the CAS group and 96% in the CEA group) were asymptomatic by the definition used for ascertaining symptomatic status.
Of the total procedures, 41% were performed in patients aged <70 years and 59% were performed among patients aged ≥70 years. Table I provides the univariate comparison of variables between patients who underwent CAS or CEA. Among patients undergoing CAS, postoperative mortality was higher but not statistically different in patients aged <70 years compared with ≥70 years significant (0.7% vs 0.5%; P = .1171). Postoperative stroke (1.9% vs 1.3%) and cardiac complication (2.4% vs 1.9%) rates were higher in patients aged ≥70 years compared with those aged <70 years. The rate of discharge to home was lower among patients aged ≥70 years undergoing CAS compared with those in <70 years. Among patients undergoing CEA, the postoperative stroke rate was not different in patients aged ≥70 years (0.95%) compared to <70 years (1.0%). Cardiac complication (2.2% vs 1.3%) and postoperative mortality (0.4% vs 0.2%) rates were higher in patients aged >70 years compared with those in <70 years. The rate of discharge to home was lower among patients aged ≥70 years undergoing CEA.
Table I.
Patient characteristics, hospital characteristics, and outcome in patients undergoing CAS or CEA in general practice (NIS 2005 to 2008)
| Patients undergoing CAS
|
Patients undergoing CEA
|
|||||
|---|---|---|---|---|---|---|
| Age < 70 years | Age ≥ 70 years | P value | Age < 70 years | Age ≥ 70 years | P value | |
| Overall number (%) | 24,063 | 33,563 | 180,827 | 256,878 | ||
| Gender | ||||||
| Women | 9710 (40.3) | 13,165 (39.2) | .2366 | 74,706 (41.3) | 110,540 (43.0) | <.0001 |
| Age (mean) | 61.1 | 77.8 | 61.9 | 77.5 | ||
| Race/ethnicitya | ||||||
| White | 15,422 (85.1) | 22,897 (87.3) | .0067 | 118,461 (88.9) | 176,168 (90.3) | <.0001 |
| African American | 764 (4.2) | 896 (3.4) | 4962 (3.7) | 5547 (2.8) | ||
| Hispanic | 967 (5.3) | 1052 (4.0) | 5182 (3.8) | 7145 (3.6) | ||
| Other | 954 (5.2) | 1370 (5.2) | 4536 (3.4) | 6121 (3.1) | ||
| Comorbid conditions | ||||||
| Hypertension | 17,086 (71.0) | 24,835 (73.9) | .0007 | 139,455 (77.1) | 203,924 (79.3) | <.0001 |
| Diabetes mellitus | 7760 (32.2) | 9256 (27.5) | <.0001 | 58,097 (28.6) | 73,511 (32.1) | <.0001 |
| Coronary artery disease | 12,313 (51.2) | 18,809 (56.1) | <.0001 | 78,235 (43.2) | 121,317 (47.2) | <.0001 |
| Chronic lung disease | 4851 (20.1) | 6474 (19.2) | .2848 | 41,446 (22.9) | 53,742 (20.9) | <.0001 |
| Congestive heart failure | 2039 (8.4) | 3823 (11.4) | <.0001 | 8142 (7.2) | 18,671 (4.5) | <.0001 |
| Renal failure | 1530 (6.3) | 2943 (8.7) | <.0001 | 7884 (4.3) | 17,475 (6.8) | <.0001 |
| Hospital bed size | ||||||
| Small | 2047 (8.5) | 3475 (10.3) | .1767 | 16,331 (9.0) | 24,619 (9.5) | .1435 |
| Medium | 4491 (18.6) | 6277 (18.7) | 40,552 (22.4) | 58,754 (22.8) | ||
| Large | 17,525 (72.8) | 23,811 (70.9) | 123,893 (68.5) | 173,479 (67.5) | ||
| Hospital location and teaching status | ||||||
| Rural | 773 (3.2) | 838 (2.5) | <.0001 | 16,783 (9.2) | 24,327 (9.4) | .0001 |
| Urban nonteaching | 8483 (35.3) | 13,929 (41.5) | 86,906 (48.1) | 129,527 (50.4) | ||
| Urban teaching | 14,808 (61.5) | 18,795 (56.0) | 77,087 (42.6) | 102,999 (40.1) | ||
| Discharge disposition | ||||||
| Home | 22,372 (92.9) | 29,843 (88.9) | <.0001 | 13,975 (92.2) | 222,695 (86.6) | <.0001 |
| Other destinations | 1691 (7.1) | 3720 (11.1) | 166,852 (7.7) | 34,183 (13.3) | ||
| In-hospital complications | ||||||
| Postoperative mortality | 184 (0.7) | 170 (0.5) | .1171 | 529 (0.2) | 1227 (0.4) | <.0001 |
| Postoperative stroke | 326 (1.3) | 663 (1.9) | .0158 | 1846 (1.0) | 2443 (0.95) | .2988 |
| Postoperative cardiac complications | 468 (1.9) | 835 (2.4) | .0481 | 2494 (1.3) | 5774 (2.2) | <.0001 |
| Composite end point | 900 (3.7) | 1513 (4.5) | .0538 | 4515 (2.4) | 8768 (3.4) | <.0001 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; NIS, Nationwide Inpatient Sample.
Race is not uniformly reported among all states.
After adjustment for potential confounders, the odds of cardiac complications were significantly higher among patients aged >70 years undergoing CAS (odds ratio [OR], 1.3) and in those undergoing CEA (OR, 1.5; Table II). The odds of postoperative stroke was significantly higher among patients aged >70 years undergoing CAS (OR, 1.7) but not in those undergoing CEA. The odds of postprocedural death was significantly higher among patients aged ≥70 years undergoing CEA (OR, 1.4) but not in those undergoing CAS. The odds of postoperative stroke or cardiac complications and composite end point (postoperative stroke, cardiac complications, and/or death) among patients aged ≥70 years was a significant factor for patients undergoing either CAS or CEA with similar effect (OR of 1.3 for both procedures). Table II provides the multivariate comparison of patients aged ≥70 years (compared with those aged <70 years) who underwent CAS or CEA.
Table II.
The adjusted odds of various outcome measures in patients aged ≥70 years (compared with those aged <70 years) among patients undergoing CAS or CEA in general practice (NIS 2005 to 2008)
| Patients undergoing CAS
|
Patients undergoing CEA
|
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Postoperative stroke | 1.7 (1.2–2.5) | .0025 | 0.9 (0.8–1.1) | .1928 |
| Postoperative cardiac complications | 1.3 (1.0–1.6) | .045 | 1.5 (1.3–1.7) | <.0001 |
| Postoperative mortality | 0.8 (0.5–1.4) | .6433 | 1.4 (1.1–1.8) | .0008 |
| Stroke/cardiac complications | 1.4 (1.1–1.7) | .0019 | 1.2 (1.2–1.4) | <.0001 |
| Composite end point | 1.3 (1.1–1.7) | .0035 | 1.3 (1.2–1.4) | <.0001 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; CI, confidence interval; NIS, Nationwide Inpatient Sample; OR, odds ratio.
The multivariate model adjusts for gender, hypertension, diabetes mellitus, coronary artery disease, chronic lung disease, renal failure, congestive heart failure, symptomatic carotid stenosis, teaching status of hospital, and hospital bed size.
We plotted the rate of various outcomes in different age strata, (by decades) (Fig 1) to identify patterns of change in rates of outcomes, and, in particular, any prominent increase in various stratas. We found that the risk of cardiac complications and mortality, but not stroke, among patients undergoing CEA gradually increased with age. In contrast, the risk of stroke but not cardiac complications, among patients undergoing CAS (Fig 2) gradually increased with age.
Fig 1.
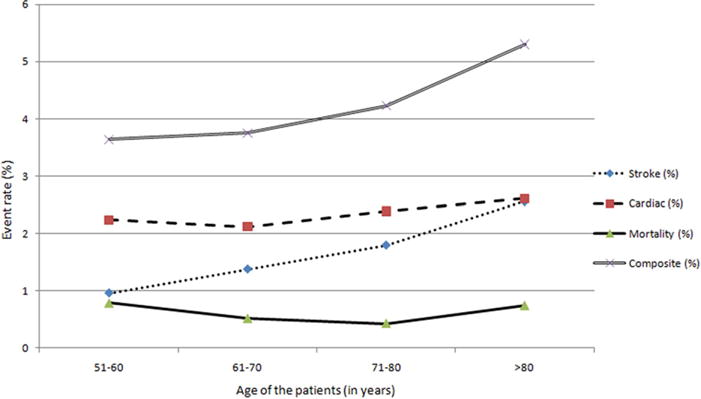
Rate of study end points among various age strata among patients undergoing carotid angioplasty and stent placement.
Fig 2.
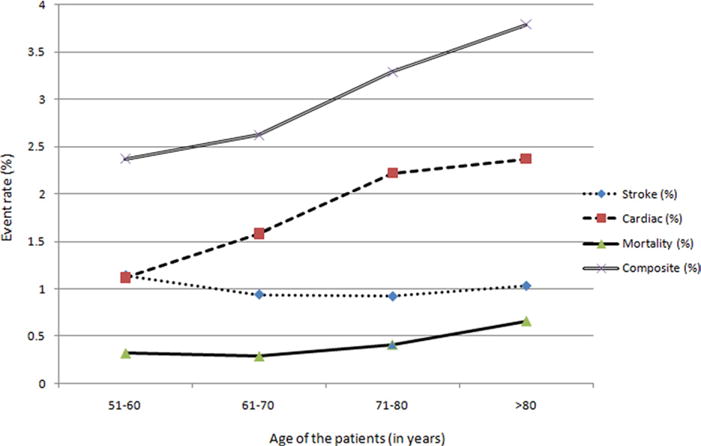
Rate of study end points among various age strata among patients undergoing carotid endarterectomy.
A direct comparison of CEA and CAS demonstrated that in patients >70 years, the unadjusted rate of postoperative stroke and composite end point were statistically favoring CEA. Similarly for patients <70 years, unadjusted in-hospital mortality and composite end point were again statistically favoring CEA (Table III).
Table III.
Univariate analysis of postoperative outcomes between CEA and CAS by age cohort (NIS 2005 to 2008)
| Age < 70 years
|
Age ≥ 70 years
|
|||||
|---|---|---|---|---|---|---|
| CAS | CEA | P value | CAS | CEA | P value | |
| Overall number (%) | 24,063 | 180,827 | 33,549 | 256,826 | ||
| In-hospital complications | ||||||
| In-hospital mortality | 184 (0.76) | 529 (0.29) | .0008 | 170 (0.50) | 1227 (0.47) | .7458 |
| Postoperative stroke | 326 (1.35) | 1846 (1.02) | .0897 | 663 (1.97) | 2443 (0.95) | <.0001 |
| Postoperative cardiac complications | 468 (1.94) | 2494 (1.37) | .0213 | 835 (2.48) | 5774 (2.24) | .3087 |
| Composite end point | 900 (3.74) | 4515 (2.49) | .0002 | 1513 (4.5) | 8768 (3.4) | .0004 |
CAS, Carotid artery stenting; CEA, carotid endarterectomy; NIS, Nationwide Inpatient Sample; OR, odds ratio.
DISCUSSION
Our analysis demonstrated that most CASs and CEAs are performed in patients aged ≥70 years in general practice and higher rates of postoperative complications are observed among these patients regardless of procedure choice. The ideal method to address the issue would be to compare outcomes between CAS and CAS among patients in the two age strata matched for prognostic characteristics. However, such a matched comparison is not possible because the characteristics of patients undergoing CAS and CEA are different in the “real-world setting.”8–11 Therefore, we used an alternative approach to determine the increase in composite end point for both CAS and CEA in the two age strata. We found that the increased odds of composite end point were similar for both CAS and CEA (OR of 1.3). The results suggest that in the general practice setting, the differential benefit of CEA over CAS among patients aged ≥70 years is blunted because of high rate of postoperative cardiac complications and mortality in patients undergoing CEA. The other finding was a higher rate of postoperative stroke among patients aged ≥70 years undergoing CAS but not in those undergoing CEA (Table II).
The age of the patient is an important consideration before planning a carotid revascularization procedure. Octogenarians were excluded from most of the prospective randomized multi-institution studies evaluating CEA, including North American Symptomatic Carotid Endarterectomy Trial (NASCET)12 and Asymptomatic Carotid Atherosclerosis Study (ACAS).13 The mean age of the included patients in these trials of CEA has been less than 70 years.14 In the NASCET, the greatest benefit of CEA compared with medical management was observed in older patients. However, several studies have suggested that the perioperative complications are higher with CEA among patients over the age of 75 in general practice.15 Therefore, CAS was initially introduced as a less invasive procedure for elderly patients with multiple medical comorbidities. The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial, in which CAS had superior outcomes to CEA in medical high-risk patients, included approximately 20% octogenarians in each treatment arm, however, the benefit was mainly because of a lower rate of myocardial infarction in the CAS group.16 In contrast to the SAPPHIRE trial, in standardrisk patients, a meta-analysis demonstrated increased risk of stroke in octogenarians undergoing CAS compared to CEA with CEA being a safer alternative.17 Similarly, the Carotid RX Acculink/Accunet Post-Approval Trial to Uncover Unanticipated or Rare Events (CAPTURE) study—a multicenter postmarketing registry also found a higher event rate in octogenarians compared with the younger cohort (7% vs 4%).18,19 Despite important implications of a potential impact of age on outcomes of CAS and CEA, little information is available from general practice settings.
The mismatch between results derived from general practice and those from randomized trials has been identified in previous studies of carotid revascularization. An analysis of 113,300 patients on Medicare undergoing CEA reported that patients’ perioperative mortality after CEA was substantially higher than that reported in the trials, even in those institutions that participated in the randomized studies.11 An audit of CEA practices in 1997 to 1998 in six hospitals9 demonstrated that 15% of the CEAs were performed for inappropriate indications; the complication rate in asymptomatic patients with high comorbidity was 6%, and those undergoing CEA with coronary artery bypass grafting was 10%, both rates incompatible with clinical benefit of the procedure. The New York Carotid Artery Surgery Study (NYCAS) was a population-based cohort study of all CEAs performed on elderly patients from January 1998 through June 1999 in New York State.10 Among the 9588 patients, 72% underwent CEA for asymptomatic stenosis. Among asymptomatic patients, those with high comorbidity had over twice the risk of death or stroke compared to those without high comorbidity (7% vs 3%). The high rate of CEA among elderly asymptomatic patients, particularly those with high comorbidities, were associated with an adverse risk/benefit ratio in practice.
In this study, the proportion of asymptomatic patients undergoing carotid revascularization is higher than expected. However, this observation is reported in other studies evaluating carotid revascularization at national level.6 The method of ascertaining symptomatic status based on ICD-9-CM codes may lead to underestimation of symptomatic patients. To minimize these inaccuracies, all secondary diagnoses (up to 15) of TIA or stroke were included as a means of further identifying the symptomatic patients. However, we are not able to comment upon the exact magnitude of this misclassification. It is unlikely that such misclassification affected our primary hypothesis testing because the trends across age groups were seen in both asymptomatic and symptomatic patients. However, the implications of such age-related increase in stroke and cardiac complications may be more pronounced in asymptomatic patients. There is lack of consensus on the optimal management of such patients and it has been suggested that up to 94% of carotid interventions among asymptomatic patients may not have benefit for the patient. It is a matter of great debate offering carotid revascularization, either CEA or CAS, to elderly asymptomatic patients is of more therapeutic value than adequate risk factor management and antiplatelet treatment. Such concerns are based on the small magnitude of benefit which can easily be offset by small increase in rates of stroke or cardiac complications. The recently published guidelines emphasize that the selection of asymptomatic patients for carotid revascularization should be guided by an assessment of comorbid conditions, life expectancy, and other individual factors, and should include a thorough discussion of the risks and benefits of the procedure with an understanding of patient preferences.1
The observations of outcome variables in different age strata demonstrate that the risk of stroke gradually increases with age in patients undergoing CAS in a manner similar to cardiac complications in patients undergoing CEA. Surprisingly, the mortality rate after CAS was highest in younger patients (less than 50 years old), however, this could be due to the low sample size (only 3% of total sample).
Even though the NIS dataset is not ideal for comparing CEA and CAS due to data limitations, we observed that the CEA procedure was better with a lower risk of composite end point in both age groups and a lower risk of in-hospital mortality in patients <70 years undergoing CEA as well as a lower rate of postoperative stroke in patients undergoing CEA in patients ≥70 years. This is consistent with previous observations as demonstrated in the NIS dataset analysis of years 2005 to 20076 where significantly higher overall rates of postoperative stroke and in-hospital mortality were observed in patients undergoing CAS compared to CEA.
The limitations of studies based on administrative datasets such as the NIS have been described previously.4 Data are lacking on the anatomic factors, including severity of carotid artery stenosis, vessel tortuosity, and vascular calcifications, which are considered high-risk features for CAS.20 The design of the data acquisition and analysis does not allow ascertainment of events after discharge. We acknowledge that such a methodology will underestimate the rate of events after CAS and CAS. In this study, the percentage of asymptomatic patients is higher than expected, however, this observation is consistent with other studies evaluating carotid revascularization at the national level.4 The method of ascertaining symptomatic status based on ICD-9-CM codes may lead to underestimation of symptomatic patients. To minimize these inaccuracies, all secondary diagnoses (up to 15) of TIA or stroke were included with the intent to further identify the symptomatic patients. Our outcome measures are reported at the time of discharge, which is suboptimal compared to a 30-day or 1-year outcome usually provided by other trials. Therefore, we may be underestimating the rates of complications. The mortality rate in our analysis is high compared to reported rates in the trials. Due to the limitation of the data, we cannot determine if the observed mortality and morbidity events were directly related to the procedure itself or a consequence of pre-existing comorbidity. We used multivariate analysis to mitigate the effect of pre-existing comorbidities.
CONCLUSION
Most CAS and CEA procedures are performed in patients aged >70 years in contemporary practice and higher rates of postoperative complications are observed among these patients regardless of the procedure choice. Age alone may not be an important factor to decide the type of revascularization procedure in patients with carotid disease. Additional studies are warranted to study the importance of age in carotid revascularization procedures in patients with carotid disease, whether it is symptomatic or asymptomatic.
Acknowledgments
Dr Adnan Qureshi has received funding from National Institutes of Health RO-1-NS44976-01A2 (medication provided by ESP Pharma) and 1U01NS062091-01A2, American Heart Association Established Investigator Award 0840053N, and Minnesota Medical Foundation, Minneapolis, Minn. Dr M. Fareed K. Suri has received funding from National Institutes of Health 5K12-RR023247-05.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: RK, AQ
Analysis and interpretation: SC, GV, MS
Data collection: SC, GV
Writing the article: RK, AQ, SC, AH, GR
Critical revision of the article: RK, AQ
Final approval of the article: RK, AQ
Statistical analysis: SC, GV, MS
Obtained funding: Not applicable
Overall responsibility: RK, AQ
Competition of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a competition of interest.
References
- 1.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/ SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients with Extracranial Carotid and Vertebral Artery Disease: Executive Summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Neuro Interventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Stroke. 2011;42:e420–63. [Google Scholar]
- 2.Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974–80. doi: 10.1161/01.str.27.11.1974. [DOI] [PubMed] [Google Scholar]
- 3.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eslami MH, McPhee JT, Simons JP, Schanzer A, Messina LM. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307–15. doi: 10.1016/j.jvs.2010.08.080. [DOI] [PubMed] [Google Scholar]
- 5.Paraskevas KI, Mikhailidis DP, Veith FJ. Carotid artery stenting may be losing the battle against carotid endarterectomy for the management of symptomatic carotid artery stenosis, but the jury is still out. Vascular. 2009;17:183–9. doi: 10.2310/6670.2009.00039. [DOI] [PubMed] [Google Scholar]
- 6.McPhee JT, Schanzer A, Messina LM, Eslami MH. Carotid artery stenting has increased rates of postprocedure stroke, death, and resource utilization than does carotid endarterectomy in the United States, 2005. J Vasc Surg. 2005;48:1442–50. doi: 10.1016/j.jvs.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Databases HCUP. Healthcare Cost and Utilization Project (HCUP) Rockville, MD: 2011. [Google Scholar]
- 8.Groeneveld PW, Yang L, Greenhut A, Yang F. Comparative effectiveness of carotid arterial stenting versus endarterectomy. J Vasc Surg. 2009;50:1040–8. doi: 10.1016/j.jvs.2009.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halm EA, Chassin MR, Tuhrim S, Hollier LH, Popp AJ, Ascher E, et al. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464–71. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 10.Halm EA, Tuhrim S, Wang JJ, Rojas M, Hannan EL, Chassin MR. Has evidence changed practice? Appropriateness of carotid endarterectomy after the clinical trials. Neurology. 2007;68:187–94. doi: 10.1212/01.wnl.0000251197.98197.e9. [DOI] [PubMed] [Google Scholar]
- 11.Wennberg DE, Lucas FL, Birkmeyer JD, Bredenberg CE, Fisher ES. Variation in carotid endarterectomy mortality in the Medicare population: trial hospitals, volume, and patient characteristics. JAMA. 1998;279:1278–81. doi: 10.1001/jama.279.16.1278. [DOI] [PubMed] [Google Scholar]
- 12.[No authors listed]; Clinical alert: benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke, Stroke and Trauma Division, North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke. 1991;22:816–7. doi: 10.1161/01.str.22.6.816. [DOI] [PubMed] [Google Scholar]
- 13.[No authors listed]; Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 14.Debing E, Van den Brande P. Carotid endarterectomy in the elderly: are the patient characteristics, the early outcome, and the predictors the same as those in younger patients? Surg Neurol. 2007;67:467–71. doi: 10.1016/j.surneu.2006.08.084. discussion 471. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein LB, McCrory DC, Landsman PB, Samsa GP, Ancukiewicz M, Oddone EZ, et al. Multicenter review of preoperative risk factors for carotid endarterectomy in patients with ipsilateral symptoms. Stroke. 1994;25:1116–21. doi: 10.1161/01.str.25.6.1116. [DOI] [PubMed] [Google Scholar]
- 16.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–501. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 17.Usman AA, Tang GL, Eskandari MK. Metaanalysis of procedural stroke and death among octogenarians: carotid stenting versus carotid endarterectomy. J Am Coll Surg. 2009;208:1124–31. doi: 10.1016/j.jamcollsurg.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 18.Gray WA, Yadav JS, Verta P, Scicli A, Fairman R, Wholey M, et al. The CAPTURE registry: predictors of outcomes in carotid artery stenting with embolic protection for high surgical risk patients in the early post-approval setting. Catheter Cardiovasc Interv. 2007;70:1025–33. doi: 10.1002/ccd.21359. [DOI] [PubMed] [Google Scholar]
- 19.Fairman R, Gray WA, Scicli AP, Wilburn O, Verta P, Atkinson R, et al. The CAPTURE registry: analysis of strokes resulting from carotid artery stenting in the post approval setting: timing, location, severity, and type. Ann Surg. 2007;246:551–6. doi: 10.1097/SLA.0b013e3181567a39. discussion 556-8. [DOI] [PubMed] [Google Scholar]
- 20.Chiam PT, Roubin GS, Iyer SS, Green RM, Soffer DE, Brennan C, et al. Carotid artery stenting in elderly patients: importance of case selection. Catheter Cardiovasc Interv. 2008;72:318–24. doi: 10.1002/ccd.21620. [DOI] [PubMed] [Google Scholar]


