Abstract
Background
To compare oral hygiene habits, oro-dental status, and dental procedures in patients with infective endocarditis (IE) according to whether the IE-causing microorganism originated in the oral cavity.
Methods
We conducted an assessor-blinded case-control study in 6 French tertiary-care hospitals. Oral hygiene habits were recorded using a self-administered questionnaire. Oro-dental status was analysed by trained dental practitioners blinded to the microorganism, using standardized clinical examination and dental panoramic tomography. History of dental procedures was obtained through patient and dentist interviews. Microorganisms were categorised as oral streptococci or non-oral pathogens using an expert-validated list kept confidential during the course of the study. Cases and controls had definite IE caused either by oral streptococci or non-oral pathogens, respectively. Participants were enrolled between May 2008 and January 2013.
Results
Cases (n=73) were more likely than controls (n=192) to be aged < 65 years (OR: 2.85; 95% CI 1.41–5.76), to be female (OR: 2.62; 95% CI 1.20–5.74), to have native valve disease (OR: 2.44; 95% CI: 1.16–5.13), to use toothpicks, dental water jet, interdental brush and/or flossing (OR: 3.48; 95% CI: 1.30–9.32), and to have had dental procedures during the prior three months (OR: 3.31; 95% CI: 1.18–9.29), while they were less likely to brush teeth after meals. Gingival inflammation, calculus and infectious dental diseases were not significantly different between groups.
Conclusions
Patients with IE caused by oral streptococci differ from patients with IE caused by non-oral pathogens regarding background characteristics, oral hygiene habits, and recent dental procedures, but not current oro-dental status.
Introduction
Infective endocarditis (IE) is a rare but severe disease with an in-hospital mortality rate of around 20% and a 5-year mortality rate of 40% [1]. It also has a high morbidity rate and cost burden: its treatment requires prolonged hospitalization; one out of two patients undergoes valve surgery during the acute phase of the disease [2]. IE antibiotic prophylaxis strategies have been therefore proposed for years to patients with IE predisposing cardiac conditions (PCC) undergoing invasive procedures responsible for bacteraemia [3, 4]. As the proof of their efficacy are lacking [5–7], guidelines have been altered towards a drastic reduction in antibiotic prophylaxis indications [3, 8–10]. An additional reason to reduce or abandon IE prophylaxis is the demonstration that “everyday low-level bacteraemia” that occur after toothbrushing, flossing, or chewing may outweigh post-dental procedure bacteraemia in terms of risk of IE [11–15]. The capability for a sustained low-grade bacteraemia as a surrogate for everyday life bacteraemia to induce IE has been confirmed experimentally, with 70% to 100% of animals developing IE, depending on the microorganism and the inoculum size [16].
On the other hand, when considering the high number of patients with IE PCC (2.5% in the general U.S. population, 1.7% in the French population and 7% in people aged > 60 years) who are exposed to daily repeated bacteraemia capable of inducing IE, the rarity of IE is quite intriguing [14, 17]. The role of dental hygiene is confusing too. Toothbrushing or flossing may increase the risk of oral streptococcal bacteraemia on a short-term basis, but may also decrease this risk of IE on a long-term basis. The development of IE as a result of everyday life bacteraemia may be determined by bacteraemia characteristics, themselves related to oral hygiene-habits and/or oro-dental status [18]. Furthermore, a trend towards an increased incidence of IE in the UK after the 2008-NICE guidelines were implemented, was recently reported, bringing back to the fore the possible role of dental procedures in the development of IE [19].
We hypothesized that oral hygiene habits and/or the oro-dental status of patients with IE caused by oral streptococci had peculiarities that could promote the development of IE, on top of everyday life bacteraemia. We therefore conducted a case-control study to examine the role of oral hygiene habits, oro-dental status, and the recent performance of dental procedures in the development of oral streptococcal IE as compared to IE caused by non-oral microorganisms.
Methods
Setting and subjects
This case-control study was conducted between May 2009 and January 2013 in 6 tertiary-care university hospitals in France. To minimize the potential role of confounders, we chose as controls instead of healthy subjects, patients with an IE caused by a non-oral microorganism. All consecutive adult patients with IE hospitalized in one of the participating centres were invited to participate in the study independently of patient and IE characteristics.
Definitions of cases and controls
Cases had a left-sided and/or right-sided definite IE due to oral streptococci, controls, IE due to a non-oral microorganism (supplementary methods) [20]. Right-sided definite IE in intravenous drug addicts were excluded in both groups. The microorganisms responsible for IE were identified in each local microbiological laboratory. Concordance between identifications in the local lab and in French national reference centre for streptococci had been assessed in a preliminary study of 162 streptococcal isolated which revealed a 97% concordance rate.
Data acquisition and definitions
Trained clinical research assistants prospectively collected clinical, biological and therapeutic IE data using a standardized case report form as previously described [21]. Each case report form was validated by an expert team as previously reported [20, 21].
Oral hygiene habits
Oral hygiene habits were recorded using a self-administered questionnaire which was filled out by the patient before the dental examination. This “oral hygiene” questionnaire collected information on frequency and conditions of toothbrushing (before or after meal) and interdental hygiene habits (use of toothpicks, of dental water jet, of flossing, and/or of interdental brush).
History of dental procedures
The history of dental procedures during the 3 months preceding IE diagnosis was obtained from the patients by a self-administered questionnaire and was cross-checked with the patient’s dentist whenever possible. We defined the day of IE diagnosis as the date of antibiotic initiation. When a subject had undergone more than one procedure during this period, that closest to the IE diagnostic date was considered.
Oro-dental status
Clinical and X-ray examinations of teeth and periodontal tissue were conducted by trained practitioners, blinded to the IE-causing microorganism and patient’s hygiene questionnaire. Using a standardized questionnaire, 12 teeth (first incisor, first premolar and first molar or adjacent teeth if absent, on each quadrant) were assessed by measuring gingival inflammation (derived from [22]), common dental plaque [23], and calculus (derived from [24]), periodontal probe above 4 mm, gingival inflammation, dental plaque and calculus and tooth status (see supplementary materials).
Statistical analysis
First, a descriptive analysis was performed, considering patients’ characteristics, hygiene, and oro-dental status. Categorical variables were summarized by frequency and percentages and compared using χ2 test. Continuous variables were summarized by mean and standard deviation (SD) and compared using Student’s t test. Non parametric Wilcoxon and Fisher’s exact tests were used to compare cases vs controls when parametric tests were not applicable.
Second, a multivariate logistic regression model was built to determine factors associated with being identified as a case. All variables with a P value of < 0.20 in the bivariate analysis were entered into a multivariate logistic regression with a forward stepwise approach with a sle=0.1 and sls=0.05 in two steps: first by subgroup for each questionnaire and then overall. Missing data for potential predictors were recorded in a modality “Unknown”. Age was categorized in two classes “<65 years of age”/”>=65 years of age” because of no adequacy with the model since Deviance test. Goodness-of-fit statistics were evaluated by Pearson Chi-square, deviance statistics. Interactions between explicative variables were tested. To estimate the proportion of IE due to a microorganism from the oral cavity that could be attributed to oral hygiene habits, oro-dental status and history of dental procedures respectively, we calculated the population-attributable risk (PAR%) for significant factors identified in multivariate analysis [25, 26]. We also performed sensitivity analyses, excluding IDU patients, or considering only dental procedures performed within 2 months prior to IE diagnosis.
Number of patients
We initially calculated that with a prevalence of a risk factor of 50%, a power of 90%, an 0.05 level of significance (2-sided), the inclusion of 450 patients (with a 1/2 case/control ratio, chosen because of an expected lower incidence of oral streptococcal IE) would allow the identification of factor associated with oral streptococci IE, with an odds ratio of 2 or above. As the accrual rate was slower than anticipated, the scientific committee advised stopping the recruitment after 3.5 years and the enrolment of 380 patients. It was then calculated that the inclusion of 73 cases and of 219 controls (1/3 ratio) would allow the identification of factors associated with oral streptococci, with an odds ratio of 2.6 or above considering the same power and level of significance.
Ethics
The study was approved by the appropriate ethics committee (Comité de Protection des Personnes Besançon, N° 09.227), and the French Data protection authority CNIL. In accordance with French law on non-interventional studies, only oral informed consent was required. The study is reported according to STROBE (Statement for Reporting case-control studies) guidelines [27].
Role of the funding source
The founders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Results
Patients’ characteristics
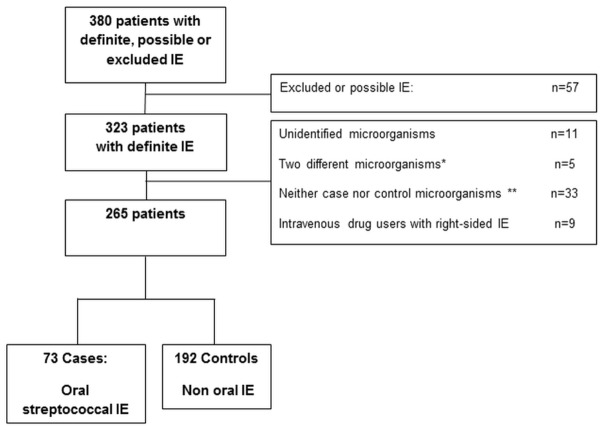
During the 57-month study period, 380 patients with IE were enrolled in the study, 323 of whom had a definite IE. Among these 323 patients, 11 had negative blood culture IE, 5 had an IE caused by two microorganisms (one oral and one non-oral), 33 patients had an IE caused by a microorganism which can be classified as oral or non-oral microorganisms and 9 patients were intravenous drug users with right-sided IE. In the remaining 265 patients, 73 patients were categorised as cases, and 192 as controls (extra oral origin) (Figure 1). Patients’ background characteristics, and IE features are presented in Table 1.
Figure 1.
Flowchart
Note:
IE: infective endocarditis
* Discordant microorganisms : Two microorganisms responsible for a single IE, from two different origins.
** See methods.
Table 1.
Background characteristics of case-patients and control-patients.
| Whole population | Cases Oral streptococcal IE | Controls Non oral IE | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 265 | 73 (27.5%) | 192 (73.4%) | |||||
| N* | %* | N* | %* | N* | %* | p | |
| Patients’ characteristics | |||||||
|
| |||||||
| Age, years (mean, SD) | 60.8 | 16.7 | 55.5 | 17.6 | 62.8 | 15.9 | 0.002 |
| Age ≥ 65 years | 123 | 46.4 | 21 | 28.8 | 102 | 53.1 | 0.001 |
|
| |||||||
| Male sex | 217 | 80.0 | 53 | 72.6 | 159 | 82.8 | 0.063 |
| At least one comorbidity | 94 | 35.5 | 15 | 20.5 | 79 | 41.1 | 0.002 |
|
| |||||||
| Diabetes mellitus | 48 | 18.1 | 9 | 12.3 | 39 | 20.3 | 0.132 |
|
| |||||||
| Cancer | 41 | 15.5 | 6 | 8.2 | 35 | 18.2 | 0.044 |
|
| |||||||
| Dialysis | 5 | 1.9 | 0 | 0 | 5 | 2.6 | 0.329 |
|
| |||||||
| Intravenous drug use | 6 | 2.3 | 0 | 0 | 6 | 3.1 | 0.019 |
|
| |||||||
| Active smoking | 51 | 19.8 | 15 | 21.7 | 36 | 19.0 | 0.631 |
|
| |||||||
| Cardiac history | |||||||
|
| |||||||
| Underlying valve diseases (HD) | 0.014 | ||||||
| Prosthetic valve | 67 | 25.3 | 18 | 24.7 | 49 | 25.5 | |
| Previously known native HD | 76 | 28.1 | 30 | 41.1 | 46 | 24.0 | |
| No previously known HD | 122 | 46.0 | 25 | 34.2 | 97 | 50.5 | |
| Pacemaker and/or implantable cardioverter defibrillator | 29 | 10.9 | 2 | 2.7 | 27 | 14.1 | 0.008 |
|
| |||||||
| History of IE | 21 | 7.9 | 7 | 9.6 | 14 | 7.3 | 0.536 |
Note:
unless otherwise specified
IE infective endocarditis
Bold value: statistically significant
Oral hygiene habits and history of dental procedures
Most patients (54.7%) reported brushing their teeth twice daily or more, 53.6% patients reported brushing teeth after meals. Interdental hygiene habits were reported by 39.6% patients. Dental procedures in the 3 months prior IE diagnosis were reported by 8.8% patients (Tables 2; S1, S2)
Table 2.
Oral hygiene habits and dental procedures in the prior 3 months in case-patients and control-patients.
| Whole population | Cases Oral streptococcal IE | Controls Non oral IE | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 265 | 73 (27.5%) | 192 (72.5%) | |||||
| N | % | N | % | N | % | p | |
| Patient oral hygiene habits | |||||||
| Tooth brushing frequency | 0.623 | ||||||
|
| |||||||
| More than twice daily | 37 | 16.2 | 9 | 13.6 | 28 | 18.1 | |
| Twice daily | 85 | 38.5 | 28 | 42.4 | 57 | 36.8 | |
| Once daily | 64 | 29.0 | 20 | 30.3 | 44 | 28.4 | |
| Less than once daily | 21 | 9.5 | 7 | 10.6 | 14 | 9.0 | |
|
| |||||||
| Tooth brushing after meal | 124 | 54.4 | 30 | 44.8 | 94 | 58.4 | 0.029 |
| Interdental hygiene habits | |||||||
|
| |||||||
| Toothpicks use | 64 | 29.2 | 24 | 36.9 | 40 | 26.0 | 0.154 |
| Dental water jet use | 10 | 4.5 | 5 | 7.6 | 5 | 3.2 | 0.194 |
| Flossing | 19 | 8.6 | 11 | 16.7 | 8 | 5.1 | 0.011 |
| Interdental brush | 23 | 10.6 | 9 | 14.1 | 14 | 9.1 | 0.274 |
| At least one of these behaviours | 89 | 39.6 | 37 | 55.2 | 52 | 32.9 | 0.006 |
|
| |||||||
| Dental procedures | |||||||
|
| |||||||
| In the 3 months prior IE* | 23 | 8.8 | 12 | 16.9 | 11 | 5.8 | 0.002 |
| In the 2 months prior IE | 19 | 7.3 | 11 | 15.5 | 8 | 4.2 | 0.002 |
| In the month prior IE | 7 | 2.7 | 3 | 4.2 | 4 | 2.1 | 0.393 |
Note:
IE infective endocarditis
Bold value: statistically significant
When considering only patients with a predisposing cardiac condition at high risk of IE (prosthetic valve, history of IE and cyanotic cardiopathy), dental procedures in the previous 3 months were performed in 5.5 % of cases vs 1% of controls (p=0.045); these figures were 12.3% and 1.5% when considering all patients with a IE predisposing cardiac condition (p<0.001) and 4.6% and 4% in patients without IE predisposing cardiac conditions (p=1).
Dental and periodontal status
Fourteen (6.2%) patients were totally edentulous. Gingival inflammation was noted in 62.3% of the patients, dental plaque in 39.9.0% and the presence of calculus in 11.8%; probing depth was above 4 mm in 28.4%. Data on dental caries, fractured teeth, impacted or partially erupted teeth are presented in Table 3.
Table 3.
Oro-dental status in case-patients and control-patients.
| Whole population | Cases Oral streptococcal IE | Controls Non oral IE | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 265 | 73 (27·5%) | 192 (72·5%) | |||||
| N | % | N | % | N | % | p | |
| Oro-dental status | |||||||
| Oral hygiene surrogate marker | |||||||
|
| |||||||
| Gingival inflammation* | 127 | 62.3 | 38 | 61.3 | 89 | 62.7 | 0.310 |
| Dental plaque* | 81 | 39.9 | 21 | 34.4 | 60 | 42.3 | 0.160 |
| Calculus* | 24 | 11.8 | 7 | 11.5 | 17 | 12.0 | 0.400 |
|
| |||||||
| Periodontal status* | |||||||
|
| |||||||
| Radiological periodontal disease | 70 | 31.8 | 22 | 32.4 | 48 | 31.6 | 0.893 |
| Probing depth > 4 mm | 58 | 28.4 | 17 | 27.4 | 41 | 28.9 | 0.359 |
|
| |||||||
| Dental diseases † | |||||||
|
| |||||||
| Dental caries | 120 | 54.3 | 35 | 51.5 | 85 | 55.6 | 0.574 |
| Apical or periapical focus of infection | 54 | 24.5 | 15 | 22.1 | 39 | 25.7 | 0.566 |
| Fractured tooth | 11 | 5.0 | 4 | 5.9 | 7 | 6.3 | 0.741 |
| Impacted tooth | 24 | 10.9 | 9 | 13.2 | 15 | 9.8 | 0.449 |
| Partially erupted tooth | 17 | 7.7 | 5 | 7.4 | 12 | 7.8 | 0.899 |
| Impacted root tooth | 39 | 17.6 | 12 | 17.6 | 27 | 17.6 | 1.000 |
| Pulpal necrosis | 9 | 4.1 | 6 | 8.8 | 3 | 2.0 | 0.026 |
| Suboptimal root canal treatment | 83 | 37.7 | 27 | 39.7 | 56 | 36.8 | 0.685 |
| Alveolar bone loss | 70 | 31.8 | 22 | 32.4 | 48 | 31.6 | 0.909 |
|
| |||||||
| Edentulous | 14 | 6.2 | 2 | 2.9 | 12 | 7.6 | 0.237 |
Note:
IE infective endocarditis
Bold value: statistically significant
See materials and methods
At least on tooth with the condition
Case-Control analysis
In bivariate analysis, cases were younger, less frequently male, more frequently had a known native valve disease, and less frequently had a pacemaker and/or implantable cardioverter defibrillator than controls (Tables 2, 3, 4). Self-reported oral hygiene habits were different in cases and controls: toothbrushing after meals was less frequent in cases than in controls (44.8% vs. 58.4%, p=0.029), whereas use of toothpicks, and/or dental water jet and/or flossing was more frequent (55.2% vs. 32.9%, p=0.006). Pulpal necrosis was more frequently observed in cases than in controls (8.8% vs. 2.0%, p=0.026). Dental procedures had been performed during the previous 3 months in 16.9% of cases vs 5.8% of controls (p=0.002) (Table S1, S2).
Table 4.
Factors associated with definite oral streptococcal infective endocarditis in 265 patients.
| Bivariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Odds ratio | 95% CI | p | Odds Ratio | 95% CI | p | |||
|
|
|
|||||||
| Inf | Sup | Inf | Sup | |||||
| Age | <0.001 | 0.004 | ||||||
|
| ||||||||
| < 65 years | 2.81 | 1.57 | 5.02 | 2.85 | 1.41 | 5.76 | ||
| ≥65 years | 1 | 1 | ||||||
|
| ||||||||
| Gender | 0.069 | 0.016 | ||||||
|
| ||||||||
| Male | 1 | 1 | ||||||
| Female | 1.82 | 0.96 | 3.44 | 2.62 | 1.20 | 5.74 | ||
|
| ||||||||
| Preexisting cardiac conditions ~ | 0.009 | 0.043 | ||||||
|
| ||||||||
| Valvular prosthesis | 1.43 | 0.71 | 2.86 | 2.13 | 0.91 | 4.96 | ||
| Native valve diseases | 2.53 | 1.34 | 4.78 | 2.44 | 1.16 | 5.13 | ||
| None | 1 | 1 | ||||||
|
| ||||||||
| Pacemaker and/or implantable cardioverter defibrillator | 0.004 | |||||||
|
| ||||||||
| No | 1 | |||||||
| Yes | 0.17 | 0.04 | 0.74 | |||||
| Patient oral hygiene habits | <0.001 | <0.001 | ||||||
| No interdental manipulation | ||||||||
| With Tooth brushing after meal | 1 | 1 | ||||||
| Without tooth brushing after meal | 3.56 | 1.45 | 8.72 | 5.51 | 2.05 | 14.82 | ||
| Interdental manipulation | ||||||||
| With tooth brushing after meal | 4.05 | 1.67 | 9.84 | 3.48 | 1.30 | 9.32 | ||
| Without tooth brushing after meal | 5.83 | 2.20 | 15.41 | 8.32 | 2.70 | 25.58 | ||
| Unknown | 1.16 | 0.38 | 3.53 | 0.63 | 0.16 | 2.49 | ||
| Edentulous | 1.09 | 0.21 | 5.71 | 2.00 | 0.35 | 11.57 | ||
|
| ||||||||
| Pulpal necrosis | 0.001 | 0.017 | ||||||
|
| ||||||||
| No | 1 | 1 | ||||||
| Yes | 4.84 | 1.17 | 19.96 | 2.80 | 0.49 | 15.99 | ||
| Unknown | 0.31 | 0.12 | 0.82 | 0.27 | 0.09 | 0.76 | ||
| Dental procedures within the 3 preceding months* | 0.005 | 0.023 | ||||||
| No | 1 | 1 | ||||||
| Yes | 3.33 | 1.40 | 7.94 | 3.31 | 1.18 | 9.29 | ||
Note: CI : confidence interval - Inf : lower limit - Sup : Upper limit; Interdental habits include toothpicks use, and/or dental water jet use and/or interdental brush and/or flossing
Dental procedures within the 2 preceding months (Odds ratio: 4.86 95% CI 1.58–14.9, p=0.00
In multivariate analysis, cases were more likely than control-patients to be aged < 65 years (OR: 2.85; CI 95% 1.41 to 5.76), to be female (OR: 2.62; CI 95%: 1.20 to 5.74), to have a known native valve disease (OR: 2.44; CI 95%: 1.16 to 5.13). To take into account the interaction between brushing teeth after meals and interdental “hygiene” habits (use toothpicks, and/or dental water jet and/or interdental brush and/or flossing), composite combined variables were created. Cases were also more likely than controls to have interdental hygiene habits, not to brush teeth after meals (Table 4), or to have undergone invasive dental procedures during the previous three months (OR: 3.31; CI 95%:1.18 to 9.29). Periodontal status (gingival inflammation, calculus, probing depth and alveolar bone loss) was not significantly different in cases and controls. Goodness of fit statistics were far from statistical significance. Considering the population-attributable risk analysis (PAR%), dental procedures within the preceding 3 months explained 16.8% of streptococcal IE whereas incorrect and/or lack of oral hygiene habits explained 63.3% of them.
Discussion
In this case control study, we assessed simultaneously the different potential risk factors for IE due to oral streptococci, i.e. patients’ background characteristics, oral hygiene habits, oro-dental status, and history of dental procedures during the previous 3 months. We showed that, compared with patients with IE due to non-oral pathogens, patients with oral streptococcal IE more frequently had a native valve disease, performed interdental oral hygiene habits, had recent history of dental procedures and less frequently brushed their teeth after meals, but did not differ in the studied oro-dental and periodontal status.
To minimise confounding factors we purposely chose controls among individuals suffering from IE. In most preceding studies, controls were individuals without IE, originating in the community or hospitalized on cardiology wards for a reason other than IE [5–7]. To have chosen as controls patients originating in the community would have led to a minimizing of the role of factors other than PCC which, while a rare condition, is nonetheless the most important IE risk factor. Conversely, to have chosen controls among known PCC patients would have eliminated patients corresponding to those developing IE without known PCC. This is crucial as approximately half of current patients with IE (46% in our study) have no previously known PCC upon IE diagnosis. The way we defined controls (a group of IE patients) made possible a global analysis of IE-associated factors, taking into account the cardiac conditions (previously known or not) which favour the implantation of the circulating microorganism onto the damaged valve. We minimised selection biases by enrolling all consecutive IE patients in the 6 participating centres. Whereas the study was conducted in tertiary-care hospitals specialized in IE with dental units, characteristics of IE cases and control-patients did not differ significantly compared to corresponding IE from the 2008 population-based study conducted in France in the 6 corresponding regions, except for age of case-patient (statistically lower in our case-control study), and rate of individuals without previously known IE PCC (lower in our case-patients - data not shown). Furthermore, patients characteristics we found associated with oral streptococci are those reported in the literature in the oral streptococci IE population [1]. Therefore, we believe that the results of the present case-control study can be extended to other IE patients.
This study enabled us to assess oral hygiene practices in a large proportion of IE-patients, which are rarely assessed to date [28]. The proportion of toothbrushing patient and daily frequency of toothbrushing did not differ between groups; however, toothbrushing after meals was less frequently reported in cases than in controls, which behaviour could favour the persistence of microorganisms carried by food in the oral cavity in cases. It can be compared to the higher rate of tooth micro-trauma such as those induced by interdental hygiene habits including uses of toothpick, dental water jet, flossing and interdental brush, all of which were more frequently reported in case-patients than in control-patients. These micro-trauma have been identified as inducing viridans streptococcal bacteraemia, in proportions which are quite similar to those of invasive oral procedures for which antibiotic prophylaxis is recommended [29]. Of note, in the case-control study conducted by Strom, which compared 287 IE patients whatever the causative microorganism (33.1 % of the total cohort had viridans streptococcal IE), authors found no statistical differences in the practice of flossing as compared to a control population of healthy US individuals [6]. Our study results support the contention that oral hygiene habits (deficiency of some, or use of others, inducing everyday life bacteraemia) can cause IE of oral origin.
We also compared oro-dental status in both groups based on thorough dental exam performed by practitioners unaware of the microorganism responsible for IE. The literature presents conflicting results concerning the relationship between gingival or periodontal disease and the risk of bacteraemia after tooth extraction [18, 30, 31]. After toothbrushing, Lockhart et al. reported in individuals without IE visiting a hospital-based dental service, a higher risk of viridans streptococcal bacteraemia in those patients with high dental plaque and calculus scores [18]; among five gingival inflammation parameters measured, only one (bleeding with toothbrushing) was also associated with viridans streptococcal bacteraemia. In our study, we found no differences concerning either calculus score or gingival inflammation between cases and controls suggesting that the increased risk of IE-associated bacteraemia noted by Lockhart in patients with poor oral hygiene may be insufficient to induce endocarditis. Pulpal necrosis, a rare condition, was more frequently noted in case-patients, albeit only statistically significant in bivariate analysis. Of note, dental plaque, the precursor of calculus, was less frequently noted in cases than in controls. As dental plaque is an overly-sensitive marker of imperfect oral-hygiene, its analysis is complex in hospitalized and bed-ridden patients who frequently modify personal oral hygiene practices during hospitalisation. We thus decided not to consider this parameter in the multivariate analysis.
Finally, we also studied history of dental procedures within the preceding 3 months, to take into account all potential IE risk factors. Three previous large case-control studies with different designs looked at the relation-ship between dental care and IE [5–7]. All were conducted before the implementation of Duke criteria. No previous study restricted their cases to IE patients with microorganisms originating in the oral cavity. Among these 3 studies, only ours performed in 1990 has found a relationship between scaling history and the occurrence of streptococcal IE [5]. In the present study, although a rare condition (16.9% of case-patients), dental procedures were statistically more frequent in cases than in control, when considering procedures performed within the 3 preceding months. Only a fraction of these procedures was performed in patients for whom antibiotic prophylaxis is currently recommended by most guidelines. In our study, the attributable risk of oral streptococcal IE due to dental procedures was far lower than that of oral hygiene. Incidentally, the numbers we report here confirm that antibiotic prophylaxis for IE during dental procedures, even if effective, could prevent only a very small proportion of all cases.
Limitations
Our study has some limitations. First, we did not assess all habits that could interfere with the risk of bacteraemia, such as the use of oral antiseptic mouthwash use, and chewing gum. Second, we assessed dental hygiene and oro-dental status after the IE onset and not before. We can wonder to what extent dental care performed before IE (and suspected of inducing IE) might have modified oral status analysed in our study; however, the sensitivity analysis excluding patients with dental care in the 3 preceding months did not modify our results concerning oral status (data not shown). Finally, we considered a time interval of 3 months between dental procedure and the IE which may be too long. However, in a study analysing the time interval between the first symptoms of IE and the IE diagnosis, we showed that this time interval was greater than 1 month in 36 % of the oral streptococcus IE which is concordant with the results of the sensitivity analyses [32].
In conclusion, this case-control study shows that several parameters independently contribute to the development of oral streptococcal IE, reconciling the partisans of the everyday-life bacteraemia theory, and those of the post-invasive procedures bacteraemia theory. Among these promoting factors, orodental hygiene (overuse of lack of hygiene) is by far the most predominant one. IE is a multifactorial disease, with multiple promoting factors which must all be considered in a global IE prevention strategy [33].
Summary.
Patients with IE caused by microorganisms originating in the oral cavity do differ from other IE patients, mainly because of different oral hygiene habits (higher use of interdental manipulations, less toothbrushing after meals) but also because of a slightly higher rate of recent dental procedures.
Acknowledgments
Funding: French ministry of Health; Société Française de Cardiologie and Fédération Française de Cardiologie (grant 2009) ; Inserm XM/GB/2009-051.
Abbreviation list
- IE
infective endocarditis
- UK
United Kingdom
- NICE
National Institute for Health and Care Excellence
- PCC
Predisposing cardiac condition
Footnotes
Conflict of interest statement:
The authors have no commercial or other associations that might pose a conflict of interest
EI-dent Study group:
Principal investigators:
X. Duval; B. Hoen; Other members: F. Alla, A. Bouvet, C. Chirouze, T. Doco-Lecompte, B. Iung, C. Selton-Suty, C. Strady, P. Tattevin.
Clinical centres:
Besançon: Catherine Chirouze, Elodie Curlier, Cécile Descottes-Genon, Edouard Euvrad, Bruno Hoen, C. Meyer, Isabelle Patry, Lucie Vettoretti. Nancy: Nejla Aissa, Aurelie Bannay, Thanh Doco-Lecompte, François Goehringer, Nathalie Keil, Lorraine Letranchant, Hepher Malela, Vanessa Moby, Christine Selton-Suty, A Westphal Paris: N. Benyounes Marie-Pierre Bretheaux, S. Broda, Xavier Duval, C. Fargou, Emila Ilic Habensus, Bernard Iung, Simone Imbert, Sarah Millot, Emmanuelle Cambau, Antoine Andremont, Clément Messeka, Catherine Leport, Daniel Thomas, François Bricaire, Jean Chastre, Jean-Louis Trouillet, Patrick Yeni, Michel Wolff. Reims: G. Bourgeois, Pierre Nazeyrollas, Véronique Vernet, Christophe Strady; Rennes : Gilbert De Mello, Caroline Piau, Audrey Brener, Hélène Martin-Thomé, Christian Michelet, Matthieu Revest, Pierre Tattevin, Elise Thébault.
Coordination and statistical analyses:
François Alla, Marie-Line Erpelding, Nelly Agrinier.
NOTES
Author contributions
XD, BH, FA designed the study
XD, SM, SM, CSS, VM, PT, CS, EE, DT, BH collected the data
FA, NA performed statistical analyses
SM, CC, CSS, VM, PT, CS, EE, NA, DT made a Critical revision of the manuscript for important intellectual content
XD, BH, CSS, FA obtained funding
XD, BH, FA wrote the article
References
- 1.Hoen B, Duval X. Infective endocarditis. N Engl J Med. 2013;368:1425–1433. doi: 10.1056/NEJMcp1206782. [DOI] [PubMed] [Google Scholar]
- 2.Verhagen DW, Hermanides J, Korevaar JC, et al. Health-related quality of life and posttraumatic stress disorder among survivors of left-sided native valve endocarditis. Clin Infect Dis. 2009;48:1559–65. doi: 10.1086/598930. [DOI] [PubMed] [Google Scholar]
- 3.Baddour LM. Prophylaxis of infective endocarditis: prevention of the perfect storm. Int J Antimicrob Agents. 2007;30(Suppl 1):S37–41. doi: 10.1016/j.ijantimicag.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015 doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 5.Lacassin F, Hoen B, Leport C, et al. Procedures associated with infective endocarditis in adults. A case control study. Eur Heart J. 1995;16:1968–74. doi: 10.1093/oxfordjournals.eurheartj.a060855. [DOI] [PubMed] [Google Scholar]
- 6.Strom BL, Abrutyn E, Berlin JA, et al. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann Intern Med. 1998;129:761–9. doi: 10.7326/0003-4819-129-10-199811150-00002. [DOI] [PubMed] [Google Scholar]
- 7.Van der Meer JT, Van Wijk W, Thompson J, Vandenbroucke JP, Valkenburg HA, Michel MF. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet. 1992;339:135–9. doi: 10.1016/0140-6736(92)90207-j. [DOI] [PubMed] [Google Scholar]
- 8.Danchin N, Duval X, Leport C. Prophylaxis of infective endocarditis: French recommendations 2002. Heart. 2005;91:715–8. doi: 10.1136/hrt.2003.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 10.Stokes T, Richey R, Wray D. Prophylaxis against infective endocarditis: summary of NICE guidance. Heart. 2008;94:930–1. doi: 10.1136/hrt.2008.147090. [DOI] [PubMed] [Google Scholar]
- 11.Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol. 2006;33:401–7. doi: 10.1111/j.1600-051X.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts GJ. Dentists are innocent! “Everyday” bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr Cardiol. 1999;20:317–25. doi: 10.1007/s002469900477. [DOI] [PubMed] [Google Scholar]
- 13.Durack DT. Prevention of infective endocarditis. N Engl J Med. 1995;332:38–44. doi: 10.1056/NEJM199501053320107. [DOI] [PubMed] [Google Scholar]
- 14.Duval X, Leport C. Prophylaxis of infective endocarditis: current tendencies, continuing controversies. Lancet Infect Dis. 2008;8:225–32. doi: 10.1016/S1473-3099(08)70064-1. [DOI] [PubMed] [Google Scholar]
- 15.Glenny AM, Oliver R, Roberts GJ, Hooper L, Worthington HV. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst Rev. 2013;10:CD003813. doi: 10.1002/14651858.CD003813.pub4. [DOI] [PubMed] [Google Scholar]
- 16.Veloso TR, Amiguet M, Rousson V, et al. Induction of experimental endocarditis by continuous low-grade bacteremia mimicking spontaneous bacteremia in humans. Infect Immun. 2011;79:2006–11. doi: 10.1128/IAI.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duval X, Alla F, Hoen B, et al. Estimated risk of endocarditis in adults with predisposing cardiac conditions undergoing dental procedures with or without antibiotic prophylaxis. Clin Infect Dis. 2006;42:e102–7. doi: 10.1086/504385. [DOI] [PubMed] [Google Scholar]
- 18.Lockhart PB, Brennan MT, Thornhill M, et al. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J Am Dent Assoc. 2009;140:1238–44. doi: 10.14219/jada.archive.2009.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet. 2015;385:1219–1228. doi: 10.1016/S0140-6736(14)62007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 21.Selton-Suty C, Celard M, Le Moing V, et al. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230–9. doi: 10.1093/cid/cis199. [DOI] [PubMed] [Google Scholar]
- 22.Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 23.Silness J, Loe H. Periodontal Disease in Pregnancy. II. Correlation between Oral Hygiene and Periodontal Condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 24.Ramfjord SP. Indices for prevalence and incidence of periodontal disease. J Periodontol. 1959;30:51–59. [Google Scholar]
- 25.https://www.hsph.harvard.edu/donna-spiegelman/software/par/.
- 26.Spiegelman D, Hertzmark E, Wand HC. Point and interval estimates of partial population attributable risks in cohort studies: examples and software. Cancer Causes Control. 2007;18:571–9. doi: 10.1007/s10552-006-0090-y. [DOI] [PubMed] [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Strom BL, Abrutyn E, Berlin JA, et al. Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation. 2000;102:2842–8. doi: 10.1161/01.cir.102.23.2842. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Daly CG, Mitchell D, Curtis B. Incidence and magnitude of bacteraemia caused by flossing and by scaling and root planing. J Clin Periodontol. 2013;40:41–52. doi: 10.1111/jcpe.12029. [DOI] [PubMed] [Google Scholar]
- 30.Lockhart PB. An analysis of bacteremias during dental extractions. A double-blind, placebo-controlled study of chlorhexidine. Arch Intern Med. 1996;156:513–20. [PubMed] [Google Scholar]
- 31.Lockhart PB, Brennan MT, Kent ML, Norton HJ, Weinrib DA. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation. 2004;109:2878–84. doi: 10.1161/01.CIR.0000129303.90488.29. [DOI] [PubMed] [Google Scholar]
- 32.N’Guyen Y, Duval X, Revest M, et al. Time Interval between Infective Endocarditis First Symptoms and Diagnosis: Relationship to Infective Endocarditis characteristics, Microorganisms and Prognosis. Ann Med. 2016:1–28. doi: 10.1080/07853890.2016.1235282. [DOI] [PubMed] [Google Scholar]
- 33.Duval X, Hoen B. Prophylaxis for infective endocarditis: let’s end the debate. Lancet. 2015;385:1164–5. doi: 10.1016/S0140-6736(14)62121-8. [DOI] [PubMed] [Google Scholar]