Abstract
The culture of mast cells from human tissues such a cord blood, peripheral blood or bone marrow aspirates has advanced our understanding of human mast cells (huMC) degranulation, mediator production and response to pharmacologic agents. However, existing methods for huMC culture tend to be laborious and expensive. Combining technical approaches from several of these protocols, we designed a simplified and more cost effective approach to the culture of mast cells from human cell populations including peripheral blood and cryopreserved cells from lymphocytapheresis. On average, we reduced by 30–50 fold the amount of culture media compared to our previously reported method, while the total MC number generated by this method (2.46 ± 0.63 × 106 vs. 2.4 ± 0.28 × 106, respectively, from 1.0 × 108 lymphocytapheresis or peripheral blood mononuclear blood cells [PBMCs]) was similar to previous method (2.36 ± 0.70 × 106), resulting in significant budgetary savings. In addition, we compared the yield of huMCs with or without IL-3 added to early cultures in the presence of stem cell factor (SCF) and interlukin-6 (IL-6) and found that the total MC number generated, while higher with IL-3 in the culture, did not reach statistical significance, suggesting that IL-3, often recommended in the culture of huMCs, is not absolutely required. We then performed a functional analysis by flow cytometry using standard methods and which maximized the data we could obtain from cultured cells. We believe these approaches will allow more laboratories to culture and examine huMC behavior going forward.
Keywords: Human mast cell culture, mast cell progenitors, lymphocytapheresis, SCF, IL3, flow cytometry
1. Introduction
The understanding of human mast cell biology has advanced in part through the study of mast cells cultured from human tissues where they are derived from precursor cells[1–4]. To obtain these human MCs (huMCs) for research, a number of groups including ours have reported methods for in vitro huMC culture using bone marrow, peripheral whole blood or umbilical cord blood as the source of progenitors [3, 5–7]. However, these methods tend to be laborious while generating relatively few mast cells for study.
Here, we present an efficient and cost effective method for generating functional huMCs from CD34+ cells isolated from peripheral blood that has been optimized to scale-down the amount of culture media and growth factors required and which requires less effort, while producing similar yields of mast cells. Furthermore, we demonstrate that huMC can be obtained in comparable numbers from cryopreserved lymphocytapheresis samples of normal subjects, a source that may be more effective and accessible over time compared to starting from fresh blood withdrawals with their associated time and cost. Cytochemistry staining of these cultures and functional analysis by flow cytometry indicated that the cell characteristics and responses were similar to mast cells obtained using our previously standardized method.
2. Methods
2.1. Human sample collection and processing
Collection of heparinized whole blood (100 ml) and lymphocytapheresis were performed on healthy adult volunteers after informed consent was obtained under protocols approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases (protocols 2009-I-0049 and 10-I-0196). Lymphocytapheresis was performed with a continuous-flow COBE Spectra cell separator (Gambro BCT, Lakewood, CO) in the Department of Transfusion Medicine (DTM), NIH, and where approximately 5 liters of blood was processed over approximately 2 hours. The final volume of depleted cells approximated 100 ml.
Peripheral blood mononuclear cells (PBMCs) from whole blood (diluted with 1x volume of PBS) and cells from lymphocytapheresis (diluted with 2x volume of HBSS [Biosource, Rockville, MD]) were isolated by density gradient centrifugation using Lymphocyte Separation Medium (MP Biomedical, Aurora, Ohio)[8]. Briefly, thirty ml of the diluted blood or cells from lymphocytapheresis was layered over 12 ml of Ficoll Paque and centrifuged at 400 × g for whole blood cells and 800 × g for cells from lymphocytapheresis for 20 min at room temp. Mononuclear cells were collected from the interphase and washed twice with PBS, centrifuging the cells each time at 300 × g for 10 min at room temp. PBMCs were cryopreserved with freeze medium (RPMI 1640 with 10% human albumin and 10% DMSO) in a freezing container (Thermo Scientific) overnight at −80°C and then transferred to a −140°C liquid nitrogen freezer (100 ×106 cells/vial) until use. Approximately 45–50 cryovials could be prepared from one lymphocytapheresis procedure while only one cryovial (100 ×106 cells/vial) could be prepared from 100 ml whole blood.
2.2. Progenitor cell enrichment
Peripheral blood progenitor cells were enriched from PBMCs with EasySep™ Human Progenitor Cell Enrichment Kit (Stemcell Technologies, Vancouver, BC) following the manufacturer’s instructions. Briefly, 100×106 PBMCs were thawed over 1 to 2 min in a 37°C water bath, washed once with 20 ml of PBS and once with reaction buffer (PBS with 2 % FBS and 1 mM EDTA) in a 50-ml conical tube. After centrifugation at 300 × g for 10 min at room temp, cells were re-suspended in 2 ml reaction buffer and incubated with 100 μl of progenitor cell enrichment antibodies cocktail (supplied in the progenitor cell enrichment kit) at room temp for 15 min. Magnetic nanoparticles (100 μl) were added to the cells, mixed and incubated at room temp for 15 min. After addition of 300 μl of reaction buffer to bring the total volume to 2.5 ml, tubes were placed into the EasySep™ magnet for 10 min at room temp for immunomagnetic separation of unwanted cells expressing the targeted surface markers. The negative, unbound fraction of the cells, which is enriched in CD34+ cells, was collected. Remaining non-CD34+ cells in this un-bound fraction were largely undefined and not identified with antibodies to CD2, CD3, CD14, CD19, and CD56.
2.3. Cell Culture
The enriched progenitor cells were suspended at 5×105 cells/ml with mast cell culture medium (MC medium) [StemPro-34 Medium (Invitrogen, Grand Island, NY) supplemented with 100 ng/ml human recombinant SCF, 100 ng/ml human rIL-6 (R&D Systems, Minneapolis, MN), 100 μg/ml penicillin/streptomycin and 2 mM L-glutamine (Invitrogen, Grand Island, NY)][9]. Then 0.5 ml of the cell suspension was added to each of the 8 inner side wells of a 24-well plate (Corning, NY) (2.5 × 105 cell/well) and 1 ml of PBS was added to each of the outer side wells to maintain humidity and prevent evaporation of the inner wells containing cells. On day 0 of the culture, 30 ng/ml human rIL-3 (PeproTech, Rocky Hill, NJ) was added to some cultures. Plates were then cultured for 2 weeks at 37°C, under 5 % CO2 with no change of media. At the end of week 2, 0.5 ml of fresh MC medium was added into each of the 8 inner wells of the culture plate and the cells cultured for 2 additional weeks with no change of media. At the end of week 4, 1 ml of MC medium was added into each of the center 8 wells of the culture plate and incubated for 2 additional weeks. At the end of week 6, cells were harvested from the center 8 wells, pooled, and centrifuged at 250 × g for 5 to 10 min. Supernatant was then removed and the pellet was resuspended in 4 ml of MC medium. Four ml of cells were then plated into a 6-well plate (Corning, NY) and incubated at 37°C and 5 % CO2 for one week to allow for increased cell expansion. At week 7, mast cells underwent analysis by flow cytometry.
2.4. Flow cytometry
An LSRII (Becton Dickinson, San Joes, CA) was used for flow cytometry. Briefly, 1 to 2×105 cells taken from the culture at indicated time points were washed once with PBS and resuspended in 200 μl of aqua staining buffer (live/dead fixable aqua dead cell stain kit, Invitrogen, CA) (1 μl aqua in 2 ml of PBS) at room temp in the dark for 20 min. Cells were washed once with 1 ml of PBS containing 3% FBS (PBS-3%FBS) and centrifuged at 300 × g for 5 min at room temp. Cells were incubated with 50 μl of an antibody cocktail containing APC-FcεRI (Biolegend, CA) and PE-CD117 (Becton Dickinson, CA) in PBS-3% FBS for 30 min at room temp. Cells were then washed twice with 1 ml of PBS and resuspended in 250 μl of PBS. Cells were acquired on an LSR II flow cytometer and analyzed using FlowJo software (TreeStar, Inc, Ashton, OR)[10].
For analysis of mast cell activation, cultured cells were sensitized overnight with 200 ng/ml human myeloma IgE (Millipore, MA) and then stimulated with 200 ng/ml anti-IgE (KPL, Gaithersburg, MD) for 1 or 2 hours at 37 °C, under 5% CO2[9]. Cells were harvested, washed once with PBS and resuspended in 200 μl aqua staining buffer (live/dead fixable aqua dead cell stain kit, Invitrogen, CA) (1 μl aqua in 2000 μl of PBS) at room temp in the dark for 20 min. Cells were washed once with 1 ml of PBS containing 3% FBS at 300 × g for 5 min at room temperature and incubated with the 50 μl of antibody cocktail containing FITC-Avidin, PE-CD117 (BD, CA), Percp-Cy5.5-CD63, and BV421-FcεRI (Biolegend, CA) in PBS-3%FBS for 30 min at room temp. Cells were washed once with 1 ml PBS and resuspended in 200 μl PBS. Some activated cells were fixed with 4% paraformaldehyde. Intracellular cytokine staining was performed with PE-Cy7-IL-8 (Biolegend), Alexa fluor700-TNFα and Brilliant Violet 421-IL-6 (BD bioscience) antibodies [10]. These cells were then analyzed by flow cytometry.
2.5. Beta-hexosaminidase assay
Cultured mast cells (2.5 × 105) were sensitized overnight with biotinylated-human IgE (100 ng/ml) in cytokine-free StemPro-34 SFM medium and rinsed with HEPES buffer (10 mM HEPES pH 7.4, 137 mM NaCl, 27 mM KCl, 0.4 mM Na2HPO4, 5.6 mM glucose, 1.8 mM CaCl2, 1.3 mM MgSO4.) containing 0.04 % bovine serum albumin. Ten thousand cells per well were plated in 96-well plates. The cells were then stimulated with 1, 10 or 100 ng/ml streptavidin in the presence or absence of 100 ng/ml SCF. After 30 min, plates were centrifuged and β-hexosaminidase (β-hex) assayed in the supernatants and cell pellets as described[11, 12]. Degranulation was calculated as the percentage of β-hex recovered from the supernatants compared to total cellular content.
2.6. Giemsa staining
Slides of huMCs were obtained using a Shandon Cytospin-3 (GMI, Ramset, MI). One hundred μl of cell suspension was added per cytofunnel (Fisher Scientific) and cytocentrifuged at 250 × g for 5 min to form a thin layer of MCs. Slides were air-dried and stained with Giemsa solution using a Hema-Tek 2000 automated slide stainer (Bayer, Elkhart, IN)[13]. Stained slides were pictured with a Zeiss microscope (Carl Zeiss, Germany) at 400× magnification.
2.7. Statistics
All results are expressed as mean ± standard deviation. Significance was determined using Students t-test or 2-way Anova analysis. P<0.05 was considered significant.
3. Results
3.1. Mast cell progenitors
The average purity of CD34+ cells after progenitor enrichment of 100 ×106 PBMCs from whole blood or lymphocytapheresis was, respectively, 5.49 ± 0.89% (lymphocytapheresis, N=5) and 4.24 ± 0.86% (whole blood, N=5) of the total enriched cells (Fig. 1A and B). The total numbers of cells after enrichment were also similar regardless of the origin of the PBMCs (2.43 ± 0.10 ×106 from lymphocytapheresis and 2.81 ± 0.72 ×106 from whole blood). Mast cell progenitors (MCP) are reported to co-express CD34, CD117 and FcεRI[14]. Among CD34+ enriched cells, no differences were observed in the number of MCP obtained from either lymphocytapheresis (4.14%; N=4) or whole blood (3.48%; N=3) (Fig 1C; gating strategy shown in Supplementary Fig 1A).
Fig. 1.
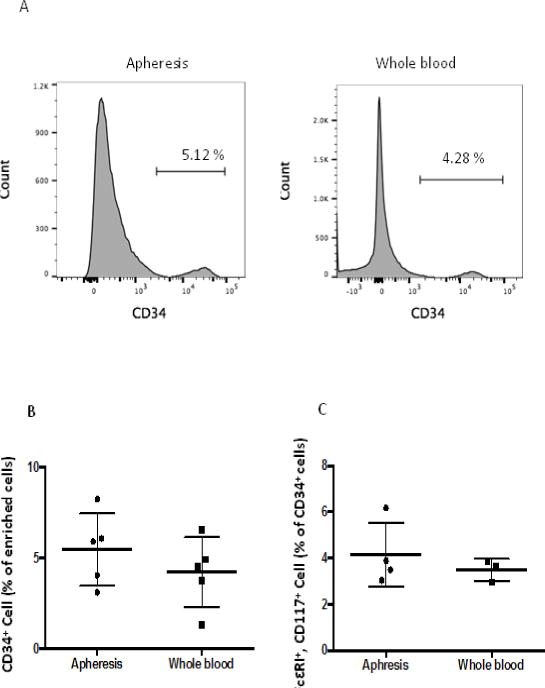
Progenitor cells in PBMCs. Progenitor cells from cryopreserved PBMCs (100 ×106 cells from lymphocytapheresis) (apheresis) and PBMCs from 100 ml of whole blood were enriched with a progenitor cell enrichment kit. N=5 (lymphocytapheresis), N=5 (whole blood). A. CD34+ cells analyzed by flow cytometry after progenitor cell enrichment. B. Statistical analysis on progenitor cells after enrichment. P=0.55. C: Percent of FceRI+, CD117+ cells in CD34+ population after progenitor cell enrichment. FcεRI+, CD117+ cells were calculated as percent of total CD34+ cells.
3.2. CD117+ FcεRI+ cells
We monitored cultures every 1–2 weeks for cells expressing the huMC markers CD117 and FcεRI after gating (Fig 2A–B). There were 10 ± 4.2% (lymphocytapheresis) and 9.2 ± 3.8% (whole blood) CD117+, FcεRI+ double positive cells at the end of the first week of culture. CD117+, FcεRI+ double positive cells increased over time (Fig. 2C). At the end of week 7, mast cell numbers were similar (2–3×106), whether starting with whole blood or lymphocytapheresis (Fig 2D) or by using previously reported method (Supplementary Fig 2A).
Fig. 2.
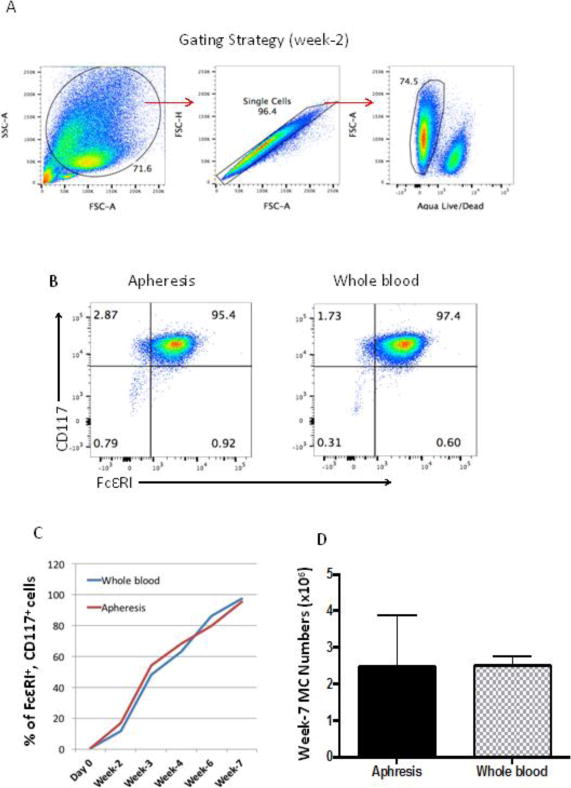
FcεRI and CD117 expression on cultured cells. N=5 (lymphocytapheresis), N=3 (whole blood). Enriched progenitor cells were suspended in MC medium at 5×105/ml with 30 ng/ml of human IL 3 and cultured as described. A. Gating strategy for analysis by flow cytometry. Cells were gated based on forward scatter and side scatter. Then singlet cells were gated followed by live cell gating. Negative cells on aqua live/dead staining represent live cells. In the culture of human mast cells, other cell types die out in the absence of specific growth factors. Mast cells survive, but total cell culture numbers always decrease over time and show as dead cells on flow. B. Flow cytometry analysis of 7-week cultured huMCs for expression of CD117 and FcεRI. C: Percent of CD117+, FcεRI+ cells over time in culture. D. Total number of mast cells at week 7 (Mean ± SD).
3.3 Morphology and functional analysis
huMCs observed at 7 weeks from whole blood or lymphocytapheresis samples were consistently round and of approximately 12.96 ± 1.18 μm in diameter. (Fig 3A). Giemsa staining of cytocentrifuged huMCs reveals granulated cells (Fig 3B) consistent with the similarities in SSC/FSC in the previous FACS analysis. To determine whether cultured huMCs are functionally reactive to stimulation through FcεRI, biotinylated human IgE was used to sensitize MCs overnight. Streptavidin (antigen) was then added to aggregate IgE receptors which resulted in β-hex release (Fig 3C). Addition of SCF enhanced β-hex release consistent with previous reports [15]. IL-8, TNFα and IL-6 cytokines in week 7 MCs were also measured by flow cytometry. IL-8 production after antigen stimulation through IgE receptor was clearly observed, with less TNFα and IL-6 detected under these conditions (Fig 3D). Degranulation was comparable between the previous culture method and the new method (Supplementary Fig 2B)
Fig. 3.
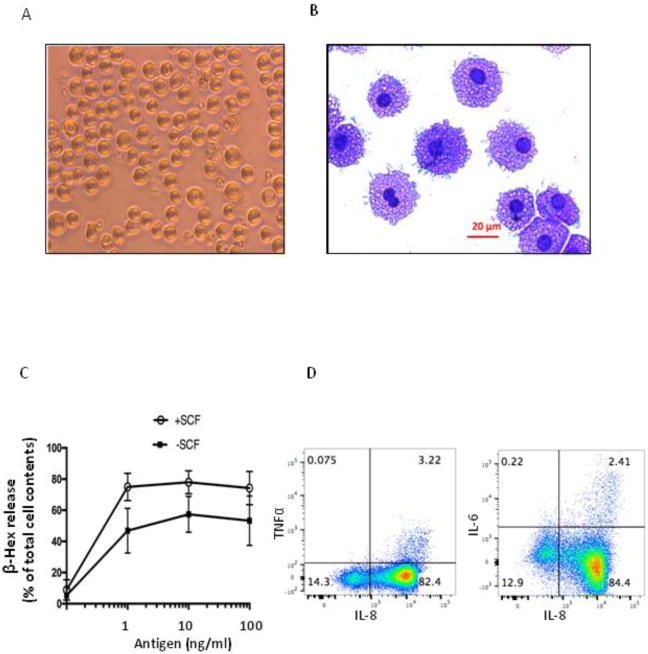
Morphology and functional assay of cells cultured for 7 weeks. A. Appearance of huMCs cultured from lymphocytapheresis at 7 weeks as visualized through an inverted scope at 200× magnification. Cultured cells were uniform in appearance. B. Cells cultured for 7 weeks and stained with Giemsa (400× magnification). Cells were again similar in appearance. C. Cells cultured for 7 weeks were sensitized overnight with biotin conjugated human IgE (100 ng/ml) and stimulated with increasing concentrations of streptavidin (antigen) for 30 min. Degranulation was monitored by β-hex release. Results are the average of three independent assays. P=0.0006 by 2-way ANOVA. D. Cells cultured for 7 weeks were sensitized overnight with human myeloma IgE (200 ng/ml) and then stimulated with 200 ng/ml anti-IgE combined with 10 μg/ml brefeldin A for 2 hours at 37 °C, under 5% CO2. Intracellular cytokine staining was performed with PE-Cy7-IL-8 (Biolegend), Alexa fluor700-TNFα and Brilliant Violet 421-IL-6 (BD bioscience). Results typical of those in two independent assays.
We also examined degranulation employing FACS analysis and which can be combined with analysis of cytokine production. As can be seen, activated MCs expressed CD63[16] and exhibited heparin on their surface as a reflection of degranulation, as has been reported [17] [18, 19](Fig 4).
Fig. 4.
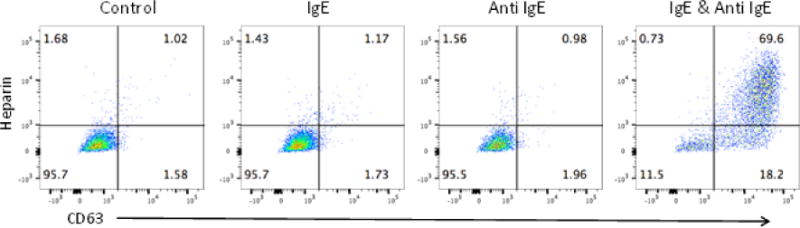
huMC activation as monitored by flow cytometry. Cells from lymphocytapheresis cultured for 7 weeks were sensitized overnight with 200 ng/ml human IgE and then stimulated with 200 ng/ml anti-IgE for 2 hours. Expression of CD63 and surface bound heparin (stained with Avidin), were measured by flow cytometry. Results shown are typical of two independent experiments.
3.4 Effects of IL-3 on huMC culture
IL-3 is reported to expand CD34+ progenitors [20] and is often added in the presence of SCF in the culture of huMCs. We thus investigated whether the addition of IL-3 had an impact on the huMC yield of the culture. After 7 weeks in culture, the majority of cells were CD117+, FcεRI+ independent of whether they had been grown in the presence of absence of IL-3 (Fig 5A). Furthermore, the average total number of huMCs was 2.46 ± 0.63 ×106 with IL-3 added at the first day of culture (N=5) and 1.11 ± 0.27 ×106 without IL-3 in the culture (N=3). Although the huMC yield trended higher with IL-3 in the culture medium, there was no overall statistical difference (p=0.165) (Fig 5B).
Fig. 5.
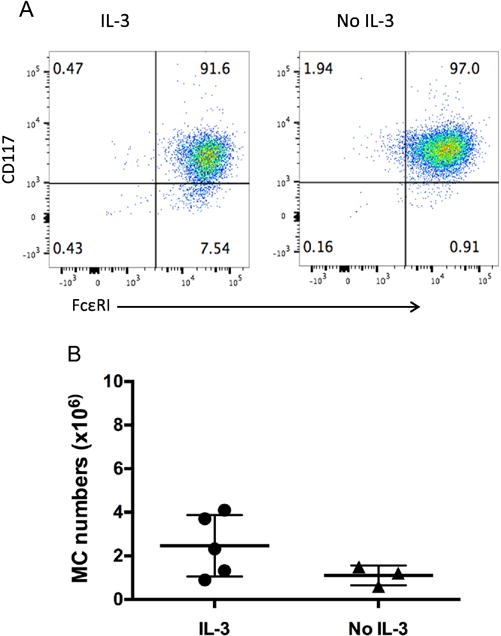
Effect of IL-3 on huMC cultures. Enriched progenitor cells from lymphocytapheresis were cultured with MC medium for 7 weeks with or without 30 ng/ml of human IL-3 added on the first day of culture only as described. A. After cells were cultured for 7 weeks, cell surface markers were analyzed with flow cytometry. (IL-3 culture, N=5; without IL-3, (N=3). B. Total mast cell numbers were counted after cells were cultured for 7 weeks with or without IL-3. P=0.165.
4. Discussion
Human peripheral blood has been used as a source for culturing huMCs[4, 14]. Current methods of culture tend to be time-consuming and costly. Furthermore, individual blood withdrawals from human subjects are in the range of 100 ml and this tends to limit the amount of cells that can be obtained from a single subject’s visit. In an attempt to reduce cost and enhance efficiency, we investigated whether the culturing technique could be revised to scale down the amount of media and consumables involved without compromising yield or quality of the cultures. We also evaluated lymphocytapheresis as source of CD34+ cells. We thus describe a method for culturing huMC that uses total culture medium volumes of 20 to 30 ml for one culture to generate MCs by 7 weeks, against as much as 1L of culture medium for some other techniques [1, 4–6, 21]. This modified method is more cost effective (for cost comparison see Supplementary Table 1) and also less labor intensive since cultures only need to be attended to biweekly during the first 6 weeks of culture vs weekly in other methods. In addition, we find that although equal numbers of enriched CD34+ progenitors obtained from equal numbers of cells placed in culture from either lymphocytapheresis or blood yielded similar numbers of huMCs at 7 weeks, one lymphocytapheresis sort resulted in sufficient cells for 50 cultures compared to one culture using 100 ml of whole blood which yields 1.0–1.4 ×108 PBMCs that are sufficient for a single culture generating 1–3 ×106 huMCs. Thus in comparison, a single lymphocytapheresis procedure generates on average 5.0 ×109 PBMCs per visit. These PBMCs may be cryopreserved and used over time as a source for huMC culture. At the end of 7 weeks in culture, huMCs derived from 100 ×106 of these cryopreserved PBMCs generated similar number and purity of FcεRI+, CD117+ double positive cells compared with those cells derived from whole blood (Fig 2). This result appears in part due to the similar starting number of MCPs (CD34+, FcεRI+, CD117+ cells) after progenitor enrichment (Fig 1).
Cultured huMCs developed granules and degranulation was induced upon FcεRI aggregation (Fig 3). The β-hex release from huMCs generated by the new method has no difference compared with previous method (Supplementary Fig 2). We also measured expression of the activation marker CD63 on 7-week cultured MCs after FcεRI-mediated activation [16] as well as heparin, which translocates to the mast cell membrane in association with degranulation [17](Fig 4). Activated huMCs also produced IL-8 and other cytokines (Fig 3D).
IL-3 is widely used in the culture of huMCs [4]. Similarly, IL-6 is known to promote huMC proliferation in vitro and thus also routinely used in the culture on huMCs [9]. In this study, IL-3 was added only once into the MC medium on the first day to some cultures. By tracking the MC surface marker expressions, we evaluated the effects of IL-3 on huMC generation. At the end of week 7 of culture, more than 90% of the generated cells expressed FcεRI and CD117 whether IL-3 was added or not (Fig 5). Although there was no difference in the huMC yield generated in cultures with and without IL-3, more cultures and further dose response studies are needed to more fully determine the consequences of IL-3 upon the in vitro culture of human mast cells in light of other reports [22].
In conclusion, our findings demonstrate that our simplified huMC culture method is less labor intensive and requires less reagents with minimal impact on the yield or functionality of the cells. In addition, cryopreserved PBMCs collected by lymphocytapheresis may be used as the source for huMC culture. Finally, such cell cultures may easily be monitored for mast cell activation and degranulation by flow cytometry using published methods [17].
Supplementary Material
Highlights.
Less labor intensive and more cost effective method to culture human mast cells.
Starting sources are cells from lymphocytapheresis and or blood.
SCF and IL-6 are the critical growth factors.
Mast cell activation and degranulation may be followed by flow cytometry.
Acknowledgments
This work was supported by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holm M, et al. Seven week culture of functional human mast cells from buffy coat preparations. J Immunol Methods. 2008;336(2):213–21. doi: 10.1016/j.jim.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 2.Kulka M, Metcalfe DD. Isolation of tissue mast cells. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im0725s90. Chapter 7: p. Unit 7 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lappalainen J, Lindstedt KA, Kovanen PT. A protocol for generating high numbers of mature and functional human mast cells from peripheral blood. Clin Exp Allergy. 2007;37(9):1404–14. doi: 10.1111/j.1365-2222.2007.02778.x. [DOI] [PubMed] [Google Scholar]
- 4.Saito H, et al. Culture of human mast cells from peripheral blood progenitors. Nat Protoc. 2006;1(4):2178–83. doi: 10.1038/nprot.2006.344. [DOI] [PubMed] [Google Scholar]
- 5.Bandara G, Metcalfe DD, Kirshenbaum AS. Growth of human mast cells from bone marrow and peripheral blood-derived CD34(+) pluripotent hematopoietic cells. Methods Mol Biol. 2015;1220:155–62. doi: 10.1007/978-1-4939-1568-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmetzer O, et al. A novel method to generate and culture human mast cells: Peripheral CD34+ stem cell-derived mast cells (PSCMCs) J Immunol Methods. 2014;413:62–8. doi: 10.1016/j.jim.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Saito H, et al. Selective growth of human mast cells induced by Steel factor, IL-6, and prostaglandin E2 from cord blood mononuclear cells. J Immunol. 1996;157(1):343–50. [PubMed] [Google Scholar]
- 8.Yin Y, et al. Rapamycin Preferentially Inhibits Human IL-5+ Th2 Cell Proliferation via an mTORC1/S6 Kinase-1 Dependent Pathway. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Desai A, et al. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J Allergy Clin Immunol. 2016;137(6):1863–1871 e6. doi: 10.1016/j.jaci.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin Y, Mitson-Salazar A, Prussin C. Detection of Intracellular Cytokines by Flow Cytometry. Curr Protoc Immunol. 2015;110:6, 24, 1–18. doi: 10.1002/0471142735.im0624s110. [DOI] [PubMed] [Google Scholar]
- 11.Chan EC, et al. Mastocytosis associated with a rare germline KIT K509I mutation displays a well-differentiated mast cell phenotype. J Allergy Clin Immunol. 2014;134(1):178–87. doi: 10.1016/j.jaci.2013.12.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolhiser MR, et al. IgG-dependent activation of human mast cells following up-regulation of FcgammaRI by IFN-gamma. Eur J Immunol. 2001;31(11):3298–307. doi: 10.1002/1521-4141(200111)31:11<3298::aid-immu3298>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27(8):677–82. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 14.Dahlin JS, et al. Lin- CD34hi CD117int/hi FcepsilonRI+ cells in human blood constitute a rare population of mast cell progenitors. Blood. 2016;127(4):383–91. doi: 10.1182/blood-2015-06-650648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smrz D, et al. Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells. Eur J Immunol. 2013;43(7):1873–82. doi: 10.1002/eji.201243214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraft S, et al. The tetraspanin CD63 is required for efficient IgE-mediated mast cell degranulation and anaphylaxis. J Immunol. 2013;191(6):2871–8. doi: 10.4049/jimmunol.1202323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joulia R, et al. Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence. Nat Commun. 2015;6:6174. doi: 10.1038/ncomms7174. [DOI] [PubMed] [Google Scholar]
- 18.Huber M. Activation/Inhibition of mast cells by supra-optimal antigen concentrations. Cell Commun Signal. 2013;11(1):7. doi: 10.1186/1478-811X-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kett WC, et al. Avidin is a heparin-binding protein. Affinity, specificity and structural analysis. Biochim Biophys Acta. 2003;1620(1–3):225–34. doi: 10.1016/s0304-4165(02)00539-1. [DOI] [PubMed] [Google Scholar]
- 20.Saeland S, et al. Combined and sequential effects of human IL-3 and GM-CSF on the proliferation of CD34+ hematopoietic cells from cord blood. Blood. 1989;73(5):1195–201. [PubMed] [Google Scholar]
- 21.Radinger M, et al. Generation, isolation, and maintenance of human mast cells and mast cell lines derived from peripheral blood or cord blood. Curr Protoc Immunol. 2010 doi: 10.1002/0471142735.im0737s90. Chapter 7: p. Unit 7 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu Y, et al. Interleukin-3 does not affect the differentiation of mast cells derived from human bone marrow progenitors. Immunol Invest. 2008;37(1):1–17. doi: 10.1080/08820130701741742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


