Abstract
Inflammation involves a cascade of cellular and molecular mediators that ultimately lead to the infiltration of immune cells into the affected area. This inflammatory process in skin is common to many diseases including acne, infection, and psoriasis, with the presence or absence of immune cells a potential diagnostic marker. Here we show that skin inflammation can be non-invasively measured and mapped using a paint-on oxygen sensing bandage in an in vivo porcine inflammation model. After injection of a known inflammatory agent, the bandage could track the increase, plateau, and decrease in oxygen consumption at the injury site over 7 weeks, as well as discern inflammation resultant from injection at various depths beneath the surface of the skin. Both the initial rate of pO2 change and the change in bandage pO2 at equilibration (CBP20) were found to be directly related to the metabolic oxygen consumption rate of the tissue in contact. Healthy skin demonstrated an initial pO2 decrease rate of 6.5 , and CBP20 of 84 . Inflamed skin had a significantly higher initial consumption rate of 55 , and a larger CBP20 of 140 . The change in the bandage pO2 before and after equilibration with tissue was found to correlate well with histological evidence of skin inflammation in the animals.
OCIS codes: (170.2655) Functional monitoring and imaging; (160.2540) Fluorescent and luminescent materials; (170.3880) Medical and biological imaging; (170.6510) Spectroscopy, tissue diagnostics
1. Introduction
Skin inflammation occurs across a wide range of dermatological conditions, from common diseases such as eczema and acne to major disorders including psoriasis, cellulitis and chronic diabetic ulcers. The skin inflammatory response typically involves a trigger that stimulates the production of inflammatory cytokines and chemokines that then mediate a cascade of cellular processes. Hallmarks of skin inflammation include erythema (redness), vasodilation, activation of mast cells, and the infiltration of immune cells such as neutrophils and macrophages into the affected tissue [1]. When triggered by pathogens such as bacteria, these reactions aid the skin in its fight against infection. However, in some cases, such as the auto-immune disease psoriasis, inflammatory responses can result in damage to the skin itself [2]. Dermatologists routinely prescribe drugs to control the inflammatory response, with corticosteroids being the most common class of compounds used. These drugs interfere with the inflammatory cascade and act to limit the severity of the inflammatory process. However, these compounds can have negative side effects and are typically not used for extended periods of time [3].
Given that inflammatory skin conditions are one of the most common problems seen by dermatologists, there is a need to visualize and quantify skin inflammation, both for the mapping and diagnosis of skin conditions, as well as to measure response to treatment. Detection and mapping of inflammation is important not only for clinical measurement, but also in the research of drugs, therapies, and cosmetic and pharmaceutical actives, where the quantitative measurement of tissue inflammation can accelerate the pace of development. There are several existing means of measuring skin inflammatory activity that include both invasive and non-invasive tools. Invasive, biopsy-based methods can provide the clearest picture of skin inflammation, with histological analysis being a standard means of confirming an inflammatory diagnosis [4,5]. While accurate, these invasive methods require the collection of skin samples and may not be ideal for long-term monitoring of skin conditions.
Noninvasive measurements hold considerably more promise, and have found use in skin inflammatory research. Tape stripping methods, such as those using celluloid or Sebutape, can be used to collect thin strips of skin for the analysis of molecular inflammatory mediators involved in the inflammatory process [6]. Recent efforts by the laboratory of John Rogers have resulted in small near-infrared tools that utilize metrics of blood flow to provide a continuous measure of local inflammatory response [7]. These noninvasive approaches, however, are largely confined to reporting inflammation within small regions of the skin. Additionally, other than the system developed by Rogers, all other methods require sample processing to extract inflammatory information. A noninvasive tool that provides instantaneous, real-time quantitative maps of skin inflammation would therefore be of great utility in both clinical and pre-clinical dermatology settings.
1.1 Oxygen consumption in inflamed tissue
Previous studies have shown that inflamed tissue consumes oxygen more rapidly compared to healthy tissue and is very often hypoxic. This is due to damage to the local vasculature and increased oxygen consumption by pathogens and infiltrating immune cells [8,9]. Tissues with ongoing inflammation are characterized by shifts towards high rates of metabolism that result from a combination of recruitment of neutrophils and monocytes as well as the high proliferation rates of the lymphocyte populations [10]. Based on these characteristics of inflamed tissue, one idea is to quantitatively measure tissue oxygenation and oxygen consumption rate as a means for tracking the degree of tissue inflammation. A device that non-invasively measures the tissue oxygenation properties across a certain area of the skin could be used to map the skin tissue inflammation.
In this work, we describe an oxygen-sensing paint-on bandage that allows for the non-invasive monitoring of skin tissue inflammation. The bandage is applied to intact skin as a liquid, before drying into a solid thin film within one minute. A barrier layer is then applied to minimize oxygen exchange between the bandage and ambient air. The oxygen within the sensing bandage equilibrates with the skin tissue underneath in about 20 minutes, during which the initial oxygen-consumption rate and the equilibrium oxygen partial pressure can be measured with a custom camera. The principles behind phosphorescence-based oxygen sensing are described in the section below.
1.2 Oxygen sensing, oxygen sensing bandage
Certain luminescent molecules, such as phosphors, have an emission that is dependent on local oxygen concentration. When the phosphor is photoexcited, it may enter a long-lived triplet excited state that can interact with its immediate environment. In the absence of any nearby molecular oxygen, the phosphor will decay back down to its ground state by emitting a red photon. In the presence of molecular oxygen, however, the excited phosphor is able to transfer this energy to oxygen through a collision. When this happens, the phosphor relaxes to its ground state without emitting a photon — its emission has been “quenched”. The relationship between emission intensity and oxygen concentration (pO2) is described by the Stern-Volmer equation, shown below [11].
where is the phosphorescence intensity at the measured oxygen concentration ; is the phosphorescence intensity in the absence of oxygen; and is the Stern-Volmer quenching constant.
This phenomenon of oxygen-dependent quenching of phosphorescence has been exploited frequently for various biomedical applications [12–19]. As has been described by Wilson and Vinogradov, this process is highly efficient and responds instantaneously to fluctuations or perturbations in oxygen concentration. Moreover, its output is essentially independent of temperature and pH within physiological ranges. Importantly, phosphorescent molecules can be modified to ensure biocompatibility [20,21]. Lastly, the optical output — usually light in the red or near infrared (NIR) — can be readily detected and processed by simple electronics and medical devices.
Recently, we described an oxygen-sensing paint-on bandage that allows for the non-invasive measurement of transdermal oxygenation [22]. This bandage relies upon a modified phosphorescent molecule contained within a liquid bandage material that makes it very conducive to skin imaging. Our previous report explored its use to study conditions of oxygen deficiency, e.g. ischemia and wound healing. The bandage was validated alongside a Clark electrode using freshly excised, viable porcine skin, as well as in live animals to demonstrate its ability to detect changes in tissue metabolism [22]. Here, we report the application of this paint-on bandage to the study of inflammation in a porcine model, and demonstrate that the bandage can serve as a non-invasive tool for the detection and quantification of inflammation in skin.
2. Materials and methods
2.1. Oxygen-sensing paint-on bandage and camera based readout system
The formulation, application, and imaging of the oxygen-sensing bandage follow the procedure detailed previously by Li et al [22]. Pd-meso-tetra-(4-carboxyphenyl)porphyrin dendrimer (Oxyphor R2, Oxygen Enterprises Ltd., Philadelphia, PA) was reacted with p-toluenesulfonic acid monohydrate (p-TsOH, Sigma Aldrich, St. Louis, MO) at a molar ratio of 1:20 in refluxing ethanol for 18 h to afford an esterified Oxyphor R2. After the reaction, any excess p-TsOH was washed out and removed. The resulting esterifed Oxyphor R2 was more compatible with the ethanol-based liquid bandage matrix. To form the final paint-on liquid-bandage mixture, esterified Oxyphor R2 in ethanol (1 mM), Coumarin 500 (Exciton, Dayton, OH) in ethanol (10 mM), and New-Skin liquid bandage (Prestige Brands, Tarrytown, NY) were mixed at a volumetric ratio of 2:1:10. This oxygen-sensing liquid bandage mixture was painted on the injection sites and allowed to dry for 5 min into a thin film. Upon drying, a transparent barrier layer of Tegaderm (1622W, 3M, Maplewood, MN) was applied over the dried liquid bandage to reduce oxygen exchange between the bandage and the ambient environment.
To ensure that sensor dye in the bandage does not leach into the skin, excised human abdominal tissue was painted with a 1 cm x 1 cm of liquid bandage containing the esterified Oxyphor R2 sensor, sealed with Tegaderm. The bandage/Tegaderm was left on for one hour before being removed. The area was then wiped with an alcohol pad. Trace metal analysis was conducted at Harvard School of Public Health using ICP-MS to measure the concentration of Pd in the samples, which is the center metal of the Oxyphor R2 molecule. 9 samples exposed to the bandage were analyzed, as well as 9 control samples. No statistically significant difference in the Pd concentration was observed between the test and the control samples, showing that the leaching of the sensor dye is negligible during a one-hour wear, which is longer than the intended exposure relevant to this study.
Oxyphor R2 (λex = 415 nm) and Coumarin 500 (λex = 392 nm), the two dyes in the bandage, were excited by brief pulses from two Thyristor(R) speedlight flash units (285 HV, Vivitar, Edison, NJ) equipped with 400/70 nm bandpass filters. The emission of these dyes was captured by a NIR CMOS camera (DCC3240N, Thorlabs, Newton, NJ) with a macro lens (AF-S Micro NIKKOR 60mm f/2.8G ED, Nikon, Melville, NY). To collect the phosphorescence of Oxyphor R2 (λem = 690 nm), a 700/70 nm bandpass filter was placed in front of the lens. For imaging of the green reference dye, Coumarin 500 (λem = 495 nm), a 510/10 nm bandpass filter was used. In this setup, the brightness of the flashes and skin autofluorescence, combined with the short lifetimes of these dyes, necessitates precise control over the timing of both the firing of the flashes and the opening of the camera shutter. To coordinate these actions and separate them by arbitrary, sub-millisecond intervals, a digital delay/pulse generator (Stanford Research System, Sunnyvale, CA) was used. Oxygen consumption data and maps were generated through analysis with MATLAB (Mathworks, Natick, MA).
The emission from the red, oxygen-sensitive dye can be divided by the emission of the green reference dye to afford a ratiometric measurement of the partial pressure of oxygen (pO2) within the bandage. The absorption of the barrier Tegaderm film within the camera captured wavelength range was tested and found to be negligible, and therefore does not affect the measurements in this study. The intensity ratio between the “red-channel” and the “green-channel” images was calibrated using a previously reported method, by placing a dried sensing film in a quartz cuvette with oxygen partial pressure controlled by a gas mixing system described [22].
2.2. In vivo tissue oxygen-consumption measurement in porcine CFA injection model
Animal housing and maintenance: Pigs ranging from 8 to 10 months in age were selected from our herd of Massachusetts General Hospital partially inbred MHC-defined miniature swine, which have been previously described [23,24]. Animals were housed at the Center for Transplantation Sciences in accordance with the Guide for the Care and Use of Laboratory Animals. All animal experiments were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital.
Anesthesia, pre- and post-injection care: Animals were nil per os the night prior to the procedure. The day of the procedure animals were anesthetized with ketamine 10 mg/kg IM. They were maintained on isofluorane at 1-1.5% throughout the procedure. Following injection of CFA, animals were gradually weaned off isofluorane and once recovered they were taken back to their cage, where they were monitored once a day for general clinical condition and for measurement of the lesions.
CFA injection in animals: After shaving the back neck of the swine, a 2 by 2 inch square was drawn, with four inner squares 1 by 1 inch, on both the right and left side. Then 0.5 mL CFA (Each mL contains 1 mg of mycobacterium tuberculosis (H37Ra, ATCC 25177), heat killed and dried, 0.85 mL paraffin oil and 0.15 mL mannide monooleate) was injected in the middle of each inner square. Each square was labeled 1 to 4. The right side was used to test the oxygen-sensing bandage, whereas the left side was reserved for biopsies.
One animal underwent intradermal injection of CFA and was used for post-injection monitoring for 7 weeks. Images of one injection site on the right side of the body were taken weekly for a total of 7 weeks. Biopsies were taken at the contralateral side on the animal both before injection, and on weeks 2 and 4 post-injection.
A second animal was used for testing the effect of injection depth on the degree of inflammation. In this animal, CFA was injected subcutaneously into both sides of the neck, at four different depths:
-
•
Site I: 15.8 mm (25g needle)
-
•
Site II: 12.7 mm (27g needle)
-
•
Site III: 8.8 mm (25g needle plus a block of 7mm)
-
•
Site IV: 5.7 mm (27g needle plus a block of 7mm)
Imaging with the oxygen-sensing bandage was carried out on both sides on day 9 post-injection, and punch biopsies were taken at each injection site immediately after imaging. Histopathology assessments of tissue inflammation: 3-6 mm punch biopsies were stored in formalin, processed, and stained with H&E. Histopathologic characteristics of inflammation were blindly assessed by a pathologist.
2.3. Inflammation assessment using the oxygen-sensing paint-on bandage
A thin layer of bandage solution was painted and left to dry over an area of skin such that the entire field of view was approximately 3 cm by 2 cm, split between inflamed and normal tissue. An image with a green filter (“green-channel” image) was obtained, as well as one with the red filter (“red-channel” image) with an 800 microsecond delay between the flash and image acquisition to avoid capture of autofluorescence. Tegaderm was placed over the area of interest and two images were acquired with the previous filter and delay settings. As the bandage equilibrated over 20 minutes, a red-channel image with an 800 microsecond delay was taken every two minutes. At the end of the 20 minute equilibration period, an image was obtained with the green filter. The red to green intensity ratios were converted to pO2 values using a previously acquired calibration curve. The initial equilibration rate and change in bandage pO2 (CBP) were calculated as below:
3. Results and discussion
3.1 Initial pO2 equilibration rate and equilibrium pO2 are related to tissue oxygen consumption rate and the degree of tissue inflammation
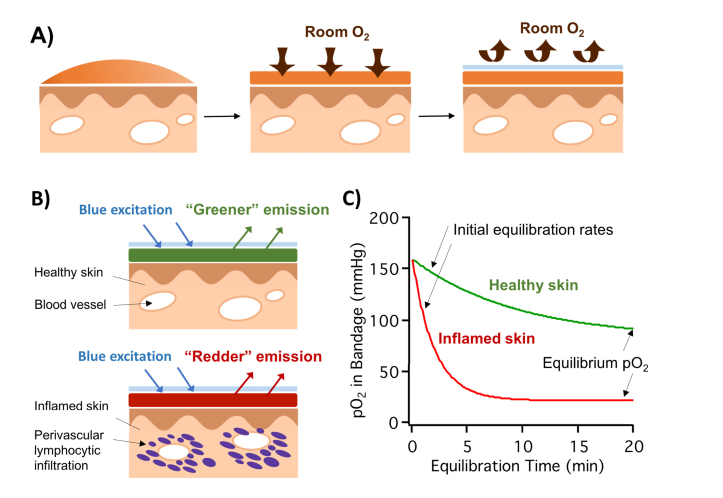
The oxygen-sensing bandage is applied to intact skin surface as a liquid, and allowed to dry into a solid thin film. As soon as the barrier layer (Tegaderm) is applied, the oxygen within the sensing bandage material starts to equilibrate with the tissue underneath, and an equilibration curve can be plotted as the change of pO2 within the bandage over time (Fig. 1). The following theoretical model demonstrates that both the initial equilibration rate and the final pO2 are directly related to the metabolic oxygen consumption rate of the tissue beneath the bandage.
Fig. 1.
A) Illustration of the oxygen-sensing paint-on bandage applied to skin as a liquid, drying into a solid thin film, and the application of the barrier layer. B) Top: bandage applied to healthy skin; bottom: bandage applied to inflamed skin with perivascular infiltrating lymphocytes actively consuming oxygen. C) Upon application, bandage pO2 equilibrates with the tissue underneath: higher equilibrium pO2 and a slower initial equilibration rate when applied to healthy skin; lower equilibrium pO2 and a faster initial equilibration rate when applied to inflamed skin.
For the purpose of setting up the model, it is assumed that the superficial skin layer of thickness d is supplied by the diffusion of oxygen from the atmosphere, and beyond this depth the tissue oxygen is supplied by circulation. Within the superficial skin layer (), the rate of metabolic oxygen consumption equals to the rate of oxygen replenishment as a result of diffusion from the atmosphere. According to Fick’s second law:
| (1) |
where is the concentration of oxygen (), is the oxygen diffusivity in skin (), is the oxygen partial pressure () as a function of tissue depth (), and is the tissue oxygen consumption rate ().
Immediately after the barrier layer is applied, the initial rate of pO2 decrease within the bandage is directly related to the oxygen consumption rate of the tissue underneath :
| (2) |
where the conversion constant for oxygen gas equals 1316 at room temperature.
In order to solve pO2 as a function of tissue depth , two boundary conditions are taken into consideration. First, oxygen flux at the surface of the skin () is equal to the volume of oxygen diffusing through a unit area multiplied by the depth of that volume:
| (3) |
where is the transcutaneous oxygen flux (), and is the thickness of skin tissue that is supplied by external oxygen ().
Second, the oxygen partial pressure at the surface of the skin () is the same as in the atmosphere:
| (4) |
With these two boundary conditions, can be solved as:
| (5) |
Once the bandage has equilibrated with the skin tissue, the equilibrium measured by the sensing bandage should equal to that of the bulk tissue ( at depth ):
| (6) |
Based on Eq. (6), the difference between the bandage initial and equilibrium oxygen partial pressures, , in the bandage is also directly related to the tissue oxygen consumption rate .
The above model sets up a predictive hypothesis that, while analyzing the equilibration curve measured by the bandage, both the initial equilibration rate and the change in bandage pO2 can be used as metrics for the metabolic oxygen consumption rate of the tissue (Eqs. (2) and (6)). For the rest of this study, the initial equilibration rate was calculated as the average rate of pO2 drop in the first 4 min of the equilibration curve, and the CBP was calculated as the difference between atmospheric pO2 and the bandage pO2 after 20 min of equilibration (denoted as CBP20). For example, the bandage-measured pO2 equilibration curves for healthy and inflamed skin are shown in Fig. 1(C). Healthy skin demonstrated an initial pO2 decrease rate of 6.5 , and CBP20 of 84 . Two weeks after injecting the pro-inflammatory CFA, the inflamed skin had a significantly higher initial consumption rate of 55 , and a larger CBP20 of 140 .
3.2 Tracking the progression of tissue inflammation in skin following CFA injection using the oxygen-sensing paint-on bandage
To demonstrate that the oxygen-sensing bandage can be used to non-invasively monitor skin inflammation over time, an in vivo porcine model was used. CFA was injected intradermally into the neck skin of one animal, and the degree of induced skin inflammation was monitored using the sensing bandage over 7 weeks after the injection. Injection of complete Freund’s adjuvant, a compound of heat killed mycobacteria, produces a local inflammatory reaction caused by the infiltration of activated immune cells which secrete inflammatory mediators leading to redness, swelling, heat and sometimes loss of function depending on the area injected [25].
It is well known that invading immune cells consume oxygen [8–10], thus making this model an adequate one to test our novel oxygen-sensing bandage to monitor skin inflammation. Bandage measurements were carried out once a week at the injection site on the right side of the animal, as well as at the control normal skin area. Biopsies were taken at the injection sites on the contralateral side at 2nd week and 4th week.
Histopathological studies confirmed the development of focal epidermal inflammation and mild, focal perivascular inflammation in the deep dermis at 2 weeks after injection. The inflammation subsided after 4 weeks. This change was not visible by regular color photography.
The inflammatory process was readily captured by the oxygen-sensing bandage. The initial equilibration rate, calculated as the average pO2 change in the bandage within the first 4 minutes of bandage application, was observed to increase during the first 3 weeks (indicated by an increase in CBP20) after intradermal CFA injection and then gradually return to the baseline by the end of 7 weeks (Fig. 2(A)). The equilibrium pO2 was observed to decrease in the first 3 weeks before returning back to normal at the end of 7 weeks (Fig. 2(B)). Since higher initial equilibration rate and lower equilibration pO2 are related to a higher tissue oxygen consumption rate, and thus to a higher degree of tissue inflammation, these results correlate with the histological-confirmed development of skin inflammation. The highest degree of inflammation was observed at week 3 after injection, after which point the inflammation receded back to normal by week 7 (Fig. 2(A), 2(B)).
Fig. 2.
Tracking the progression of tissue inflammation in skin following intradermal CFA injection using the oxygen-sensing paint-on bandage. A) initial equilibration rate, calculated as the average pO2 change in the bandage within the first 4 minutes of bandage application, changes over the course of 7 weeks after CFA injection. B) CBP20 calculated as the difference between the initial and equilibrium oxygen partial pressure within the sensing bandage, tracked over 7 weeks post-injection. C) Correlating bandage equilibrium pO2 with the degree of tissue inflammation assessed by histological analyses. Top: equilibrium pO2 map captured by the oxygen-sensing bandage for skin with no CFA injection, 2nd week and 4th week after CFA injection. Equilibrium pO2 drops significantly after 2 weeks of injection, and returns after 4 weeks. Arrows pointing to outer square of the injection area marked by ink (also seen in the regular photograph); injection site is 0.5 inch to the right side of the line. Middle: regular photographs of skin with no CFA injection, 2nd week and 4th week after CFA injection. Bottom: histology of skin no CFA injection, 2nd week and 4th week after CFA injection. Focal epidermal and dermal inflammation was observed after 2 weeks of injection, which subsides after 4 weeks. Arrows pointing to perivascular inflammatory infiltrate.
The top panel in Fig. 2(C) shows the pO2 maps captured by the sensing bandage before injection, at 2 weeks post-injection, and at 4 weeks post-injection. An ink line was drawn around the boundary of the injection site. The oxygenation images show a significant decrease in equilibrium pO2 at 2 weeks after injection that corresponded to the appearance of immune infiltrates within the tissue via histology. This equilibrium pO2 level is restored after 4 weeks when immune cells leave the tissue region. Based on the 2D map, we could see that the tissue on the inside of the boundary, closest to the injection site, experienced a higher degree of inflammation compared to areas outside of the boundary further from the injection site.
3.3 Evaluating the effect of CFA injection depth on transcutaneous oxygenation using oxygen-sensing paint-on bandage
The bandage was then used to assess the effect of CFA injection depth on the level of transcutaneous oxygenation. To test this, the pro-inflammatory CFA was injected subcutaneously into the neck skin of a pig at four different skin depths, as described in the methods section (Fig. 3(A)). On day 9 after injection, equilibrium pO2 values were measured for the all injection sites as well as the control skin (Fig. 3(B)).
Fig. 3.
A) Illustration of the different subcutaneous injection depths used in the experiment. B) CBP20 measured by the oxygen-sensing bandage on day 9 after CFA injection at the different injection depths. C) Confirming the degree of tissue inflammation by histological analysis. From top to bottom: regular photographs, histology (epiderm/derm), histology (derm/subcutaneous) at injection sites I, II, III and IV.
The CBP20 after application of the dressing for the healthy control skin on day 9 was 116 ± 4 mmHg. The corresponding values for injection sites I, II, II and IV were 140 ± 2 mmHg, 148 ± 1, 148 ± 3 and 148 ± 5 mmHg, respectively. The CBP20 was plotted as a function of injection depth (Fig. 3(B)). Since the CBP20 is directly related to the oxygen consumption rate of the tissue, it can be observed that the injection sites at all depths demonstrated higher oxygen consumption than the control skin, as anticipated. Interestingly, the deepest injection depth (site I) demonstrated a lower oxygen consumption rate compared to the other three injection depths. This effect could arise either due to depth-dependent differences in the immune response to the injection, or due to the increased distance of the immune response to the bandage.
To investigate this difference, biopsy samples were taken from injection sites I-IV, and histopathological analysis was performed to identify tissue inflammatory states in the epidermis/dermis as well as subcutaneous layers (Fig. 3(C)). At injection site I, granulomatous inflammation in dermis and subcutaneous tissue was observed. Injection sites II and III demonstrated moderate inflammation in the dermis and subcutaneous tissue, and focal mild inflammation in the epidermis. At injection site IV, moderate to severe inflammatory infiltrate was observed in the epidermis and dermis, with diffuse granulomatous inflammation in deep dermis and subcutaneous tissue.
These histology results confirmed that the depth at which local inflammatory response occurs in the skin is highly dependent on the injection depth of the pro-inflammatory agent. Moreover, the magnitude of the inflammatory response was histologically confirmed to be linked to the injection depth: as the injection depth became shallower, a higher degree of inflammatory response was observed in the superficial layers of the skin. This observation is therefore consistent with the transcutaneous oxygenation measured by the bandage, as greater levels of oxygen consumption were observed at shallower injection depths.
4. Conclusions
Inflammatory responses are accompanied by a series of shifts in metabolism and deviation from the tissue’s normal oxygenation profile. Such deviations, including an increased rate of oxygen consumption and lower static pO2, can therefore be used to characterize the extent of tissue inflammation. We have demonstrated a non-invasive method for monitoring skin inflammation using an oxygen-sensing bandage. The bandage contains phosphorescent sensor molecules whose emission intensity is dependent on oxygen concentration (pO2). When applied to the surface of the skin, the pO2 within the bandage equilibrates with the tissue underneath, and the oxygenation profile across the bandage can be captured and tracked over time using a camera device. A mathematical model was set up to describe the oxygen equilibration process upon bandage application, and demonstrated that both the initial rate of pO2 change and the CBP20 are directly related to the metabolic oxygen consumption rate of the tissue in contact.
After injection of a known inflammatory agent, the bandage could track the increase, plateau, and decrease in oxygen consumption at the injury site over 7 weeks, as well as discern inflammation resultant from injection at various depths beneath the surface of the skin. These measurements all correlate quite well with histology; thus, we conclude that this oxygen-sensing bandage technology makes for a sensitive and robust indicator of inflammation in skin and could hold enormous diagnostic and prognostic value in various dermatological and cosmeceutical applications.
This imaging tool could have significant benefits in the routine assessment of skin inflammation, particularly for conditions that are difficult to diagnose in the primary care and emergency medicine setting. Cellulitis, for example, is an infection of the skin that presents similarly to a number of other dermatological conditions. Out of an abundance of caution, many patients are held at the hospital settings until cellulitis can be confirmed by an expert dermatologist, both of which add up to significant costs to the healthcare system. A simple-to-use tool such as the paint-on oxygen sensing bandage could potentially be used to detect the inflammation caused by the local skin infection, aiding in the rapid diagnosis of conditions like cellulitis and reducing hospital costs.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
Acknowledgments
The authors would like to acknowledge funding from the Air Force Office for Scientific Research (FA9550-13-1-0068) for supporting the development of the oxygen sensing bandage technology and support from CO6RR020135-01 for construction of the facility utilized for production and maintenance of miniature swine. We would also like to thank Mr. Edward Harrington, Dr. George Abraham and Ms. Aurore Pruneveille for excellent animal care & technical assistance, and Dr. Ivy Rosales for histology assistance during the study.
References and links
- 1.Pasparakis M., Haase I., Nestle F. O., “Mechanisms regulating skin immunity and inflammation,” Nat. Rev. Immunol. 14(5), 289–301 (2014). 10.1038/nri3646 [DOI] [PubMed] [Google Scholar]
- 2.Lowes M. A., Suárez-Fariñas M., Krueger J. G., “Immunology of psoriasis,” Annu. Rev. Immunol. 32(1), 227–255 (2014). 10.1146/annurev-immunol-032713-120225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchman A. L., “Side effects of corticosteroid therapy,” J. Clin. Gastroenterol. 33(4), 289–294 (2001). 10.1097/00004836-200110000-00006 [DOI] [PubMed] [Google Scholar]
- 4.Sina B., Kao G. F., Deng A. C., Gaspari A. A., “Skin biopsy for inflammatory and common neoplastic skin diseases: optimum time, best location and preferred techniques. A critical review,” J. Cutan. Pathol. 36(5), 505–510 (2009). 10.1111/j.1600-0560.2008.01175.x [DOI] [PubMed] [Google Scholar]
- 5.Yoshinaga Y., Higaki M., Terajima S., Ohkubo E., Nogita T., Miyasaka N., Kawashima M., “Detection of inflammatory cytokines in psoriatic skin,” Arch. Dermatol. Res. 287(2), 158–164 (1995). 10.1007/BF01262325 [DOI] [PubMed] [Google Scholar]
- 6.Robinson M. K., Mills K. J., Handbook of Cosmetic Science and Technology, Fourth Edition, Chapter 34 Noninvasive Clinical Assessment of Skin Inflammation, (CRC Press, Boca Raton, FL, 2014). [Google Scholar]
- 7.Webb R. C., Ma Y., Krishnan S., Li Y., Yoon S., Guo X., Feng X., Shi Y., Seidel M., Cho N. H., Kurniawan J., Ahad J., Sheth N., Kim J., Taylor J. G., 6th, Darlington T., Chang K., Huang W., Ayers J., Gruebele A., Pielak R. M., Slepian M. J., Huang Y., Gorbach A. M., Rogers J. A., “Epidermal devices for noninvasive, precise, and continuous mapping of macrovascular and microvascular blood flow,” Sci. Adv. 1(9), e1500701 (2015). 10.1126/sciadv.1500701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frede S., Berchner-Pfannschmidt U., Fandrey J., “Regulation of hypoxia-inducible factors during inflammation,” Methods Enzymol. 435, 403–419 (2007). 10.1016/S0076-6879(07)35021-0 [DOI] [PubMed] [Google Scholar]
- 9.Imtiyaz H. Z., Simon M. C., “Hypoxia-inducible factors as essential regulators of inflammation,” Curr. Top. Microbiol. Immunol. 345, 105–120 (2010). 10.1007/82_2010_74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kominsky D. J., Campbell E. L., Colgan S. P., “Metabolic shifts in immunity and inflammation,” J. Immunol. 184(8), 4062–4068 (2010). 10.4049/jimmunol.0903002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turro N. J., Modern Molecular Photochemistry (University Science Books, Sausalito, CA, 1991). [Google Scholar]
- 12.Amao Y., “Probes and Polymers for Optical Sensing of Oxygen,” Mikrochim. Acta 143(1), 1–12 (2003). 10.1007/s00604-003-0037-x [DOI] [Google Scholar]
- 13.Borisov S. M., Zenkl G., Klimant I., “Phosphorescent platinum(II) and palladium(II) complexes with azatetrabenzoporphyrins-new red laser diode-compatible indicators for optical oxygen sensing,” ACS Appl. Mater. Interfaces 2(2), 366–374 (2010). 10.1021/am900932z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu C. S., Lo Y. L., Sung T. W., “Enhanced oxygen sensing properties of Pt(II) complex and dye entrapped core-shell silica nanoparticles embedded in sol-gel matrix,” Talanta 82(3), 1044–1051 (2010). 10.1016/j.talanta.2010.06.020 [DOI] [PubMed] [Google Scholar]
- 15.Winter M. B., McLaurin E. J., Reece S. Y., Olea C., Jr, Nocera D. G., Marletta M. A., “Ru-porphyrin protein scaffolds for sensing O2.,” J. Am. Chem. Soc. 132(16), 5582–5583 (2010). 10.1021/ja101527r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C., Bull B., Christensen K., McNeill J., “Ratiometric single-nanoparticle oxygen sensors for biological imaging,” Angew. Chem. Int. Ed. Engl. 48(15), 2741–2745 (2009). 10.1002/anie.200805894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunphy I., Vinogradov S. A., Wilson D. F., “Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence,” Anal. Biochem. 310(2), 191–198 (2002). 10.1016/S0003-2697(02)00384-6 [DOI] [PubMed] [Google Scholar]
- 18.Niedermair F., Borisov S. M., Zenkl G., Hofmann O. T., Weber H., Saf R., Klimant I., “Tunable phosphorescent NIR oxygen indicators based on mixed benzo- and naphthoporphyrin complexes,” Inorg. Chem. 49(20), 9333–9342 (2010). 10.1021/ic100955z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebedev A. Y., Cheprakov A. V., Sakadzić S., Boas D. A., Wilson D. F., Vinogradov S. A., “Dendritic phosphorescent probes for oxygen imaging in biological systems,” ACS Appl. Mater. Interfaces 1(6), 1292–1304 (2009). 10.1021/am9001698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols A. J., Roussakis E., Klein O. J., Evans C. L., “Click-Assembled, Oxygen-Sensing Nanoconjugates for Depth-Resolved, Near-Infrared Imaging in a 3D Cancer Model,” Angew. Chem. Int. Ed. Engl. 53(14), 3671–3674 (2014). 10.1002/anie.201311303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roussakis E., Li Z., Nowell N. H., Nichols A. J., Evans C. L., “Bright, “Clickable” Porphyrins for the Visualization of Oxygenation under Ambient Light,” Angew. Chem. Int. Ed. Engl. 54(49), 14728–14731 (2015). 10.1002/anie.201506847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Z., Roussakis E., Koolen P. G., Ibrahim A. M., Kim K., Rose L. F., Wu J., Nichols A. J., Baek Y., Birngruber R., Apiou-Sbirlea G., Matyal R., Huang T., Chan R., Lin S. J., Evans C. L., “Non-invasive transdermal two-dimensional mapping of cutaneous oxygenation with a rapid-drying liquid bandage,” Biomed. Opt. Express 5(11), 3748–3764 (2014). 10.1364/BOE.5.003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sachs D. H., MHC Homozygous Miniature Swine. In: Swindle M. M., Moody D. C., Phillips L. D., eds. Swine as Models in Biomedical Research. 1992: 3–15 (Ames, Iowa: Iowa State University Press; ). [Google Scholar]
- 24.Sachs D. H., Leight G., Cone J., Schwarz S., Stuart L., Rosenberg S., “Transplantation in miniature swine. I. Fixation of the major histocompatibility complex,” Transplantation 22(6), 559–567 (1976). 10.1097/00007890-197612000-00004 [DOI] [PubMed] [Google Scholar]
- 25.Fehrenbacher J. C., Vasko M. R., Duarte D. B., “Models of Inflammation: Carrageenan- or Complete Freund’s Adjuvant-Induced Edema and Hypersensitivity in the Rat,” Curr. Protoc. Pharmacol., 2012. March; 0 5: Unit5.4. [DOI] [PMC free article] [PubMed]