Abstract
The purpose of this study was to develop and test a nonlinear optical device to photoactivate riboflavin to produce spatially controlled collagen crosslinking and mechanical stiffening within the cornea. A nonlinear optical device using a variable numerical aperture objective was built and coupled to a Chameleon femtosecond laser. Ex vivo rabbit eyes were then saturated with riboflavin and scanned with various scanning parameters over a 4 mm area in the central cornea. Effectiveness of NLO CXL was assessed by evaluating corneal collagen auto fluorescence (CAF). To determine mechanical stiffening effects, corneas were removed from the eye and subjected to indentation testing using a 1 mm diameter probe and force transducer. NLO CXL was also compared to standard UVA CXL. The NLO CXL delivery device was able to induce a significant increase in corneal stiffness, comparable to the increase produced by standard UVA CXL.
OCIS codes: (190.0190) Nonlinear optics, (170.0170) Medical optics and biotechnology
1. Introduction
UVA-riboflavin crosslinking (UVA CXL), developed by Spoerl and Wollensak [1–3], is commonly used to induce crosslinking and stiffening of the corneal stroma as a therapeutic treatment for keratoconus and post LASIK ectasia that cause progressive, severe astigmatism. Photo-activation of riboflavin, which has been diffused into the corneal stroma, induces the production of oxygen free radicals which in turn induce covalent crosslinking of collagen fibrils [3, 4]. Collagen crosslinking has been shown to enhance blue collagen auto fluorescence (CAF) [5] and increase mechanical stiffness of the cornea in humans up to 300% [2]. Furthermore, recent clinical studies indicate that crosslinking can halt the progression of corneal disease and in some cases reverse steepening by an average of one diopter after six months of treatment, and lasting to at least 24 months [2, 6–11]. While various changes have been implemented to shorten and improve the crosslinking procedure, including accelerated crosslinking using high power UVA light and enhanced riboflavin penetration through the corneal epithelium to avoid epithelial debridement, the procedure is not without disadvantages [8, 12, 13].
First and foremost, UVA irradiation and oxygen free radical generation is toxic to cells and therefore poses a risk of damaging keratocytes, endothelial cells, and underlying structures. The parameters of standard UVA CXL were carefully chosen to reduce these risks, and the risk of corneal endothelial damage is only reached in corneas thinner than 400 µm [14]. This means that patients who have progressed further, and whose corneas are thinner than 400 µm, are not eligible for standard treatment. Another weakness of this technique is its lack of precision. It is difficult to control the lateral position of crosslinking and the volume of crosslinking begins at the corneal surface, going only as deep as UVA light can penetrate into the tissue, diminishing in effectiveness with depth [3].
Many of these weaknesses are due to the single photon excitation process using UVA light to excite riboflavin. Single photon excitation occurs when one photon of a specific wavelength is used to excite a molecule to a higher energy level. In the case of UVA CXL, one photon of UVA light holds enough energy to excite a molecule of riboflavin to the excited singlet state. If the excited singlet riboflavin undergoes intersystem crossing to an excited triplet state then a free oxygen radical can be produced. The free radical then induces covalent crosslinking of collagen fibrils. Without intersystem crossing the excited riboflavin returns to the ground state energy level via green fluorescence [4]. There are several disadvantages to single photon excitation. First, UVA light will excite all of the riboflavin in the optical path. Besides the absorption of UVA light by the stroma, absorption by riboflavin also leads to attenuation of quenching of UVA light as a function of depth reducing the efficiency of crosslinking deeper within the cornea. Prolonged riboflavin excitation also leads to photo-bleaching and loss of riboflavin excitation and collagen crosslinking. Finally the prolonged riboflavin excitation and generation of free radicals contributes not only to collagen crosslinking but also cell damage within the tissue. All of these disadvantages are enhanced by increasing the UVA power as occurs in accelerated crosslinking.
By contrast, two photon or nonlinear excitation of riboflavin uses a longer wavelength, lower energy light to excite riboflavin within a defined optical volume. Two photon excitation occurs when two photons of half the original excitation energy simultaneously (10−15 s) excite the same molecule [15]. This event occurs within an area of exceptionally high photon density, such as that created by focusing high intensity, very short pulsed (femtosecond) laser light.
The substitution of nonlinear excitation for UVA irradiation has many benefits over single photon excitation. First, nonlinear optical crosslinking (NLO CXL) uses highly focused 760 nm femtosecond (fs) laser light to induce two photon excitation of the riboflavin within the corneal tissue. This near infrared light has a much higher transmission through the cornea, and therefore a deeper depth of penetration than UVA light, eliminating attenuation of the light at deeper stromal depths. Furthermore, since riboflavin can only be activated in the focal volume there is no photo-bleaching of the riboflavin above or below the region being crosslinked. Also, there is no generation of free radicals outside the region of crosslinking leading to greatly reduced oxidative cellular damage. Finally, limiting photoactivation to the focal volume allows for highly controllable crosslinking in x, y, and z directions, and therefore precise regional stiffening within the cornea using patterns that can be designed based on individual patient topographies. The focal volume can then be scanned through a preprogrammed pattern to stiffen large volumes of corneal tissue, without causing collateral damage to nearby tissue outside the treatment zone. Also, near infrared 760 nm fs laser light is nontoxic to cells allowing for the focal volume to be positioned much deeper into the tissue without damaging the endothelium or underlying structures for safe treatment of thinner corneas.
Previous studies have shown that it is possible to mechanically stiffen compressed type I collagen hydrogels to a degree similar to that achieved by standard UVA CXL using NLO CXL [16]. It has also been shown that a similar degree of enhanced CAF, used as a measure of collagen CXL, can be detected in ex vivo rabbit eyes treated with both NLO CXL and UVA CXL, depending on the scanning parameters [17]. In these studies a Zeiss LSM 510 confocal microscope was used to perform NLO CXL. While it was effective, a higher degree of control is needed over parameters such as numerical aperture (NA), scanning speed, scanning pattern, line separation, etc. The purpose of this study was to build and use a customized device with a higher degree of control over these variables and capable of delivering a nonlinear focal volume into both ex vivo and live rabbit corneas, and to determine the combination of scanning parameters which most effectively stiffen the corneal stroma.
2. Methods
2.1 Device design
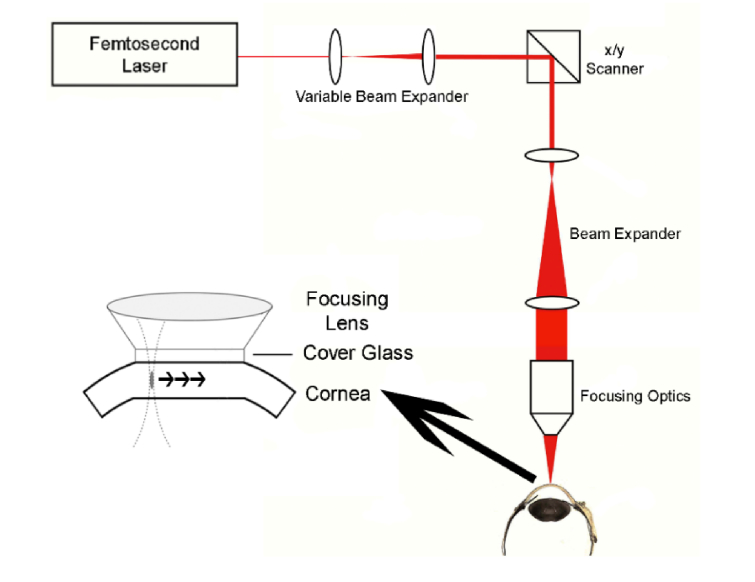
A device capable of delivering a nonlinear focal volume of continuously variable size into rabbit corneas was built, according to the design seen in Fig. 1. In this design, the 760 nm fs beam (Chameleon, Coherent Inc., Santa Clara, CA) is directed into a variable beam expander which allows for control of the effective NA of the device, and therefore control of the size of the focal volume. For example, when the beam is fully widened it completely fills the back aperture of the objective producing the highest NA and smallest focal volume. The widened beam is then directed onto software controlled x, y scanning mirrors (GSI Lumonics, Bedford, MA) which scan the focal volume through a preprogrammed pattern. A second fixed beam expander widens the beam again before it hits the back aperture of the objective. Depth is controlled by a computerized motor on the objective. A removable cone with contact glass flattens the cornea during treatment, stabilizing the eye and establishing a zero plane.
Fig. 1.
Schematic of designed delivery device with software controlled x, y scanners, variable beam expander, and objective with attached cone and contact glass.
2.2 Measuring effect of NA on focal volume
After the delivery device was designed and ready for use, the effect of NA on focal volume size was characterized. The effective NA of the system was calculated for five designated positions of the variable beam expander, labeled 1-5. A tank of 0.5% riboflavin-5-phosphate solution (Sigma-Aldrich, St. Louis, MO) in phosphate-buffered saline (PBS; pH 7.2) was placed beneath the objective and raised to the focal plane level, so that a fluorescent focal volume would be visible within the solution. A SPOT RT3 camera (SPOT Imaging Solutions, Sterling Heights, MI) with an attached 10X objective was positioned in front of the tank and images were taken of the riboflavin fluorescence by all five beam expander positions using an IR blocking filter. The pixel size of the camera and objective together was 0.74 µm. Within each image a line was drawn axially and laterally in Metamorph digital image processing software (Metamorph; Molecular Devices, Sunnyvale, CA) through the focal volume. Intensity values along these lines were exported into Excel and plotted as intensity vs. distance. Full width half max (FWHM) values were calculated using these plots to estimate the lateral and axial dimensions of the focal volume at each setting. Axial measurements were then used to calculate the effective NA based on Zipfel’s equation [18] shown in Eq. (1), where ω is axial length of the focal volume, λ is the excitation wavelength, and n is the refractive index of the surrounding medium. Images were also taken as the depth was increased in steps to estimate the maximum depth available.
| (1) |
2.3 Preparation of eyes
Eyes were prepared in the same manner as in our previous work [17]. Briefly, whole New Zealand albino rabbit eyes (65) were shipped overnight to the laboratory (Pel-Freez, Rogers, AR) for immediate use. Eyes were inspected for damage before having the corneal epithelium removed in an 8 mm diameter region of the central cornea. Drops of 0.5% or 0.1% riboflavin-5-phosphate solution, for NLO CXL or UVA CXL respectively, with 20% high-fraction dextran, molecular weight of 450-650 KDa, (Sigma-Aldrich, St. Louis, MO) in PBS were applied every two minutes for 30 minutes, in the manner outlined by Spoerl and Wollensak [1, 2]. Eyes treated with NLO CXL were also marked near the limbus for orientation during crosslinking.
2.4 Treatment of eyes
A total of 65 eyes were divided into 3 different experimental groups. Twelve eyes were treated with NLO CXL using a variable beam expander setting of 1. The NA at this setting was measured to be 0.12, the closest reliable setting to our previous studies which used a NA of 0.1 [17] These 12 eyes were then placed underneath of the objective of the delivery device with the positioning mark placed to the far left and the pupil aligned in the center of the contact glass. The corneas were then exposed to 760 nm fs laser light at 900 mW of power, at 50 µm below the corneal surface. Using custom Labview software the laser beam was raster scanned over a 4 mm diameter circular area at 5.4 mm/s and 3 µm line separation. These treatment setting would result in a cylindrical volume of treated area of approximately 1.4 mm3. Total exposure time for each NLO CXL treatment was roughly 11 minutes. For comparison, the remaining eyes were used as controls (38), with no soaking and no treatment, or were treated with UVA CXL (15) using 370 nm excitation at 3 mW/cm2 for 30 minutes.
2.5 Mechanical stiffness measurements
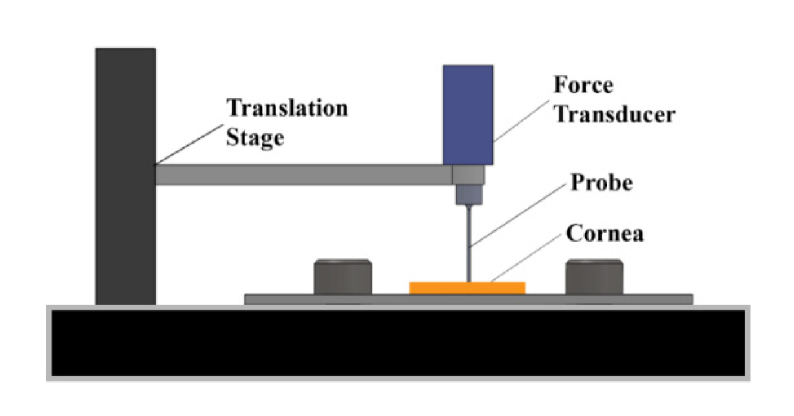
After treatment, eyes were prepared for mechanical stiffness testing using the indentation approach described by Chai et al. and Levental et al. [16, 19] An indentation approach was used because of the need to measure local tissue stiffness in the region of NLO CXL. Specifically, indentation allows for mechanical stiffness testing in a 1 mm diameter region whereas more standard tensiometry or stretch testing would require using a strip of cornea, for which much of the tissue would have been un-crosslinked. A 5 x 5 mm area of cornea, centered around the pupil was removed from the globe and its thickness measured using a pachymeter (Reichert Technologies, Depew, NY) Corneal thickness was then adjusted to within 400-450 µm by dropping either PBS to thicken or 20% dextran solution to thin the cornea. It was necessary to control thickness because the dextran solution used on treated eyes caused marked corneal thinning, which could make them appear to be artificially stiffer than control corneas. Preparation for mechanical measurements took roughly 30 minutes for each sample. Each excised portion of cornea was then placed directly under a 1 mm diameter, flat tip probe with attached force transducer which was manually moved down to contact the anterior surface of the tissue, as shown in Fig. 2. The probe was then cycled up and down ten times at a rate of 5 µm/s, indenting up to 10% of the measured thickness. Equations outlined by Hayes et al. [19, 20] were used to convert the force value reported at the peak of the tenth cycle to elasticity measurements of the corneal sample. Damaged eyes or inability to find the treated area before dehydration occurred forced the omission of mechanical measurements on 2 control eyes, 2 UVA CXL treated eyes, and 1 NLO CXL treated eye. Also seven control eyes were used only for CAF analysis, and did not undergo mechanical testing. Elasticity values were then obtained on a total of 29 control eyes, 13 UVA CXL treated eyes, and 11 NLO CXL treated eyes. After measuring the stiffness, corneas were fixed overnight in 2% paraformaldehyde (PFA, Mallinckrodit Baker, Inc., Phillipsburg, NJ) in PBS at 4° C.
Fig. 2.
Schematic of indentation device used to measure the mechanical stiffness of the crosslinked region of the corneal stroma.
2.6 Sectioning and imaging
Fixed corneas were sectioned using a vibratome (Campden Instruments, Loughborough, England), and 250 µm thick sections collected perpendicular to the line of crosslinking at the center of the cornea. Induced crosslinking has been shown to enhance blue CAF [5], which has an emission peak of 450 nm when using a two photon excitation of 760 nm. Images were taken of the crosslinked regions by using a Zeiss LSM 510 (Carl Zeiss, Jena, Germany) and Chameleon femtosecond laser tuned to 760 nm to induce two photon excited CAF, and collected over a 400-450 nm spectrum using the meta detector of the Zeiss LSM 510. Intensity differences were then measured within the images of each sample (one section per eye) using Metamorph imaging software to calculate the average intensity value within three different 100 x 100 pixel regions of interest within the crosslinked region (central anterior). Crosslinked region intensities were averaged together for each sample and compared to intensities from the same location of control eyes. One NLO CXL sample was not able to be sectioned perpendicularly due to error in orientation during treatment and was excluded from CAF measurements. Four UVA CXL samples were also excluded due to improper sectioning. In this report CAF intensity is reported in a total of 7 control eyes, 11 UVA CXL treated eyes, and 10 NLO CXL treated eyes.
2.7 Statistics
Statistical analysis was performed in all cases using the Tukey-Kramer method for a multiple comparison, one-way analysis of variance (ANOVA) in Matlab (Mathworks, Natick, MA).
3. Results
3.1 Control of NLO CXL focal volume and depth
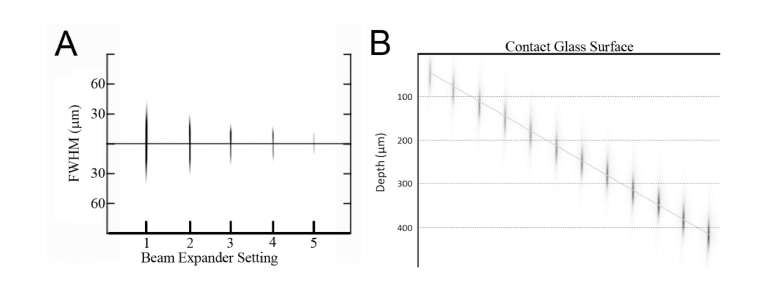
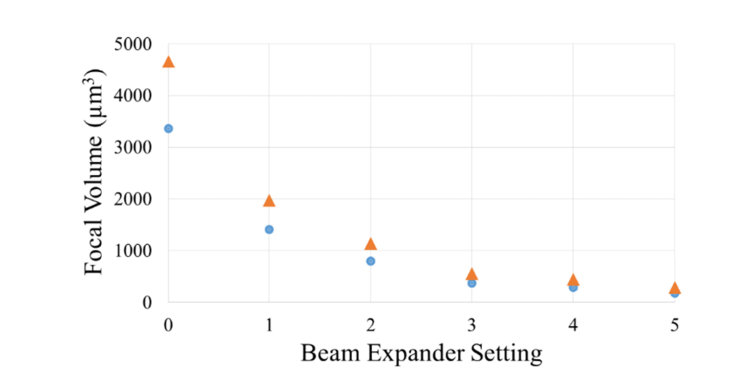
Two-photon excited fluorescent images of riboflavin taken with the SPOT RT3 camera are shown in Fig. 3, and demonstrate control of fluorescent focal volume (Fig. 3(A)) and depth (Fig. 3(B)). To determine the NA of different beam expander settings, the lateral and axial lengths FWHM were measured. These empirical values were then used to calculate the focal volume and effective NA of each setting using the theoretical equation, Eq. (1). As quantified in Table 1, changes in the beam expander setting had a dramatic effect on both axial length and focal volume, such that increasing the beam expander setting from its minimum to maximum position, 1-5, produced a more than 7.5 fold decrease in volume and 2.5 fold decrease in axial length, Fig. 4. As the beam expander setting increased from 1 to 5 the axial FWHM length decreased from 79.5 µm to 28.6 µm. Using Zipfel’s equation this would correspond to a range of 109.5 µm to 39.5 µm in corneal tissue for corresponding NA’s. The lengths are increased in corneal tissue compared to water because the refractive index of corneal tissue is 1.376, larger than that of water, 1.33. A range of depth of up to 500 µm below the contact glass was also reached, Fig. 3(B).
Fig. 3.
Images taken of the two photon focal volume in a tank of riboflavin solution as the beam expander setting (A) and depth (B) were increased in steps, used to measure the size of the focal volume and depth below the contact glass.
Table 1. Calculated axial and lateral length, focal volume, and NA.
| Beam Expander Setting | FWHM (μm) | Volume (μm3) |
Theoretical volume in Cornea (μm3) |
Calculated NA | |
|---|---|---|---|---|---|
| Axial | Lateral | ||||
| 1 | 79.5 | 2.9 | 1407.1 | 1939.4 | 0.12 |
| 2 | 59.9 | 2.5 | 800.7 | 1104.3 | 0.14 |
| 3 | 41.0 | 2.3 | 375.6 | 518.6 | 0.17 |
| 4 | 36.3 | 2.2 | 294.9 | 407.3 | 0.18 |
| 5 | 28.6 | 2.5 | 182.8 | 252.8 | 0.20 |
Fig. 4.
As the beam expander setting increased from 0 to 5 the volume of the focal spot decreased more than 18 fold in both water (blue circles) and corneal tissue (orange triangles).
3.2 Change in mechanical stiffness
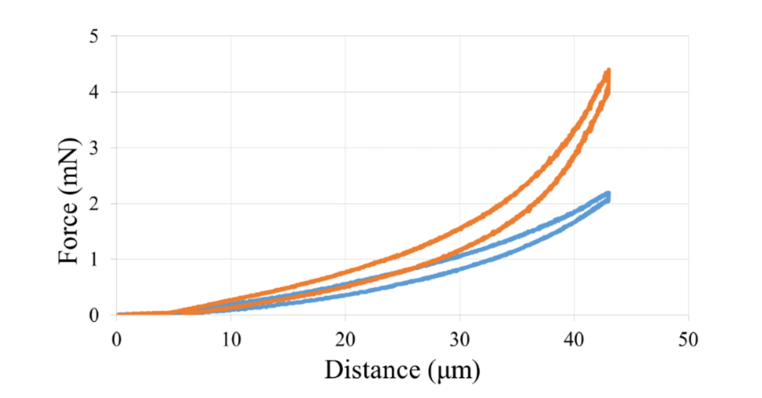
As shown in Fig. 5, as the indenting probe moved deeper into the normal corneal tissue (blue), the force reported by the transducer, in mN, increased, and moving the probe out of the tissue resulted in decreasing force measurement, which was lower at every value due to hysteresis of the tissue. By comparison, indentation of NLO crosslinked corneal tissue (orange) showed increased peak force compared to control corneas indicating mechanical stiffening. The value used for calculations was the force value taken at the peak of the tenth cycle, at 10% strain.
Fig. 5.
Plotted force required to indent the corneal stroma to 10% thickness. The orange, taller, graph shows the data for a single indentation cycle of a sample crosslinked with 900 mW, and the blue, shorter, graph shows the same data for a control cornea.
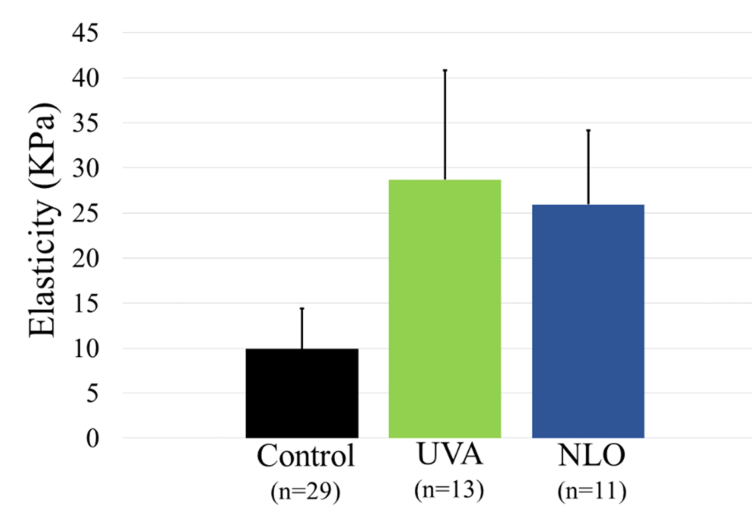
Figure 6 shows the results from the force measurements converted to the elasticity moduli for control, UVA CXL, and NLO CXL. Both UVA CXL (green) and NLO CXL (blue) were shown to be significantly stiffer than controls (black) with P values of less than 0.001 (Power = 0.9 for a twofold difference between NLO CXL and control, and 0.8 for a twofold difference between UVA CXL and control). Comparison of the elasticity measured for NLO CXL was not significantly different from that obtained following UVA CXL (P = 0.66). Corneas treated with NLO CXL (11) had an average elasticity of 25.95 ± 8.23 KPa, 2.6 times the 9.91 ± 4.54 KPa of the control corneas. In comparison, corneas treated with UVA CXL (13) had an elasticity of 28.72 ± 12.11 KPa on average, 2.9 times the force needed to indent control corneas.
Fig. 6.
Both UVA (green) and NLO (blue) treated eyes were significantly stiffer than controls (black) with P values less than 0.001.
3.3 NLO CXL induced CAF
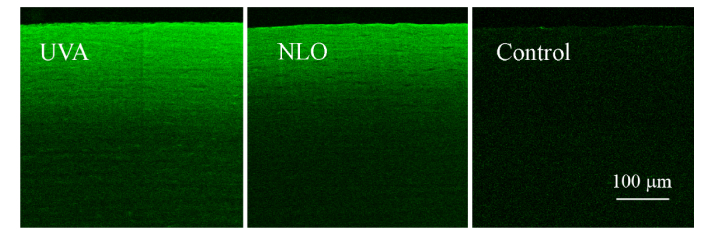
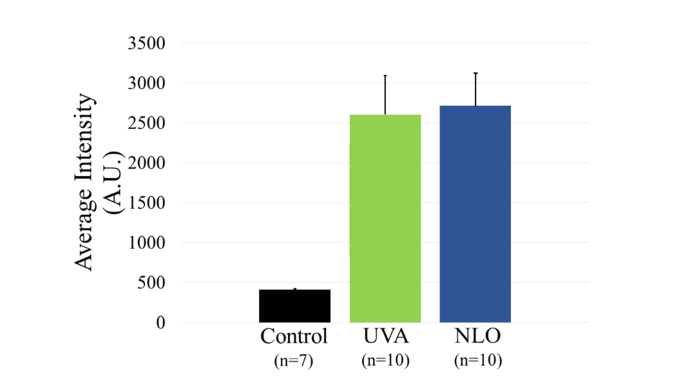
Other studies have linked increased CAF intensity to increased mechanical stiffness [5]. Images of CAF showed a similar intensity of emission for NLO CXL treated eyes as achieved by UVA CXL. Figure 7 provides examples of CAF images used to take intensity measurements. As shown in Fig. 8 the average intensity per 0.4 µm2 area of eyes treated with UVA CXL (10) and NLO CXL (10) were 2603.61 ± 490.47 and 2717.78 ± 403.01 respectively in the anterior crosslinked stroma compared to an intensity of 409.47 ± 10.14 in the comparable anterior stroma of control eyes. No significant difference was seen between the intensities of any of the treatment groups, P = 0.6, but both groups were significantly brighter than controls, P < 0.001. The statistical analysis had a power of 0.86 for a 30% difference.
Fig. 7.
Examples of CAF images for UVA CXL, NLO CXL, and control samples.
Fig. 8.
CAF intensity was not significantly different between UVA (green) or NLO (blue) treatment groups.
4. Discussion
Chai et al. has shown that it is possible to mechanically stiffen collagen hydrogels using two-photon, nonlinear excitation of riboflavin comparable to that achieved using UVA CXL [16]. However, in this earlier study a high NA lens (0.8) was used that crosslinked only a very small volume. Crosslinking a larger volume using a high NA lens required scanning the hydrogels through multiple planes, resulting in very long exposure times. More recently we have shown that a low NA lens (0.1) can generate a CAF signal in ex vivo rabbit eyes that has an expanded axial and lateral dimension suggesting a dramatic increase in crosslinking volume compared to high NA lenses [17]. This increased CAF volume suggests that by using a low NA lens one might be able to use NLO CXL to more rapidly and precisely crosslink large volumes of cornea to increase tissue mechanical stiffness. For this study we designed a custom device which allows control over lens NA, focal volume size, depth within the cornea, scanning speed, and pattern of crosslinking. It also has the ability to perform NLO CXL in living rabbits, as well as ex vivo eyes, for use in future studies.
In this report we demonstrated that NLO CXL treatment using this device is capable of mechanically stiffening corneal tissue comparable to that using UVA CXL. Corneas treated with NLO CXL, using 900 mW of laser power, were significantly stiffer than controls. In fact, the elasticity of these samples was 2.6 fold higher than that of control samples, comparable to the 2.9 fold increase following UVA CXL. Also both treatments showed a similar increase in CAF intensity of the treated region. While this study shows potential for this NLO CXL design, there is still much that needs to be improved to achieve safe and effective NLO CXL of live corneas.
One obstacle to consider is the total power being used. The laser that was used as a source for this device required a full 900 mW of laser power using a 0.1 NA lens and multiple overlapping of pulses to achieve the crosslinking effect. The American National Standards Institute (ANSI) retinal thermal power limit for 760 nm fs light focused with a 0.1 NA lens is 46.1 mW, well below the 900 mW used in this study. Recently we have reported that a single amplified fs pulse can generate CAF within rabbit corneas [21]. The amplified laser that was used had a repetition rate of 5 KHz delivering 2.4 µJ/pulse for a total energy of 12 mW, in contrast to the 76 MHz fs laser used in this study that delivered 11.8 nJ/pulse at a total energy of 900 mW. The increase in pulse energy using a 5 KHz fs laser allows for a dramatic decrease in the overall power, satisfying ANSI limits and getting rid of the need for overlapped pulses. Also when a single pulse is used, instead of overlapping multiple pulses per spot of tissue, the volume can be scanned much faster using higher repetition rate lasers (10-50 KHz), reducing the overall procedure time. While additional experiments are necessary to validate these early findings, the use of regeneratively amplified fs lasers appears to overcome a major hurdle toward the clinical applicability of NLO CXL.
More improvements may also be made by experimenting with the pattern of crosslinking. The versatility of focal volume size and placement allows for more regionally defined crosslinking. For example, this technique could allow for crosslinking of the area around the LASIK flap as a preventative measure against post LASIK ectasia. This versatility could also be used to design customized treatment patterns for Keratoconus patients based on their corneal topography and regional thickness. Also, since crosslinking treatment has been shown to flatten corneas as much as one-two diopters, modifying corneal shape using NLO CXL may have the potential to correct refractive errors [10, 11].
In conclusion, we have developed a device that provides for nonlinear optical crosslinking of the cornea that is as effective as UVA CXL. Further development of this novel technology may help expand the applicability domain for corneal crosslinking by providing for faster, safer, and more controlled regional stiffening.
Disclosures
The authors declare that there are no conflicts of interest related to this article.
Acknowledgements
National Eye Institute (NEI), and Research to Prevent Blindness (RPB), Inc.
Funding
Supported by NEI EY024600
References and links
- 1.Spoerl E., Huhle M., Seiler T., “Induction of cross-links in corneal tissue,” Exp. Eye Res. 66(1), 97–103 (1998). [DOI] [PubMed] [Google Scholar]
- 2.Wollensak G., Spoerl E., Seiler T., “Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus,” Am. J. Ophthalmol. 135(5), 620–627 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Raiskup F., Spoerl E., “Corneal crosslinking with riboflavin and ultraviolet A. I. Principles,” Ocul. Surf. 11(2), 65–74 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Kamaev P., Friedman M. D., Sherr E., Muller D., “Photochemical kinetics of corneal cross-linking with riboflavin,” Invest. Ophthalmol. Vis. Sci. 53(4), 2360–2367 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Chai D., Gaster R. N., Roizenblatt R., Juhasz T., Brown D. J., Jester J. V., “Quantitative assessment of UVA-riboflavin corneal cross-linking using nonlinear optical microscopy,” Invest. Ophthalmol. Vis. Sci. 52(7), 4231–4238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malik S., Humayun S., Nayyar S., Ishaq M., “Determining the efficacy of corneal crosslinking in progressive keratoconus,” Pak. J. Med. Sci. 33(2), 389–392 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hersh P. S., Stulting R. D., Muller D., Durrie D. S., Rajpal R. K., “United States Multicneter Clinical Trial of Corneal Collagen Crosslinking for Keratoconus Treatment,” Ophthalmology 124, 1259–1270 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Shalchi Z., Wang X., Nanavaty M. A., “Safety and efficacy of epithelium removal and transepithelial corneal collagen crosslinking for keratoconus,” Eye (Lond.) 29(1), 15–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanellopoulos A. J., Asimellis G., “Combined laser in situ keratomileusis and prophylactic high-fluence corneal collagen crosslinking for high myopia: two-year safety and efficacy,” J. Cataract Refract. Surg. 41(7), 1426–1433 (2015). [DOI] [PubMed] [Google Scholar]
- 10.De Bernardo M., Capasso L., Lanza M., Tortori A., Iaccarino S., Cennamo M., Borrelli M., Rosa N., “Long-term results of corneal collagen crosslinking for progressive keratoconus,” J. Optom. 8(3), 180–186 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elling M., Kersten-Gomez I., Dick H. B., “Photorefractive intrastromal corneal crosslinking for the treatment of myopic refractive errors: Six-month interim findings,” J. Cataract Refract. Surg. 43(6), 789–795 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Hagem A. M., Thorsrud A., Sandvik G. F., Råen M., Drolsum L., “Collagen crosslinking with conventional and accelerated ultraviolet-A irradiation using riboflavin with hydroxypropyl methylcellulose,” J. Cataract Refract. Surg. 43(4), 511–517 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Lombardo G., Micali N. L., Villari V., Leone N., Serrao S., Rusciano D., Lombardo M., “Assessment of stromal riboflavin concentration-depth profile in nanotechnology-based transepithelial corneal crosslinking,” J. Cataract Refract. Surg. 43(5), 680–686 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Spoerl E., Mrochen M., Sliney D., Trokel S., Seiler T., “Safety of UVA-riboflavin cross-linking of the cornea,” Cornea 26(4), 385–389 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Kaiser W., Garrett C. G. B., “Two-photon excitation in CaF2:Eu2+,” Phys. Rev. Lett. 7(6), 229–231 (1961). [Google Scholar]
- 16.Chai D., Juhasz T., Brown D. J., Jester J. V., “Nonlinear optical collagen cross-linking and mechanical stiffening: a possible photodynamic therapeutic approach to treating corneal ectasia,” J. Biomed. Opt. 18(3), 038003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford S. M., Brown D. J., Juhasz T., Mikula E., Jester J. V., “Nonlinear optical corneal collagen crosslinking of ex vivo rabbit eyes,” J. Cataract Refract. Surg. 42(11), 1660–1665 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zipfel W. R., Williams R. M., Webb W. W., “Nonlinear magic: multiphoton microscopy in the biosciences,” Nat. Biotechnol. 21(11), 1369–1377 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Levental I., Levental K. R., Klein E. A., Assoian R., Miller R. T., Wells R. G., Janmey P. A., “A simple indentation device for measuring micrometer-scale tissue stiffness,” J. Phys. Condens. Matter 22(19), 194120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes W. C., Keer L. M., Herrmann G., Mockros L. F., “A mathematical analysis for indentation tests of articular cartilage,” J. Biomech. 5(5), 541–551 (1972). [DOI] [PubMed] [Google Scholar]
- 21. Mikula E. R., Bradford S., Brown D. J., Juhasz T., Jester J., “Precise corneal crosslinking (CXL) using a 5 KHz amplified femtosecond laser,” presented at The Association for Research in Vision and Ophthalmology annual meeting, Baltimore, Maryland, 7–11 May 2017. [Google Scholar]