ABSTRACT
Organ-like microenviroment and 3-dimensional (3D) cell culture conformations have been suggested as promising approaches to mimic in a micro-scale a whole organ cellular functions and interactions present in vivo. We have used this approach to examine biologic features of hepatocellular carcinoma (HCC) cells. In this study, we demonstrate that hepatocellular carcinoma (HCC) cells, fibroblasts, endothelial cells and extracellular matrix can generate organoid-like spheroids that enhanced numerous features of human HCC observed in vivo. We show that the addition of non-parenchymal cells such as fibroblast and endothelial cells is required for spheroid formation as well as the maintenance of the tissue-like structure. Furthermore, HCC cells cultured as spheroids with non-parenchymal cells express more neo-angiogenesis-related markers (VEGFR2, VEGF, HIF-α), tumor-related inflammatory factors (CXCR4, CXCL12, TNF-α) and molecules-related to induced epithelial-mesenchymal transition (TGFβ, Vimentin, MMP9) compared with organoids containing only HCC cells.
These results demonstrate the importance of non-parenchymal cells in the cellular composition of HCC organoids. The novelty of the multicellular-based organotypic culture system strongly supports the integration of this approach in a high throughput approach to identified patient-specific HCC malignancy and accurate anti-tumor therapy screening after surgery.
Keywords: endothelial cell, fibroblast, hepatocellular carcinoma, organoid, tumor microenvironment
Introduction
Liver cancer is the fifth most frequently diagnosed cancer worldwide and the third leading cause of cancer mortality, and China shares the burden of almost half of new cases and deaths.1,2 Although in recent years, hepatocellular carcinoma (HCC), the most common form of liver cancer, accomplished outstanding clinical prognosis based on the rapidly advancing of early stage diagnosis technologies and comprehensive treatment strategies, HCC is frequently considered as highly malignant tumor phenotype and its subsequent intrahepatic recurrence make the 5-year survival rate at around 20% in the US.3 Due to the heterogeneity of HCC tumors, investigations have been undertaken to identify molecular targets for particular groups of HCC.4 However, to effectively rank, identify and evaluate genomic alterations in each tumor, a simple and near-physiologic 3D ex-vivo model that can both well predict HCC malignancy and provide therapy predictions may help to improve the HCC outcomes.
Traditionally, investigators have approached ex vivo culture systems using 2-dimensional (2D) cultures and animal models of HCC.5-8 However, 2D cultures lack the necessary cell-cell and cell-matrix contacts found in vivo. Additionally, animal models of HCC, do not reflect the genomic alterations seen in man, especially due to the heterogenicity of each tumor which plays an important role in malignancy degree and drug treatment such as diffusion and development of drug resistance.4,6 A viable alternative is the implementation of organoid-based technologies across academia as near-physiologic models for use in both basic and translational research. Unlike more traditional in vitro cultures, organoids are similar to primary tissue in their composition and architecture, harboring small populations of different types of cells. For instance, hepatocyte organoids from both primary or stem cell-derived hepatocytes have shown characteristics seen only in vivo (e.g. acinar structure, polarity, bile canaliculi).9-11 Moreover, it is well known that heterotypic cell interactions are required for the phenotypic stability of the liver parenchymal cells as well as for proper liver function and to recapture liver structure.12
In this study, we sought to determine if supportive cell types could influence the outcome and tumor HCC microenvironment. Here, we demonstrate the ability of liver non-parenchymal cells including endothelial cells and fibroblast to support HCC tumor-organoid formation in an in vitro –Matrigel- assay. The formed HCC-based organoid dramatically retained and enhanced angiogenesis-related markers, tumor-related inflammatory factors and molecules-related to cellular invasion and induced epithelial-mesenchymal transition markers compared with organoids containing only HCC cells. Our ability to establish a tumor-organoid system that enhances HCC malignancy-related hallmarks offers the possibility to study the heterotypic cell–cell interactions shown to be important for proper HCC-like microenvironment, under carefully controlled conditions, and suggests a new and reliable method to design high throughput therapeutic screenings.
Materials and Methods
Cell line and primary cell culture
The human HCC cell line HCCLM3 used in this study was kindly provided by Dr. Yan Li from Fudan University, Shanghai, China. The human HCC cell line Hep3B was purchased from ATCC, Manassas, VA, USA. For maintaining and expanding, cell lines were cultured in standard medium [1:1 mixture of DMEM and F-12 (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS (Gibco, Carlsbad, CA, USA), 1% penicillin- streptomycin (Gibco, Carlsbad, CA, USA)]. The human primary fibroblasts were isolated and expanded from the fetal liver as described by Tobita T et.al.13 under a protocol approved by the Human Research Review Committee of the University of Pittsburgh (Honest broker approval number HB015 and HB000836). HUVECs were purchased from LONZA Walkersville, MD, USA and maintained in endothelial growth medium (Lonza, Walkersville, MD, USA). All the cells were cultured under 37°C in a humidified 5% CO2 incubator.
Multi-cellular tumor-organoid self-assembly
To construct the tumor-organoids in 24 wells plate, briefly, Matrigel™ (Corning) was thaw overnight at 4°C, then a total of 2 million cells suspended in 200μl of culture medium were mixed with 200 μl Matrigel™ (Corning). This configuration allowed a thick layer (250mm) of Matrigel™ (Corning) for each well. The cell ratio of HCC cells and non-parenchymal cells that allowed tumor-organoid condensation was 1:1 (per every million HCC cells, 0.6 × 106 endothelial cells and 0.4 × 106 fibroblast were added). Assembly culture medium used was a mix 1:1 ratio of standard medium: endothelial growth medium for each well and cultured for 24 hours and 7 days for. Matrigel™ (Corning) was solidified in 37°C for more than 2 hours, then 1 ml assembly culture medium was added into each well and changed every 2 d.
Immunofluorescence Staining
Tumor-organoids cultured for 24 h were washed 3 times with PBS and then harvested into Tissue-Tek O.C.T. Compound (Sakura Finetek, Torrance, CA, USA) on dry ice, followed by 10 mm sections performed on cryostat from each group and fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS), then stored in −80°C for future staining. To illustrate only HCC cells in tumor-organoids we used Glypican 3 (GPC-3) surface marker. Next, samples were blocked and permeabilized with blocking buffer containing Triton X-100 and 1% BSA for one hour at room temperature, the samples are dually labeled with the primary antibody GPC-3 (1:50 dilution, Santa Cruz Biotechnology, Dallas, TX, USA) as a marker for HCC, Vimentin (1:200 dilution), Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) (1:100 dilution) and the 7 transmembrane G-protein coupled receptor (CXCR4) (1:200 dilution) (Abcam, Cambridge, MA, USA) as markers of HCC malignancy. As a cell proliferation marker Ki67 (BD Biosciences) was used. von Willebrand factor (vWF) (1:200 dilution Abcam, Cambridge, MA, USA) was used to locate the endothelial cells. Samples were exposed in blocking buffer overnight at 4°C, then samples were incubated with 2 secondary antibodies with different fluorescence colors (1:250 dilution, Invitrogen, Eugene, OR, USA) for one hour at room temperature in the dark. The nuclei were stained by VECTASHIELD Antifade Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Negative controls were performed in the absence of primary antibodies. As positive controls human HCC tissue was used. Paraffin embedded slide samples were kindly provided by Dr. Satdarshan Singh Monga from the Department of Pathology at the University of Pittsburgh. The digital images were taken using the Eclipse Ti-E microscope equipped with an NIS-Elements software (Nikon, Japan). Acquired images were processed and quantified using ImageJ 1.48 v software. For proliferation marker (Ki67) quantification; 3 sections from 3 different areas for each culture condition were examined by 2 independent observers. The number of positive cells was assessed in 4 randomized high-power fields for each section. All the labeled nuclei, regardless of the staining intensity, were considered positive. A mean percentage for every sample was obtained and data were reported as means SD.
RNA extraction, Reverse Transcription and quantitative Polymerase Chain Reactions
To extract total RNA from the cultured tumoroids, we used the RNeasy mini kit (Qiagen, Valencia, CA, USA) with the manufacturer's protocol. Quantification of RNA was performed by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). RNA was pre-mixed with random hexamers (Invitrogen, Carlsbad, CA, USA) and heated to 70°C for 5 minutes and then reverse transcribed with the Superscript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA), following the manufacturer's protocol. Subsequently, qPCR was performed using TaqMan Fast Advanced Master Mix (Life Technologies, CA, USA) and the TaqMan Gene Expression Assays (Assay #: Hs01052961_m1; Hs00900055_m1; Hs00958111_m1; Hs00607978_s1; Hs00936781_m1; Hs00820148_g1; Hs00233987_m1; Hs03676656_mH; Hs00153153_m1; Hs01060665_g1. (Life Technologies, CA, USA) in StepOnePlus system (Applied Biosystems, Foster City, CA, USA). Expression of specific genes was normalized to an internal control Hypoxanthine-guanine phosphoribosyltransferase (HPRT) mRNA expression.
Statistical analysis
Quantitative data are demonstrated as mean ± standard deviation. Experiments were performed in triplicates or more. All the values were compared through Student's t test between groups and only p < 0.05 were considered statistically significant.
Results
Co-seeding of HCC cells and non-parenchymal cells formed tumor-organoid-like structures and maintained viability
Since tumor microenvironment (TME) is composed of extracellular matrix (ECM) elements and from different type of cells, especially fibroblast and endothelial cells14; we attempted to provide and merge these important factors in an in-vitro organoid-based approach. First, to recapitulate the HCC-TEM, HCC cells (Hep3B15 cell line and HCCLM316) were cultivated alone or in combination with stromal cell population primary human fetal fibroblast and human microvascular endothelial cells. Notably, cells were plated in 3-dimensional conditions using thick layer of Matrigel™, human HCC cells and non-parenchymal cells self-organized into macroscopically visible 3-dimensional cell clusters by an intrinsic organizing capacity up to 24 h after seeding (Fig. 1A). The cell ratio of HCC cells and non-parenchymal cells that allowed tumor-organoid condensation was 1:1 (per every million HCC cells, 0.6 × 106 endothelial cells and 0.4 × 106 fibroblast were added). The tumor-organoids were stable and could be manipulated easily. We visualized the formation of the tumor-organoids for replicating HCC cells and found that HCC cells homogeneously distributed through the organoid (Fig. 1 B). Moreover, immune fluorescence studies for HCC cells and Ki67 revealed that the addition of non-parenchymal cells and the 3D organoid formation did not impact the replicative capacity of HCC cells (Fig. 1 C).
Figure 1.
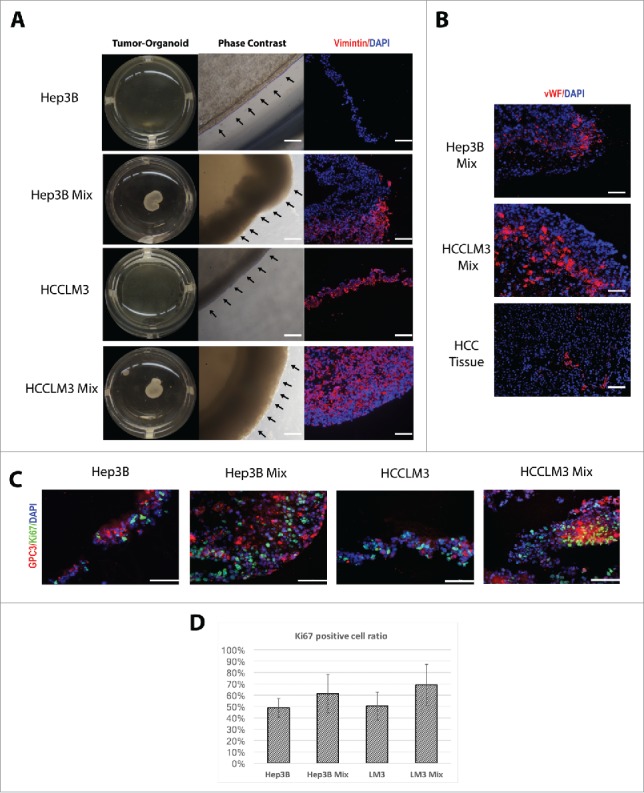
Self-assembly of tumor-organoids and proliferation assay. (A) Phase contrast microscopy of tumor-organoid in a 24-well culture plate. Time-lapse of HCC cells and human non-parenchymal cells (fibroblast and endothelial cells) or only HCC cells cultured on matrigel at 24h. Immunofluorescence analysis of the resulting tumor-organoids, Vimentin; red, nuclei; blue. Scale bar 100 µm. (B) Immunofluorescence stainings of endothelial cells in 2 mixed cells tumor-organoids and human HCC tissue, vWF; red, nuclei; blue. Scale bar 100 µm. (C) Proliferation analysis by Ki67 immunefluorescence staining, GPC-3; red, nuclei; blue, Ki67; green. Scale bar 200 µm. (n = 3) (D) Ki67-GPC3 positive cell quantification (n = 3).
Tumor-organoids enhanced the expression of markers related to Epithelial–mesenchymal transition and malignancy
The epithelial–mesenchymal transition (EMT) has a pivotal role in tumor invasion and dissemination. To characterize the expression profiles of the formed tumor-organoids and determine whether addition of stromal components would aid to mimic the TME, we performed an immune flourecence analyses of vimentin, a known marker of EMT in HCC (Fig. 2A). The addition of non-parenchymal cellular component induces a stronger expression of vimentin in both types of HCC cell lines analyzed. We corroborated this data by examining the mRNA expression using real time qPCR for Vimentin (Fig. 2B). Moreover, we examined the expression of other pathways related to malignancy and metastasis of HCC tumors. It is recognized that matrix metallopeptidases (MMP) are highly expressed in HCC tumors; especially MMP-9 and that citokines like Transforming growth factor (TGF-β) could accelerate tumor aggressiveness by inducing metastases.14,17 Indeed, the tumor-organoids expressed high levels of MMP9 and TGFβ (Fig. 2B).
Figure 2.
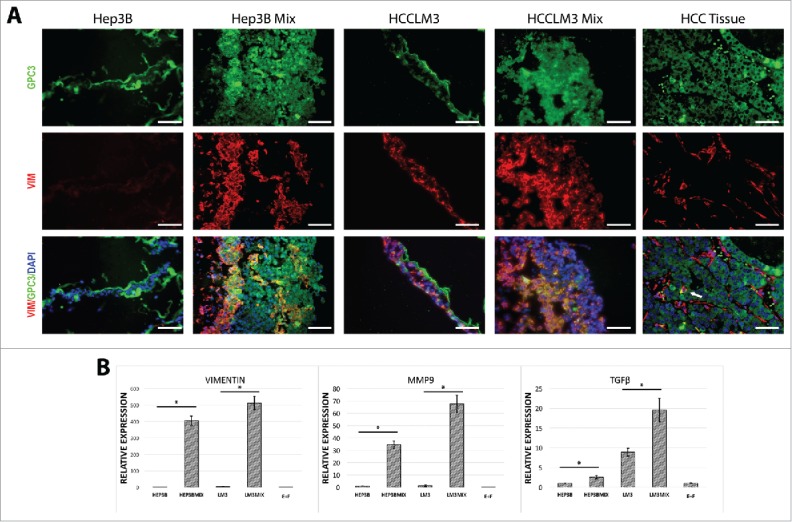
Characterization of tumor-organoids for epithelial-mesenchymal transition associated markers. (A) Immunofluorescence analysis of the resulting tumor-organoids and human HCC tissue, white arrow; strong double positive cells in human HCC tissue, GPC-3; green, nuclei; blue, vimentin; red. Scale bar 200 µm. (n = 3). (B) Quantitative PCR analysis of Vimentin, MMP9 and TGFβ in human tumor-organoids and controls at 24 h after self-assembly. Results represent mean ± s.d., (n = 3) * p < 0.05.
Tumor-organoids enhanced the expression of markers related to neo-angiogenesis
Angiogenesis is essential for tumor growth.14 To test whether non-parenchymal cells especially endothelial cells were capable of inducing expression of markers related to neo-angiogenesis, we tested the expression of VEGFR2 by immune staining. VEGFR2 was significantly increased in tumor-organoids made from HCC cell line Hep3B but not in HCCLM3-based organoids (Fig. 3A). Similar results were obtained when mRNA was analyzed for VEGFR2 (Fig. 3B). Hypoxia plays a significant role in the development of HCC and is an inductor of pathways related to neo-angiogenesis. For instance, Hypoxia-inducible factor 1-α (HIF-1α) is a key transcription factor involved in the hypoxic response of cancer cells.18 Next, we examined the gene expression for VEGF and HIF-1α in tumor-organoids. As expected in TME, both VEGF and HIF-1α were significantly upregulated (Fig. 3B). Altogether, these results indicate that the tumor-organoid formation enhances some aspects of HCC cells growth seen in normal tumors.
Figure 3.
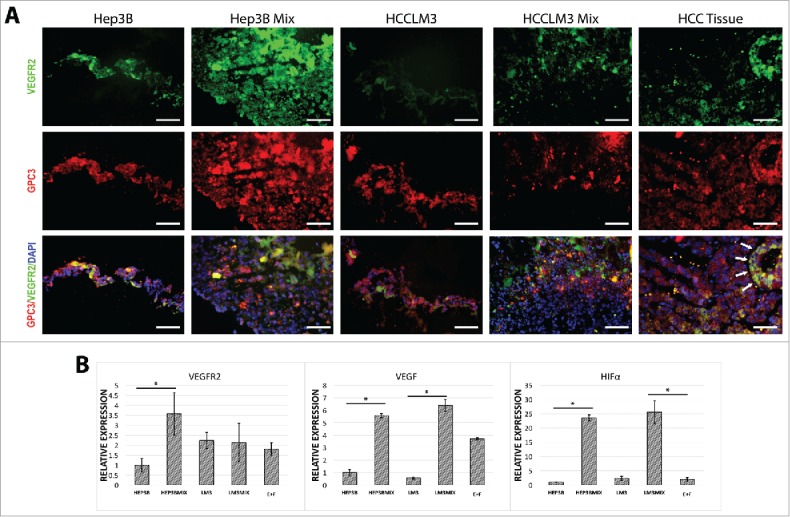
Characterization of tumor-organoids for neo-angiogenesis-related markers. (A) Immunofluorescence analysis of the resulting tumor-organoids and human HCC tissue, white arrows; strong double positive cells in human HCC tissue, GPC-3; red, nuclei; blue, VEGFR2; green. Scale bar 200 µm. (n = 3). (B) Quantitative PCR analysis of VEGFR2, VEGF, HIF-α in human tumor-organoids and controls at 24 h after self-assembly. Results represent mean ± s.d., (n = 3) * p < 0.05.
Tumor-organoids enhanced the expression of markers associated to inflammation
In HCC, chemokines have shown to affect leukocyte recruitment, neovascularization and tumor progression.19 Stromal-derived factor 1 α- SDF-1 (CXCL12) is the main ligand for CXCR4. The CXCR4/CXCL12 axis exerts a variety of functions of HCC tumor progression, using autocrine and/or paracrine mechanisms to sustain tumor cell growth, to induce angiogenesis and to facilitate tumor escape through evasion of immune surveillance.20 To evaluate this axis, we performed immune staining of CXCR4 and found up regulation in tumor-organoids (Fig. 4A). qPCR revealed that the expression of CXCR4, CXCL12 as well as the inflammatory cytokine tumor necrosis factor (TNF)-α in HCC cells with non-parenchymal cells were significantly higher than those without non-parenchymal cells (Fig. 4B).
Figure 4.
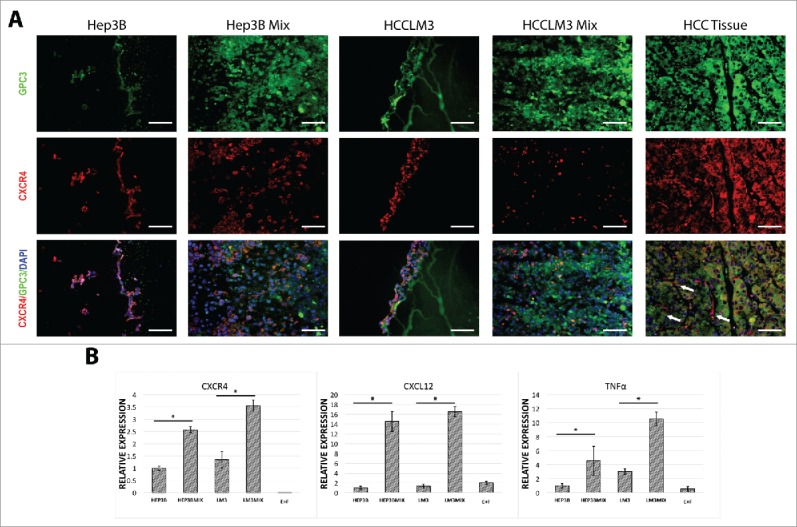
Characterization of tumor-organoids for tumor-related inflammatory factors. (A) Immunofluorescence analysis of the resulting tumor-organoids and human HCC tissue, white arrows; CXCR4 positive fibroblasts in human HCC tissue, GPC-3; green, nuclei; blue, CXCR4; red. Scale bar 200 µm. (n = 3). (B) Quantitative PCR analysis of CXCR4, CXCL12, TNF-α in human tumor-organoids and controls at 24 h after self-assembly. Results represent mean ± s.d., (n = 3) * p<0.05.
To evaluate the capacity of tumor-organoids to maintain HCC-TME for long-term compared with cultivation of only HCC cells, we performed a gene characterization of all the markers analyzed and found that even after 7 d in culture these genes associated to neo-angiogenesis-related markers (VEGFR2, VEGF, HIF-α), tumor-related inflammatory factors (CXCR4, CXCL12, TNF-α) and molecules-related to induced epithelial-mesenchymal transition (TGFβ, Vimentin, MMP9) were upregulated compared with the cultivation of only HCC cells (Fig. 5A).
Figure 5.
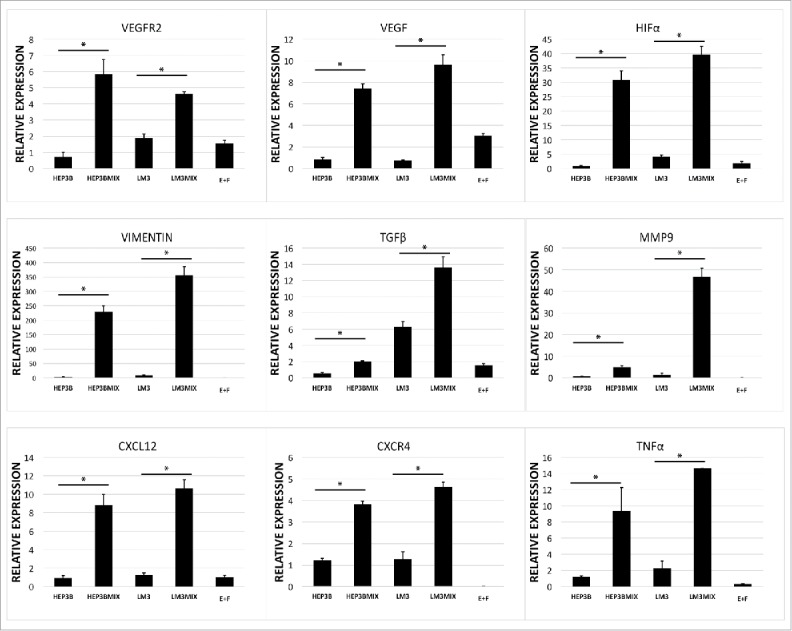
Expression of long-term cultured tumor-organoids. (A) Quantitative PCR analysis of Vimentin, MMP9, TGFβ, VEGFR2, VEGF, HIF-α, CXCR4, CXCL12 and TNF-α in human tumor-organoids and controls at 7 d after self-assembly. Results represent mean ± s.d., (n = 3) * p<0.05.
Discussion
There is evidence suggesting that interaction between endothelium and surrounding stromal tissue plays an intimate role in tissue differentiation, maintenance, and organization.21-23 However, when a cell starts to be cancerous, its surrounding matrix changes in a way to support cancer development and these modifications in the tumor microenvironment also supports the malignant cells growth.24 Tumor microenvironment is composed of fibroblasts, myofibroblasts, endothelial cells, pericytes, adipose cells, immune and inflammatory cells, and extracellular matrix (ECM) elements. It is also enriched with cytokines and chemokines, which are secreted from both cancerous and noncancerous cells.14 In such milieu, the crosstalk of all these compartments eventually decides how the tumors growth and differentiate. Mimicking this milieu has been demonstrated to be useful to study human physiology in vitro as organoids.9,25,26 Cancer cell-derived organoid models from liver, prostate, lung and pancreatic cancers have also been established as an in vitro system to model an in vivo tumor pathophysiological state, such as tumor-associated signaling pathways and most importantly chemoresistance .17,27 However, HCC organoid models have incorporated only artificial ECM in the organotypic cultures.17 In this study, we have developed an HCC organoid model based on human primary fibroblast and microvascular endothelial cells and Matrigel™ (gelatinous protein mixture from sarcoma cells). HCC cells can efficiently form tumor-organoids in less than 24 h with features of glandular epithelium with elevated cell turnover. We also found that the addition of human primary non-parenchymal cells to the organoids enhanced the expression of HCC cells for angiogenesis-related markers (VEGFR2, VEGF, HIF-α), tumor-related inflammatory factors (CXCR4, CXCL12, TNF-α) and molecules-related to induced epithelial-mesenchymal transition (TGFβ, Vimentin, MMP9).
The stabilization of hepatocyte function by fibroblasts has been previously demonstrated,28 also, there are few reports describing the fate of hepatocytes cocultured with bone marrow-derived stem cells29 and endothelial cells22 and even a combination of them including cholangiocytes12 but the actual organization of microvascular endothelial cells and fibroblast combined with HCC cells is unique to our system. A growing body of literature indicates that a subpopulation of fibroblasts can modulate cancer progression. These carcinoma-associated fibroblasts have been isolated from human tumors (e.g., prostate, breast, esophagus).30-32 Carcinoma-associated fibroblasts secrete vast amounts of ECM (e.g., collagen type I, fibronectin) and in the other hand it is documented that they can also secrete matrix-metalloproteinases including MMP-2, MMP-3 and MMP-9. This ability to remodel surrounding ECM facilitates tumor invasion.33 Increased expression of MMP-9 in serum of HCC patients make it as a candidate diagnostic marker.34 Moreover, It is demonstrated that the frequency of carcinoma-associated fibroblasts around HCC region is positively correlated with the tumor size. Additionally these cells secrete the hepatocyte growth factor in a level higher than the normal fibroblasts.35 A common theory of the origins of cancer-associated fibroblasts points to the resident tissue fibroblasts. Recent studies have shown that cancer cells reprogram fibroblasts to become cancer-associated fibroblasts through the actions of miRNAs (miR-31, miR-214, and miR-155). It has been described that cancer-associated fibroblasts are a source of inflammatory cytokines (e.g., IL6) and contribute to drug-resistance acquisition in cancer cells.14,33 Indeed, in our study we observed that in HCC tumor-organoids induction of mRNA expression of MMP9 was increased when non-parenchymal cells were used.
It is well known that endothelial cells play a critical role in tumor angiogenesis. The interaction of endothelial cells with ECM and basement membrane proteins such as collagen, laminin and fibronectin is also important for the angiogenesis process. Such environment is important during endothelial cell stability, morphogenesis, proliferation and neoangiogenesis. One described mechanism for neoangiogenesis; a critical factor to supply tumors with nutrients and oxygen; is simply the exposure of endothelial cells to collagen.36 HCC cells display normal cell cycles despite hypoxia, HIF-1α upregulated growth factors, such as VEGF, which promotes tumor proliferation, and hexokinases, which help generate ATP to provide an energy source for HCC cells.37 In fact, tumor progression and metastasis are connected to angiogenesis through VEGF signaling pathway.38 Studies showed that VEGF—an endothelial-specific marker—has been increased in serum of patients having HCC and strongly related to the degree of invasiveness, metastasis and shorter survival.39 Consistently with this knowledge, tumor-organoids expressed angiogenesis-related factors (VEGFR2, VEGF, HIF-α) when non-parenchymal cells were added. Demonstrating that tumor-organoids can mimic some important aspects of HCC tumor characteristics.
The epithelial-mesenchymal transition (EMT) has a pivotal role in tumor invasion and dissemination. A number of pathways, which are involved in EMT, have been detected in a variety of tumors. A hallmark of EMT is the loss of epithelial characteristics such as a decrease in the expression of the cell adhesion molecular E-cadherin and acquisition of a mesenchymal phenotype accompanied by increased expression of vimentin.40 Moreover, TGF-β is a key member of the TGF-β superfamily that is known to induce EMT. In this study, we have explored the roles of EMT in HCC organoid formation by analyzing the expression of vimentin, TGF-β and also other inflammation-associated factors such as CXCR4, CXCL12 and TNF-α. Tumor-organoids dramatically unregulated the expression of all these markers of EMT and inflammation. Further experiments will include investigating the effect or possible treatments aimed to regulate these pathways. Taken together, these in vitro results suggested that non-parenchymal cells might utilize mechanisms to stabilize hepatocellular function and enhancing the tumoral capabilities of HCC cells, although those warrants further investigation for long-term culture. Studies have demonstrated that human liver non-parenchymal-derived molecules include many growth factors, cytokines and chemokines that collectively can enhance hepatocyte replication and protect against hepatocyte death.23 A comprehensive proteomic analysis of the conditioned medium is underway to identify the active ingredient(s) within human liver non-parenchymal conditioned medium.
In conclusion, these findings demonstrate the importance of non-parenchymal cells in the cellular composition of HCC tumor-organoids. We therefore suggest that this system and the resulting organoids may serve as a unique in vitro model of HCC for therapeutic screenings when using personalized patient-derived HCC cells. We believe this method is useful to effectively test various candidate driver genes and screen new therapeutic molecule that block HCC process in a high throughput fashion.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This work was supported by the following grants: Natural Science Foundation of China grant # 81502509, 81470874; Natural Science Foundation of Zhejiang Province grant # LY13H030009; Funding of Beijing Key Laboratory of Liver Cancer and Fibrosis.
REFERENCES
- [1].Organization WH. World Cancer Report 2014. World Health Organization: International Agency for Research on Cancer; 2014. [Google Scholar]
- [2].Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology 2014; 60(6):2099-108; PMID:25164003; https://doi.org/ 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012; 379(9822):1245-55; PMID:22353262; https://doi.org/ 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- [4].Shibata T, Aburatani H. Exploration of liver cancer genomes. Nat Rev Gastroenterol Hepatol 2014; 11(6):340-9; PMID:24473361; https://doi.org/ 10.1038/nrgastro.2014.6 [DOI] [PubMed] [Google Scholar]
- [5].Tao J, Xu E, Zhao Y, Singh S, Li X, Couchy G, Chen X, Zucman-Rossi J, Chikina M, Monga SP. Modeling a human hepatocellular carcinoma subset in mice through coexpression of met and point-mutant beta-catenin. Hepatology 2016; 64(5):1587-605; PMID:27097116; https://doi.org/ 10.1002/hep.28601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bakiri L, Wagner EF. Mouse models for liver cancer. Mol Oncol 2013; 7(2):206-23; PMID:23428636; https://doi.org/ 10.1016/j.molonc.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deng G, Zeng S, Ma J, Zhang Y, Qu Y, Han Y, Yin L, Cai C, Guo C, Shen H. The anti-tumor activities of Neferine on cell invasion and oxaliplatin sensitivity regulated by EMT via Snail signaling in hepatocellular carcinoma. Sci Rep 2017; 7:41616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Takeishi K, Taketomi A, Shirabe K, Toshima T, Motomura T, Ikegami T, Yoshizumi T, Sakane F, Maehara Y. Diacylglycerol kinase alpha enhances hepatocellular carcinoma progression by activation of Ras-Raf-MEK-ERK pathway. J Hepatol 2012; 57(1):77-83; PMID:22425622; https://doi.org/ 10.1016/j.jhep.2012.02.026 [DOI] [PubMed] [Google Scholar]
- [9].Takebe T, Koike N, Sekine K, Fujiwara R, Amiya T, Zheng YW, Taniguchi H. Engineering of human hepatic tissue with functional vascular networks. Organogenesis 2014; 10(2):260-7; PMID:24451152; https://doi.org/ 10.4161/org.27590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hamilton GA, Westmorel C, George AE. Effects of medium composition on the morphology and function of rat hepatocytes cultured as spheroids and monolayers. In Vitro Cell Dev Biol Anim 2001; 37(10):656-67; PMID:11776971; https://doi.org/ 10.1290/1071-2690(2001)037%3c0656:EOMCOT%3e2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- [11].Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res 2002; 274(1):56-67; PMID:11855857; https://doi.org/ 10.1006/excr.2001.5467 [DOI] [PubMed] [Google Scholar]
- [12].Soto-Gutierrez A, Navarro-Alvarez N, Yagi H, Nahmias Y, Yarmush ML, Kobayashi N. Engineering of an hepatic organoid to develop liver assist devices. Cell Transplant 2010; 19(6):815-22; PMID:20573303; https://doi.org/ 10.3727/096368910X508933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tobita T, Guzman-Lepe J, Takeishi K, Nakao T, Wang Y, Meng F, Deng CX, Collin de l'Hortet A, Soto-Gutierrez A. SIRT1 Disruption in Human Fetal Hepatocytes Leads to Increased Accumulation of Glucose and Lipids. PLoS One 2016; 11(2):e0149344; PMID:26890260; https://doi.org/ 10.1371/journal.pone.0149344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang JD, Nakamura I, Roberts LR. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin Cancer Biol 2011; 21(1):35-43; PMID:20946957; https://doi.org/ 10.1016/j.semcancer.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hiron M, Daveau M, Arnaud P, Bauer J, Lebreton JP. The human hepatoma Hep3B cell line as an experimental model in the study of the long-term regulation of acute-phase proteins by cytokines. Biochem J 1992; 287(Pt 1):255-9; PMID:1384466; https://doi.org/ 10.1042/bj2870255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, Chen J, Gao DM, Bao WH. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001; 7(5):630-6; PMID:11819844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Takai A, Fako V, Dang H, Forgues M, Yu Z, Budhu A, Wang XW. Three-dimensional Organotypic Culture Models of Human Hepatocellular Carcinoma. Sci Rep 2016; 6:21174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lin D, Wu J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J Gastroenterol 2015; 21(42):12171-8; PMID:26576101; https://doi.org/ 10.3748/wjg.v21.i42.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Aleksandrova K, Boeing H, Nothlings U, Jenab M, Fedirko V, Kaaks R, Lukanova A, Trichopoulou A, Trichopoulos D, Boffetta P, et al.. Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology 2014; 60(3):858-71; PMID:24443059; https://doi.org/ 10.1002/hep.27016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res 2014; 6(4):340-52: PMID:25075251 [PMC free article] [PubMed] [Google Scholar]
- [21].Michalopoulos GK, Bowen WC, Zajac VF, Beer-Stolz D, Watkins S, Kostrubsky V, Strom SC. Morphogenetic events in mixed cultures of rat hepatocytes and nonparenchymal cells maintained in biological matrices in the presence of hepatocyte growth factor and epidermal growth factor. Hepatology 1999; 29(1):90-100; PMID:9862855; https://doi.org/ 10.1002/hep.510290149 [DOI] [PubMed] [Google Scholar]
- [22].Nahmias Y, Schwartz RE, Hu WS, Verfaillie CM, Odde DJ. Endothelium-mediated hepatocyte recruitment in the establishment of liver-like tissue in vitro. Tissue Eng 2006; 12(6):1627-38; PMID:16846358; https://doi.org/ 10.1089/ten.2006.12.1627 [DOI] [PubMed] [Google Scholar]
- [23].Soto-Gutierrez A, Kobayashi N, Rivas-Carrillo JD, Navarro-Alvarez N, Zhao D, Okitsu T, Noguchi H, Basma H, Tabata Y, Chen Y, et al.. Reversal of mouse hepatic failure using an implanted liver-assist device containing ES cell-derived hepatocytes. Nat Biotechnol 2006; 24(11):1412-9; PMID:17086173; https://doi.org/ 10.1038/nbt1257 [DOI] [PubMed] [Google Scholar]
- [24].Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med 2015; 13:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Workman MJ, Mahe MM, Trisno S, Poling HM, Watson CL, Sundaram N, Chang CF, Schiesser J, Aubert P, Stanley EG, et al.. Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 2017; 23(1):49-59; PMID:27869805; https://doi.org/ 10.1038/nm.4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].McCracken KW, Cata EM, Crawford CM, Sinagoga KL, Schumacher M, Rockich BE, Tsai YH, Mayhew CN, Spence JR, Zavros Y, et al.. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 2014; 516(7531):400-4; PMID:25363776; https://doi.org/ 10.1038/nature13863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al.. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014; 159(1):176-87; PMID:25201530; https://doi.org/ 10.1016/j.cell.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol 2008; 26(1):120-6; PMID:18026090; https://doi.org/ 10.1038/nbt1361 [DOI] [PubMed] [Google Scholar]
- [29].Isoda K, Kojima M, Takeda M, Higashiyama S, Kawase M, Yagi K. Maintenance of hepatocyte functions by coculture with bone marrow stromal cells. J Biosci Bioeng 2004; 97(5):343-6; PMID:16233641; https://doi.org/ 10.1016/S1389-1723(04)70217-0 [DOI] [PubMed] [Google Scholar]
- [30].Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003; 107(1):1-10; PMID:12925950; https://doi.org/ 10.1002/ijc.11335 [DOI] [PubMed] [Google Scholar]
- [31].Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005; 121(3):335-48; PMID:15882617; https://doi.org/ 10.1016/j.cell.2005.02.034 [DOI] [PubMed] [Google Scholar]
- [32].Zhang C, Fu L, Fu J, Hu L, Yang H, Rong TH, Li Y, Liu H, Fu SB, Zeng YX, et al.. Fibroblast growth factor receptor 2-positive fibroblasts provide a suitable microenvironment for tumor development and progression in esophageal carcinoma. Clin Cancer Res 2009; 15(12):4017-27; PMID:19509166; https://doi.org/ 10.1158/1078-0432.CCR-08-2824 [DOI] [PubMed] [Google Scholar]
- [33].Shiga K, Hara M, Nagasaki T, Sato T, Takahashi H, Takeyama H. Cancer-Associated Fibroblasts: Their Characteristics and Their Roles in Tumor Growth. Cancers (Basel) 2015; 7(4):2443-58; PMID:26690480; https://doi.org/ 10.3390/cancers7040902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hayasaka A, Suzuki N, Fujimoto N, Iwama S, Fukuyama E, Kanda Y, Saisho H. Elevated plasma levels of matrix metalloproteinase-9 (92-kd type IV collagenase/gelatinase B) in hepatocellular carcinoma. Hepatology 1996; 24(5):1058-62; PMID:8903375; https://doi.org/ 10.1002/hep.510240513 [DOI] [PubMed] [Google Scholar]
- [35].Liu J, Chen S, Wang W, Ning BF, Chen F, Shen W, Ding J, Chen W, Xie WF, Zhang X. Cancer-associated fibroblasts promote hepatocellular carcinoma metastasis through chemokine-activated hedgehog and TGF-beta pathways. Cancer Lett. 2016; 379(1):49-59; PMID:27216982; https://doi.org/ 10.1016/j.canlet.2016.05.022 [DOI] [PubMed] [Google Scholar]
- [36].Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 2005; 97(11):1093-107; PMID:16306453; https://doi.org/ 10.1161/01.RES.0000191547.64391.e3 [DOI] [PubMed] [Google Scholar]
- [37].Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol 2005; 42(3):358-64; PMID:15710218; https://doi.org/ 10.1016/j.jhep.2004.11.020 [DOI] [PubMed] [Google Scholar]
- [38].Pralhad T, Madhusudan S, Rajendrakumar K. Concept, mechanisms and therapeutics of angiogenesis in cancer and other diseases. J Pharm Pharmacol 2003; 55(8):1045-53; PMID:12956893; https://doi.org/ 10.1211/0022357021819 [DOI] [PubMed] [Google Scholar]
- [39].Poon RT, Ho JW, Tong CS, Lau C, Ng IO, Fan ST. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg 2004; 91(10):1354-60; PMID:15376182; https://doi.org/ 10.1002/bjs.4594 [DOI] [PubMed] [Google Scholar]
- [40].Scheel C, Weinberg RA. Cancer stem cells and epithelial-mesenchymal transition: concepts and molecular links. Semin Cancer Biol 2012; 22(5–6):396-403; PMID:22554795; https://doi.org/ 10.1016/j.semcancer.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


