ABSTRACT
Endometriosis is defined by the presence of endometrial ectopia. Multiple hypotheses have been postulated to explain the etiology of endometriosis to understand various clinical evidences. The etiology of endometriosis is still unclear.The primary question to understanding the etiology of endometrial ectopia (endometriosis) is determining the origin of eutopic (normally cited) endometrium.According to the new theory, primordial germ cells migrate from hypoblast (yolk sac close to the allantois) to the gonadal ridges. The gonadal ridges which composed of primordial germ cells derive to the: eutopic endometrium, ovary, ovarian ligament and ligamentum teres uteri.There are 2 principal processes in uterine organogenesis: the intersection of gonadal ridges with mesonephral ducts to form the uterine folds with an endometrial cavity and the fusion of the both uterine folds together to form the unicavital (normal) uterus. In the uterine folds there are closer cell-to-cell communications, polypotential germ cells differentiate and grow into myometrium and endometrial layers.Some of the polypotential germ cells fail to reach the ridges and stay in the peritoneal cavity, where they may be transforming into external endometrial heterotopies.The main insight in the etiology of endometriosis is polypotential germ cells origin, which may explain its potency, pathogenesis and expansion.
KEYWORDS: adenomyosis, congenital anomaly, ectopic endometrium, endometriosis, endometriosis aetiology, eutopic endometrium, genital ridge, germ cells, stem cells, uterovaginal embryogenesis
INTRODUCTION
Endometriosis is defined as the presence of ectopic endometrial glands and stroma outside of the uterine cavity.1,2
External endometrial lesions are frequently found in the pelvic peritoneum (extra-genital endometriosis) and ovaries (ovarian cyst or superficial heterotopies). Most often, external endometriosis is located on the peritoneal surface of the recto-vaginal space: at the retrocervical area, on the uterosacral ligaments and in the recto-sigmoid region of the colon. Endometrial lesions can also be found at scars of the abdominal wall; on the bladder and ureters, and on the bowels and appendix. Rarely, endometriosis is recognized in other extra abdominal organs, such as the brain and oculus.1,2
Internal endometriosis (adenomyosis) is characterized by the presence of heterotopic endometrial glands and stroma in the uterine intramural muscular layer (deep in the myometrium) and is surrounded by reactive fibrosis of the myocytes. For a review see refs.1-3.
Endometriosis is a proliferative estrogen dependent disorder; symptoms may begin in adolescence. The prevalence of endometriosis in the female population is estimated to be 6 to 10%, and the frequency increases to 24–35% in females with pain and up to 50–60% in women with infertility. For a review see refs. 1-3.
The most often symptoms are refers to endometriosis: chronic pelvic pain, algodysmenorrhea, dyspareunia and primary infertility. Congenital uterovaginal anomalies defined as rare conditions that represent in 4–7% females,1-4 however the concomitant endometriosis revealed in 46% of those cases.
Signorile et al. investigated the autopsies of (101) human female fetuses at different gestational ages and found ectopic endometrium in 9% of cases.5,6 The authors suggested that endometriosis developed during organogenesis by dislocation (ectopia) of primitive endometrial tissue outside of the uterine cavity.7
Jean Bouquet de Jolinière et al. researched the reproductive organs from 7 female fetuses at autopsy between 18 and 36 weeks of gestation. Serial sections revealed numerous ectopic endometrial glands and embryonic duct remnants inside the myometrium, uterine broad and ovarian ligaments and under the fallopian tube serosa in 2 fetuses with low levels of expression of estrogen receptor-α (ER-a) and progesterone receptors (PR). Authors have supported the theory that some subtypes of endometrioid lesions may be related to anomalies occurring during embryogenesis.8
Male endometriosis of the prostate9 and male “uterus-like mass” are 2 unusual manifestations of endometriosis. González RS et al.10 reported a case of male uterus-like mass (endomyometriosis) in the right inguinal area at the site of a prior hernia repair. The lesion was tubular in shape, with a thick muscular wall and a central blood-filled lumen. Microscopically, the tissue showed layers of concentric smooth muscle, with endometrial glands and stroma lining the lumen.9,10
How explain the presence of endometriosis: in female fetuses, in females with complete utero-vaginal aplasia without functional endometrial cavity and even in males or DSD (disorders of sex development) patients with 46,XY karyotype?
The goal of this article is to understanding the etiology of endometriosis and presence of endometrioid lesions in unusual cases.
Endometriosis theories
Multiple hypotheses have been postulated to explain the etiology of endometriosis to understand various clinical evidences (Table 1) in females.
TABLE 1.
Various theories of endometriosis etiology.
| Theory | Mechanism |
|---|---|
| Implantation theory | Retrograde menstruation allowing implantation of endometrial glands in pelvic peritoneum |
| Coelomic metaplasia | Transformation of peritoneal mesothelium or other cell types into endometrial tissue |
| Hormonal induction | Oestrogen-driven proliferation of endometrial lesions or metaplasia by hormonal stimulation |
| Inflammation and oxidative stress | Recruitment of immune cells and their production of cytokines which promotes endometrial growth |
| Immune dysfunction or deficiency | Prevention of eliminating menstrual debris and promotion of implantation and growth of endometrial lesions |
| Apoptosis suppression | Survival of endometrial cells by suppression of apoptotic pathways |
| Genetic | Alteration of cell types to endometriosis lesions |
| Stem cells | Transformation of haematopoietic, bone marrow or mesenchymal stem cells to endometriosis |
| Lymphatic, haematogenous metastasis | Spread of endometrial cells by lymphatic or haematogenous vessels |
| Embryonic cell remnants | Mullerian duct rests, Wolffian duct rests |
| Multiple factors | Combination of in situ development and endometrial transplantation and implantation |
| New theory of endometriosis aetiology from primordial germ cells | The eutopic and ectopic endometrium originating from primordial germ cells |
The Implantation theory of endometriosis is the most widely accepted. Sampson proposes that reflux or retrograde menstruation allowing outflow of endometrial tissue through the Fallopian tubes into the abdominal cavity is followed by implantation of endometrial glands and stroma at extra-uterine sites.11
The coelomic metaplasia theory suggests metaplastic processes of the peritoneal mesothelium in endometriosis.12
The metaplasia theory supports the transformation of the germinal epithelium into ovarian endometriosis.13
Circulating stem cells are proposed to be involved in the transformation of pluripotent haematopoietic stem cells in endometrial cells in the peritoneal cavity.14,15
Knapp postulated that endometriosis is caused by small defects of embryogenesis. Mullerian duct maldevelopment during embryogenesis could cause the spread of endometriotic cells across the posterior pelvic floor and the persistence of embryonic cell rests.4
Lymphatic and vascular dissemination theories have been proposed to explain the presence of endometrial lesions in lymphatic vessels and the spread to lymph nodes and rare sites.16
Immune dysfunction and deficiency impair the survival of endometrial cells and lesion establishment, and high reoccurrence rates occur following treatment. Additionally, lymph-angiogenesis in endometriotic lesions may contribute to lesion growth and persistence of endometrial cells in the draining lymph nodes.17,18
Adenomyosis may originate from the invagination of the basalis of the endometrium into the myometrium. As a second theory, this basalis invagination proceeds along the intra-myometrial lymphatic system. A third theory suggests that a metaplastic process initiating from ectopic intramyometrial endometrial tissue is produced de novo. For a review see refs. 3,18-20.
Many theories have been proposed regarding the etiology of both endomyometriosis and endometriosis in males, including remnant areas of Müllerian tissue and metaplasia.3,9,10,13,15
Various theories have also been combined to understand the unusual types of endometriosis. Although its underlying cause is uncertain, it is likely to be multifactorial and include genetic factors with possible epigenetic influences, hormonal induction and apoptosis suppression. For a review see refs. 18-20
Germ cells in embryo
Zygote is totipotent, because a single cell has ability to generate all differentiated cells in the entirely embryo. During first weeks of gestation the zygote undergoes to a series of asynchronous holoblastic divisions, increasing the numbers of cells - blastomeres, which form animal pole and vegetative pole. For a review see refs. 21-25.
Due to asynchronous division the initial population of blastomeres in the vegetative pole preserves their potency.
The blastomeres are pluripotent, refers to differentiate into 3 germ layers: endoderm, mesoderm and ectoderm. Gastrulation begins with formation of the trilaminar germ disk, the extraembryonic epyblast and hypoblast. For a review see refs. 21-25.
In human embryos, the germline epithelium of the indifferent gonads originates from primordial germ cells at the time of gastrulation (at 3 weeks of gestation, equal to Carnegie stages 7 to 9). Initially, the population of primordial germ cells is located in the posterior endoderm that forms the hindgut and yolk sac close to the allantois. At 4–5 weeks of development (Carnegie stages 8–15) the primordial germ cells migrate by amoeboid movement via the hindgut to the dorsolateral gonadal ridges, arriving at the indifferent genital ridges during the 6th week. The indifferent gonad is differentiating into an ovary at 6–8 weeks (Carnegie stages 16–19), and the embryo becomes female. Primordial germ cells subsequently develop into oogonia, but they can enter meiosis or become granulosa cells in primordial follicles. The pathway of primordial germ cell migration has been defined: from the caudal wall of the yolk sac close to the allantois, along the wall of the hindgut and the dorsal mesentery followed by bilateral migration into the genital ridges. For a review see refs. 21-25.
There is no evidence of sex-specific differences during primordial germ cells migration in male or female embryos. The maintenance of germ cell potency may be suppressed while they migrate to the somatic gonadal ridges and generate mature sex-specific gametes. For a review see refs. 21-25.
New theory of endometriosis origination from primordial germ cells
The primary question to understanding the etiology of endometrial ectopia (endometriosis) is determining the origin of eutopic (normally cited) endometrium.
According to the “New theory of uterovaginal anomalies,” published in Organogenesis 2016,26 the eutopic endometrium develops inside of the uterine folds from gonadal ridges, which composed of primordial germ cells. The uterus develops in the area of intersection between the mesonephral ducts and the gonadal ridges by the fusion of the 2. The gonadal ridges composed of primordial germ cells derive to the: eutopic endometrium, ovary, ovarian ligament and ligamentum teres uteri.26,27
Germ cells at the gastrulation stage appear among endoderm cells in the wall of the yolk sac close to the allantois. They migrate by amoeboid movement from the hypoblast back to the dorsolateral gonadal ridges along the mesentery of the hindgut (Fig. 1).
FIGURE 1.
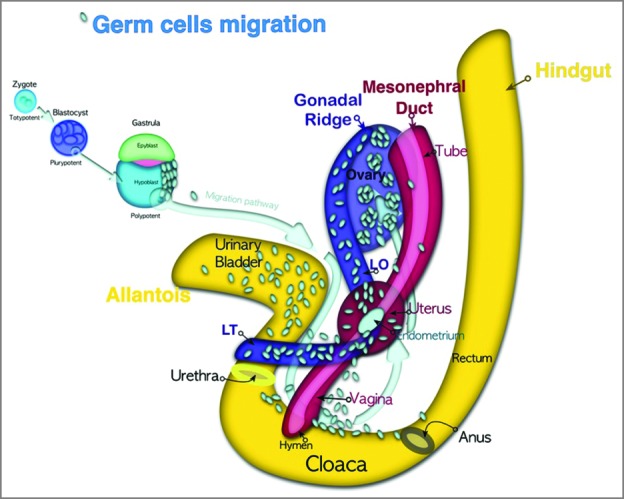
Primordial germ cells migration pathway. Zygote is totipotent. The blastomeres are pluripotent. Due to asynchronous division the initial population of blastomeres in the vegetative pole preserves their potency. Primordial germ cells are located first at the vegetative pole of blastocyst, then in the hypoblast (yolk sac) to avoid of differentiation signals and maintain the polypotency. After gastrulation the primordial germ cells migrate by amoeboid movement back to the embryo through the peritoneum to the dorsolateral gonadal ridges.
The hypothetic possibility that the ovary and endometrium derive from the gonadal ridges could be the key point to understanding the aetiologies of external (extragenital) and ovarian endometriosis. For a review see refs. 26-28.
Primordial germ cells derivation in female reproductive organs (Table 2):
Germ cells in the ovary differentiate into germline derivatives - oocytes and granulosa cells.
Eutopic endometrium derives from germ cells in crossing area between gonadal ridges with mesonephral ducts.
Ovarian ligamentum proprium and ligamentum teres uteri are remnants of gonadal ridges.
TABLE 2.
Primordial germ cells derivation in female reproductive organs
| Organs | Cell lines |
|---|---|
| Ovary | Germinal epithelium: oocytes, granulosa cells |
| Ovarian ligamentum proprium | Remnants of gonadal ridges |
| Uterus | Endometrium |
| Ligamentum teres uteri | Remnants of gonadal ridges |
Speculations about the etiology of endometriosis with clinical evidence supporting the new theory
Ovarian endometriosis (Table 3) derives by metaplasia of germline epithelium occurring during luteinization (Fig. 2).
TABLE 3.
New theory of endometriosis etiology
| Endometriosis localization | Derivation |
|---|---|
| Ovarian cysts | Luteinization of unovulated (unruptured) follicle |
| Superficial ovarian endometriosis in the cortical layer | On the stigma after ovulation |
| Extra-genital endometriosis on the peritoneum | Persistence of primordial germ cells on the embryonal pathway |
| Endometriosis on the utero-sacral ligaments | Most common area of embryonal pathway migration from hindgut |
| Retrocervical endometriosis | The early pathway area of migration from hindgut |
| Adenomyosis | Endometrium derives from the intersection of gonadal ridges, containing primordial germ cells, with mesonephral ducts. In the uterine folds there are closer cell-to-cell communications. |
| Endometriosis in the other area | Primordial germ cells may migrate in the other somatic area from the hypoblast, through the endodermal gut to derivative organs |
| Endometriosis in urinary bladder | Bladder is derived from the hindgut, especially part of the allantois |
| Endometriosis in males | Male prostatic glands are analogues of female endometrium. The gubernaculum testis is similar to the uterine broad ligament |
FIGURE 2.
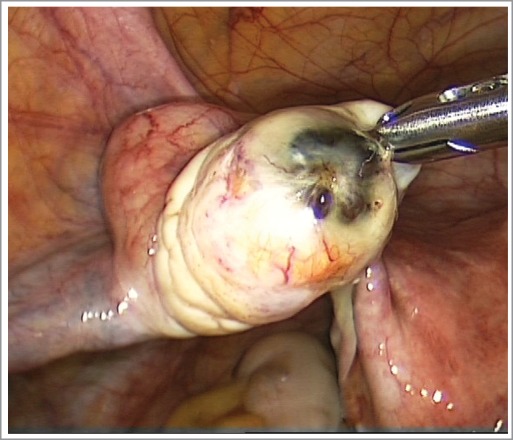
The luteinization of unovulated follicle (LUF syndrome) in the left ovary: a corpus luteum with endometriotic lesions on the upper pole without ovulatory stigma.
Interesting evidence we found during laparoscopy in patients with primary infertility. In the left ovary, there is a corpus luteum with endometriotic lesions on the upper pole without ovulatory stigma. This is luteinization of the unovulated (or unruptered) follicle (LUF syndrome). Apparently, LUF may be the early stage of aetio-pathological development of the endometriotic cyst.
The cases of LUF may be key to understanding infertility in patients with ovarian endometriosis.
External endometriosis derives from primordial germ cells during the migration pathway from the yolk sac to the gonadal ridges (Fig. 1). The germ cells pathway passes through the recto-vaginal and vesico-uterine surface, which explains the most common localization of ectopic endometrial lesions. Some of the polypotential germ cells fail to reach the ridges and stay in the peritoneal cavity, where they may be transformed into external endometrial heterotopy.
The new theory may explain the presence of endometriosis under the peritoneum in fetuses and females with complete utero-vaginal aplasia (Fig. 3).
FIGURE 3.
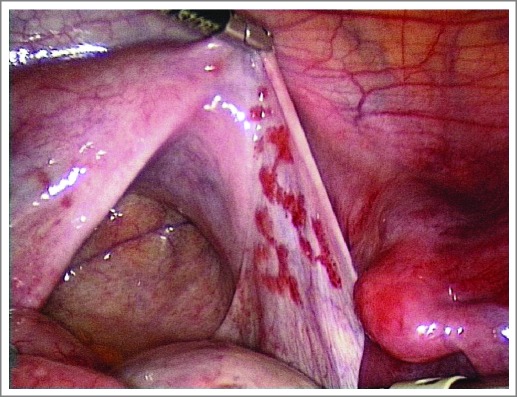
Patient with uterovaginal aplasia (Mayer-Rokitansky-Kuster-Hauser syndrome) and non-functioning uterine rudiments had extra-genital endometriosis on the peritoneal surface.
Patient with uterovaginal aplasia (Mayer-Rokitansky-Kuster-Hauser syndrome). The uterine rudiments are non-functional and located at the intersection of the Fallopian tubes with broad and ovarian ligaments. The endometriotic lesions are located superficially on the peritoneum, to the right of the utero-sacral ligament. The presence of lesions in this area seems to show similarity to the migration pathway (Fig. 1).
External endometriosis originates from the primordial germ cells before organogenesis of the reproductive system, and disturbances in germ cell migration to the gonadal ridges can occur.
There are 2 principal processes in uterine organogenesis: intersection of gonadal ridges with mesonephral ducts to form uterine folds with endometrial cavity (eutopic endometrium) and fusion of the both uterine folds together. There are closer cell-to-cell communications in the uterine folds. Polypotential germ cells differentiate and grow into myometrium and endometrial layers.
Endometrial glands may persist at the ovarian ligamentum proprium and ligamentum teres uteri, as they are remnants of the gonadal ridges after complete uterine embryogenesis.
Disorders of these intersections may result in internal endometriosis (adenomyosis). Disorders of uterine fold fusion may result in congenital uterine anomalies. These related processes may explain the high rate of congenital uterine anomalies with concomitant endometriosis.
According to our research published in the Organogenesis 2016(12), volume 1 (“New theory of uterovaginal anomalies”),26 extra-genital endometriosis was the major concomitant pathology in 46% of patients with various uterovaginal anomalies.
We analyzed the correlation of endometriosis with anomaly type and menstrual outflow obstruction. There were no significant differences between obstructive anomalies (such as partial or complete vaginal aplasia, functional uterine horn) and in non-obstructive symmetric malformations (with uterus duplex, septate uterus).
Histological investigation revealed internal endometriosis in 37% of cases of removed functional uterine rudiments and uterine horns.26
Estrogens influence the eutopic and ectopic endometrium survival and proliferation. This effect explains the initiation of manifestation symptoms in females in puberty and progression in reproductive age, especially in females with hyperoestrogenia.
Patients with luteinization of unovulated follicle may also have hyperoestrogenia, which may stimulate progression of extragenital lesions.
Estrogen levels are lower in the male embryo than in the female embryo but are sufficient for the survival of some germ cell populations. Endometriosis in males and 46,XY DSD patients may persist as they migrate at an early stage before sexual differentiation.
CONCLUSION
Primordial germ cells initially do not have sex-specific features and have polypotential characteristics during migration.24
In female embryo germ cells, those arriving the gonadal ridges give rise to germinal epithelium of the gonads (oocytes, granulosa cells in ovary) and eutopic endometrium.
The ectopic endometrium (endometriosis) originates from primordial germ cells and preserves polypotency (stem cell properties) such as: proliferation, germination, invasion, spread or metastasis by lymphatic and haematogenous pathways.
Germ cell properties of endometrioid lesions should be considered to better understanding the pathogenesis, as they may evolve into a novel target treatment.29
The main insight in the etiology of endometriosis is polypotential germ cells origin, which may explain its potency, pathogenesis and expansion.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
REFERENCES
- [1].Johnson NP, Hummelshoj L, Adamson GD, Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R, Sharpe-Timms KL, et al.. World Endometriosis Society consensus on the classification of endometriosis. Hum Reprod 2017; 32(2):315-24; PMID:27920089; https://doi.org/ 10.1093/humrep/dew293 [DOI] [PubMed] [Google Scholar]
- [2].Rogers PA, Adamson GD, Al-Jefout M, Becker CM, D'Hooghe TM, Dunselman GA, Fazleabas A, Giudice LC, Horne AW, Hull ML, et al.. WES/WERF consortium for research priorities in endometriosis. research priorities for endometriosis. Reprod Sci 2017; 24(2):202-26; https://doi.org/ 10.1177/1933719116654991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ferenczy A. Pathophysiology of adenomyosis. Hum Reprod Update 1998; 4:312-22; PMID:9825847; https://doi.org/ 10.1093/humupd/4.4.312 [DOI] [PubMed] [Google Scholar]
- [4].Knapp VJ. How old is endometriosis? Late 17th and 18th century European descriptions of the disease. Fertil Steril 1999; 72:10-4; PMID:10428141; https://doi.org/ 10.1016/S0015-0282(99)00196-X [DOI] [PubMed] [Google Scholar]
- [5].Signorile PG, Baldi A. Endometriosis: New concepts in the pathogenesis. Int J Biochem Cell Biol 2010; 42(6):778-80; PMID:20230903; https://doi.org/ 10.1016/j.biocel.2010.03.008 [DOI] [PubMed] [Google Scholar]
- [6].Signorile PG, Baldi F, Bussani R, D'Armiento MR, De Falco M, Boccellino M, Quagliuolo L, Baldi A. New evidence sustaining the presence of endometriosis in the human foetus. Reprod Biomed Online 2010; 21(1):142-7. [DOI] [PubMed] [Google Scholar]
- [7].Signorile PG, Baldi F, Bussani R, D'Armiento MR, De Falco M, Baldi A. Ectopic endometrium in human fetuses is a common event and sustains the theory of mullerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res 2009; 28:49; PMID:19358700; https://doi.org/ 10.1186/1756-9966-28-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].De Jolinière JB, Ayoubi JM, Lesec G, Validire P, Goguin A, Gianaroli L, Dubuisson JB, Feki A, Gogusev J. Identification of Displaced Endometrial Glands and Embryonic Duct Remnants in Female Fetal Reproductive Tract: Possible Pathogenetic Role in Endometriotic and Pelvic Neoplastic Processes. Front Physiol 2012; 3:444; PMID:23227010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beckman EN, Pintado SO, Leonard GL, Sternberg WH. Endometriosis of the prostate. Am J Surg Pathol 1985; 9(5):374-9; https://doi.org/ 10.1097/00000478-198505000-00008 [DOI] [PubMed] [Google Scholar]
- [10].González RS, Vnencak-Jones CL, Shi C, Fadare O. Endomyometriosis (“Uterus-like mass”) in an XY male: case report with molecular confirmation and literature review. Int J Surg Pathol 2014; 22(5):421-6; https://doi.org/ 10.1177/1066896913501385 [DOI] [PubMed] [Google Scholar]
- [11].Sampson J. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927; 14:442-69; https://doi.org/ 10.1016/S0002-9378(15)30003-X [DOI] [Google Scholar]
- [12].Gruenwald P. Origin of endometriosis from the mesenchyme of the celomic walls. Am JObstetrics Gynecol 1942; 44(3):470-4; https://doi.org/ 10.1016/S0002-9378(42)90484-8 [DOI] [Google Scholar]
- [13].Von Numers Observations on metaplastic changes in the germinal epithelium of the ovary and on the aetiology of ovarian endometriosis. Acta Obstet Gynecol Scand 1965; 44:107-16; PMID:14299344; https://doi.org/ 10.3109/00016346509153982 [DOI] [PubMed] [Google Scholar]
- [14].Hufnagel D, Li F, Cosar E, Krikun G, Taylor HS. The Role of Stem Cells in the Etiology and Pathophysiology of Endometriosis. Semin Reprod Med 2015; 33(5):333-40; PMID:26375413; https://doi.org/ 10.1055/s-0035-1564609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sasson E, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008; 1127:106-15; PMID:18443337; https://doi.org/ 10.1196/annals.1434.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jerman LF, Hey-Cunningham AJ. The role of the lymphatic system in endometriosis: a comprehensive review of the literature. Biol Reprod 2015; 92(3):64; PMID:25588508; https://doi.org/ 10.1095/biolreprod.114.124313 [DOI] [PubMed] [Google Scholar]
- [17].Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG. Immune interactions in endometriosis. Expert Rev Clin Immunol 2011; 7(5):611-26; PMID:21895474; https://doi.org/ 10.1586/eci.11.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laganà AS, Sturlese E, Retto G, Sofo V, Triolo O. Interplay between misplaced Müllerian-derived stem cells and peritoneal immune dysregulation in the pathogenesis of endometriosis. Obstet Gynecol Int 2013; 2013:527041 ID-527041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sahin AA, Silva EG, Landon G, et al.. Endometrial tissue in myometrial vessels not associated with menstruation. Int J Gynecol Pathol 1989; 8:139-46; PMID:2469659; https://doi.org/ 10.1097/00004347-198906000-00007 [DOI] [PubMed] [Google Scholar]
- [20].Sourial S, Tempest N, Hapangama DK. Theories on the pathogenesis of endometriosis. Int J Reprod Med 2014; 2014:179515; PMID:25763392; https://doi.org/ 10.1155/2014/179515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bukovsky A. Ovarian Stem Cell Niche and Follicular Renewal in Mammals. Anat Rec 2011; 294(8):1284-306; https://doi.org/ 10.1002/ar.21422 [DOI] [PubMed] [Google Scholar]
- [22].Heeren AM, He N, de Souza AF, Goercharn-Ramlal A, van Iperen L, Roost MS, Gomes Fernandes MM, van der Westerlaken LA, Chuva de Sousa Lopes SM. On the development of extragonadal and gonadal human germ cells. Biol Open 2016; 5(2):185-94; PMID:26834021; https://doi.org/ 10.1242/bio.013847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Seydoux G, Braun RE. Pathway to totipotency: lessons from germ cells. Cell 2006; 127(5):891-904; PMID:17129777; https://doi.org/ 10.1016/j.cell.2006.11.016 [DOI] [PubMed] [Google Scholar]
- [24].Wear HM, McPike MJ, Watanabe KH. From primordial germ cells to primordial follicles: a review and visual representation of early ovarian development in mice. J Ovarian Res 2016; 9:36; PMID:27329176; https://doi.org/ 10.1186/s13048-016-0246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hill MA. UNSW Embryology 2017. Primordial Germ Cell Migration Movie. https://embryology.med.unsw.edu.au/embryology/index.php/Primordial_Germ_Cell_Migration_Movie [Google Scholar]
- [26].Makiyan Z. New theory of uterovaginal embryogenesis. Organogenesis 2016; 12(1):33-41; PMID:26900909; https://doi.org/ 10.1080/15476278.2016.1145317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Makiyan Z. Studies of gonadal sex differentiation. Organogenesis 2016; 12(1):42-51; PMID:26950283; https://doi.org/ 10.1080/15476278.2016.1145318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Makiyan Z. Systematization of ambiguous genitalia. Organogenesis 2016; 12(4):169-82; PMID:27391116; https://doi.org/ 10.1080/15476278.2016.1210749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Binda MM, Donnez J, Dolmans MM. Targeting mast cells: a new way to treat endometriosis. Expert Opin Ther Targets 2017; 21(1):67-75; PMID:27841046; https://doi.org/ 10.1080/14728222.2017.1260548 [DOI] [PubMed] [Google Scholar]


