ABSTRACT
Periodontal ligament stem cells (PDLSCs) have mesenchymal-stem-cells-like qualities, and are considered as one of the candidates of future clinical application in periodontal regeneration therapy. Enamel matrix derivative (EMD) is widely used in promoting periodontal regeneration. However, the effects of EMD on the proliferation and osteogenic differentiation of human PDLSCs grown on the Ti implant surface are still no clear. Therefore, this study examined the effects of EMD on human PDLSCs in vitro. Human PDLSCs were isolated from healthy participants, and seeded on the surface of Ti implant disks and stimulated with various concentrations of EMD. Cell proliferation was determined with Cell Counting Kit-8 (CCK-8). The osteogenic differentiation of PDLSCs was evaluated by the measurement of alkaline phosphatase (ALP) activity, Alizarin red staining, and real-time polymerase chain reaction (qRT-PCR) and Western blotting, respectively. The results indicated that EMD at concentrations (5–60 µg/ml) increased the viability and proliferation of PDLSCs. The treatment with 30 and 60 µg/ml of EMD significantly elevated ALP activity, augmented mineralized nodule formation and calcium deposition, and upregulated the mRNA and protein levels of Runx-2 and osteocalcin (OCN) in the PDLSCs grown on the Ti surface. Further investigation found that EMD treatment did not change the protein levels of phosphatidylinositol-3-kinase (PI3K), p-PI3K, Akt and mTOR, but significantly upregulated the phosphorylated levels of Akt and mTOR. Collectively, these results suggest that EMD stimulation can promote the proliferation and osteogenic differentiation of PDLSCs grown on Ti surface, which is possibly associated with the activation of Akt/mTOR signaling pathway.
KEYWORDS: Akt, Enamel matrix derivative, mTOR, osteogenic differentiation, osteoblasts, periodontal ligament stem cells
INTRODUCTION
Dental implant therapy is one of the most important and effective prosthodontic therapy options for partially and completely edentulous patients.1 Currently, the use of dental implants has widely become an accepted treatment in many clinical situations.2 Titanium (Ti) devices have been widely used as major biomaterials in dental implant therapy for many years and their rough surface can stimulate the differentiation of bone-forming osteoblasts.3,4 Although many evidence indicates that implant therapy is an efficient and safe choice and the brilliant success of dental implants has been made, low bone quality and unpredictable ossteointegration still result in the failure of dental implant therapy.5,6
Periodontal disease is a chronic inflammation that leads to the destruction of periodontal ligament, cementum and bone.7 PDLSCs discovery provides a new prospect for periodontal tissue regeneration because they were found to form a cementum/PDL-like structure after in vivo transplantation.8 Additionally, many studies have indicated that PDLSCs have multipotent differentiation ability toward a chondrogenic, osteoblastic, and adipogenic phenotype under defined conditions.9-11 Chemical induction of osteoblastic phenotype in PDLSCs through the application of various growth factors and biomaterials, such as platelet-derived growth factor (PDGF) and enamel matrix derivative (EMD), has been studied extensively.12,13
Enamel matrix derivative is an extracellular matrix derivative obtained from porcine tooth buds,14 and has been reported to be very effective in regeneration of cementum, periodontal ligament and bone.15 Although it was found that EMD can stimulate cellular proliferation and mineralization of preosteoblasts and periodontal ligament cells, and plays an important role in the cell differentiation necessary for periodontal regeneration,16,17 other studies have pointed out that EMD also reduces the differentiation of osteoblasts.18,19 In addition, some studies indicated that the application of EMD contributing to osteoblasts differentiation and bone formation around Ti implants remains controversial.20,21 Therefore, in this study, we investigated the effect of EMD on the proliferation and osteogenic differentiation of PDLSCs grown on the surface of Ti disks.
RESULTS
Isolation and identification of human PDLSCs
Light microscopy demonstrated that the primary human PDLSCs were spindle-shape (Fig. 1A). The results of flow cytometry analysis revealed that these isolated cells strongly expressed STRO-1 and CD146 (48.67% and 86.29%, respectively) cell surface antigens, and were negative for CD34 (0.83%) and CD45 (1.20%), as shown in Fig. 1B. These data suggested that these cells isolated from human periodontal ligament tissues belonged to PDLSCs.
Figure 1.
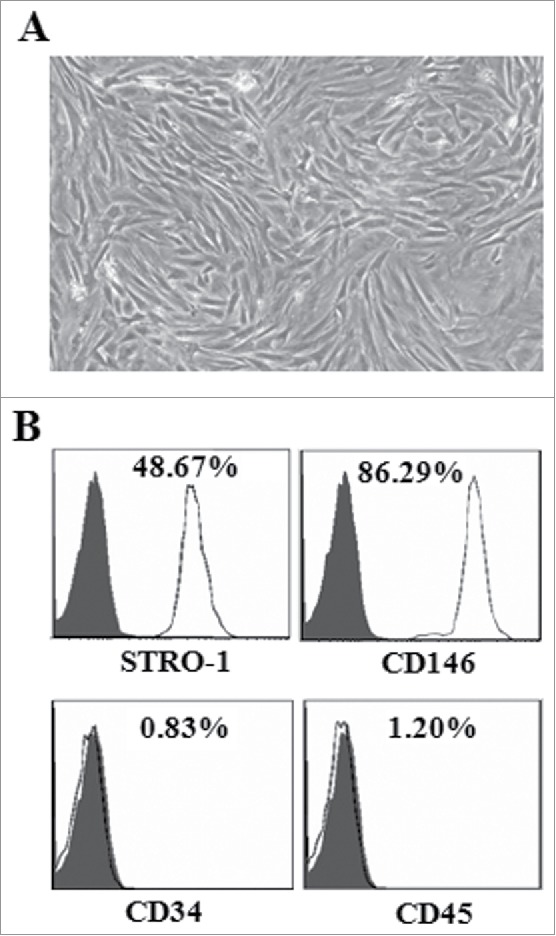
Morphologic and phenotypic analysis of PDLSCs. (A) PDLSCs showed a fibroblast-like spindle shape under the microscope view. Original magnification: × 400. (B) Flow cytometric analysis of surface markers in the isolated PDLSCs.
Effect of EMD on the proliferation of PDLSCs
Cell proliferation was evaluated using CCK-8 assay to detect the viability of PDLSCs grown on Ti implant surfaces. The results showed that 5–60 µg/ml of EMD treatment of 24 h resulted in a dose-dependent increase in the viability of PDLSCs, but more higher doses (such as 90–120 µg/ml) of EMD led to an inhibitory effect on cell viability (Fig. 2A). Additionally, a time-dependent increase of cell viability was observed when the PDLSCs were incubated with 30 and 60 µg/ml of EMD for 24, 48 and 72 h (Fig. 2B).
Figure 2.
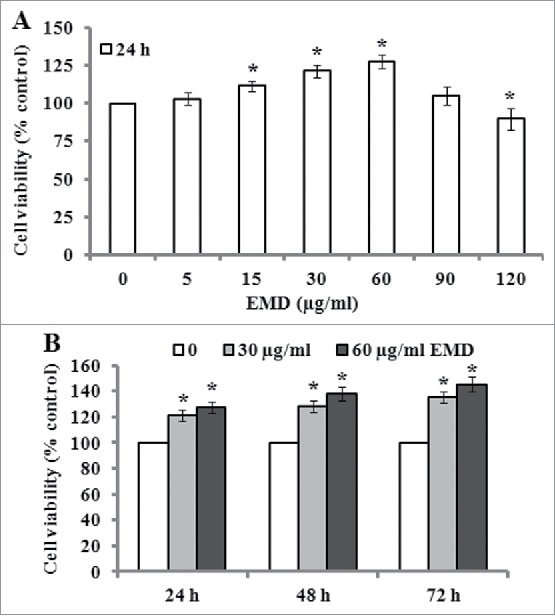
Effect of EMD on the viability of PDLSCs. (A-B) The viability of PDLSCs was determined by CCK-8 assay after the cells were treated with various concentrations (0, 5, 15, 30, 60, 90 and 120 µg/ml) of EMD for 24 h or with 30 and 60 µg/ml of EMD for 24, 48 and 72 h. Values of changes caused by EMD treatment in the viability of PDLSCs were normalized to the control, and the representative results from 3 independent experiments are shown. *P < 0.05 vs. the control.
EMD promotes the differentiation of PDLSCs into osteoblasts
ALP is an early marker for dental mesenchymal cell differentiation toward osteoblastic phenotype. Therefore, ALP activity was determined after 7 d of induction culture containing 30 and 60 µg/ml of EMD. The results indicated that EMD significantly enhanced ALP activity in the PDLSCs when compared with the untreated cells (Fig. 3). In addition, Alizarin red staining and a quantitative biochemical colorimetric assay displayed that the EMD-enhanced calcium deposition and mineralized nodule formation were observed after 21 d of culture (Fig. 4). On the other hand, the gene expression levels of osteogenic markers such as Runx-2 and OCN in the PDLSCs were detected after 14 d culture. The results of real-time PCR indicated that the mRNA levels of Runx-2 and OCN were significantly elevated in comparison to the control (Fig. 5A). The results of western blot analysis confirmed that the protein levels o Runx-2 and OCN were significantly increased by 30 and 60 µg/ml of EMD treatment (Fig. 5B and C).
Figure 3.
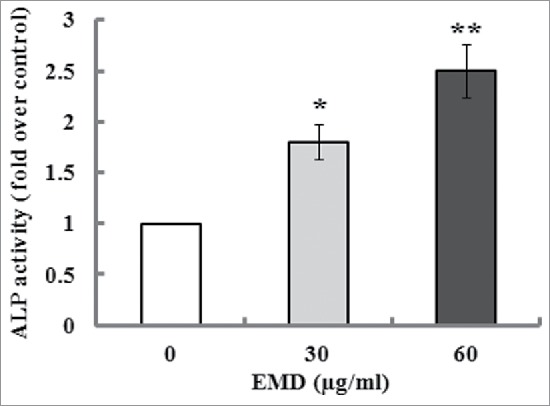
Effect of EMD on the ALP activity of PDLSCs. After 7 d of incubation in the induction medium added with 30 and 60 µg/ml of EMD, the ALP activity of PDLSCs was determined and normalized to the control (untreated cells), which was arbitrarily set to 1. *P< 0.05 and **P < 0.01 vs. the control.
Figure 4.
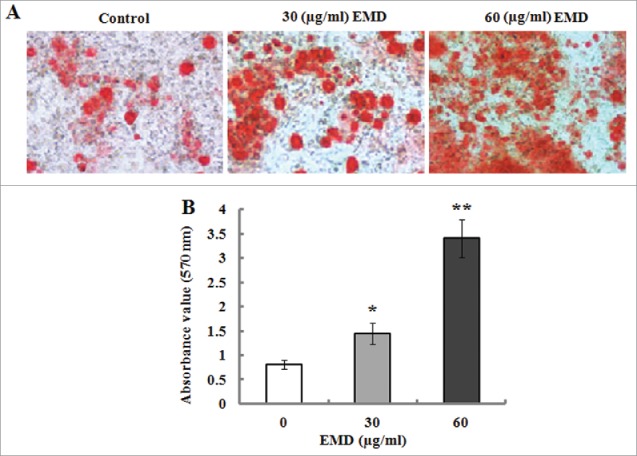
Effect of EMD on osteogenic differentiation of PDLSCs. (A) After 21 d of incubation in the induction medium added with 30 and 60 µg/ml of EMD, mineralized deposits was evaluated by Alizarin red S staining. (B) The deposition of Alizarin staining was extracted, and the absorbance value of released dye was determined at 562 nm using a microplate reader. *P< 0.05 and **P < 0.01 vs. the control.
Figure 5.
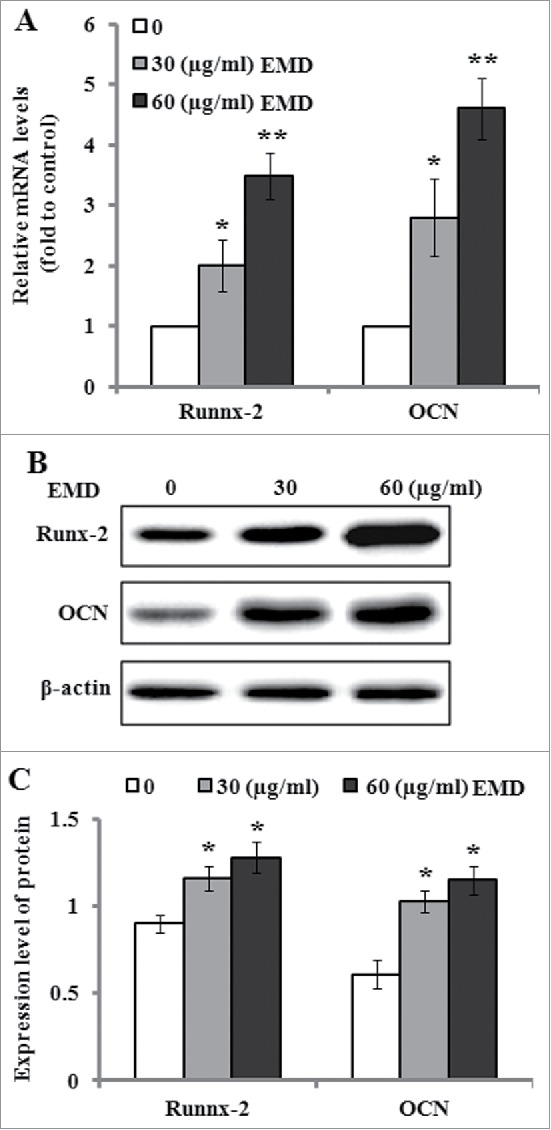
Effects of EMD on the expression levels of Runx-2 and OCN. (A) The relative mRNA level of Runx-2 and OCN in the PDLSCs was detected by real time PCR after 14 d of induction culture containing 30 and 60 µg/ml of EMD. (B) Representative western blot images for the expression of Runx-7 and OCN in the PDLSCs after 14 d of osteogenic induction containing 30 and 60 µg/ml of EMD. (C) Relative protein level of Runx-2 and OCN were quantified by the densitometry of each band and normalized to β-actin signal. *P < 0.05 vs. the control.
EMD activates Akt signaling in PDLSCs
Previous studies have indicated phosphatidylinositol-3-kinase/protein kinase B (PI3K/Akt) play an important role in the regulation of cell survival, proliferation, and cell differentiation.22,23 Accordingly, we examined the protein levels of PI3K/Akt and their downstream mTOR and their phosphorylation states in the PDLSCs after 48 h incubation with 30 and 60 µg/ml of EMD. The results demonstrated that EMD treatment did not change the expression levels of PI3K, p-PI3K, Akt and mTOR, but significantly upregulated the phosphorylated levels of Akt and mTOR (Fig. 6), which suggested that the activation of Akt/mTOR signaling was correlated with EMD-induced osteogenic differentiation of PDLSCs.
Figure 6.
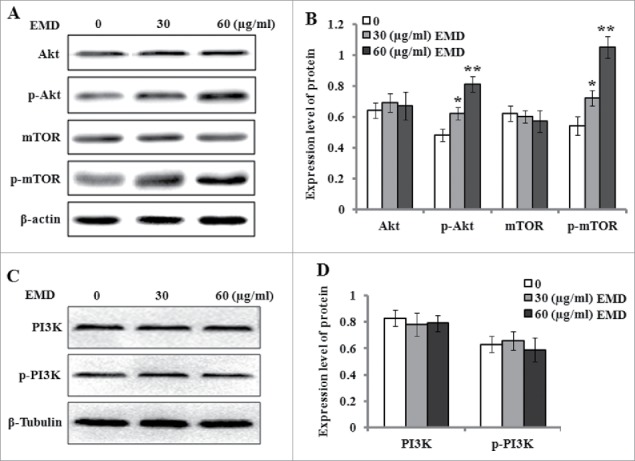
EMD activates the Akt signal pathway in the osteogenic differentiation of PDLSCs. PDLSCs were incubated in differentiation medium without or with 30 and 60 µg/ml EMD, and cell lysates were then prepared and subjected to SDS-PAGE analysis. (A) Representative western blot images for the expression of Akt, p-Akt, mTOR and p-mTOR. (B) Relative protein levels of Akt, p-Akt, mTOR and p-mTOR were quantified by the densitometry of each band and normalized to β-actin signal. (C-D) Representative western blot images for the expression of PI3K and p-PI3K, and their relative protein levels were quantified by the densitometry of each band and normalized to β-Tubulin signal.*P < 0.05 and **P < 0.01 vs. the control.
DISCUSSION
To determine the applicability of EMD for enhancing PDLSCs-based bone regeneration modalities, we evaluated the effects of EMD on proliferation and osteogenic differentiation of human PDLSCs grown on the surface of Ti implant disks. EMD at various concentration was added in the medium in which PDLSCs were cultured. The results showed that the proliferation of PDLSCs was significantly increased by 15, 30 and 60 µg/ml of EMD after 24 h of incubation. However, more than 90 µg/ml of EMD treatment resulted in an inhibitory role on PDLSCs viability. Therefore, 30 and 60 µg/ml of EMD were chosen for evaluation on osteogenic differentiation of PDLSCs based on these findings. The results indicated that EMD promoted osteogenic differentiation of PDLSCs, upregulated the expression of Runx-2 and OCN, and activated Akt/mTOR signaling pathway.
PDLSCs are known to possess multi-differentiation potential and exhibit similar properties to those described for bone marrow-derived mesenchymal stem cells (MSCs).24 In this study, the PDLSCs were isolated from healthy participants, and these cells positively expressed the mesenchymal stem-cell markers STRO and CD146, did not express haematopoietic stem-cell markers CD34 and CD45. These results confirmed that these isolated PDLSCs possess similar characteristics as bone marrow-derived MSCs, and are also consistent with previous reports.25,26
EMD is a purified extract of porcine enamel matrix, and has been documented as a promising biomaterial for in vivo periodontal regeneration.27 However, the influences of EMD on different cell types and osteogenic differentiation of MSCs remain still controversial.12,19,28 Additionally, the combined application of Ti implants and EMD seems to be a promising approach for a further improvement in the clinical implant outcome.29 However, a lack of agreement exists regarding the effect of EMD on bone formation around Ti implants.20,30 In the present study, the influence of EMD on osteogenic differentiation of PDLSCs seeded on the surface of Ti implants was evaluated. The results of Alizarin red staining showed that the formation of mineralized and calcium deposition was apparently increased after 21 d of incubation in the osteogenic induction medium supplemented with EMD. ALP is an indicator of new bone formation, and its high-level expression is induced during early osteogenic differentiation,31 and so it has been commonly studied as an early maker of osteogenic differentiation. Our findings demonstrated that ALP activity was significantly elevated in the PDLSCs after 7 d of incubation in the presence of EMD in comparison with the control cells. In addition, the expression levels of osteogenic differentiation related genes such as Runx-2 and OCN were upregulated as determined using real time PCR and Western blot analysis after osteogenic induction with EMD. Runx-2 is the bone specific transcription factor, and has a major impact on osteogenesis by stimulating osteoblast differentiation gene transcription.32 Additionally, OCN is also an osteoblast-specific marker, and it is present during the late stages of osteoblast differentiation.33 Furthermore, previous studies have confirmed that OCN is the target gene of Runx-2 and is modulated by Runx-2.34,35 Therefore, the upregulation of OCN expression tended to correlated positively with the expression of Runx-2.36 In this study, the changes in Runx-2 and OCN expression suggested that EMD could promote the osteogenic differentiation of PDLSCs grown on Ti implant surface.
Many studies have indicated that PI3K/Akt/mTOR signaling pathway, which can be activated by various extracellular stimuli, plays an important role in the regulation of cell motility, proliferation, cell cycle progression, and cell differentiation.37,38 Recent studies showed that Mechano-growth factor promoted the growth rate and osteogenic differentiation of mesenchymal stem cells via PI3K/Akt pathway.23 In the present study, our results demonstrated that EMD treatment did not change the protein levels of PI3K, p-PI3K, Akt and mTOR, but upregulated the phosphorylated forms of p-Akt and p-mTOR. These results indicated that the activation of Akt/mTOR signaling pathway occurred in the EMD-mediated osteogenic differentiation of PDLSCs grown on the Ti surface. Interestingly, some studies demonstrate that mTOR activation contributes to osteoblast differentiation in mouse or rat bone marrow stromal cells,39,40 as opposed to suppression in human embryonic stem cells.41 These apparent discrepancies are probably associated with cell-type or various methodological differences.
In conclusion, our results indicate that EMD can increase the viability and proliferation of PDLSCs at a proper concentration range, and enhance the osteogenic differentiation of human PDLSCs grown on Ti implant surface. Additionally, EMD treatment elevates ALP activity and up-regulates Runx-2 and OCN expression and the phosphorylated levels of Akt and mTOR. These data seem to suggest that the activation of Akt/mTOR signal pathway involves in the EMD-mediated osteogenic differentiation of PDLSCs. Therefore, EMD could be a useful tool for further promoting implant osseointegration and enhancing clinical outcome.
MATERIALS AND METHODS
Materials
EMD powder was obtained from Institut Straumann (Basel, Switzerland), and dissolved in 0.1% acetic acid to prepare a concentration of 10 mg/ml of EMD as the stock solution that was diluted to the working concentration with culture medium. The final dose of acetic acid was less than 0.001% throughout the following studies, and did not display any effect on the parameters investigated in the present study. Pure Ti disks with a cylindrical shape with 1 mm thickness and 15 mm diameter were commercially available from Institut Straumann. The Ti disks with coarse-grit-blasted and acid-etched surface were prepared as described previously.42,43
PDLSCs isolation and cultivation
PDLSCs isolation and culture were performed as described previously,44 with some modifications. Briefly, periodontal ligament tissues from healthy teeth extracted for orthodontic purpose were immediately washed and minced into small pieces, and enzymatically digested with type I collagenase (0.75 mg/ml) for 30 min. Single-cell suspensions were then obtained and incubated with Dulbecco's modified Eagle's medium (DMEM; Gibco, Life Technologies) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. The informed consents were obtained from the subjects who donated the periodontal ligament tissues, and reviewed and approved by the Ethics Committee of Chinese PLA General Hospital (Beijing, China).
To identify the phenotype of PDLSCs, the cells (Passage 3) were incubated with FITC or PE-conjugated monoclonal antibodies against human CD34, CD45, CD146 and STRO-1 (Cell Signal Technology, USA) for 30 min in the dark, and then subjected to flow cytometric analysis using CellQestPro acquisition software.
The isolated PDLSCs were then seeded on the Ti disks in 24-well cell culture plates at a density of 1 × 104 cells in 500 µl culture medium added without or with 5, 15, 30, 60, 90 and 120 µg/ml of EMD, and cultured for indicated time to evaluate cell proliferation/viability and osteogenic differentiation.
Cell viability
Cell Counting Kit 8 (CCK-8; Genomeditech, Shanghai, China) was used to evaluate the effect of EMD on cell viability. Briefly, PDLSCs were incubated with various concentrations (0, 5, 15, 30, 60, 90 and 120 µg/ml) of EMD for 24 h or with 30 and 60 µg/ml of EMD for 24, 48 and 72 h, respectively. 50 µl of CCK-9 reagents were then added into each well and incubated for another 4 h at 37°C. Subsequently, 100 µl of the resulting suspension containing water-soluble formazan produced by WST-8 tetrazolium salt upon reduction by cellular mitochondria respiratory chain, were transferred into 96-well plates. Absorbance value was then determined at 570 nm using automatic reader for microtiter plates (BMG Labtech PHERA star FS, Germany). The experiments were performed 3 times.
Osteogenic differentiation of PDLSCs
The cells at passage 3 were seeded in 24-well plates at a density of 1 × 104 cells/ml in 500 µl of growth medium, and incubated overnight. Growth medium was replaced with induction medium consisting of DMEM, 10% FBS, 10 nM dexamethasone (Sigma-Aldrich, USA), 10 mM β-glycerophosphate (Sigma-Aldrich), 200 µM ascorbate-2-phosphate (Sigma-Aldrich), 100 U/ml penicillin/streptomycin. The medium was replaced every 2 d. EMD (0, 30 and 60 µg/ml) was added and kept in the cell culture. For the analysis of alkaline phosphatase (ALP) activity, the cells were differentiated for 7 d. The real-time polymerase chain reaction (PCR) analysis and the western blot analysis were performed at day 14, and mineralized nodules by Alizarin red S staining were evaluated at 21.
Measurement of alkaline phosphatase (ALP) activity
The cells were incubated in the induction medium for 7 days, and were then lysed by 0.1% Triton X-100. The activity of ALP was determined using a commercially available assay kit (Nanjing Jiancheng Bioengnineering Institute, Nanjing, China) according to the manufacturer's instructions. Enzyme activity was measured by absorbance at 520 nm using a microplate reader (BMG Labtech PHERA star FS, Germany).
Alizarin red S staining-based calcium deposition analysis
After induction culture for 3 weeks, the cells were washed with PBS and fixed with 4% paraformaldehyde, and then stained using 2% Alizarin red for 20 min at room temperature to evaluate calcium deposition. Alizarin red quantification was performed as described previously.45 Briefly, 1 ml of 10% cetylpyridinium chloride (Sigma-Aldrich, USA) was added into each well to extracted the deposition, followed by collection of the solution, and the light absorbance at 562 nm was determined with a microplate reader (BMG Labtech PHERA star FS, Germany).
Real-time polymerase chain reaction
Total RNA was extracted with Trizol reagent (Invitrogen, CA, USA) according to the manufacturer's instructions, and cDNA was then reversely synthesized using OligodT primer and PrimeScript® RTase (Takara, Dalian, China) following to the manufacturer's instructions. Quantitative RT-PCR was performed with SYBR Green Premix Ex Taq™ kit (Takara) using a specific thermal cycler (Applied Biosystems, NY, USA). The specific primers used for Runx-2, OCN, and GAPDH in this study were as follows: Runx-2 forward 5′-GCAGTTCCCAAGCATTTCAT-3′, and reverse 5′-CACTCTGGCTTTGGGAAGAG-3′; OCN forward 5′-GTGCAGAGTCCAGCAA
AGGT-3′, and reverse 5′-AGACTGCGCCTGGTAGTTGT-3′; GAPDH forward 5′-CATCAAGAAGGTGGTGAAGCAGG-3′, and reverse 5′- AAAGGTGGAGGAGT
GGGTGTCG-3′. The samples were subjected to 40 cycles of amplification at 95°C for 30 sec, 55°C for 45 sec, and 70°C for 30 sec. Critical threshold (CT) values obtained from qPCR were analyzed using the 2−ΔΔCT method for relative gene expression level. The results were presented relative to the control value, which was arbitrarily set to 1.
Western blot analysis
After culture in the indicated conditions for 2 weeks, total proteins were extracted from the PDLSCs with lysis buffer, and the protein concentration of each sample was determined with BCA assay kit (Bio-Rad Laboratories, USA). Subsequently, lysate proteins were resolved by electrophoresis, followed by transfer to polyvinylidene difluoride (PVDF) membranes, and blotted with antibodies against Runx-2, OCN, PI3K, p-PI3K, Akt, p-Akt, mTOR, p-mTOR and β-actin (dilution,1: 500). The integrated optical density values of each blot were analyzed using an ImageQuant LAS 4000 (Fujifilm, Tokyo, Japan), and expressed as a relative expression level against β-actin protein.
Statistical analysis
All of the data were presented as the mean ± SD. All statistical analyses were performed using a SPSS software (version 11.0). The comparisons among different groups were performed by Student's t-test. P value < 0.05 was considered statistically significant.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
FUNDING
This study was granted from National Natural Science Foundation of China (No.51472270) and Military Logistics Research Programs (No.BWS11C009).
REFERENCES
- [1].Kondo R, Atsuta I, Ayukawa Y, Yamaza T, Matsuura Y, Furuhashi A, Tsukiyama Y, Koyano K. Therapeutic interaction of systemically-administered mesenchymal stem cells with peri-implantmucosa. PLoS One 2014; 9(3):e90681; PMID:24651408; https://doi.org/ 10.1371/journal.pone.0090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Birang R, Shahabooei M, Mashhadiabbas F, Atabaki M, Naghsh N, Kavosh K, Birang E, Mogharehabed A. Effect of enamel matrix derivative on bone formation around intraosseous titanium implant: An experimental study in canine model. Dent Res J 2012; 9:790-6 [PMC free article] [PubMed] [Google Scholar]
- [3].Freitas GP, Lopes HB, Martins-Neto EC, de Oliveira PT, Beloti MM, Rosa AL. Effect of surface nanotopography on bone response to Titanium implant. J Oral Implantol 2016; 42:240-7; PMID:26390195; https://doi.org/ 10.1563/aaid-joi-D-14-00254 [DOI] [PubMed] [Google Scholar]
- [4].Zhang W, Cao H, Zhang X, Li G, Chang Q, Zhao J, Qiao Y, Ding X, Yang G, Liu X, Jiang X. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016; 8:5291-301; PMID:26881868; https://doi.org/ 10.1039/C5NR08580B [DOI] [PubMed] [Google Scholar]
- [5].Schwartz-Arad D, Laviv A, Levin L. Failure causes, timing, and cluster behavior: an 8-year study of dental implants. Implant Dent 2008; 17:200-7; PMID:18545052; https://doi.org/ 10.1097/ID.0b013e3181777906 [DOI] [PubMed] [Google Scholar]
- [6].Sakka S, Coulthard P. Implant failure: etiology and complications. Med Oral Patol Oral Cir Bucal 2011; 16:42-4; https://doi.org/ 10.4317/medoral.16.e42 [DOI] [PubMed] [Google Scholar]
- [7].Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, Gamonal J, Diaz PI. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J 2013; 7:1016-25; PMID:23303375; https://doi.org/ 10.1038/ismej.2012.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004; 364:149-55; PMID:15246727; https://doi.org/ 10.1016/S0140-6736(04)16627-0 [DOI] [PubMed] [Google Scholar]
- [9].Lindroos B, Mäenpää K, Ylikomi T, Oja H, Suuronen R, Miettinen S. Characterisation of human dental stem cells and buccal mucosa fibroblasts. Biochem Biophys Res Commun 2008; 368:329-35; PMID:18230338; https://doi.org/ 10.1016/j.bbrc.2008.01.081 [DOI] [PubMed] [Google Scholar]
- [10].Wen Y, Lan J, Huang H, Yu M, Cui J, Liang J, Jiang B, Xu X. Application of eGFP to label human periodontal ligament stem cells in periodontal tissue engineering. Arch Oral Biol 2012; 57:1241-50; PMID:22410147; https://doi.org/ 10.1016/j.archoralbio.2012.02.017 [DOI] [PubMed] [Google Scholar]
- [11].Xu J, Wang W, Kapila Y, Lotz J, Kapila S. Multiple differentiation capacity of STRO-1+/CD146+ PDL mesenchymal progenitor cells. Stem Cells Dev 2009; 18:487-96; PMID:18593336; https://doi.org/ 10.1089/scd.2008.0113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Houshmand B, Behnia H, Khoshzaban A, Morad G, Behrouzi G, Dashti SG, Khojasteh A. Osteoblastic differentiation of human stem cells derived from bone marrow and periodontal ligament under the effect of enamel matrix derivative and transforming growth factor-beta. Int J Oral Maxillofac Implants 2013; 28:e440-50; PMID:24278943; https://doi.org/ 10.11607/jomi.te24 [DOI] [PubMed] [Google Scholar]
- [13].Kato H, Katayama N, Taguchi Y, Tominaga K, Umeda M, Tanaka A. A synthetic oligopeptide derived from enamel matrix derivative promotes the differentiation of human periodontal ligament stem cells into osteoblast-like cells with increased mineralization. J Periodontol 2013; 84:1476-83; PMID:23173824; https://doi.org/ 10.1902/jop.2012.120469 [DOI] [PubMed] [Google Scholar]
- [14].Grandin HM, Gemperli AC, Dard M. Enamel matrix derivative: a review of cellular effects in vitro and a model of molecular arrangement and functioning. Tissue Eng Part B Rev 2012; 18:181-202; PMID:22070552; https://doi.org/ 10.1089/ten.teb.2011.0365 [DOI] [PubMed] [Google Scholar]
- [15].Matarasso M, Iorio-Siciliano V, Blasi A, Ramaglia L, Salvi GE, Sculean A. Enamel matrix derivative and bone grafts for periodontal regeneration of intrabony defects. A systematic review and meta-analysis. Clin Oral Investig 2015; 19:1581-93; https://doi.org/ 10.1007/s00784-015-1491-7 [DOI] [PubMed] [Google Scholar]
- [16].Aimetti M, Ferrarotti F, Mariani GM, Romano F. A novel flapless approach versus minimally invasive surgery in periodontal regeneration with enamel matrix derivative proteins: a 24-month randomized controlled clinical trial. Clin Oral Investig 2017; 21:327-37; PMID:27044318; https://doi.org/ 10.1007/s00784-016-1795-2 [DOI] [PubMed] [Google Scholar]
- [17].Lossdörfer S, Sun M, Götz W, Dard M, Jäger A. Enamel matrix derivative promotes human periodontal ligament cell differentiation and osteoprotegerin production in vitro. J Dent Res 2007; 86:980-5; PMID:17890675; https://doi.org/ 10.1177/154405910708601012 [DOI] [PubMed] [Google Scholar]
- [18].Hama H, Azuma H, Seto H, Kido J, Nagata T. Inhibitory effect of enamel matrix derivative on osteoblastic differentiation of rat calvaria cells in culture. J Periodontal Res 2008; 43:179-85; PMID:18302620; https://doi.org/ 10.1111/j.1600-0765.2007.01010.x [DOI] [PubMed] [Google Scholar]
- [19].Wada Y, Yamamoto H, Nanbu S, Mizuno M, Tamura M. The suppressive effect of enamel matrix derivative on osteocalcin gene expression of osteoblasts is neutralized by an antibody against TGF-beta. J Periodontol. 2008; 79:341-7; PMID:18251649; https://doi.org/ 10.1902/jop.2008.070197 [DOI] [PubMed] [Google Scholar]
- [20].Franke Stenport V, Johansson CB. Enamel matrix derivative and titanium implants. J Clin Periodontol 2003; 30:359-63; PMID:12694436; https://doi.org/ 10.1034/j.1600-051X.2003.00326.x [DOI] [PubMed] [Google Scholar]
- [21].Kadonishi Y, Deie M, Takata T, Ochi M. Acceleration of tendon-bone healing in anterior cruciate ligament reconstruction using an enamel matrix derivative in a rat model. J Bone Joint Surg Br 2012; 94:205-9; PMID:22323687; https://doi.org/ 10.1302/0301-620X.94B2.26904 [DOI] [PubMed] [Google Scholar]
- [22].Lee JE, Lim MS, Park JH, Park CH, Koh HC. S6K promotes dopaminergic neuronal differentiation through PI3K/Akt/mTOR-dependent signaling pathways in human neural stem cells. Mol Neurobiol 2016; 53:3771-82; PMID:26143260; https://doi.org/ 10.1007/s12035-015-9325-9 [DOI] [PubMed] [Google Scholar]
- [23].Tong Y, Feng W, Wu Y, Lv H, Jia Y, Jiang D. Mechano-growth factor accelerates the proliferation and osteogenic differentiation of rabbit mesenchymal stem cells through the PI3K/AKT pathway. BMC Biochem. 2015; 16:1; PMID:25588515; https://doi.org/ 10.1186/s12858-015-0031-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S. Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev 2014; 23:1001-11; PMID:24351050; https://doi.org/ 10.1089/scd.2013.0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Moshaverinia A, Chen C, Xu X, Akiyama K, Ansari S, Zadeh HH, Shi S. Bone regeneration potential of stem cells derived from periodontal ligament or gingival tissue sources encapsulated in RGD-modified alginate scaffold. Tissue Eng Part A 2014; 20:611-21; PMID:24070211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Osathanon T, Vivatbutsiri P, Sukarawan W, Sriarj W, Pavasant P, Sooampon S. Cobalt chloride supplementation induces stem-cell marker expression and inhibits osteoblastic differentiation in human periodontal ligament cells. Arch Oral Biol 2015; 60:29-36; PMID:25244616; https://doi.org/ 10.1016/j.archoralbio.2014.08.018 [DOI] [PubMed] [Google Scholar]
- [27].Rathe F, Junker R, Chesnutt BM, Jansen JA. The effect of enamel matrix derivative (Emdogain) on bone formation: a systematic review. Tissue Eng Part B Rev 2009; 15:215-24; PMID:18710336; https://doi.org/ 10.1089/ten.teb.2008.0065 [DOI] [PubMed] [Google Scholar]
- [28].Jue SS, Lee WY, Kwon YD, Kim YR, Pae A, Lee B. The effects of enamel matrix derivative on the proliferation and differentiation of human mesenchymal stem cells. Clin Oral Implants Res 2010; 21:741-6; PMID:20636728; https://doi.org/ 10.1111/j.1600-0501.2009.01901.x [DOI] [PubMed] [Google Scholar]
- [29].Qu Z, Andrukhov O, Laky M, Ulm C, Matejka M, Dard M, Rausch-Fan X. Effect of enamel matrix derivative on proliferation and differentiation of osteoblast cells grown on the titanium implant surface. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011; 111:517-22; PMID:21232997; https://doi.org/ 10.1016/j.tripleo.2010.10.011 [DOI] [PubMed] [Google Scholar]
- [30].Casati MZ, Sallum EA, Jr Nociti FH, Caffesse RG, Sallum AW. Enamel matrix derivative and bone healing after guided bone regeneration in dehiscence-type defects around implants. A histomorphometric study in dogs. J Periodontol 2002; 73:789-96; https://doi.org/ 10.1902/jop.2002.73.7.789 [DOI] [PubMed] [Google Scholar]
- [31].Cao FY, Fan JX, Long Y, Zeng X, Zhang XZ. A smart fluorescence nanoprobe for the detection of ellular alkaline phosphatase activity and early osteogenic differentiation. Nanomedicine 2016; 12:1313-22; PMID:26961462; https://doi.org/ 10.1016/j.nano.2016.01.010 [DOI] [PubMed] [Google Scholar]
- [32].Kwun IS, Cho YE, Lomeda RA, Shin HI, Choi JY, Kang YH, Beattie JH. Zinc deficiency suppresses matrix mineralization and retards osteogenesis is transiently with catch-up possibly through Runx 2 modulation. Bone 2010; 46:732-41; PMID:19913120; https://doi.org/ 10.1016/j.bone.2009.11.003 [DOI] [PubMed] [Google Scholar]
- [33].zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation 2003; 71:18-27; PMID:12558600; https://doi.org/ 10.1046/j.1432-0436.2003.700602.x [DOI] [PubMed] [Google Scholar]
- [34].Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today 2005; 75:213-225; PMID:16187316; https://doi.org/ 10.1002/bdrc.20043 [DOI] [PubMed] [Google Scholar]
- [35].Ziros PG, Basdra EK, Papavassiliou AG. Runx2: of bone and stretch. Int J Biochem Cell Biol 2008; 40:1659-1663; PMID:17656144; https://doi.org/ 10.1016/j.biocel.2007.05.024 [DOI] [PubMed] [Google Scholar]
- [36].Shen T, Qiu L, Chang H, Yang Y, Jian C, Xiong J, Zhou J, Dong S. Cyclic tension promotes osteogenic differentiation in human periodontal ligament stem cells. Int J Clin Exp Pathol 2014; 7:7872-80; PMID:25550827 [PMC free article] [PubMed] [Google Scholar]
- [37].Nii T, Marumoto T, Kohara H, Yamaguchi S, Kawano H, Sasaki E, Kametani Y, Tani K. Improved hematopoietic differentiation of primate embryonic stem cells by inhibition of the PI3K-AKT pathway under defined conditions. Exp Hematol 2015; 43:901-11; PMID:26073521; https://doi.org/ 10.1016/j.exphem.2015.06.001 [DOI] [PubMed] [Google Scholar]
- [38].Yang HJ, Wang L, Wang M, Ma SP, Cheng BF, Li ZC, Feng ZW. Serine/threonine-protein kinase PFTK1 modulates oligodendrocyte differentiation via PI3K/AKTpathway. J Mol Neurosci 2015; 55:977-84; PMID:25355490; https://doi.org/ 10.1007/s12031-014-0454-9 [DOI] [PubMed] [Google Scholar]
- [39].Isomoto S, Hattori K, Ohgushi H, Nakajima H, Tanaka Y, Takakura Y. Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J Orthop Sci 2007; 12:83-8; PMID:17260122; https://doi.org/ 10.1007/s00776-006-1079-9 [DOI] [PubMed] [Google Scholar]
- [40].Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G. Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1cells and primary mouse bone marrow stromal cells. J Cell Biochem 2008; 103:434-46; PMID:17516572; https://doi.org/ 10.1002/jcb.21411 [DOI] [PubMed] [Google Scholar]
- [41].Lee KW, Yook JY, Son MY, Kim MJ, Koo DB, Han YM, Cho YS. Rapamycin promotes the osteoblastic differentiation of human embryonic stem cells by blocking the mTOR pathway and stimulating the BMP/Smad pathway. Stem Cells Dev 2010; 19:557-68; PMID:19642865; https://doi.org/ 10.1089/scd.2009.0147 [DOI] [PubMed] [Google Scholar]
- [42].An N, Schedle A, Wieland M, Andrukhov O, Matejka M, Rausch-Fan X. Proliferation, behavior, and cytokine gene expression of human umbilical vascular endothelial cells in response to different titanium surfaces. J Biomed Mater Res A 2010; 93:364-72; PMID:19569217 [DOI] [PubMed] [Google Scholar]
- [43].Rausch-Fan X, Qu Z, Wieland M, Matejka M, Schedle A. Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent Mater 2008; 24:102-10; PMID:17467048; https://doi.org/ 10.1016/j.dental.2007.03.001 [DOI] [PubMed] [Google Scholar]
- [44].Zhang L, Liu W, Zhao J, Ma X, Shen L, Zhang Y, Jin F, Jin Y. Mechanical stress regulates osteogenic differentiation and RANKL/OPG ratio in periodontal ligament stem cells by the Wnt/β-catenin pathway. Biochim Biophys Acta 2016; 1860:2211-9; PMID:27154288; https://doi.org/ 10.1016/j.bbagen.2016.05.003 [DOI] [PubMed] [Google Scholar]
- [45].Manescu A, Giuliani A, Mohammadi S, Tromba G, Mazzoni S, Diomede F, Zini N, Piattelli A, Trubiani O. Osteogenic potential of dual blocks cultured with human periodontal ligament stem cells: in vitro and synchrotron microtomography study. J Periodontal Res 2016; 51:112-24; PMID:26094874; https://doi.org/ 10.1111/jre.12289 [DOI] [PubMed] [Google Scholar]


