ABSTRACT
A real-time PCR (RT-PCR) assay was designed for the simultaneous identification of Neisseria gonorrhoeae and its ciprofloxacin susceptibility status. A SYBR green-based multiplex RT-PCR format was used; it comprised two different forward primers and a common reverse primer to detect single nucleotide polymorphisms (SNPs) in gyrA of N. gonorrhoeae. The primer pairs were evaluated for their sensitivity and specificity using genomic DNA from 254 N. gonorrhoeae isolates (82 were ciprofloxacin susceptible and 172 were ciprofloxacin resistant) and 23 non-N. gonorrhoeae species isolates. The performance of the primers was validated using genomic DNA from 100 different N. gonorrhoeae isolates (46 were ciprofloxacin susceptible and 54 were ciprofloxacin resistant) and 52 non-N. gonorrhoeae isolates. The latter panel was revalidated by testing 99 (46 isolates were ciprofloxacin susceptible and 53 isolates were ciprofloxacin resistant) of the N. gonorrhoeae isolates and 23 non-N. gonorrhoeae isolates. These primers detected N. gonorrhoeae and its ciprofloxacin susceptibility status with over 99% sensitivity and specificity for all panels tested. This assay has the potential to be an inexpensive and rapid test for the simultaneous identification of N. gonorrhoeae and its ciprofloxacin susceptibility status.
KEYWORDS: Neisseria gonorrhoeae, antimicrobial susceptibility (AMS), ciprofloxacin, gyrA, multiplex RT-PCR assay
INTRODUCTION
Gonorrhea remains an important global public health concern, with 78 million new infections worldwide each year (1). Cases of gonorrhea are underreported, especially because most infections (almost half of the women infected) are asymptomatic; such infections can have major long-term and negative impacts on reproductive health, especially in women (2). Gonococcal infections can only be cured with antibiotics; there are no vaccines against this disease (3, 4). Over time, Neisseria gonorrhoeae isolates have developed resistance to every class of antibiotic introduced for therapy, including third-generation cephalosporins, which were the last class of antibiotic used in single-dose therapy (3, 5). The recommended treatment in many countries for gonococcal infections now comprises co-therapy with two antibiotics, azithromycin coupled with ceftriaxone (6). There is increased urgency for new strategies to treat gonococcal infections, either with new antibiotics, antibiotic combinations, or possibly the use of older antimicrobials where susceptibility to the agent is known.
The identification of N. gonorrhoeae in high-income countries is usually accomplished by nucleic acid amplification tests (NAATs) (7–10). A limitation of NAATs is that susceptibility testing cannot be completed at present, as such testing requires the pathogen to be cultured and molecular testing for AMS is still under evaluation (3, 5, 11, 12). Due to the widespread use of NAATs, AMS testing of N. gonorrhoeae isolates has significantly declined in resource-rich countries (12, 13). Such testing is often beyond the technical capability of resource-limited settings.
Ciprofloxacin, a fluoroquinolone antibiotic, was first introduced for the treatment of gonococcal infections in 1985; by 2006, in the United States, ciprofloxacin resistance was observed in 13.8% of isolates (11, 14). The population-based recommendation of the World Health Organization is that when resistance to an antibiotic exceeds 5% of isolates tested, the antibiotic should be discontinued for treatment (5, 6). Consequently, from 2007 ciprofloxacin has not been recommended for the treatment for N. gonorrhoeae infections in the United States (15). In Canada, between 2004 and 2014, ciprofloxacin resistance in N. gonorrhoeae isolates increased from 6.3% to 34.0% and is no longer recommended for the primary treatment of gonorrhea (16, 17). It is notable, however, that in in the United States ∼80% of N. gonorrhoeae isolates are susceptible to ciprofloxacin (18). The issue is that individual patients infected with a susceptible isolate must be identified before this antibiotic can be considered a treatment option. Thus, there is a need for a point-of-care test (POCT) that might identify those infected with ciprofloxacin-susceptible N. gonorrhoeae isolates.
Ciprofloxacin resistance in N. gonorrhoeae is caused by single nucleotide polymorphisms (SNPs) within DNA gyrase A (gyrA; amino acid positions S91 and D95) and parC (amino acid positions S88 and E91) (3, 5, 19, 20). Studies have shown that more than 99% of ciprofloxacin-resistant N. gonorrhoeae isolates carry gyrA S91 and/or D95 mutations, making these targets potentially diagnostic for resistance (21–23).
We report on the design and evaluation of a test that can be used simultaneously to identify N. gonorrhoeae and to determine its ciprofloxacin susceptibility status in a single multiplex assay. Our test was over 99% sensitive and specific for the identification of the ciprofloxacin susceptibility status of cultured N. gonorrhoeae.
RESULTS
Specificity and sensitivity of gyrA primers for identification of N. gonorrhoeae either in single PCR or multiplex formats.
Individually and in multiplex, gyrA-W and gyrA-M primers did not amplify DNA from any of the non-N. gonorrhoeae isolates (Table 1), indicating 100% specificity. A nonspecific melt curve was produced by the gyrA-M primer pair for Lactobacillus jensenii that was not reproduced on the repeated analyses. Similarly, the gyrA-W primer pair produced nonspecific melt curves for Neisseria animaloris and Neisseria meningitidis, which were not observed in repeated experiments. Nonspecific melt curves were considered negative amplifications, as these melt curve temperatures fell beyond the expected range and did not interfere with the melt curve interpretation of the ciprofloxacin susceptibility status of N. gonorrhoeae. The gyrA-W primer pair and gyrA-M primer pair amplified 57% and 58%, respectively, of the 254 N. gonorrhoeae isolates (Table 1). In the multiplex format with three primers (2 forward primers and a common reverse primer), all 254 N. gonorrhoeae isolates were amplified and no non-N. gonorrhoeae isolates were amplified (Table 1). Therefore, the multiplex assay showed 100% sensitivity and specificity for the identification of N. gonorrhoeae.
TABLE 1.
Sensitivity and specificity of gyrA-W and gyrA-M primer pairs in multiplex format for identification of N. gonorrhoeae isolates and determination of their ciprofloxacin susceptibility status
| Primer type | Isolate group | No. of isolates amplified/total no. of isolates | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| Single gyrA-W | N. gonorrhoeae | 144/254 | 57 | NAb |
| Single gyrA-W | Non-N. gonorrhoeae | 0/23 | NA | 100 |
| Single gyrA-M | N. gonorrhoeae | 148/254 | 58 | NA |
| Single gyrA-M | Non-N. gonorrhoeae | 0/23 | NA | 100 |
| Multiplex gyrA-M and gyrA-W | N. gonorrhoeae | 254/254 | 100 | NA |
| Non-N. gonorrhoeae | 0/23 | NA | 100 | |
| Ciprofloxacin-susceptiblea N. gonorrhoeae | 82/82 | 100 | 99c | |
| Ciprofloxacin-intermediate/resistanta N. gonorrhoeae | 171/172 | 99 | 100 |
Per ciprofloxacin MIC analyses of N. gonorrhoeae: 82 isolates susceptible, 7 isolates intermediate, and 165 isolates resistant.
NA, not applicable.
One ciprofloxacin-resistant isolate was incorrectly identified as ciprofloxacin susceptible.
Multiplex primers in ascertaining ciprofloxacin susceptibility status.
A total of 94 N. gonorrhoeae isolates (a subset of panel 1) was analyzed by multiplex RT-PCR; 47 isolates were previously determined to be ciprofloxacin susceptible (MIC 0.002 to 0.063 μg/ml) and another 47 isolates were determined as ciprofloxacin intermediate/resistant (MIC 0.125 to 64 μg/ml). The putative ciprofloxacin susceptibility status of the isolates was precisely ascertained by melt curve analyses, which distinctly differentiated between ciprofloxacin susceptible and intermediate/resistant N. gonorrhoeae isolates. Susceptible N. gonorrhoeae produced melt curve values of ∼78.0°C and intermediate/resistant N. gonorrhoeae produced melt curves values of 80.0 to 83.0°C. Mutations in the gyrA sequence resulting in S91 and D95 amino acid alterations were distinguished using the multiplex assay (Fig. 1A). Furthermore, the multiplex assay performed equally well in differentiating WHO N. gonorrhoeae reference strains into ciprofloxacin susceptible and intermediate/resistant groups (Fig. 1B). The gyrA primers in multiplex format successfully identified WHO-B, C, F, O, and P strains as ciprofloxacin sensitive (melt curve, ∼78.0°C), and WHO-K, L, and M strains as intermediate/resistant (melt curve, 80.0 to 83.0°C).
FIG 1.
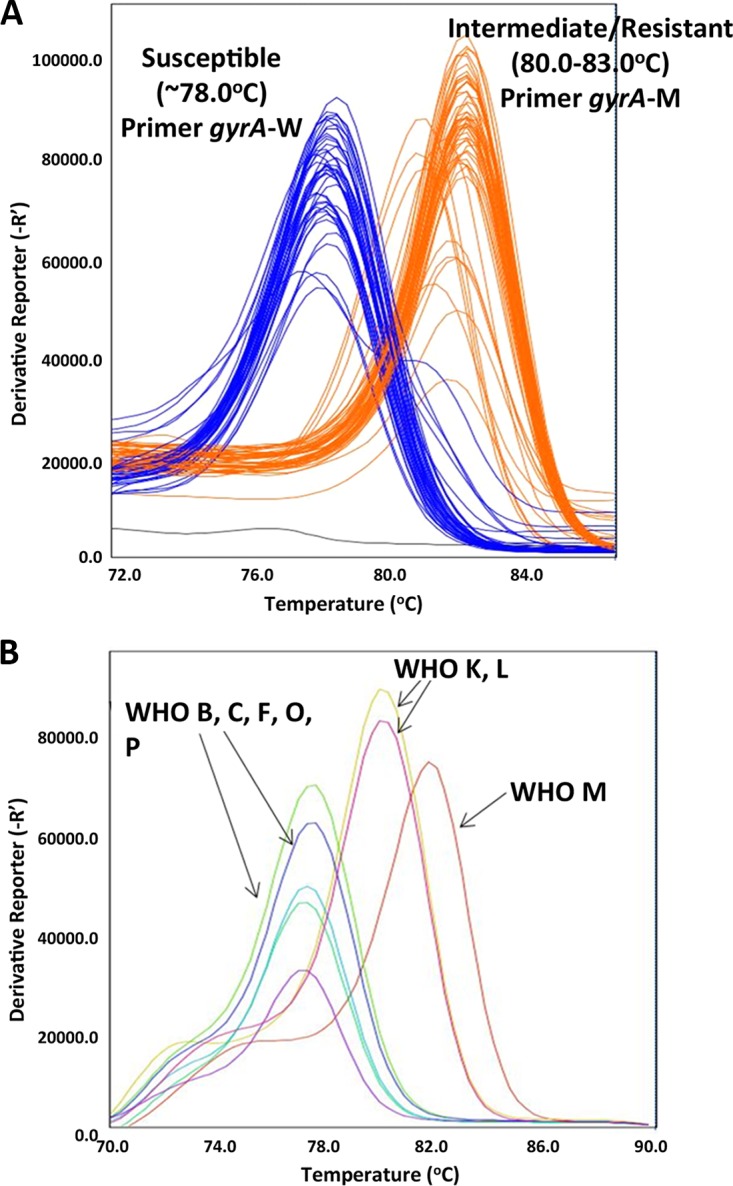
Multiplex RT-PCR melt curve representation of ciprofloxacin-susceptible and intermediate/resistant N. gonorrhoeae isolates. (A) Ciprofloxacin-susceptible (n = 47) and intermediate/resistant (n = 47) N. gonorrhoeae isolates (subset of panel 1). Blue lines represent susceptible isolates, orange lines represent intermediate/resistant isolates, and black lines represent no template negative controls. (B) WHO N. gonorrhoeae reference strains (n = 8). Strains B, C, F, O, and P are susceptible (melt curve ∼78°C), and strains K, L, and M are intermediate/resistant (melt curve 80.0-83.0°C).
The ciprofloxacin susceptibility status of panel 1 isolates had previously been determined with MIC analyses: 82 isolates were susceptible (MIC 0.002 to 0.063 μg/ml), while 172 isolates were intermediate (n = 7; MIC 0.125 to 0.5 μg/ml) or resistant (n = 165; MIC 2 to 64 μg/ml). With multiplex RT-PCR, 171/172 isolates were characterized as ciprofloxacin intermediate/resistant and 82/82 isolates were characterized as ciprofloxacin susceptible (Table 1). One resistant isolate was incorrectly classified as susceptible after amplification. Thus, gyrA primers in multiplex were 100% sensitive and 99% specific for the identification of ciprofloxacin susceptibility status. Similarly, the primers were 99% sensitive and 100% specific in the identification of ciprofloxacin intermediate/resistant N. gonorrhoeae isolates (Table 1).
Validation of RT-PCR multiplex tests for N. gonorrhoeae identification and ciprofloxacin susceptibility status determination.
The gyrA primers were used to ascertain gonococcal identification and susceptibility status with panel 2 isolates. Used as single primer pairs, gyrA-W was 94% sensitive and 100% specific for the identification of N. gonorrhoeae and gyrA-M was 100% sensitive and specific for N. gonorrhoeae identification (Table 2). Some nonspecific peaks were observed with the gyrA-M primer pair and interpreted to be nonspecific amplifications: short peak melt curves with temperatures 84.1°C, 86.9°C, and 87.2°C were observed for Atopobium vaginae, N. animaloris, and Salmonella enterica serovar Typhimurium, respectively. Neisseria perflava produced a melt curve at 76.7°C for the gyrA-W primer pair but this observation was not reproduced in repeated experiments (data not shown).
TABLE 2.
Validation and re-validation of primers gyrA-W and gyrA-M by RT-PCR using panels 2 and 3 N. gonorrhoeae and non-N. gonorrhoeae isolatesa
| Primer type | Isolate group | Panel 2 |
Panel 3b |
||||
|---|---|---|---|---|---|---|---|
| No. of isolates amplified/total no. of isolates | Sn (%) | Sp (%) | No. of isolates amplified/total no. of isolates | Sn (%) | Sp (%) | ||
| Single gyrA-W | N. gonorrhoeae | 94/100 | 94 | NA | 92/99 | 93 | NA |
| Single gyrA-W | Non-N. gonorrhoeae | 0/52 | NA | 100 | 0/23 | NA | 100 |
| Single gyrA-M | N. gonorrhoeae | 100/100 | 100 | NA | 98/99 | 99 | NA |
| Single gyrA-M | Non-N. gonorrhoeae | 0/52 | NA | 100 | 0/23 | NA | 100 |
| Multiplex gyrA-M and gyrA-W | N. gonorrhoeae | NP | NP | NP | 99/99 | 100 | NA |
| Non-N. gonorrhoeae | NP | NP | NP | 0/23 | NA | 100 | |
| Ciprofloxacin-susceptible N. gonorrhoeaec | NP | NP | NP | 47/47 | 100 | 100 | |
| Ciprofloxacin-intermediate/resistant N. gonorrhoeaec | NP | NP | NP | 52/52 | 100 | 100 | |
Sn, sensitivity; Sp, specificity; NA, not applicable; NP, not performed.
The primary purpose of panel 3 was to assess the performance of multiplex primers using a majority of panel 2 isolates.
Per ciprofloxacin MIC analyses of N. gonorrhoeae: 47 isolates susceptible and 52 isolates resistant.
With panel 3, which comprised DNA from isolates in panel 2, primer pair gyrA-W was 93% sensitive and 100% specific for N. gonorrhoeae identification while gyrA-M was 99% sensitive and 100% specific for N. gonorrhoeae identification (Table 2). In multiplex analyses, the primers were 100% sensitive and specific for N. gonorrhoeae identification. Amplification of N. animaloris and N. meningitides produced melt curves at 76.7°C for gyrA-W, but was not reproduced on replication and the result was interpreted as negative amplification.
DISCUSSION
A method for the simultaneous identification of the pathogen N. gonorrhoeae and its ciprofloxacin susceptibility status has been described. The gyrA-M forward primer is unique to N. gonorrhoeae and specific to ciprofloxacin-resistant isolates. The gyrA-W forward primer is specific to ciprofloxacin-susceptible isolates. These primers, paired with the common reverse primer, were not anticipated to be highly sensitive for gonococcal identification when used as individual pairs; however, the multiplex test format had high sensitivity and specificity (>99%) for detecting N. gonorrhoeae and its ciprofloxacin susceptibility status in a single amplification.
The gyrA and parC genomic regions of N. gonorrhoeae are associated with ciprofloxacin resistance (19). parC mutations in association with gyrA mutations maintain higher levels of overall resistance (MIC of ≥1) whereas lower levels of resistance are associated with gyrA mutations only. On the other hand, parC mutations alone are not associated with ciprofloxacin resistance (22, 24, 25). In our study, we considered gyrA mutations alone and clearly identified the ciprofloxacin-susceptible and resistant isolates in a single multiplex RT-PCR assay. Thus, our results support the findings by others and demonstrate that the gyrA region alone is sufficient for the determination of ciprofloxacin susceptibility status (13, 18, 22, 26). We determined through DNA sequencing that the majority of isolates with intermediate susceptibility in our study carried mutations only at the S91 or D95 position, and not both (Table 3); one isolate with intermediate susceptibility in panel 2 had mutations at both S91 and D95 positions. Similarly, the majority of resistant isolates carried mutations at both S91 (S91F) and D95 positions (either D95G, D95A, or D95N). One resistant isolate in panel 1 had a mutation only at the D95 position. Thus, we showed that ciprofloxacin resistance can arise due to mutations at both S91 and D95 amino acid positions, and analysis of the S91 position alone is not sufficient for comprehensive determination of ciprofloxacin susceptibility status.
TABLE 3.
Single nucleotide polymorphisms observed at amino acid positions S91 and D95 of N. gonorrhoeae isolates based on gyrA amplicon sequencinga
| No. of SNPs observed at amino acid position | Panel 1b |
Panel 2 |
||
|---|---|---|---|---|
| No. of isolates | Susceptibility status | No. of isolates | Susceptibility status | |
| Susceptible, S91/D95 | 35 | S | 46 | S |
| Intermediate | ||||
| S91/D95G | 1 | I | ||
| S91F/D95 | 2 | I | 2 | I |
| S91Y/D95 | 2 | I | 1 | I |
| Resistant | ||||
| S91/D95N | 1 | R | ||
| S91F/D95G | 44 | R | 43 | I, Rc |
| S91F/D95A | 56 | R | 5 | R |
| S91F/D95N | 12 | R | 3 | R |
| Total no. of isolates tested | 154 | 100 | ||
SNP, single nucleotide polymorphism; S, susceptible; I, intermediate; R, resistant.
gyrA sequencing information from panel 1 is presented for 154/254 isolates. Sequencing information is not available for older isolates. Ciprofloxacin susceptibility status of panel 1 and panel 2 isolates was confirmed with MIC testing.
1/43 isolates intermediate and 42/43 isolates resistant.
To our knowledge, this is the first report of simultaneous detection of N. gonorrhoeae as well as of its ciprofloxacin susceptibility status in a single and simple test that is highly specific, sensitive, inexpensive, and rapid. The costs associated with specimen culturing and training of laboratory personnel, as well as the current costs of NAATs, are beyond the economic capabilities of many resource-limited settings. The DNA extraction and PCR cost per sample in the assay discussed in our study (Can$11.07) is about 2 times less than the cost per sample incurred by the current N. gonorrhoeae diagnostic NAATs or the costs associated with identification and SNP testing at the National Microbiology Laboratory (estimated at Can$75/sample, inclusive of labor). Thus, the assay discussed in our work is far more affordable than the current NAATs for N. gonorrhoeae diagnosis. One study demonstrated detection of N. gonorrhoeae and its ciprofloxacin susceptibility status, but this assay required three consecutive tests for a clear interpretation (27). Several recent publications introduced RT-PCR-based methods for detection of mutations at the gryA S91 locus from clinical specimens (18, 26, 28). However, these analyses were restricted only to the S91 locus and the assays could be more likely to have errors if ciprofloxacin resistance arose through mutations in the D95 locus. In our work, we have observed through amplicon sequencing that ciprofloxacin resistance primarily arose due to mutations in both S91 and D95 loci, while intermediate resistance mainly arose due to mutations in either S91 or D95 loci (Table 3).
Although the results of this study highlight the utility of this multiplex RT-PCR method for identifying N. gonorrhoeae and determining its ciprofloxacin susceptibility status, caution is needed, as only a very limited number of non-N. gonorrhoeae isolates were tested. In addition, further studies are needed to investigate if the assay has clinical applicability by testing clinical specimens directly with the aim of personalized care (18).
Our findings show promise for the development of a POCT for the identification of N. gonorrhoeae and its ciprofloxacin susceptibility status. POCTs facilitate the diagnosis at the health care provider's office and avoid unnecessary treatment delays. This eliminates the need for patients to return for post diagnosis treatments and prevents unwanted complications and disease transmission (27). Given its probable low cost and relative ease of use, this assay could have major implications for resource-poor, high disease burden countries (29, 30). Furthermore, our assay may prove helpful to clinicians and laboratories to respond to infectious disease control organizations, increase surveillance for ciprofloxacin resistance, and present coherent findings for epidemiological and surveillance purposes. With gyrA-based diagnosis of gonococcal AMS status, the utilization of ciprofloxacin could be increased, thereby reducing the use of last-resort antimicrobials (18). Thus, a rapid and reliable assay, such as the platform discussed by our work, would improve the understanding of the extent of the disease worldwide.
MATERIALS AND METHODS
Bacterial strains.
Two different N. gonorrhoeae strain panels were used and comprised DNA extracted from clinically derived cultured specimens. Panel 1 comprised 254 gonococcal and 23 nongonococcal isolates (Table 4). Panel 1 N. gonorrhoeae isolates were chosen randomly from the Dillon Culture Collection, which consisted of isolates previously reported by Vidovic et al. (31) and Liao et al. (33). A core feature of all selections was their different susceptibilities to ciprofloxacin. The WHO reference isolates were reported in Unemo et al. (34). A random collection of non-Neisseria species isolates and other Neisseria species isolates were obtained from the National Microbiology Laboratory (NML) (Winnipeg, MB, Canada). The second unrelated panel comprised 100 gonococcal and 52 nongonococcal isolates from across Canada and was selected at the NML to include isolates having different susceptibilities to ciprofloxacin. A subsample of panel 2 (panel 3) containing DNA from 99 gonococcal and 23 nongonococcal isolates was used for revalidation. One isolate from panel 2 was eliminated in panel 3 due to inconsistent results. WHO N. gonorrhoeae reference strains for ciprofloxacin susceptibility determination included strains F, O, P, B, and C (susceptible), G (intermediate), and M, N, K, and L (resistant) (34).
TABLE 4.
N. gonorrhoeae and non-N. gonorrhoeae isolates used in this study
| Isolate selection panel no. | Organism(s) | Geographic source | No. of isolates | Reference(s) or source |
|---|---|---|---|---|
| 1 | Neisseria gonorrhoeae | Saskatchewan | 110 | 31, 32 |
| China | 96 | 33 | ||
| WHO | 10 | 34 | ||
| USA | 13 | Dillon Culture Collection | ||
| South America and the Caribbean | 25 | Dillon Culture Collection | ||
| All sources | 254 | |||
| Non-N. gonorrhoeaeb Neisseria species | Canada | 13 | NMLa | |
| Non-Neisseria speciesb | Canada | 10 | NML | |
| All non-N. gonorrhoeae species | All sources | 23 | ||
| All species | 278 | |||
| 2 | N. gonorrhoeae | Canada | 100 | NML |
| Non-N. gonorrhoeae and non-Neisseria speciesc | Canada | 52 | NML | |
| All species | 152 | |||
| 3 | N. gonorrhoeae | Canada | 99 | NML |
| Non-N. gonorrhoeae & non-Neisseria speciesb | Canada | 23 | NML | |
| Total species | 122 |
NML, National Microbiology Laboratory.
Neisseria animaloris, Neisseria elongata, Neisseria flava, Neisseria lactamica, Neisseria meningitides, Neisseria mucosa, Neisseria perflava, Neisseria polysaccharea, Neisseria sicca, Neisseria subflava, Neisseria wadsworthii, Neisseria weaveri, Neisseria cinerea, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Klebsiella oxytoca, Lactobacillus jensenii, Moraxella catarrhalis, Pseudomonas aeruginosa, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, and Staphylococcus epidermis.
Atopbium vaginae, Bacteroides ureolyticus, Candida albicans, Corynebacterium glucoronolyticum, Corynebacterium urealyticum, Corynebacterium xerosis, Cryptococcus neoformans, Enterobacter aerogenes, E. faecalis, E. faceium, E. coli, Gardnerella vaginalis, K. oxytoca, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus iners, L. jensenii, Leptotrichia buccalis, Listeria monocytogenes, Mobiluncus curtisii, M. catarrhalis, Neisseria animalis, N. animaloris, Neisseria cinerea, N. elongata, N. flava, Neisseria flavescens, N. lactamica, N. meningitidis, N. mucosa, N. perflava, N. polysaccharea, N. sicca, N. subflava, N. wadsworthii, N. weaveri, Peptococcus niger, Peptostreptococcus anaerobius, Prevotella bivia, Proteus mirabilis, P. aeruginosa, S. Typhimurium, S. aureus, S. epidermidis, Streptococcus agalactiae, Streptococcus gordonii, Streptococcus infantis, Streptococcus oralis, Streptococcus pyogenes, Ureaplasma urealyticum, Ureaplasma parvum, and Mycoplasma hominis.
N. gonorrhoeae isolates were stored at −80°C in brain heart infusion broth (Difco BD Bioscience) with 20% glycerol. Aliquots from frozen inoculum were cultured on GC medium base (Difco BD Bioscience) supplemented with 1% Kellogg's defined supplement. Plates were incubated at 35°C with 5 to 7% CO2 in a humid environment for 18 to 24 h (35).
DNA extraction was performed using a QIAamp DNA minikit (catalog no. 51306; Qiagen, Inc.) according to the manufacturer's protocol. DNA was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc.) and 50 ng/μl of DNA from each isolate was used for RT-PCRs.
Antimicrobial susceptibility testing.
The MICs of all N. gonorrhoeae isolates to ciprofloxacin (Sigma-Aldrich, Oakville ON, Canada) were assessed by the agar dilution method (35). AMS criteria recommended by the Clinical and Laboratory Standards Institute (CLSI) were used to interpret ciprofloxacin MICs as follows: sensitive (≤0.06 μg/ml), intermediate (0.125 to 0.50 μg/ml), and resistant (≥1.0 μg/ml) (35).
Primers and real-time (RT) PCR.
Two different forward primers and one common reverse primer with homology to N. gonorrhoeae gyrA were designed (U.S. utility patent no. 62/088,332) using Primer-BLAST (36). Primer pair gyrA-W comprised forward primer gyrA-W-F (5′GCGATTCCGCAGTTTACGA3′) and the common reverse primer gyrA-R (5′CGAAATTTTGCGCCATACGGACGAT3′). Primer pair gyrA-M comprised forward primer gyrA-M-F (5′ TACCACCCCCACGGCGATTT3′) and the common reverse primer gyrA-R.
RT-PCR (for panels 1 and panel 3) was performed using an Applied Biosystems (AB) StepOnePlus RT-PCR system (catalog no. 4376600; Life Technologies, Inc.) on a 96-well platform (catalog no. 4346907) and for panel 2 using a ViiA 7 RT-D2 RT-PCR instrument (serial number 278880994; Life Technologies, Inc.) on a 96-well platform with the SYBR Select Master Mix Reagent kit (catalog no. 4472908; Life Technologies, Inc.). PCR mixtures contained 5 μl of 2× SYBR green master mix, 0.25 μl of each primer (10 μM), and 2 μl of DNA template (50 ng/μl). The final reaction volume was adjusted to 10 μl with deionized water. PCR was conducted according to the manufacturer's guidelines with the following modifications: initial holding and activation at 50°C for 2 min followed by a secondary holding at 95°C for 2 min. PCR was performed for 25 cycles at 95°C for 15 s and 60°C for 30 s. The post-PCR melt curve was performed between temperatures of 60°C to 95°C with 0.3°C temperature increments. DNA from WHO strain F was used as a positive control and an aliquot without any template was used as a negative control. Data were collected at 50°C in the holding stage, at 60°C in the annealing stage (for amplification data), and during the melt curve process (for melt curve data).
Multiplex RT-PCR analyses.
For multiplex RT-PCR analyses, reaction mixtures contained 5 μl of 2× SYBR green master mix, 0.25 μl of each of the forward primers (10 μM), 0.25 μl of the common reverse primer (10 μM), and 2 μl of the DNA template (50 ng/μl). The final reaction volume was adjusted to 10 μl with deionized water. The method for multiplex RT-PCR was the same as described above. Initially, the sensitivity and specificity of the primers were analyzed using purified DNA from 254 N. gonorrhoeae and 23 non-N. gonorrhoeae isolates (panel 1 in Table 4) using single primer pairs and in multiplex format.
These tests were validated with the same primers and methods but using panel 2 isolates. A subset of DNA from panel 2 isolates was revalidated as panel 3 (Table 4).
Calculation of specificity and sensitivity.
Specificity and sensitivity were calculated using the formulas described before (37).
DNA sequencing.
The gyrA genomic region of N. gonorrhoeae was PCR-amplified with primers gyrA-F (ACTGTACGCGATGCACGAGC) and gyrA-R (TCTGCCAGCATTTCATGTGAG) (19). Sanger sequencing of the amplicon was performed at Eurofins Genomics (Louisville, KY).
ACKNOWLEDGMENTS
This work was supported by a Grand Challenges Canada Grant (S5-0398-01) and a Saskatchewan Health Research Foundation (SHRF) Collaborative Innovation Grant (no. 3380) to J.R.D., as well as by funding from the University of Saskatchewan (no. 417069). S.R.P. was partially supported by a University of Saskatchewan College of Medicine scholarship.
The authors declare no conflict of interests.
REFERENCES
- 1.Newman l. Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker CK, Sweet RL. 2011. Gonorrhea infection in women: prevalence, effects, screening, and management. Int J Womens Health 3:197–206. doi: 10.2147/IJWH.S13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dillon JR, Parti RP, Thakur SD. 2015. Antibiotic resistance in Neisseria gonorrhoeae: will infections be untreatable in the future? Culture 35:1–8. [Google Scholar]
- 4.Unemo M, Nicholas RA. 2012. Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 7:1401–1422. doi: 10.2217/fmb.12.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Unemo M, Shafer WM. 2014. Antimicrobial resistance in Neisseria gonorrhoea in the 21st century: past, evolution and the future. Clin Microbiol Rev 27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2016. WHO guidelines for the treatment of Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland. http://www.who.int/reproductivehealth/publications/rtis/gonorrhoea-treatment-guidelines/en/. [PubMed] [Google Scholar]
- 7.Tabrizi SN, Unemo M, Limnios AE, Hogan TR, Hjelmevoll SO, Garland SM, Tapsall J. 2011. Evaluation of six commercial nucleic acid amplification tests for detection of Neisseria gonorrhoeae and other Neisseria species. J Clin Microbiol 49:3610–3615. doi: 10.1128/JCM.01217-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pope CF, Hay P, Alexander S, Capaldi K, Dave J, Sadiq ST, Ison CA, Planche T. 2010. Positive predictive value of the Becton Dickinson VIPER system and the ProbeTec GC Qx assay, in extracted mode, for detection of Neisseria gonorrhoeae. Sex Transm Infect 86:465–469. doi: 10.1136/sti.2010.044065. [DOI] [PubMed] [Google Scholar]
- 9.Moncada J, Clark CB, Holden J, Hook EW III, Gaydos CA, Schachter J. 2017. Stability studies on dry swabs and wet mailed swabs for detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Aptima assays. J Clin Microbiol 55:971–977. doi: 10.1128/JCM.02235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham RMA, Doyle CJ, Jennison AV. 2017. Epidemiological typing of Neisseria gonorrhoeae and detection of markers associated with antimicrobial resistance directly from urine samples using next generation sequencing. Sex Transm Infect 93:65–67. doi: 10.1136/sextrans-2015-052422. [DOI] [PubMed] [Google Scholar]
- 11.Goire N, Lahra MM, Chen M, Donovan B, Fairley C, Guy R, Kaldor J, Regan D, Ward J, Nissen MD, Sloots TP, Whiley DM. 2014. Molecular approaches to enhance surveillance of gonococcal antimicrobial resistance. Nat Rev Microbiol 12:223–229. doi: 10.1038/nrmicro3217. [DOI] [PubMed] [Google Scholar]
- 12.Public Health Agency of Canada. 2016. National surveillance of antimicrobial susceptibilities of Neisseria gonorrhoeae annual summary 2014. Public Health Agency of Canada, Ottawa, ON, Canada: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/drugs-products-medicaments-produits/2014-neisseria/alt/surveillance-gonorrhoeae-2014-eng.pdf. [Google Scholar]
- 13.Peterson SW, Martin I, Demczuk W, Bharat A, Hoang L, Wylie J, Allen V, Lefebvre B, Tyrell G, Horsman G, Haldane D, Garceau R, Wong T, Mulvery MR. 2015. Molecular assay for detection of ciprofloxacin resistance in Neisseria gonorrhoeae isolates from cultures and clinical nucleic acid amplification test specimens. J Clin Microbiol 53(11):3606–3608. doi: 10.1128/JCM.01632-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein E, Kirkcaldy RD, Reshef D, Berman S, Weinstock H, Sabeti P, Del Rio C, Hall G, Hook EW, Lipsitch M. 2012. Factors related to increasing prevalence of resistance to ciprofloxacin and other antimicrobial drugs in Neisseria gonorrhoeae, United States. Emerg Infect Dis 18(8):1290–1297. doi: 10.3201/eid1808.111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 2016. Antibiotic-resistant gonorrhea basic informatio. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/std/gonorrhea/arg/basic.htm/. Accessed 7 July 2016. [Google Scholar]
- 16.Kropp RY, Steben M. 2006. Canadian guidelines on sexually transmitted infections, 2006 edition. Public Health Agency of Canada, Ottawa, ON, Canada: http://www.phac-aspc.gc.ca/std-mts/pdf/whatsnew-quoineuf_e.pdf. [Google Scholar]
- 17.Public Health Agency of Canada. 2013. Section 5-6: Canadian guidelines on sexually transmitted infections—management and treatment of specific infections—gonococcal infections. Public Health Agency of Canada, Ottawa, ON, Canada. http://www.phac-aspc.gc.ca/std-mts/sti-its/cgsti-ldcits/section-5-6-eng.php Accessed 7 July 2016.
- 18.Allan-Blitz LT, Humphries RM, Hemarajata P, Bhatti A, Pandori MW, Siedner MJ, Klausner JD. 2017. Implementation of a rapid genotypic assay to promote targeted ciprofloxacin therapy of Neisseria gonorrhoeae in a large health system. Clin Infect Dis 64:1268–1270. doi: 10.1093/cid/ciw864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belland RJ, Morrison SG, Ison C, Huang WM. 1994. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone resistant isolates. Mol Microbiol 14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 20.Giles JA, Falconio J, Yuenger JD, Zenilman JM, Dan M, Bash MC. 2004. Quinolone resistance-determining region mutations and por type of Neisseria gonorrhoeae isolates: resistance surveillance and typing by molecular methodologies. J Infect Dis 189:2085–2093. doi: 10.1086/386312. [DOI] [PubMed] [Google Scholar]
- 21.Zhao LH, Zhao SP. 2012. TaqMan real-time quantitative PCR assay for the detection of fluoroquinolone-resistant Neisseria gonorrhoeae. Curr Microbiol 65:692–695. doi: 10.1007/s00284-012-0212-6. [DOI] [PubMed] [Google Scholar]
- 22.Siedner MJ, Pandori M, Castro L, Barry P, Whittington WLH, Liska S, Klausner JD. 2007. Real-time PCR assay for detection of quinolone-resistant Neisseria gonorrhoeae in urine samples. J Clin Microbiol 45(4):1250–1254. doi: 10.1128/JCM.01909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trembizki E, Guy R, Donovan B, Kaldor JM, Lahra MM, Whiley DM. 2016. further evidence to support the individualised treatment of gonorrhoeae with ciprofloxacin. Lancet Infect Dis 16(9):1005–1006. doi: 10.1016/S1473-3099(16)30271-7. [DOI] [PubMed] [Google Scholar]
- 24.Li Z, Yokoi S, Kawamura Y, Maeda S, Ezaki T, Deguchi T. 2002. Rapid detection of quinolone resistance-associated gyrA mutations in Neisseria gonorrhoeae with a LightCycler. J Infect Chemother 8:145–150. doi: 10.1007/s101560200025. [DOI] [PubMed] [Google Scholar]
- 25.Lindback E, Gharizadeh B, Ataker F, Airell A, Jalal S, Nyren P, Wretlind B. 2005. DNA gyrase gene in Neisseria gonorrhoeae as indicator for resistance to ciprofloxacin and species verification. Int J STD AIDS 16(2):142–147. doi: 10.1258/0956462053057675. [DOI] [PubMed] [Google Scholar]
- 26.Allan-Blitz LT, Wang X, Klausner J. 2017. wild-type gyrase A genotype of Neisseria gonorrhoeae predicts in vitro susceptibility to ciprofloxacin: a systematic review of the literature and meta-analysis. Sex Transm Dis 44(5):261–265. doi: 10.1097/OLQ.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaydos CA, Van Der Pol B, Jett-Goheen M, Barnes M, Quinn N, Clark C, Daniel GE, Dixon PB, Hook EW. 2013. Performance of the Cepheid CT/NG Xpert Rapid PCR test for detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol 51:1666–1672. doi: 10.1128/JCM.03461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buckley C, Trembizki E, Donovan B, Chen M, Freeman K, Guy R, Kundu R, Lahra MM, Regan DG, Smith H, Whiley DM. 2016. A real-time PCR assay for direct characterization of the Neisseria gonorrhoeae GyrA 91 locus associated with ciprofloxacin susceptibility. J Antimicrob Chemother 71:353–356. doi: 10.1093/jac/dkv366. [DOI] [PubMed] [Google Scholar]
- 29.Watchirs Smith LA, Hillman R, Ward J, Whiley DM, Causer L, Skov S, Donovan B, Kaldor J, Guy R. 2013. Point-of-care tests for the diagnosis of Neisseria gonorrhoeae infection: a systematic review of operational and performance characteristics. Sex Transm Infect 89:320–326. doi: 10.1136/sextrans-2012-050656. [DOI] [PubMed] [Google Scholar]
- 30.Herbst de Cortina S, Bristow CC, Davey DJ, Klausner JD. 2016. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis. Infect Dis Obstet Gynecol 2016:4386127. doi: 10.1155/2016/4386127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vidovic S, Caron C, Taheri A, Thakur SD, Read TD, Kusalik A, Dillon JA. 2014. Using crude whole-genome assemblies of Neisseria gonorrhoeae as a platform for strain analysis: clonal spread of gonorrhea infection in Saskatchewan, Canada. J Clin Microbiol 52:3772–3776. doi: 10.1128/JCM.01502-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidovic S, Horsman GB, Liao M, Dillon JR. 2011. Influence of conserved and hypervariable genetic markers on genotyping circulating strains of Neisseria gonorrhoeae. PLoS One 6:e28259. doi: 10.1371/journal.pone.0028259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao M, Helgeson S, Gu WM, Yang Y, Jolly AM, Dillon JR. 2009. Comparison of Neisseria gonorrhoeae multiantigen sequence typing and porB sequence analysis for identification of clusters of N. gonorrhoeae isolates. J Clin Microbiol 47(2):489–491. doi: 10.1128/JCM.01612-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unemo M, Fasth O, Fredlund H, Limnios A, Tapsall J. 2009. Phenotypic and genetic characterization of the 2008 WHO Neisseria gonorrhoeae reference strain panel intended for global quality assurance and quality control of gonococcal antimicrobial resistance surveillance for public health purposes. J Antimicrob Chemother 63:1142–1151. doi: 10.1093/jac/dkp098. [DOI] [PubMed] [Google Scholar]
- 35.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing, 27th ed CLSI supplement M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 36.Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. 2012. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 2012:134. doi: 10.1186/1471-2105-13-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lalkhen AG, McCluskey A. 2008. Clinical tests: sensitivity and specificity. Contin Educ Anaesth Crit Care Pain 8:221–223. doi: 10.1093/bjaceaccp/mkn041. [DOI] [Google Scholar]


