ABSTRACT
The purpose of this study was to perform a multisite evaluation to establish the performance characteristics of the BD Max extended enteric bacterial panel (xEBP) assay directly from unpreserved or Cary-Blair-preserved stool specimens for the detection of Yersinia enterocolitica, enterotoxigenic Escherichia coli (ETEC), Vibrio, and Plesiomonas shigelloides. The study included prospective, retrospective, and prepared contrived specimens from 6 clinical sites. BD Max xEBP results were compared to the reference method, which included standard culture techniques coupled with alternate PCR and sequencing, except for ETEC, for which the reference method was two alternate PCRs and sequencing. Alternate PCR was also used to confirm the historical results for the retrospective specimens and for discrepant result analysis. A total of 2,410 unformed, deidentified stool specimens were collected. The prevalence in the prospective samples as defined by the reference method was 1.2% ETEC, 0.1% Vibrio, 0% Y. enterocolitica, and 0% P. shigelloides. Compared to the reference method, the positive percent agreement (PPA) (95% confidence interval [CI]), negative percent agreement (NPA) (95% CI), and kappa coefficient (95% CI) for the BD Max xEBP assay for all specimens combined were as follows: ETEC, 97.6% (87.4 to 99.6), 99.8% (99.5 to 99.9), and 0.93 (0.87 to 0.99); Vibrio, 100% (96.4 to 100), 99.7% (99.4 to 99.8), and 0.96 (0.93 to 0.99); Y. enterocolitica, 99.0% (94.8 to 99.8), 99.9% (99.8 to 99.9), and 0.99 (0.98 to 1); P. shigelloides, 100% (96.4 to 100), 99.8% (99.5 to 99.9), and 0.98 (0.95 to 1), respectively. In this multicenter study, the BD Max xEBP showed a high correlation (kappa, 0.97; 95% CI, 0.95 to 0.98) with the conventional methods for the detection of ETEC, Vibrio, Y. enterocolitica, and P. shigelloides in stool specimens from patients suspected of acute gastroenteritis, enteritis, or colitis.
KEYWORDS: BD Max, extended enteric bacterial panel, gastrointestinal panel, multiplex PCR, enteric pathogens, Yersinia enterocolitica, enterotoxigenic Escherichia coli, Vibrio, Plesiomonas shigelloides
INTRODUCTION
Diarrheal syndrome-based, gastrointestinal (GI) molecular panels are becoming a popular alternative to traditional microscopy, culture, and antigen detection methods for the detection of enteric pathogens (EPs). These multiplex molecular panels have the advantage of increased sensitivity with reduced turnaround time and improved detection of mixed infections in comparison to traditional methods (1, 2). Ultimately, this methodology allows for more rapid diagnosis and decisions regarding treatment and infection control measures.
Two approaches to multiplex syndromic panels have been observed. The first approach is to have a comprehensive panel that covers all potential enteric pathogens, including bacteria, viruses, and/or parasites and/or Clostridium difficile targets within a single panel. Examples of these include the BioFire FilmArray gastrointestinal (GI) panel (BioFire Diagnostics, Inc., Salt Lake City, UT), the Luminex Nanosphere Verigene enteric pathogen (EP) panel (Luminex Corporation, Toronto, ON, Canada), and the Luminex xTAG gastrointestinal pathogen panel (GPP). The second approach is to have smaller molecular panels that target microorganism-specific groups, i.e., a bacterial panel, a parasite panel, a viral panel, and a C. difficile assay. The BD Max system (BD Diagnostics, Sparks, MD, USA) uses the latter for a more targeted approach.
The BD Max system is a fully automated PCR instrument with sample-to-result capability. In 2014, the BD Max enteric bacterial panel (EBP) for the detection of Salmonella spp., Shigella spp., Campylobacter jejuni/coli, and Shiga toxin genes (stx1 and stx2) was U.S. FDA cleared, European CE marked, and Health Canada IVD approved (3). The panel allowed for the partial transition of a bacterial culture-specific stool bench to a less labor-intensive molecular approach. However, the panel did not cover the full spectrum of enteric bacterial pathogens. Thus, the BD Max extended enteric bacterial panel (xEBP) was created to be used in conjunction with the BD Max EBP assay as an optional master mix addition to simultaneously detect Yersinia enterocolitica, enterotoxigenic Escherichia coli (ETEC), Vibrio (Vibrio parahaemolyticus, Vibrio cholerae, and Vibrio vulnificus), and Plesiomonas shigelloides.
The purpose of this study was to perform a large, multisite evaluation to establish the performance characteristics of the BD Max xEBP assay for the direct, qualitative presence of Y. enterocolitica, ETEC, Vibrio, and P. shigelloides directly from unpreserved or Cary-Blair-preserved stool specimens in comparison to the reference methods.
RESULTS
Demographic data.
A total of 2,410 unformed, deidentified stool specimens were evaluated, including 2,264 (93.9%) prospective fresh/frozen and 146 retrospective (6.1%) specimens. The majority (55.9%) of specimens were collected in the outpatient setting with the remaining 31.0% from the inpatient setting, 9.5% from emergency departments, and 0.1% from long-term-care facilities. The specimens were evenly distributed between patients aged ≤18 years (42.6%) and ≥19 years (56.6%) and between males (48%) and females (51%). For some specimens, demographic information was unknown.
Prospective specimens.
A total of 2,264 prospective specimens including 1,382 (61.0%) Cary-Blair-preserved and 882 (39.0%) unpreserved specimens were collected. These specimens included 853 (37.7%) prospective fresh specimens and 1,411 (62.3%) prospective frozen specimens. The results of the BD Max xEBP assay for prospective fresh and prospective frozen specimens compared to those of culture and ETEC PCRs (reference method) are summarized in Table 1 for each target. There were no statistically significant differences for the positive percent agreement (PPA) and negative percent agreement (NPA) between prospective fresh and prospective frozen results for each target (when applicable for PPA), nor were there any statistically significant differences between Cary-Blair-preserved and unpreserved specimens observed (Table 2). The prevalence in the prospective samples as defined by the reference method was 1.2% (26/2,218) ETEC, 0.1% (2/2,250) Vibrio, 0% Y. enterocolitica, and 0% P. shigelloides.
TABLE 1.
Performance of the BD Max xEBP by specimen origin
| Organism and specimen type | BD Max xEBP performance characteristic compared to the reference methodc,g |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. of specimens with result: |
PPA (95% CI) | NPA (95% CI) | Kappaf (95% CI) | |||||
| True pos | False neg | False pos | True neg | Totalb | ||||
| Vibrio | ||||||||
| Prospective fresh | 0 | 0 | 1 | 837 | 838 | No data | 99.9 (99.3–100) | |
| Prospective frozen | 2 | 0 | 6 | 1,380 | 1,388 | 100 (32.4–100) | 99.6 (99.1–99.8) | |
| Retrospectivea | 4 | 0 | 1 | 61 | 66 | 100 (51.0–100) | 98.4 (91.4–99.7) | |
| Contrived | 96 | 0 | 0 | 288 | 384 | 100 (95.2–100) | 100 (98.4–100) | |
| Total | 102 | 0 | 8 | 2,566 | 2,676 | 100 (96.4–100) | 99.7 (99.4–99.8) | 0.96 (0.93–0.99) |
| Plesiomonas shigelloides | ||||||||
| Prospective fresh | 0 | 0 | 0 | 834 | 834 | No data | 100 (99.5–100) | |
| Prospective frozen | 0 | 0 | 3 | 1,384 | 1,387 | No data | 99.8 (99.4–99.9) | |
| Retrospectivea | 7 | 0 | 1 | 84 | 92 | 100 (64.6–100) | 98.8 (93.6–99.8) | |
| Contrived | 96 | 0 | 1d | 287 | 384 | 100 (96.2–100) | 99.7 (98.1–99.9) | |
| Total | 103 | 0 | 5 | 2,589 | 2,697 | 100 (96.4–100) | 99.8 (99.5–99.9) | 0.98 (0.95–1) |
| Yersinia enterocolitica | ||||||||
| Prospective fresh | 0 | 0 | 0 | 823 | 823 | No data | 100 (99.5–100) | |
| Prospective frozen | 0 | 0 | 1 | 1,381 | 1,382 | No data | 99.9 (99.6–100) | |
| Retrospectivea | 9 | 0 | 0 | 79 | 88 | 100 (70.1–100) | 100 (95.4–100) | |
| Contrived | 95 | 1e | 0 | 287 | 384 | 99.0 (94.3–99.8) | 99.7 (97.8–99.9) | |
| Total | 104 | 1 | 1 | 2,570 | 2,676 | 99.0 (94.8–99.8) | 99.96 (99.8–1) | 0.99 (0.98–1) |
| ETEC | ||||||||
| Prospective fresh | 9 | 0 | 0 | 821 | 830 | 100 (70.1–100) | 100 (99.5–100) | |
| Prospective frozen | 17 | 0 | 4 | 1,345 | 1,366 | 100 (81.6–100) | 99.7 (99.2–99.9) | |
| Retrospectivea | 14 | 1 | 1 | 54 | 70 | 93.3 (70.2–98.8) | 98.2 (90.4–99.7) | |
| Total | 40 | 1 | 5 | 2,220 | 2,266 | 97.6 (87.4–99.6) | 99.8 (99.5–99.9) | 0.93 (0.87–0.99) |
Results of all retrospective samples were confirmed by alternate PCR and bidirectional sequencing prior to testing with the BD Max xEBP.
Number of specimens varies by target due to differences in compliance with study protocol and unresolved results.
Abbreviations: pos, positive; neg, negative; PPA, positive percent agreement; NPA, negative percent agreement; CI, confidence interval.
One false-positive result occurred initially for P. shigelloides from an unpreserved specimen at 4 times the LoD but was found negative upon repeat testing from the SBT.
One false-negative result was observed for Y. enterocolitica among a Cary-Blair-preserved specimen at 4 times the LoD but was positive upon repeat testing from the SBT.
Kappa coefficient, correlation of BD Max result with reference methods.
Reference methods were standard culture techniques and/or alternate PCR followed by sequencing.
TABLE 2.
Performance of the BD Max xEBP by specimen type
| Organism and specimen type | BD Max xEBP performance characteristics compared to the reference methodb,f |
||||||
|---|---|---|---|---|---|---|---|
| No. of specimens with result: |
PPA (95% CI) | NPA (95% CI) | |||||
| True pos | False neg | False pos | True neg | Totala | |||
| Vibrio | |||||||
| Clinical specimensc | |||||||
| Cary-Blair preserved | 4 | 0 | 5 | 1,367 | 1,376 | 100 (51–100) | 99.6 (99.1–99.8) |
| Unpreserved | 2 | 0 | 3 | 911 | 916 | 100 (34.2–100) | 99.7 (99–99.9) |
| Contrived specimens | |||||||
| Cary-Blair preserved | 48 | 0 | 0 | 144 | 192 | 100 (92.6–100) | 100 (97.4–100) |
| Unpreserved | 48 | 0 | 0 | 144 | 192 | 100 (92.6–100) | 100 (97.4–100) |
| Total | 102 | 0 | 8 | 2,566 | 2,676 | 100 (95.4–100) | 99.7 (99.4–99.9) |
| Plesiomonas shigelloides | |||||||
| Clinical specimensc | |||||||
| Cary-Blair preserved | 4 | 0 | 2 | 1,393 | 1,399 | 100 (51–100) | 99.9 (99.5–100) |
| Unpreserved | 3 | 0 | 2 | 909 | 914 | 100 (43.9–100) | 99.8 (99.2–99.9) |
| Contrived specimens | |||||||
| Cary-Blair preserved | 48 | 0 | 0 | 144 | 192 | 100 (92.6–100) | 100 (97.4–100) |
| Unpreserved | 48 | 0 | 1d | 143 | 192 | 100 (92.6–100) | 99.3 (96.2–99.9) |
| Total | 103 | 0 | 5 | 2,589 | 2,697 | 100 (95.5–100) | 99.8 (99.5–99.9) |
| Yersinia enterocolitica | |||||||
| Clinical specimensc | |||||||
| Cary-Blair preserved | 0 | 0 | 1 | 1,373 | 1,374 | No data | 99.9 (99.6–100) |
| Unpreserved | 9 | 0 | 0 | 910 | 919 | 100 (70.1–100) | 100 (99.6–100) |
| Contrived specimens | |||||||
| Cary-Blair preserved | 47 | 1e | 0 | 144 | 192 | 97.9 (89.1–99.6) | 100 (97.4–100) |
| Unpreserved | 48 | 0 | 0 | 144 | 192 | 100 (92.6–100) | 100 (97.4–100) |
| Total | 104 | 1 | 1 | 2,570 | 2,676 | 99.0 (94.0–99.9) | 99.9 (99.7–99.9) |
| ETEC (clinical specimensc) | |||||||
| Cary-Blair preserved | 15 | 0 | 3 | 1,376 | 1,394 | 100 (79.6–100) | 99.8 (99.4–99.9) |
| Unpreserved | 25 | 1 | 2 | 844 | 872 | 96.2 (81.1–99.3) | 99.8 (99.1–99.9) |
| Total | 40 | 1 | 5 | 2,220 | 2,266 | 97.6 (85.6–99.9) | 99.8 (99.4–99.9) |
Number of specimens varies by target due to differences in compliance with protocol and unresolved results.
Abbreviations: pos, positive; neg, negative; PPA, positive percent agreement; NPA, negative percent agreement; CI, confidence interval.
Clinical specimens include the prospective fresh, prospective frozen, and retrospective specimens.
The sample was initially positive for P. shigelloides but was negative on repeat testing from the SBT.
The sample was initially negative for Y. enterocolitica but was positive on repeat testing from the SBT.
Reference methods were standard culture techniques and/or alternate PCR followed by sequencing.
Retrospective specimens.
Overall, a total of 146 retrospective specimens were enrolled and included 59 (40.4%) Cary-Blair-preserved and 87 (59.6%) unpreserved specimen. The results of the BD Max xEBP assay for retrospective specimens compared to an alternate PCR and bidirectional sequencing for each target are summarized in Table 1. Similarly to the prospective specimens, there was no statistically significant difference for the PPA and NPA between Cary-Blair-preserved and unpreserved specimens (Table 2).
Contrived specimens.
The contrived specimen results for Y. enterocolitica, Vibrio, and P. shigelloides are summarized in Table 1 and further divided by specimen type (Cary-Blair versus unpreserved) in Table 2. Two discrepant results were observed. One false-positive result occurred initially for P. shigelloides from an unpreserved specimen at 4 times the limit of detection (LoD) but was found negative upon repeat testing from the sample buffer tube (SBT). One false-negative result was observed for Y. enterocolitica among a Cary-Blair-preserved specimen at 4 times the LoD but was positive upon repeat testing from the SBT.
Discrepant results.
Discrepant results are summarized in Table 3. Overall, 19 (0.8%) discrepant results were observed including 8 (42.1%) Vibrio results, 6 (31.6%) ETEC results, 4 (21.1%) P. shigelloides results, and 1 (5.3%) Y. enterocolitica result. Overall, 18/19 (94.7%) were false-positive (FP) results with threshold cycle (CT) values greater than 32 (except 3 positive Vibrio results with CT values of 20.9, 22.2, and 31.9; CT values are not available to the end user). A single false-negative (FN) result occurred for a retrospective unpreserved specimen positive for ETEC. Discrepant results occurred in both prospective and retrospective specimens and among both Cary-Blair-preserved and unpreserved specimens. Three discrepant retrospective results were not available for discrepant analysis due to limited specimen volume (one ETEC FN, one ETEC FP, and one Vibrio FP). Of the 16 FP discrepant specimens tested by discrepant analyses, there were 7 Vibrio, 4 P. shigelloides, 4 ETEC, and 1 Y. enterocolitica result. Three of 4 FP P. shigelloides results were also positive for a second target on the BD Max (1 ETEC, 1 Y. enterocolitica, and 1 Vibrio).
TABLE 3.
BD Max discrepant results for prospective and retrospective specimens
| Target | No. (%) of discrepant results (n = 19) | Specimen originb | Specimen type | Type of discrepancy | Discrepant analysis resulta |
|---|---|---|---|---|---|
| Vibrio | 8 (42.1) | 7 prospective, 1 retrospective | 5 Cary-Blair preserved, 3 unpreserved | False positive | 5/8 were negative by both xEBP and alternate PCR, 2/8 were repeat positive by the xEBP and negative by alternate PCR, 1/8 was unavailable for retesting |
| Plesiomonas shigelloides | 4 (21.1) | 3 prospective, 1 retrospective | 2 Cary-Blair preserved, 2 unpreserved | False positive | 1/4 was negative by both xEBP and alternate PCR, 3/4 were repeat positive by the xEBP and negative by alternate PCR |
| Yersinia enterocolitica | 1 (5.3) | 1 prospective | 1 Cary-Blair preserved | False positive | 1/1 was negative by both xEBP and alternate PCR |
| ETEC | 6 (31.6) | 4 prospective, 2 retrospective | 3 Cary-Blair preserved, 3 unpreserved | 5 false positive, 1 false negative | 1/6 was negative by both xEBP and alternate PCR, 1/6 was repeat positive by the xEBP and negative by alternate PCR, 2/6 were repeat positive by the xEBP and positive by alternate PCR and sequencing, 2/6 were unavailable for retesting |
Discrepant analysis included repeat testing on the xEBP assay and an alternate PCR followed by bidirectional sequencing.
Three discrepant retrospective results were not available for discrepant analysis due to limited specimen volume (one ETEC false negative, one ETEC false positive, and one Vibrio false positive).
Unresolved results.
Unresolved results due to failure of the internal control could be caused by inhibitory substances in the stool specimens or reagent or instrument failure. For Cary-Blair-preserved specimens, the unresolved rate was 2.4%, and for unpreserved specimens, the unresolved rate was 2.2%. After repeat testing, the unresolved rate fell below 0.3% for both specimen types.
DISCUSSION
The fully automated BD Max system takes a microorganism group-specific approach to the detection of gastrointestinal pathogens. Currently, there are four panels that are FDA cleared for the BD Max system: a C. difficile toxin B gene assay, an enteric bacterial panel (EBP), an enteric parasite panel (EPP), and last, the most recent panel to receive FDA clearance, the extended EBP (xEBP) that was evaluated in this multicenter study (3, 4). To cover the full spectrum of enteric pathogens on the BD Max system, an enteric virus panel (EVP) is currently undergoing FDA clinical trials and should be available soon.
Syndrome-based GI panels cast a broad net for clinically indistinguishable diseases to ultimately help achieve a more timely diagnosis. One study evaluating the xTAG GPP assay found that physician ordering practices missed up to 65% of pathogens detected by the panel (5). However, some argue that these comprehensive panels should be restricted for use in only certain patient populations such as the critically ill, immunocompromised hosts, patients with a travel history, and patients with prolonged diarrhea (1, 3). The BD Max microorganism group-specific panel-based method provides a happy medium between the comprehensive panels and traditional techniques. The BD Max panels allow for a tailored approach to GI pathogen test ordering and detection, enabling clinicians to order based on the patient's risk factors, such as community-acquired versus hospital-acquired diarrhea, an immunocompetent versus immunocompromised host, and/or pediatric versus adult patients (6). Furthermore, the expense of smaller, focused panels is lower than that of broad, comprehensive panels, and thus, the costs are more likely to be reimbursed by insurance companies (1).
The BD Max xEBP evaluated in this study was designed to be used in conjunction with the BD Max EBP assay as an optional master mix addition to simultaneously detect Y. enterocolitica, ETEC, Vibrio, and P. shigelloides. The addition of the xEBP master mix to the EBP allows for the complete transition of a bacterial culture-specific stool bench to a less labor-intensive molecular approach (7). That being said, there is one bacterial enteric pathogen not included in the panels—Aeromonas species. Thus, laboratories that convert completely to a molecular biology-based approach using the BD Max system for the detection of bacterial enteric pathogens must decide if they are still going to offer Aeromonas species culture, as Aeromonas is known to be an enteric pathogen in both pediatric and adult populations (8, 9). However, the detection of Aeromonas in fecal specimens should be interpreted with caution as it may not always be associated with disease and has been found among healthy patient controls (10, 11). One additional disadvantage of moving to a molecular biology-based approach is increased laboratory expenses. However, these expenses might be overcome by decreasing technologist hands-on time (2) or by net cost savings for the hospital by shortening patient isolation days due to more rapid results than conventional methods, as demonstrated in a recent study (12).
The prevalences of ETEC, Vibrio, Y. enterocolitica, and P. shigelloides in the prospective samples were 1.2%, 0.1%, 0%, and 0%, respectively. Although it is well documented that these pathogens are less likely to be encountered (with the exception of ETEC), the prevalences of Y. enterocolitica and P. shigelloides in this multicenter study were surprisingly low. These observations are similar, however, to a recent multicenter trial evaluating the FilmArray GI panel in the United States that found the prevalence of ETEC, P. shigelloides, Vibrio, and Y. enterocolitica to be 1.4% (22/1,556), 0.2% (3/1,556), 0% (0/1,556), and 0.1% (1/1,556), respectively (13). Seasonality was considered an explanation for the low prevalence of targets; however, study enrollment spanned a calendar year and removed this as a variable. Our result for ETEC was also consistent with that of a recent U.S. study describing a prevalence of 1% (5). Interestingly, ETEC was the sixth most commonly encountered GI pathogen in two recent studies evaluating multiplex molecular GI panels from the United States (1%) and Europe (4.23%) (6, 14).
Due to the low prevalence of pathogens in the prospective arm (with the exception of ETEC), both retrospective and contrived samples were included in the study. Compared to the reference method, the positive percent agreement, negative percent agreement, and kappa coefficient for the BD Max xEBP assay for all specimens combined and for all targets were ≥97.6%, ≥99.7%, and 0.97, respectively. These results are similar to those reported by the multicenter clinical trial for the FilmArray gastrointestinal panel (13). In contrast, a multicenter study evaluating the xTAG GPP demonstrated a sensitivity of 0% (0/2 specimens) for detection of Yersinia enterocolitica and was not able to assess the sensitivity for ETEC and V. cholerae due to the lack of positive specimens (5). A subsequent study, comparing the FilmArray GI panel to the xTAG GPP, further confirmed the poor sensitivity of the xTAG GPP for the detection of Yersinia enterocolitica, detecting less than half of previously positive stool samples (13/27; 48.1%) which were all detected by the FilmArray GI panel (14). Overall, the sensitivity of these multiplex panels for the detection of the enteric organisms covered by the xEBP assay from prospective stool specimens has not been well defined due to the low prevalence of these pathogens in developed countries.
Nineteen (0.8%) discrepant results were observed in this study, of which the majority (18/19) were false-positive results and mostly occurred in prospective frozen samples (16/18). Two of the false-positive ETEC results were confirmed by discordant analysis. The remainder were not confirmed by alternate PCR. Despite these false-positive results, the specificities for these targets were ≥99.7% in the prospective cohort. These false-positive results could be due to cross-reactivity of primers and probes with other off-panel targets. For example, a few false-positive results for ETEC by the FilmArray study were attributed to cross-reactivity with Citrobacter koseri and Hafnia alvei in the specimens (13). However, analytical specificity studies revealed that of 184 organisms tested for cross-reactivity, only two strains of Vibrio mimicus were found to cross-react with the Vibrio target on the xEBP assay (data not presented). Laboratory contamination is another possible cause of the false-positive results, even though environmental testing was performed at each site to assess and control contamination weekly during testing. Finally, the limit of detection of the BD Max assay could potentially be lower than those of the reference and discrepant analysis methods. As with all diagnostic assays, positive predictive value is a function of the disease prevalence; therefore, when the prevalence is low, the predictive value is expected to be lower. Therefore, in settings where disease prevalence is very low, laboratories may consider the possibility of repeat testing or confirmation of the test result by another method.
The unresolved rates among preserved (2.4%) and unpreserved (2.2%) specimens in this study were similar. This is in contrast to what was observed in the BD Max EBP multisite evaluation, where there was a higher unresolved rate among unpreserved specimens (7.1% compared to 3.7% for preserved specimens). It was thought that dilution of the stool in Cary-Blair medium reduced the effects of inhibitory substances (3). No changes in the extraction or internal controls were made between the EBP and xEBP assays to account for the lower unresolved rates between the two specimen types. However, changes were made to the pipetting/mixing protocol on the BD Max system that have significantly reduced the occurrence of bubbles in the PCR cartridge, consequently reducing the unresolved rate.
A limitation of this study is the low number of positives for Vibrio, Y. enterocolitica, and P. shigelloides in the prospectively collected cohort, requiring the use of both retrospective and contrived specimens to determine the sensitivity for detection of these targets. Strengths of this study include the multicenter analysis including sites in the United States and Canada, the large number of stool specimens enrolled, and the inclusion of both Cary-Blair-preserved and unpreserved specimens.
In conclusion, in this large, multicenter study, the BD Max xEBP showed a very high correlation with conventional and molecular methods for the detection of ETEC, Vibrio, Y. enterocolitica, and P. shigelloides in stool specimens of patients suspected of or having acute gastroenteritis, enteritis, or colitis.
MATERIALS AND METHODS
Study design and specimen types.
The study was conducted between June 2015 and May 2016 and included 6 clinical sites. Five sites were located in the United States, and one was in Canada. Overall, 2,410 unformed, deidentified stool specimens were obtained from unique pediatric or adult patients suspected of or having acute gastroenteritis, enteritis, or colitis. Acceptable specimens included unpreserved stool specimens and Cary-Blair-preserved stool specimens. This study included prospectively collected fresh specimens (prospective fresh) as well as specimens collected earlier in the season (June to December 2015) and frozen (≤−70°C) prior to testing on the BD Max system (prospective frozen). The prospective frozen specimens were tested at the clinical trial sites within the stability period as established by the manufacturer for the xEBP assay (9 months) and reference methods (9 to 10 months depending on the target). The vast majority of these specimens were remnant specimens from patients suspected of acute gastroenteritis, enteritis, or colitis. For one site, three stool specimens were also collected under informed consent for the purpose of this study. Considering the low prevalence of the assay targets, retrospectively archived specimens (including specimens from one site in Uganda) and a contrived specimen study were included to supplement the number of positives.
Contrived specimens.
The contrived specimen study was performed to supplement the number of positive results for Y. enterocolitica (12 strains), Vibrio (V. parahaemolyticus, V. vulnificus, and V. cholerae; 4 strains each of 3 species), and P. shigelloides (12 strains), for both unpreserved and Cary-Blair-preserved stools (15). Overall, a total of 384 contrived samples were tested, 128 at each of three testing sites. Each strain was tested at 2 times, 4 times, 5 to 10 times, 15 to 25 times, and 500 to 1,000 times the limit of detection (LoD). For information on the LoD for each target, we refer readers to the BD Max xEBP package insert (15).
Reference methods for prospective specimens.
The prospective specimens were cultured within 96 h of collection for Cary-Blair-preserved specimens and 24 h for unpreserved specimens. Clinical and Laboratory Improvement Amendment (CLIA)-compliant culture methods for standard patient care were used at each site, and appropriate quality control was documented according to the Clinical and Laboratory Standards Institute (CLSI) M22-A3 guidelines. Each site had to pass a culture proficiency panel prior to culture enrollment. Stools were cultured directly using cefsulodin (15 mg)-Irgasan-novobiocin (CIN; BD BBL prepared medium; Sparks, MD), thiosulfate-citrate-bile salts-sucrose agar (TCBS; BD BBL prepared medium), and Trypticase soy agar with 5% sheep blood (blood agar; BD BBL prepared medium). Culture combined with standard identification laboratory practice (visual inspection and oxidase testing) was used for Y. enterocolitica, the Vibrio group, and P. shigelloides. An additional characterization (e.g., validated alternate PCR and bidirectional sequencing) was also performed on all presumptive positive isolates. For ETEC, the xEBP results were compared to two sets of validated alternate PCRs (a total of six PCRs for three toxins) performed directly from the stool, followed by bidirectional sequencing of the amplicon from PCR set 1 only. Heat-labile toxin (LT), heat-stable porcine-type variant toxin (STp), and heat-stable human-type variant toxin (STh) were the three toxins detected. The same proprietary alternate PCRs that were used to confirm prospective results were also used to confirm the presence of targets in the retrospective specimens and to assess discrepant results, as described below.
Reference method for retrospective specimens.
Historical results obtained from standard routine methods were used as the first portion of a composite reference method. In addition, all specimens underwent testing with one alternate PCR (one set of PCRs for ETEC with one PCR for each toxin type) followed by a bidirectional sequencing method to confirm historical routine testing results as targets may have degraded during storage (4). Only specimens with at least 1 ml of remaining volume for which historical results had been confirmed with the alternate PCR and bidirectional sequencing were included in the retrospective study.
BD Max testing.
Prospective fresh specimens were tested within 120 h of collection if stored at 4°C and 48 h if kept at room temperature. The prospective frozen specimens were tested within 9 months from collection. When thawed, prospective frozen and retrospective specimens were tested within the same time frame as those required for prospective fresh specimens as described above. Each site was required to pass a BD Max xEBP proficiency panel prior to testing specimens on the BD Max system. The BD Max xEBP assay was performed according to the investigational-use-only (IUO) package insert. The setup of the xEBP assay is identical to that of the FDA-cleared BD Max EBP assay with the exception of adding the additional xEBP master mix tube to the four-snap EBP unitized reagent strip (URS) (Fig. 1). Briefly, 10 μl of homogenized stool specimen was transferred each into a sample buffer tube (SBT) by use of a calibrated loop and vortexed. Then, the URS was placed onto a BD Max rack along with the extraction tubes and the EBP and xEBP master mix tubes by simply snapping them into the individual URS for each specimen. Last, the SBTs were loaded on the BD Max rack with the URS and reagents and the rack was placed onto the BD Max system. The BD Max system automates the extraction, real-time TaqMan-based PCR amplification, fluorophore-labeled probe detection, and automatic result interpretation at each PCR cycle. Proprietary targets and primers included in the BD Max xEBP assay detect Y. enterocolitica, Vibrio (V. cholerae, V. vulnificus, and V. parahaemolyticus), and P. shigelloides. Vibrio is reported as a composite positive result and does not differentiate among the three species. In addition, primers and probes detect three toxins produced by ETEC including the heat-labile toxin (LT), the heat-stable porcine-type variant toxin (STp), and the heat-stable human-type variant toxin (STh). Only one of the toxin genes needs to be present to be considered a positive result for ETEC. The total run time for the EBP and xEBP assays together on a single URS is 3.5 h for a batch of 24 samples.
FIG 1.
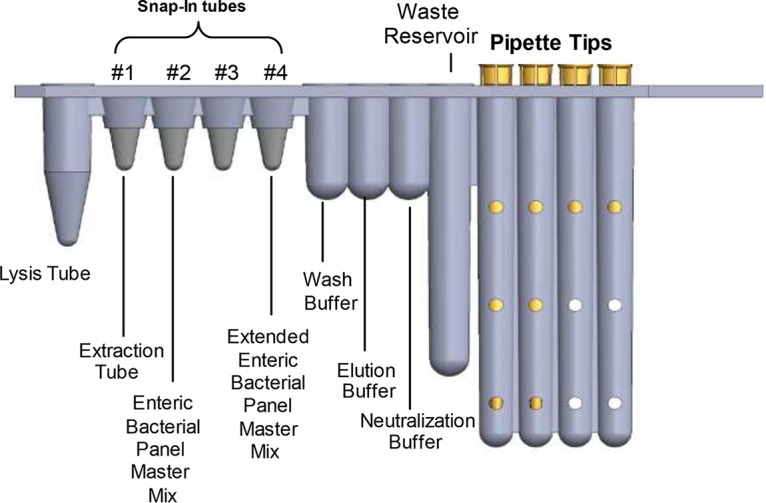
The unitized reagent strip for the BD Max EBP and xEBP assays. The xEBP master mix is added to the unitized reagent strip of the EBP assay. The setup of the xEBP assay is identical to that of the FDA-cleared BD Max EBP assay with the exception of adding the additional xEBP master mix tube to the four-snap EBP unitized reagent strip.
Environmental testing.
Environmental testing was performed prior to the start of the BD Max testing and then regularly until the study concluded; the work area and equipment were monitored for the presence of target DNA contamination. Environmental swab samples were collected and tested with the BD Max EBP and xEBP assays. If contamination occurred, the work area and surfaces were properly decontaminated prior to further specimen testing.
BD Max controls and unresolved results.
A positive and negative external control were included with each run. The positive control was cycled on a daily basis and contained 1.5 × 105 CFU/ml of ATCC 9610 Y. enterocolitica, ATCC 14033 V. cholerae, ATCC 35401 ETEC, or ATCC 14029 P. shigelloides spiked in at 5 times the LoD (15). An internal control was included in each extraction tube to monitor extraction, amplification, and detection steps. If results were not reportable due to lack of amplification of the internal control or either of the external controls, the test was repeated using the initially inoculated SBT within 5 days of inoculation. If the controls failed on repeat, a second SBT was prepared and up to 2 additional extraction and amplification reactions could be performed.
Data analysis.
Results obtained from the BD Max xEBP assay for the prospective and retrospective specimens were compared to those obtained with the reference methods as described above. Results obtained for the contrived specimens were tested by the BD Max xEBP assay and compared to the expected results. The positive percent agreement (PPA) and negative percent agreement (NPA) were calculated with 95% confidence intervals. Prevalence rates for each target were calculated as the number of prospective specimens that tested positive by the reference method divided by the total number of compliant trial specimens.
Discrepant analysis.
Prospective samples with discrepant results between the reference method and the BD Max xEBP assay were retested by the BD Max xEBP and by an alternate PCR directly from the stool followed by bidirectional sequencing of the amplicon. The alternate PCRs used distinct targets for identification of the organisms and targeted different regions of the toxin genes for ETEC than did the BD Max xEBP assay.
ACKNOWLEDGMENTS
We thank the dedicated laboratory professionals across all study sites, without whom this work would have not been possible.
REFERENCES
- 1.Binnicker MJ. 2015. Multiplex molecular panels for diagnosis of gastrointestinal infection: performance, result interpretation, and cost-effectiveness. J Clin Microbiol 53:3723–3728. doi: 10.1128/JCM.02103-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mortensen JE, Ventrola C, Hanna S, Walter A. 2015. Comparison of time-motion analysis of conventional stool culture and the BD MAX Enteric Bacterial Panel (EBP). BMC Clin Pathol 15:9. doi: 10.1186/s12907-015-0010-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrington SM, Buchan BW, Doern C, Fader R, Ferraro MJ, Pillai DR, Rychert J, Doyle L, Lainesse A, Karchmer T, Mortensen JE. 2015. Multicenter evaluation of the BD Max enteric bacterial panel PCR assay for rapid detection of Salmonella spp., Shigella spp., Campylobacter spp. (C. jejuni and C. coli), and Shiga toxin 1 and 2 genes. J Clin Microbiol 53:1639–1647. doi: 10.1128/JCM.03480-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madison-Antenucci S, Relich RF, Doyle L, Espina N, Fuller D, Karchmer T, Lainesse A, Mortensen JE, Pancholi P, Veros W, Harrington SM. 2016. Multicenter evaluation of BD Max enteric parasite real-time PCR assay for detection of Giardia duodenalis, Cryptosporidium hominis, Cryptosporidium parvum, and Entamoeba histolytica. J Clin Microbiol 54:2681–2688. doi: 10.1128/JCM.00765-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. 2013. Performance of the xTAG gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol 23:1041–1045. doi: 10.4014/jmb.1212.12042. [DOI] [PubMed] [Google Scholar]
- 6.Spina A, Kerr KG, Cormican M, Barbut F, Eigentler A, Zerva L, Tassios P, Popescu GA, Rafila A, Eerola E, Batista J, Maass M, Aschbacher R, Olsen KE, Allerberger F. 2015. Spectrum of enteropathogens detected by the FilmArray GI panel in a multicentre study of community-acquired gastroenteritis. Clin Microbiol Infect 21:719–728. doi: 10.1016/j.cmi.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Beal SG, Mesfin M, Ciurca J, Smith G, Gander RM. 2013. Evaluation of costs, technologists time and turn-around times for conventional stool cultures, abstr 113. Abstr 113th Gen Meet Am Soc Microbiol. [Google Scholar]
- 8.George WL, Nakata MM, Thompson J, White ML. 1985. Aeromonas-related diarrhea in adults. Arch Intern Med 145:2207–2211. [PubMed] [Google Scholar]
- 9.Gracey M, Burke V, Robinson J. 1982. Aeromonas-associated gastroenteritis. Lancet ii:1304–1306. [DOI] [PubMed] [Google Scholar]
- 10.Surek M, Vizzotto BS, Souza EM, Pedrosa FDO, Dallagassa CB, Farah SM, Fadel-Picheth CM. 2010. Identification and antimicrobial susceptibility of Aeromonas spp. isolated from stool samples of Brazilian subjects with diarrhoea and healthy controls. J Med Microbiol 59:373–374. doi: 10.1099/jmm.0.014258-0. [DOI] [PubMed] [Google Scholar]
- 11.Tompkins DS, Hudson MJ, Smith HR, Eglin RP, Wheeler JG, Brett MM, Owen RJ, Brazier JS, Cumberland P, King V, Cook PE. 1999. A study of infectious intestinal disease in England: microbiological findings in cases and controls. Commun Dis Public Health 2:108–113. [PubMed] [Google Scholar]
- 12.Goldenberg SD, Bacelar M, Brazier P, Bisnauthsing K, Edgeworth JD. 2015. A cost benefit analysis of the Luminex xTAG gastrointestinal pathogen panel for detection of infectious gastroenteritis in hospitalised patients. J Infect 70:504–511. doi: 10.1016/j.jinf.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Buss SN, Leber A, Chapin K, Fey PD, Bankowski MJ, Jones MK, Rogatcheva M, Kanack KJ, Bourzac KM. 2015. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53:915–925. doi: 10.1128/JCM.02674-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khare R, Espy MJ, Cebelinski E, Boxrud D, Sloan LM, Cunningham SA, Pritt BS, Patel R, Binnicker MJ. 2014. Comparative evaluation of two commercial multiplex panels for detection of gastrointestinal pathogens by use of clinical stool specimens. J Clin Microbiol 52:3667–3673. doi: 10.1128/JCM.01637-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becton, Dickinson and Company. 2017. BD Max extended enteric bacterial package insert, reference 443812. Becton, Dickinson and Company, Sparks, MD. [Google Scholar]


