LETTER
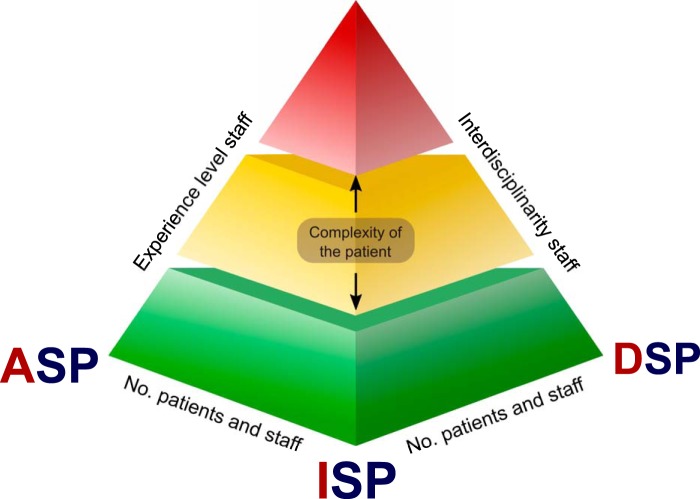
With great interest, we have read the minireview by Messacar et al. (1). The authors state that they “… introduce […] the role of diagnostic and antimicrobial stewardship ….” They emphasize the importance of correct use, timing, and interpretation of molecular diagnostics and the importance of this for antimicrobial stewardship. We could not agree more with this viewpoint and the importance of this topic. However, we are convinced that the focus of stewardship activities should be broader and integrate all areas of infection management: diagnosis, treatment, and prevention of infections. Thus, we proposed the concept of an integrated stewardship model comprising antimicrobial, infection prevention, and diagnostic stewardship, or AID stewardship (Fig. 1) (2).
FIG 1.
Multistakeholder platform of the AID stewardship model. Pyramid platform showing the interdisciplinary stakeholder connections between the antimicrobial stewardship program (ASP), infection prevention stewardship program (ISP), and diagnostic stewardship program (DSP) within the AID stewardship model as published in reference 2. (Republished from reference 2 with permission of the publisher.)
Molecular diagnostics add important rapid tools for detection and identification of microorganisms, including selected resistance determinants important in clinical decision making and infection prevention. They are currently increasingly used in routine diagnostics (3). However, molecular testing (still) lacks important aspects in bacteriology, like antimicrobial susceptibility testing and attributing mobile genetic elements carrying resistance genes to a certain species. Next to rapid and high-quality molecular testing, automation of culture-based diagnostics in bacteriology and mycology, together with identification by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS), has substantially reduced time to result and improved quality and work flow efficiency as well. Diagnostic stewardship should include all relevant modalities for optimal diagnosis of infections: detection and identification (old and new) but also clinical chemistry (leukocytes, C-reactive protein [CRP]/procalcitonin [PCT], etc.), imaging (radiology, nuclear medicine, ultrasound, etc.), and pharmacy/pharmacology (therapeutic drug monitoring, including pharmacokinetic [PK]/pharmacodynamic [PD]-based dosing regimens) where appropriate.
We strongly believe in a broader framework, required for optimization of patient-centered infection management, which also includes infection prevention and/or control besides antimicrobial and diagnostic stewardship. Rapid molecular diagnostics, for example, can play a vital role in hospital infection prevention and control as well as patient management by enabling tailor-made diagnostics and control (4, 5). Reducing the time-to-result can have the effect of shorter isolation of positive patients or even avoiding isolation completely (6). Performing appropriate diagnostics, such as blood cultures, is associated with a reduced length of stay for infectious patients (J. W. Dik, J. R. Lo-Ten-Foe, B. Sinha, P. Nannan Panday, R. Hendrix, M. J. Postma, and A. W. Friedrich, presented at the 25th ECCMID, Copenhagen, Denmark, 25 to 28 April 2015). During an outbreak, the impact of rapid diagnostics can be substantial and directly influence infection control and treatment options as well as having a positive financial effect on health care.
In summary, we are convinced that all three aspects in an integrated AID stewardship model are essential for optimal benefits. This integral view is also supported by a recent review on diagnostic stewardship (10) with the examples of acute respiratory tract infections (7) and quinolone resistance (8). Thus, we strongly advocate the implementation of integrated stewardship programs as a standard approach to optimize patient management and patient care, increase cost-effectiveness, and address the urgent problem of rising antimicrobial resistance (2, 9).
Footnotes
For the author reply, see https://doi.org/10.1128/JCM.01348-17.
REFERENCES
- 1.Messacar K, Parker SK, Todd JK, Dominguez SR. 2017. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol 55:715–723. doi: 10.1128/JCM.02264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dik JW, Poelman R, Friedrich AW, Panday PN, Lo-Ten-Foe JR, van Assen S, van Gemert-Pijnen JE, Niesters HG, Hendrix R, Sinha B. 2016. An integrated stewardship model: antimicrobial, infection prevention and diagnostic (AID). Future Microbiol 11:93–102. doi: 10.2217/fmb.15.99. [DOI] [PubMed] [Google Scholar]
- 3.Bauer KA, West JE, Balada-Llasat JM, Pancholi P, Stevenson KB, Goff DA. 2010. An antimicrobial stewardship program's impact with rapid polymerase chain reaction methicillin-resistant Staphylococcus aureus/S. aureus blood culture test in patients with S. aureus bacteremia. Clin Infect Dis 51:1074–1080. doi: 10.1086/656623. [DOI] [PubMed] [Google Scholar]
- 4.Zhou K, Lokate M, Deurenberg RH, Arends J, Lo-Ten Foe J, Grundmann H, Rossen JW, Friedrich AW. 2015. Characterization of a CTX-M-15 producing Klebsiella pneumoniae outbreak strain assigned to a novel sequence type (1427). Front Microbiol 6:1250. doi: 10.3389/fmicb.2015.01250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deurenberg RH, Bathoorn E, Chlebowicz MA, Couto N, Ferdous M, García-Cobos S, Kooistra-Smid AM, Raangs EC, Rosema S, Veloo AC, Zhou K, Friedrich AW, Rossen JW. 2017. Application of next generation sequencing in clinical microbiology and infection prevention. J Biotechnol 243:16–24. doi: 10.1016/j.jbiotec.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Poelman R, van der Meer J, van Leer-Buter C, Riezebos-Brilman A, Niesters HGM. 2015. Point-of-impact testing in the emergency department: rapid diagnostics for respiratory viral infections. J Clin Virol 70(Suppl 1):S48. doi: 10.1016/j.jcv.2015.07.114. [DOI] [Google Scholar]
- 7.Brink AJ, Van Wyk J, Moodley VM, Corcoran C, Ekermans P, Nutt L, Boyles T, Perovic O, Feldman C, Richards G, Mendelson M. 2016. The role of appropriate diagnostic testing in acute respiratory tract infections: an antibiotic stewardship strategy to minimise diagnostic uncertainty in primary care. S Afr Med J 106(6):30–37. doi: 10.7196/SAMJ.2016.v106i6.10857. [DOI] [PubMed] [Google Scholar]
- 8.Pitiriga V, Vrioni G, Saroglou G, Tsakris A. 2017. The impact of antibiotic stewardship programs in combating quinolone resistance: a systematic review and recommendations for more efficient interventions. Adv Ther 34:854–865. doi: 10.1007/s12325-017-0514-y. [DOI] [PubMed] [Google Scholar]
- 9.Oberjé EJM, Tanke MAC, Jeurissen PPT. 2017. Antimicrobial stewardship initiatives throughout Europe: proven value for money. Infect Dis Rep 9(1):6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morgan DJ, Malani P, Diekema DJ. 2017. Diagnostic Stewardship—leveraging the laboratory to improve antimicrobial use. JAMA 318:607–608. doi: 10.1001/jama.2017.8531. [DOI] [PubMed] [Google Scholar]