Abstract
Objective
Patients with third window syndrome and superior semicircular canal dehiscence (SSCD) symptoms whose surgical outcomes placed them as outliers were systematically studied to determine comorbidities that were responsible for their poor outcomes due to these confounding factors.
Study Design
Observational analytic case‐control study in a tertiary referral center.
Methods
Twelve adult patients with clinical SSCD syndrome underwent surgical management and had outcomes that did not resolve all of their subjective symptoms. In addition to one of the neurotologists, 2 neurologists (one specializing in migraine and the other a neuro‐ophthalmologist), and a psychologist clinician‐investigator completed comprehensive evaluations. Neuropsychology test batteries included: the Millon Behavioral Medicine Diagnostic; Patient Health Questionnaire (PHQ‐9) and Generalized Anxiety Disorder Screener (GAD‐7); Adverse Childhood Experiences Scale; the Wide Range Assessment of Memory and Learning, including the 3 domains of verbal memory, visual memory, and attention/concentration; Wechsler Adult Intelligence Scale; and the Delis‐Kaplan Executive Function System. The control cohort was comprised of 17 participants who previously underwent surgery for third window syndrome that resulted in the expected outcomes of resolution of their third window syndrome symptoms and cognitive dysfunction.
Results
There was a high rate of psychological comorbidity (n = 6) in the outlier cohort; multiple traumatic brain injuries were also a confounding element (n = 10). One patient had elevated cerebrospinal fluid (CSF) pressure requiring ventriculoperitoneal shunting to control the recurrence of dehiscence and one patient with a drug‐induced Parkinson‐like syndrome and idiopathic progressive neurological degenerative process.
Conclusions
Components of the Millon Behavioral Medicine Diagnostic, PHQ‐9 and GAD‐7 results suggest that these instruments would be useful as screening tools preoperatively to identify psychological comorbidities that could confound outcomes. The identification of these comorbid psychological as well as other neurological degenerative disease processes led to alternate clinical management pathways for these patients.
Level of Evidence
2b.
Keywords: Cognitive dysfunction, conversion disorder, CSF, depression, factitious disorder, functional neurological symptom disorder, memory, migraine, otic capsule dehiscence syndrome, perilymph fistula, somatic symptom disorder, superior semicircular canal dehiscence syndrome, third window syndrome, traumatic brain injury
INTRODUCTION
Clinicians managing patients with peripheral vestibular disorders are challenged with signs and symptoms of altered cognitive function, which often introduce difficulties when trying to elicit a cogent history. Cognitive alterations appear to be associated with many vestibular asymmetries1 and in particular with otic capsule defects (third window syndrome [TWS]).2, 3 A quarter century ago, Black et al. reported that the majority of patients with perilymph fistula (PLF) experience altered cognitive status.4 Similar cognitive changes have recently been described in patients with superior semicircular canal dehiscence (SSCD) syndrome (SSCDS) symptoms.2, 3 Video recordings of consenting patients before and after intervention help to further document these obvious alterations in ways that complement standardized neuropsychology testing.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 Other investigators have also explored the relationship between vestibular dysfunction and cognitive dysfunction;19, 20, 21, 22, 23, 24, 25, 26, 27, 28 however, Gizzi and coworkers used the Neurobehavioral Symptom Inventory, and the Beck Depression Inventory and reported no causal connection between vestibular disorders and cognitive dysfunction.28 They studied 200 patients with “dizziness”"–half with a history of brain trauma and half without. They concluded that in patients with postconcussive dizziness, cognitive complaints are likely due to neurologic injury or affective disturbance; and in dizzy patients without brain trauma, cognitive complaints are likely due to concurrent affective disturbance.
The CT positive (CT+) SSCD first described by Minor et al. is well‐recognized;29, 30 however, we recently recognized that a CT negative (CT‐) TWS with exactly the same clinical phenotype also exists.2, 3 We reported a prospective cohort of 12 patients with long‐term follow‐up and with SSCDS, 6 with radiographic evidence (CT+ TWS) of SSCD treated with a middle fossa approach and plugging; and 6 with a CT‐ TWS (no imaging visible otic capsule dehiscence) treated with round window reinforcement (RWR).2 In our 2 publications related to this topic, we included data showing that CT‐ TWS is also associated with a pseudoconductive hearing loss and abnormal cVEMP findings of reduced threshold and increased amplitude.2, 3 Dennis Poe's group included reporting 4 cases of CT‐ TWS among their series of CT+ SSCD who had also had abnormally low cVEMP thresholds.31 Because of this diagnostic dilemma, they did not manage these patients with surgical intervention. Currently they are preparing a report of a CT‐ TWS patient cohort surgically managed with RWR (personal communication, Dr. Dennis S. Poe, March 11, 2017).
We have suggested that the term SSCDS be replaced with otic capsule dehiscence syndrome (OCDS) or third window syndrome (TWS) because SSCD symptoms and diagnostic findings can occur with posterior semicircular canal dehiscence, internal carotid artery‐cochlea dehiscence, posterior semicircular canal‐jugular bulb dehiscence, wide vestibular aqueduct in children (personal communication, Dr. Soumit Dasgupta, March 3, 2017), posttraumatic hypermobile stapes footplate (personal communication, Dr. Arun Gadre, August 1, 2015) and in patients with CT‐ TWS.2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 We have reported the development of CT‐ TWS developing in a delayed manner after surgical plugging and resurfacing of CT+ SSCD TWS.2, 3 John Carey's group has also noted that in a series of near‐SSCD patients undergoing plugging and resurfacing procedures all patients noted initial improvement in at least one presenting TWS symptom; however, 5 subjects (45%) had persistence or recurrence of at least 1 TWS symptom at greater than 1 month after surgery.40 They have also reported their experience with revision surgery for SSCD.41 Of the 222 patients who underwent plugging procedures for SSCD, there were 21 patients who underwent 23 revision surgeries for failure to resolve their TWS symptoms. After revision surgery, TWS symptoms were completely resolved in 8 (35%), partially resolved in 7 (30%), and not resolved in 7 (30%).41 One possible explanation of these findings is that in 14 (61%) of these patients, they also had CT‐ TWS. We have suggested that the modiolus may be one site for a CT‐ TWS,3 and Ilmari Pyykkö's demonstration that intratympanic injection of gadolinium subsequently fills the perilymphatic space in humans and then exits the inner ear via the modiolus and into the internal auditory canal supports this possibility (personal communication, Dr. Ilmari Pyykkö, March 4, 2017). Manzari and Scagnelli reported a patient with bilateral SSCD and bilateral dehiscent modiolus experiencing bilateral TWS; however, the patient was lost to follow‐up before surgical intervention.42
We have used a battery of neuropsychology tests to provide the first quantitative characterization of the preoperative and postoperative cognitive function changes in patients undergoing surgical management of their TWS.3 This systematic study of the cognitive dysfunction and recovery in this cohort of patients revealed statistically significant improvements in several domains and also documented the video descriptions of many of these patients' experiences over time.3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Many of the TWS patients also have experienced traumatic brain injury (TBI) and mild TBI (mTBI), which can also produce cognitive dysfunction and dizziness highlighting the role of this comorbidity.3, 21, 43, 44 TBI and mTBI are a significant health issue which affects service members and veterans during times of both peace and war. The high rate of TBI and blast‐related concussion events resulting from current combat operations directly impacts the health and safety of individual service members and subsequently the level of unit readiness and troop retention. The impacts of TBI are felt within each branch of the service and throughout both the Department of Defense (DoD) and the Department of Veterans Affairs (VA) health care systems. The DoD reports that 361,092 service members have been diagnosed with TBI since 2000.45
The clinical picture of mTBI and TBI is further complicated because the same type of mechanisms producing TBI from blast injuries and head trauma can produce a TWS resulting in inner ear dysfunction.2, 3 These TWS patients experience sound‐induced nausea and dizziness, as well as being able to hear internal sounds unusually well; such as their voice resonating and for some even hearing their eyes move.2, 3 These TWS patients also experience chronic migraine headaches and cognitive dysfunction.2, 3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18
As one of the neurotologists (P.A.W.) and the psychologist (H.T.M‐P.) began seeing a few patients whose outcomes following surgery for TWS were not as expected with resolution of TWS symptoms, migraine headaches, postural dyscontrol, and cognitive dysfunction, we designed a prospective and retrospective study of a consecutive series of these patients using an extensive neuropsychology test battery and comprehensive multidisciplinary clinical evaluations.
Since familiarity with neuropsychology testing may be limited in our academic and clinical communities, a review of the instruments used in this study is included.
NEUROPSYCHOLOGY ASSESSMENT INSTRUMENTS
Millon Behavioral Medicine Diagnostic
The Millon Behavioral Medicine Diagnostic (MBMD) is a 165‐item, self‐report inventory with 29 clinical scales, 3 response pattern scales, 1 validity indicator, and 6 negative health habits indicators.46 It is intended to assess psychological factors that can influence the course of treatment of medically ill patients. “Psychological factors that influence almost every general medical condition includes Axis I disorders, Axis II disorders, psychological symptoms or personality traits that do not meet the full criteria for a specific mental disorder, maladaptive health behaviors, or physiological responses to environmental or social stressors.”46
In statistics and research, internal consistency is a measure based on the correlations between different items on the same test (or the same subscale on a larger test). It measures whether several items that propose to measure the same general construct produce similar scores. Cronbach alpha was used to analyze internal consistency (n = 726).47 This, and test–retest results were obtained over a 7‐ to 30‐day interval for the general medical population. Internal consistency attained a median value of 0.79, and test–retest reached a median value of 0.83, both results demonstrate an acceptable level of item stability. There are 7 domains for the MBMD, each composed of various subscales. Two domains assess response patterns that allude to problematic behavior; the other 5 assess psychiatric elements that may shape the way patients cope with their health issues, as well as highlight characterological (trait) and attitudinal (state) factors that may interfere with their overall prognosis. The 7 domains are: Response Patterns, Negative Health Habits, Psychiatric Indications, Coping Styles, Stress Moderators, Treatment Prognostics, and Management Guides. An interpretive report is provided for clinical psychologists, as well as a one‐page Healthcare Provider Summary composed of assessment findings and treatment recommendations for health care providers. The 7 domains of the MBMD, and 3 of its 29 subscales are outlined below. Scales typically included in analyses reveal prevalence scores equal to or greater than 60; however, for the purposes of this study, we used a cut‐off score of 75 in order to maintain data manageability prior to statistical analysis.
Response Patterns
This scale is comprised of 3 subscales: Disclosure, Desirability, and Debasement, developed to point out distorted response styles and to correct their effects on the instrument's clinical scales. Also, a validity indicator was devised to detect random responses, confusion, and reading difficulties.
Negative Health Habits
Six indicators constitute this domain: Alcohol, Drug, Eating, Caffeine, Inactivity, and Smoking. Information about these negative lifestyle habits are thought to be of considerable utility to health care providers in planning pre‐ and post‐interventions.
Coping Styles
These scales were devised to appraise tendencies that “reflect the cognitive, behavioral, and interpersonal strategies patients use to acquire rewards and to avoid discomfort not only in medical settings, but in other spheres of their lives as well.”46
Coping Styles: Dejected
Those who record a high score on the Dejected scale are inclined to be persistently and characteristically disheartened, unable to experience the pleasures of joys of life. Notably disconsolate and with a somewhat hopeless orientation, they are easily disposed to give up trying to work through their emotional or physical problems. This pessimistic inclination will call for greater effort than usual from health care staff. It should be noted that 20% of general medical patients have Dejected Coping Style.46
Stress Moderators
These are factors that may exacerbate or protect against the impact of stressful events on the psycho‐physiological functioning of patients, as well as the course of their recovery from medical conditions, illness or disease.
Stress Moderators: Functional Deficits versus Functional Competence
This scale assesses the degree to which patients perceive that they are unable to carry out the vocational and avocational activities, roles, and responsibilities of daily life. Like a quality of life indicator, this scale focuses specifically on a patient's sense of loss of independence and freedom to engage in pleasurable, meaningful, and necessary activities. Information from this scale may inform the health care provider of current illness burdens in a patient's life that could act as barriers to treatment adherence and adjustment to stressful medical procedures. It should be noted that 35% of general medical patients exhibit significant reductions in their capacity to carry out life functions as well as they once could.46
Stress Moderators: Pain Sensitivity versus Pain Tolerance
Pain is undoubtedly a very distressing symptom for a significant number of medical patients. It is well known that pain colors a patient's overall outlook and management, as is evident in the increasing number of pain clinics and rehabilitation programs in the country. This scale addresses the tendency to be overly sensitized and reactive to mild/moderate bodily sensation and the degree to which symptoms are likely to dominate the clinical picture and potentially affect adjustment and recovery following treatment. It should be noted that 30% of general medical patients have an appreciable degree of pain or pain sensitivity that may, or may not, be related to their medical condition.46
Stress Moderators: Future Pessimism versus Future Optimism
This scale assesses patients' perceptions of future health status. Based on a large body of research on optimism and learned helplessness, this patient characteristic was hypothesized to influence a number of medical outcomes including adherence to and confidence in medical regimens, emotional reactions to diagnostic test results, and possibly the actual physical course of disease. A high score on this scale may reflect a patient's response to his/her current medical problems rather than a lifelong tendency to be pessimistic (as assessed by the Dejected Scale). Patients with high scores on the Future Pessimism Scale probably do not anticipate a productive life. They often consider their medical state serious and potentially life‐threatening. Their bleak outlook may require considerable support on the part of health care personnel. It should be noted that 25% of general medical patients exhibit this pessimistic outlook.46
Management Guides
The Management Guides are comprised of 2 subscales, Adjustment Difficulties and Psych Referral, which together, provide summary information regarding the patient's major problem areas.
Management Guides: Adjustment Difficulties
This scale assesses the risk of treatment complications due to the patient's coping styles, current psychological issues operating in the patient's life, their available resources for managing stress, and their risk of engaging in unhealthy behavior. In general, this scale assesses problems that may call for the services of physicians, nurses, health psychologists, and other counseling and behavioral medicine specialists. It should be noted that 30% of general medical patients have psychosocial handicaps that may benefit from special attention.46
Management Guides: Psych Referral
The Psych Referral scale indicates whether the patient might benefit from psychosocial intervention and the likelihood that they would respond well to a specific type of intervention. There are certain classes of patients who are likely to benefit from the therapeutic intervention of psychologists or psychiatrists. It should be noted that 25% of general medical patients tend to have high scores on the Psych Referral scale.46
Patient Health Questionnaire‐9
The Patient Health Questionnaire (PHQ‐9)48 is a self‐report measure used for diagnosis, screening, monitoring and measuring the severity of depression. It incorporates the Diagnostic and Statistical Manual, 4th Edition (DSM‐IV) depression diagnostics criteria along with other leading major depressive symptoms. The PHQ‐9 uses the frequency of the symptoms to factor into the scoring severity index. It can also be administered repeatedly, which helps clinicians in tracking improvement or regression of the depressive state of the patient. The PHQ‐9 scores of 5, 10, 15, and 20 represent mild, moderate, moderately severe, and severe depression, respectively. The diagnostic validity of the PHQ‐9 was established in studies that involved 8 primary care and 7 obstetrical clinics across the United States.49 PHQ‐9 scores less than or equal to 10 had a sensitivity of 88% and a specificity of 88% for major depression. The PHQ‐9 demonstrated excellent internal reliability with a Cronbach alpha = 0.89 in the primary care study. Test‐retest reliability was also excellent with a correlation of 0.84 within a 48‐hour period.48 In a study looking at the validity of the PHQ‐9 in the general population done in 2005, it proved to be reliable and valid in not only recognizing major depression but also subthreshold depressive disorders in the general population.49
Generalized Anxiety Disorder‐7
The Generalized Anxiety Scale (GAD‐7) is a brief, self‐report measure designed to assess symptoms of generalized anxiety disorder.50 Although GAD‐7 and depression symptoms frequently co‐occur, factor analysis confirmed them as distinct dimensions. Moreover, generalized anxiety disorder and depression symptoms have differing but independent effects on functional impairment and disability. The GAD‐7 reflects all of the DSM‐IV symptom criteria for generalized anxiety disorder, as well as 4 items on the basis of review of existing anxiety scales. The diagnostic validity of the GAD‐7 was established in studies across a network of 15 primary care sites located in 12 states (13 family practice, 2 internal medicine). Internal consistency of the GAD‐7 was excellent (Cronbach alpha = 0.92). Test‐retest reliability was also good (intra‐class correlation coefficient = 0.83). Scores of 5, 10, and 15 might be interpreted as representing mild, moderate, and severe levels of anxiety on the GAD‐7, similar to levels of depression on the PHQ‐9.
Adverse Childhood Experiences Rating Scale
The Adverse Childhood Experiences Rating Scale (ACEs) measures 10 types of childhood trauma. The ACEs included only those 10 childhood traumas because those were mentioned as most common by a group of about 300 Kaiser members; those traumas have also been well studied individually in the literature.51, 52 The questionnaire asks the examinee to answer the questions relative to their experiences prior to their eighteenth birthday. Five are personal: physical abuse, verbal abuse, sexual abuse, physical neglect, and emotional neglect. Five are related to other family members: a parent who's an alcoholic, a mother who's a victim of domestic violence, a family member in jail, a family member diagnosed with a mental illness, and the disappearance of a parent through divorce, death, or abandonment. Each type of trauma counts as 1 point. For example, a person who has been physically abused, with 1 alcoholic parent, a family member with mental illness, and a mother who was beaten up has an ACEs score of 4. The Centers for Disease Control (CDC) and Kaiser Permanente conducted The Adverse Childhood Experiences Study, which included 9,508 participants.51 They found a link between childhood trauma and some of the chronic diseases that develop as adults, as well as social and emotional problems. These chronic diseases included heart disease, lung cancer, diabetes, and many autoimmune diseases, as well as depression, violence, being a victim of violence, and suicide.51, 52
Wide Range Assessment of Memory and Learning‐2
Wide Range Assessment of Memory and Learning, Second Edition (WRAML2) was designed to provide a psychometrically sound measure of important core memory components.53 There are 6 core subtests comprising the WRAML2, with 9 optional subtests available for participants aged 18–89. The core subtests measure the Verbal, Visual, and Attention/Concentration Indices, while 2 optional subtests encompass the Working Memory Index. Additional optional subtests measure verbal and visual memory delay recall and verbal and visual memory recognition. The Working Memory Index of the WRAML2 was not utilized in this study as a Working Memory Index was obtained within the WAIS IV. The Verbal Index is comprised of 2 subtests, Story Memory and Verbal Learning. In Story Memory, 2 short stories are read to the participant, who is then asked to recall as many parts to the story as can be remembered. The stories are constructed with differing levels of cognitive and linguistic complexity. In Verbal Learning, the participant is read a list of 1‐syllable words and is asked to recall as many words as possible. Three more trials of list presentation are given, all of which are followed by immediate recall. This task evaluates an individual's ability to learn unrelated verbal information.
The Visual Index also consists of 2 subtests: Design Memory and Picture Memory. In Design Memory participants are shown 5 cards with different geometric forms for a 5‐second period. They are then asked to draw as much of each figure as can be remembered. Picture Memory is similar in concept to Design Memory. Participants are shown 4 typical, but complex scenes, for 10 seconds each. They are then shown a similar alternate scene and asked to circle any item that has been “moved, changed, or added.”
The Attention/Concentration Index is made up of 2 core subtests and 2 optional subtests. The 2 core subtests are Finger Windows and Number Letter. In Finger Windows, an 8.5” x 11” card with asymmetrical holes punched into it, is held vertically in front of the participant. The examiner presents a visual sequence using the card, which the participant duplicates the sequence. The sequences gradually become longer throughout the subtest. In Number Letter, the participant is orally presented with a sequence of mixed‐up numbers and letters and is asked to verbally repeat the sequence exactly.
Wechsler Adult Intelligence Scale IV
The Wechsler Adult Intelligence Scale IV (WAIS IV) is a comprehensive intellectual abilities assessment for individuals ranging in age from 16–90.11 years; for normative data, the mean score is 100; standard deviation is 15.54, 55 Composite scores obtained from a full battery represent intellectual functioning in 4 cognitive domains: Verbal Comprehension Index (VCI); Perceptual Reasoning Index (PRI); Working Memory Index (WMI); and Processing Speed Index (PSI). The Core Battery consists of ten subtests: Block Design, Similarities, Digit Span, Matrix Reasoning, Vocabulary, Arithmetic, Symbol Search, Visual Puzzles, Information, and Coding.
The Similarities and Vocabulary subtests comprise the Verbal Comprehension Index. In Similarities, the participant is presented 2 words that represent common objects or concepts and explains how they are similar, and in turn, primarily measuring verbal concept formation, abstract reasoning, and associative and categorical thinking. For the Vocabulary subtest, the participant defines words that are presented orally, and is designed to measure crystallized intelligence–information that a person has stored in memory about people, places, and things (this fund of stored memories, or knowledge, increases with education); and degree of language development.
The Perceptual Reasoning Index is comprised of the Block Design, Matrix Reasoning, and Visual Puzzles subtests. Block Design is a time‐limited test in which the participant views a model and illustration of a block design and uses blocks to recreate the design as quickly as possible. “It is designed to measure the ability to analyze and synthesize abstract visual information, non‐verbal concept formation and reasoning, and the ability to separate figure–ground in visual stimuli. In Matrix Reasoning, the participant views an incomplete matrix design, then from a series of possible answers, chooses a response that completes the design correctly. This subtest measures perceptual organization: knowledge of part‐whole relationships, classification and spatial ability. Visual Puzzles is a timed test in which the participant views a completed puzzle and selects 3 options that, when combined, reconstruct the puzzle. It is designed to measure the ability to analyze and synthesize abstract visual stimuli, to anticipate relationships among parts of a whole.
Working Memory subtests are Digit Span and Arithmetic. Digit Span is subdivided into 3 tasks: Digit Span Forward, Digit Span Backward, and Digit Span Sequencing. In Digit Span Forward, the participant is read a sequence of numbers and is then asked to repeat the numbers back in the same order. In the second division of this subtest, the participant is asked to recall a sequence of numbers in reverse order in Digit Span Backwards. And, lastly, in Digit Span Sequencing, after being read a series of numbers, the participant is asked to recall the numbers in ascending order. This subtest is designed to measure rote learning, attention, encoding, auditory processing, visuo–spatial imaging, and mental manipulation. Arithmetic is a timed subtest in which the participant solves a series of mathematical story problems delivered orally by the examiner. It measures concentration, short‐ and long‐term memory, numerical reasoning ability, and like the Digit Span subtests, mental manipulation. All 4 subtests of this index are measures of fluid intelligence–the ability to form concepts, reason, and identify similarities; it is intuitive and embodies the activity involved when forming new mental constructs, seeing complex relationships, and solving problems.
Contemporary research has shown that the speed of information processing is dynamically related to mental capacity, reading performance and development, reasoning by conservation of cognitive resources, and the efficient use of working memory for higher order fluid tasks.54, 55 The Processing Speed Index is made up of 2 subtests, Symbol Search and Coding, both of which are still performed using paper and pencil rather via iPad (Apple, Cupertino, CA) administration. The Symbol Search subtest is a timed test in which the participant scans a search group and specifies whether one of the symbols in the target group matches. This test involves speed of processing of short‐term visual memory, visual discrimination, psychomotor speed and coordination, and perceptual organization. Coding is also a timed subtest. The participant uses a key to copy symbols under corresponding numbers as accurately and quickly as possible. Both of these subtests involve speed of processing of short‐term visual memory, visual discrimination, psychomotor speed and coordination, and perceptual organization.
All 10 of the core subtests of the WAIS IV involve the ability to attend and concentrate on the task at hand. Except for the Perceptual Reasoning subtests, all involve receptive language ability, and various aspects of memory, e.g., short‐, long‐, and working memory. Internal consistency and reliability for the Wide Range Intelligence Test Full Scale Intelligence Quotient (WRIT FSIQ) is provided as well.
Wide Range Intelligence Test and Wechsler Adult Intelligence Scale IV Correlation
Results from the Wide Range Intelligence Test (WRIT) and the WAIS IV can be used interchangeably in the literature and was so used in this study; however, it is important to discuss psychometric equivalence between these two cognitive batteries. As of this the time of this publication, the WRIT has not been updated. The most recent data correlating the WRIT and the WAIS is from the year 2000, when the WAIS was in its third edition (WAIS III). The WRIT and WAIS III demonstrated a correlation of 0.976.56 Glutting and coworkers assert, “… it seems reasonable to infer that the 4 subtests WRIT and the 11‐subtest version of the WAIS III are evaluating phenomena that are so common that each of the respective constructs, i.e., general ability, verbal ability, and visual/performance ability, are virtually the same across tests!” The WAIS IV was published in 2008. Only one change to the Core Battery of 10 subtests was made; the Object Assembly subtest (a visuo–motor task) of the WAIS III was replaced by the Visual Puzzles subtest (a visual task without a motor component) in the WAIS IV. Correlational data obtained during normative analysis (n = 240) demonstrate a strong correlation (r = 0.94) between the WAIS III and the WAIS IV, suggesting it is reasonable to assume that correlation between the WRIT and the WAIS IV is equal, or very nearly equal to that of the correlation between the WRIT and WAIS III.
Reliability for special groups supports the generalizability of the WAIS IV. Ten of the experimental participants and 6 of the comparator participants acknowledged a history of TBI, therefore internal consistency reliability was acquired for that group. Data was obtained from a sample of 22 adults with traumatic brain injury. The average range for group mean comparison of a TBI group with a matched control group was found to be r = 0.87–0.98.54
Delis‐Kaplan Executive Functions System
Trail‐Making Tests
The Delis‐Kaplan Executive Functions System (DKEFS) Trail‐Making Tests (TMT) Conditions 4 and 5 were designed as a measure of executive functioning,57 specifically in relation to higher level skills such as multitasking, simultaneous processing, and divided attention.58 There are 5 trails in all. However, for the purposes of this study, patients were only assessed with 2, Number‐Letter Switching (Condition 4) and Motor Speed (Condition 5). Number‐Letter Switching requires the examinee to mentally shift from one task to another, specifically shifting back and forth connecting dots from numerical to alphabetical order. Motor Speed measures how quickly the examinee can connect a series of dots, providing a baseline level of motor functioning and visual scanning. The contrast between these 2 conditions provide normative data regarding the extent to which difficulty on the switching condition may be related to a motor deficit.57 The Number‐Letter Switching task is a measure of cognitive flexibility, one's ability to shift quickly from one paradigm to another.
The DKEFS assessment battery was standardized on a nationally representative, stratified sample of 1,750 nonclinical children, adolescents, and adults, ages 8–89 years old; the mean score is 10 with a standard deviation of 3 for all subtests in the battery. Test‐Retest reliability studies were based on 101 participants across all age ranges, with time between administrations 9–74 days, with an average of 25 days.59 Internal consistency is based on a composite score of Number Sequencing and Letter Sequencing conditions. The internal consistency of this composite score is analyzed by utilizing performance on each condition as an equivalent half test. The Spearman–Brown formula was used to correct correlation, deriving reliability coefficient of 0.66 for Combined Number Sequencing and Letter Sequencing for all ages. Specific measures to this study, Motor Speed and Number/Letter Switching, demonstrated reliability of 0.77 and 0.38, respectively. Correlations for the ages represented in this study range from 0.68 (12 years of age) to 0.80 (60–69 years of age).57
METHODS
Participants
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration. Our Institutional Review Board approved these studies.
For the outlier cohort, the inclusion criteria included: CT+ TWS SSCD patients who had middle cranial fossa (MCF) craniotomies and plugging of the SSCD; CT‐ TWS patients who had treatment as a vestibular migraine patient for at least 6 months before RWR; failure to resolve TWS symptoms and no objective evidence of a third window, migraine headaches, postural dyscontrol and cognitive dysfunction; and conforming to the ages associated with the normative data for each neuropsychology test. For the outlier cohort, the exclusion criteria included: resolution of TWS symptoms, migraine headaches, postural dyscontrol, and cognitive dysfunction; known psychiatric illness; not willing to participate in the research study; dementia; confirmed brain injury; history of cerebrovascular accident; and not conforming to the ages associated with the normative data for each neuropsychology test.
One of the neurotologist authors performed 162 MCF craniotomies and plugging of the CT+ SSCD between February 2010 and April 2017. Twelve participants with TWS and SSCD symptoms had surgical management of CT+ SSCDS TWS, CT‐ TWS or both, whose surgical outcomes placed them as outliers met the inclusion and exclusion criteria and were recruited consecutively and agreed to participate in and completed the study as the outlier cohort. All 12 were adults. The outlier cohort had a mean age of 49.8 years (range 22.6–62.4 years) at the time of manuscript submission, with 4 males and 8 females (66.7% female). These 12 subjects were systematically studied prospectively to determine comorbidities that contributed to their poor outcomes. Retrospective analysis of their clinical features and detailed clinical course was also completed.
Seventeen, healthy subjects who successfully had surgical management of CT+ SSCDS TWS, CT‐ TWS or both agreed to participate in and completed the study as the control cohort. These participants were part of a study published previously.3 Their neuropsychology testing was performed prospectively and they also underwent a retrospective analysis of their clinical features and detailed clinical course as reported previously. 3 There were 16 adults and 1 child. The control cohort had a mean age of 38.2 years (range 16.1–64.0 years) at the time of manuscript submission, with 3 males and 14 females (82.4% female). The demographic, diagnostic and surgical management of this cohort have been reported previously.3 Of the controls, published in the 2016 paper reporting the cognitive dysfunction and recovery after surgery, there were CT‐ TWS patients (n = 8), CT+ TWS SSCD patients who then went on to develop a CT‐ TWS and had surgery (n = 4), as well as CT+ SSCD patients (n = 5).3 For this study, we invited the participants from the previous study to undergo a new set of neuropsychology tests and they were willing to do so.
The patient demographics, clinical features, diagnostic studies, and surgical histories for each participant in the outlier cohort are summarized in Tables 1 and 2.
Table 1.
Participant History, Symptoms, Physical Findings and Results of Diagnostic Studies Before Initial Surgical Intervention.
| Participant (Age) | Sound‐induced | Hearing Internal Sounds | 256 Hz Tuning Fork to Knees and Elbows | Cognitive Dysfunction | Spatial Disorientation | Anxiety | Nausea | Ability to Listen to More than One Person Speaking | Migraine Character | Trauma | Pseudoconductive Hearing Loss | Endolymphatic Hydrops | cVEMP | Moving Platform Pressure Test | High‐Resolution TB CT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (22.6) | Increased nausea, body twitching | Autophony, heel strike, left ear | Positive, left heard and felt buzzing | Yes, memory, word finding | Yes, trouble judging distances, detachment, occasional out of body experience | No | Yes, constant | Lost this ability | Frequent ocular migraines, rare migraine headaches | MVA 5 years before, truck slid sideways dropped 6 feet before coming to rest | No, right low frequency conductive HL | Bilateral | Positive, left | Positive, left greater than right, small response | Normal |
| 2 (30.3) | Dizziness, nausea, drop attacks, perceived floor moving and walls narrowing | Autophony (intense), heartbeat | Positive, could hear in both ears | Yes, memory, concentration, word finding, name finding, occasional slurred speech | Yes, trouble judging distances, detachment, occasional out of body experience | Yes | Yes | Lost this ability | Severe, frequent migraine headaches | Multiple concussions, MVA, thrown off of a horse with blow to the head resulting in LOC | Bilateral | Bilateral | Positive, bilateral | Positive, bilateral, small responses | Bilateral SSCD |
| 3a (39.8) | Dizziness, nausea, headache, light sensitivity | Autophony, extremely loud right ear | Positive, right greater than left, 128 Hz tilting, and vibration of skull base | Yes, memory, word finding, concentration, reading | Yes, detachment | No | Yes | Never had this ability | Right‐sided, frequent headaches, light sensitive | Long history of boxing, plus 3 years prior to presentation drawing heavily from a bong, heard loud pop right ear | Right | Bilateral | Positive, left greater than right | Positive, right, small response | Right near SSCD |
| 4a (48.2) | Dizziness, nausea, makes her upset | Autophony, heartbeat, joints moving | Positive, could hear in both ears | Yes, memory, word finding, concentration | Yes, detachment, trouble judging distances when driving | Mild | Mild | Lost this ability | Frequent headaches | Multiple concussions, onset of symptoms 14 months before presentation slipped on ice struck back of her head | Right | Left | Positive, right | Negative | Left SSCD, right near‐SSCD |
| 5a (52.9) | Dizziness, nausea, headache | Autophony, heartbeat, left greater than right | Positive, could hear in both ears | Yes, memory, concentration, word finding, name finding, occasional slurred speech | Yes, detachment, distorted environment | Yes | Yes, constant | Lost this ability | Daily headache, clusters of left hemiplegic migraine, frequent ocular migraine, rare vestibular migraine, constant light sensitivity | Multiple concussions, onset of symptoms after prolonged period of vomiting | No | Right | Positive, left | Negative | Right SSCD, left near SSCD |
| 6 (54.2) | Dizziness, nausea, legs buckle, falls, especially with low frequency sounds | Autophony, hearing eyes move, right greater than left | Positive, could hear in both ears, right greater than left | Yes, memory, concentration, trouble finding nouns and frequently reverted to pronouns | Yes, disconnected | Rarely | Yes, frequent | Never had this ability | No migraine headaches or light sensitivity, “sinus headaches” with normal paranasal sinus CT | No | Bilateral | Left | Small response right, absent left | Positive, right much greater than left | Right SSCD |
| 7a (55.3) | Dizziness, headache, blurred vision | Autophony, heartbeat, eyes moving, left ear only | Positive, could hear only in left ear | Yes, memory, concentration, word finding | Yes, detached | No | Frequent | Lost this ability | Migraine headache 24/7 “low grade” worsens with activity/exertion, light sensitivity | Post‐concussive syndrome after struck by motorcycle requiring resuscitation age 6, 1996 fall with concussion, 2001 fall with cervical spine injury | Left | No | Positive left | Positive, left, small response | Left SSCD |
| 8 (56.7) | Decreased cognitive function | No | Positive, could hear only in left ear | Yes, others note that he is a “different person” | Yes, detachment, trouble judging distances when driving | No | No | Never had this ability | Chronic migraine headaches | Three concussions and an additional MVA | Left | No | Normal right, absent left | Negative | Left SSCD |
| 9 (57.1) | Dizziness, nausea, irritating | Hearing neck facets popping with head turns | Positive, could hear only in left ear | Yes, progressive cognitive dysfunction | Yes, detached | No | Mild | Never had this ability | Chronic migraine headaches, light sensitivity | Multiple concussions; including MVA with airbag deployment and totaling his car after all otologic surgical procedures | Bilateral | Bilateral | Positive, left | Negative | Bilateral near‐SSCD, left greater than right |
| 10 (57.5) | Confusion, nausea | Autophony, chewing, eyes blinking, eyes moving | Positive, could mildly hear only in right ear when on right elbow only | Yes, concentration, organizing difficulty, word finding | Yes, detachment, trouble judging distances when in a car | Yes, mild | Mild | Lost this ability | Frequent migraine headaches, ocular migraine | Multiple concussions, onset of symptoms 31 years before presentation during difficult childbirth, traumatic loss of vision right eye | Bilateral | Left | Positive, right | Positive, left, small response | Bilateral SSCD |
| 11 (60.1) | Dizziness, nausea | Autophony, chewing, fluid moving in neck, right greater than left | Positive, could hear only in right ear | Yes, memory, executive function, word finding, reading | Yes, trouble judging distances | Yes | Intermittent | Lost this ability | Severe migraine headaches, occasional ocular migraines | Multiple falls with concussions, 2 MVAs | Right | No | Positive, right | Negative | Right SSCD |
| 12 (62.4) | No symptoms | Autophony | Positive, could hear only in left ear | Yes, memory, concentration, occasional word finding | Yes, detachment, trouble judging distances | Yes | Severe, progressive | Lost this ability | No migraine headaches, had migraine headaches in her 30s | No | Bilateral | Bilateral | Absent right, positive left | Negative | Left SSCD, right near SSCD |
See video links in references3–7; 24/7 = migraine headache present constantly, 24 hours per day and 7 days per week while awake; 256 Hz = ability to hear or feel the vibration of the tuning fork when applied to knees and elbows; cVEMP positive = increased amplitude response and decreased threshold to 70 dB SPL; Dizziness = gravitational receptor asymmetry type of vertigo (e.g., as if on a boat, rocky, wavy, tilting, being pushed, tilting, or sense of floor falling out from under them); Endolymphatic hydrops = abnormal summating potential/action potential ratio with electrocochleography; HA = headache; LOC = loss of consciousness; MVA = motor vehicle accident; SCD = superior canal dehiscence; TB = temporal bone; TMJ = temporomandibular joint.
Table 2.
Surgical Histories of the Outlier Study Participants.
| Participant | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery 1 | L RWR* 5/4/16 | L SSCD 6/7/11 | R SSCD + RWR 2/12/14 | R SSCD 5/7/14 | R SSCD 11/19/14 | R SSCD 5/1/13 | L SSCD 11/12/14 | L SSCD 4/9/14 | L IT corticosteroids 5/23/13 | R SSCD 9/3/14 | R SSCD 12/23/14 | L SSCD 3/5/14 |
| Surgery 2 | R SSCD 7/6/11 | R RWR 8/6/14 | L SSCD 8/13/14 | R RWR* 7/14/15 | R RWR 12/11/13 | L RWR* 2/11/15 | L RWR* 12/29/14 | L SSCD 12/4/13 | L SSCD 11/19/14 | R RWR* 2/10/16 | R SSCD 8/13/14 | |
| Surgery 3 |
L RWR 12/9/11 Out of State Surgeon (G.J.G.) |
R RWR* 12/30/14 | L RWR* 11/12/14 | L RWR* 12/7/15 | L RWR* 9/9/15 | R SSCD 11/12/14 | L RWR* 4/15/15 | R RWR 12/30/14 | ||||
| Surgery 4 |
R RWR 3/9/12 Out of State Surgeon (G.J.G.) |
R RWR* 6/10/15 | L RWR* 12/30/14 | R RWR* 8/12/15 | R RWR* 6/3/15 | R RWR* 6/2/15 | ||||||
| Surgery 5 |
L SSCD resurfacing + L RWR 9/13/12 Out of State Surgeon (G.J.G.) |
R RWR glass ionomer cement 12/9/15 | L RWR* 5/13/15 | L RWR* 10/7/15 | L RWR* 11/11/15 | |||||||
| Surgery 6 | VP shunt 7/3/13 Pseudotumor cerebri | L RWR glass ionomer cement 2/10/16 | ||||||||||
| Surgery 7 | R RWR 3/12/13 | R epidural abscess 4/6/16 | ||||||||||
| Surgery 8 | L RWR 5/14/13 | |||||||||||
| Surgery 9 | L RWR 2/5/14 | |||||||||||
| Surgery 10 | L RWR* 8/6/14 | |||||||||||
| Surgery 11 | R RWR* 12/30/14 | |||||||||||
| Surgery 12 | R RWR* 3/11/15 | |||||||||||
| Surgery 13 | R RWR* 3/12/15 | |||||||||||
| Surgery 14 | L RWR glass ionomer cement 8/12/15 |
CSF = cerebrospinal fluid; IT = intratympanic perfusion over three hours; L = left; R = right; RWR = round window reinforcement with loose areolar tissue; RWR* = round window reinforcement with perichondrium, cartilage graft and loose areolar tissue; TBI = traumatic brain injury; VP = ventriculoperitoneal.
None of the clinical interventions with either cohort affected the neuropsychology test results. All of the control and outlier participants received vestibular rehabilitation therapy. All of the participants received the same antinausea and vestibular suppressant medications during the perioperative period. None of the participants received counseling or divergent care–until the study was completed and the comorbidities were identified. At that point, and not a part of this study, the outliers were referred for cognitive therapy or therapy targeted to address their specific neuropsychiatric pathology.
Diagnostic Studies
Comprehensive audiometric testing, electrocochleography (ECoG), cVEMP, vestibular autorotation testing (VAT), moving platform pressure test, and computerized dynamic posturography were performed pre‐ and postoperatively. The methods associated with performing these studies have been reported previously.2, 3 In the outlier group, for those studies summarized in Table 1, the methods are described below.
Tuning Fork Testing
As a screening study in patients with SSCDS/TWS symptoms, a low frequency tuning fork was applied to a patient's knees and elbows and they were asked if they could hear or feel the vibration in their head.10 Both 256 Hz and 128 Hz tuning forks were used.
Audiometry
Pure‐tone audiometry was performed over the frequency ranges of 250 to 8,000 Hz for air conduction and 250 to 3,000 Hz for bone conduction. Testing was performed in a soundproof booth. Appropriate masking was used for bone conduction and, when needed, for air conduction. Tympanometry was performed. Acoustic reflexes were tested for ipsilateral and contralateral presentation of tones.
Electrocochleography
Preoperative ECoG was performed using gold foil tiptrodes (Etymotic Research Inc., Elk Grove, IL, USA), which were placed adjacent to the tympanic membrane in the external auditory canal and stabilized at the foam tip of the insert audio transducer. Unfiltered clicks of 100 µs duration were presented at an intensity of 85 dB nHL. Two replications of averaged responses elicited by 1,500 clicks presented at a rate of 11.7 per second were obtained. Responses were band pass filtered (20–1,500 Hz) and averaged, and the summating potential to action potential (SP/AP) ratio was calculated. SP/AP ratio of greater than 0.4 was defined as abnormal for purposes of this study, based on commonly used standards for clinical testing.60
Acoustic cVEMP Stimuli and Recording Techniques
A commercial auditory evoked potential system (Bio‐logic Systems Corp, Software version 6.2.1d, Mundelein, IL) was used for acoustic cVEMP testing. Sound stimuli were delivered monaurally via intra‐auricular transducer with foam E‐A‐R Link Inset Earphones (Aearo Company Auditory Systems, Indianapolis, IN) as described previously.61
During the recording protocol, the subjects were seated upright. The skin, in areas of electrode placement, was cleansed with alcohol preps prior to electrode placement. cVEMP measurements were recorded using disposable, self‐adhesive, pre‐gelled, electrodes (3M Red Dot Ag/AgCl, London, Ontario) and lead wires from the Bio‐logic Corp. The electrode montage consisted of an active electrode on the top third of the sternocleidomastoid muscle, a reference electrode on the sternoclavicular junction, and a ground electrode placed on the sternal notch.
During the cVEMP instruction, patients were asked to rotate their head towards the contralateral shoulder from the stimulus, and tilt/angle approximately 30 degrees maximizing the contraction of the sternocleidomastoid muscle. The clinician applied the maximum amount of manual resistance that each subject could tolerate while visually confirming the SCM contraction during stimulus delivery.
During the cVEMP and measurements, air conducted stimuli were delivered with 1000 Hz, 90 dB nHL tone burst of positive polarity at a repetition rate of 4.3 per second (2 ms rise/fall time, 2 ms plateau). The air‐conduction stimuli were also presented at 80, 70 and 60 dB nHL. Evoked myogenic potentials were amplified by 1000x and band‐pass filtered (10–1500 Hz). Average sweeps per test were approximately 80–150.
The response parameters were defined as the cVEMP p13 potential being the first distinctive trough in the waveform, occurring approximately at the anticipated 10–14 ms, post stimulus, and the n23 potential being the first distinctive peak in the waveform, occurring approximately 19–23 ms after stimulus onset. Peak‐to‐peak amplitude was calculated using the Bio‐logic software, after peaks were labeled and encompassing the amplitude difference between the 2 peaks. The lowest dB SPL at which a p13 and n23 response could be recorded was the threshold. For reporting purposes, the cVEMP was considered positive when an increased amplitude and decreased threshold (70 dB nHL) was observed.
Moving Platform Pressure Test
Most of the patients underwent moving platform pressure testing (fistula test) preoperatively and those who developed a CT‐ TWS after SSCD plugging had this performed postoperatively as described by Black and coworkers.62, 63 To summarize, the moving platform pressure test was performed in the vision‐denied, sway‐referenced surface condition (i.e., Sensory Organization Test 5 [SOT 5]). During the test, a probe was placed in the ear that alternately applied positive pressure, negative pressure and no pressure. The pressure used was ± 500 dekapascal (daPa). The outcome was a measurement of sway energy (SE) derived from the change in position. A baseline SE during no pressure application was measured and compared to the SE during pressure application (positive or negative). Outcomes were expressed as a percentage increase from the baseline SE. Further, an assessment was made of sway synchronization during stimulus to assess temporal relation of output to stimulus. The test requires adequate performance of SOT 5 on CDP, therefore patients unable to complete SOT 5 would not be able to complete the moving platform pressure test; however, all 12 participants were able to do so.
Computed Tomography of the Temporal Bone
The patients underwent helical high‐resolution computed tomography (CT) of the temporal bone. This was performed using a Siemens Somatom Sensation 64 slice scanner (Siemens Corporation, Malvern, PA) with a collimation of 12 x 0.6 mm and a reconstruction increment of 0.3 mm. Axial imaging was obtained with reconstructions in sagittal and coronal planes. The images were optimized using a very sharp kernel and a Siemens software‐specific window level dedicated to the inner ear.
Next, the axial 0.6 mm raw data set was loaded onto a TeraRecon AquariusNET Viewer (TeraRecon, Inc., Foster City, CA) in 3D mode. Using 3D manipulation, the left and right superior semicircular canals were manipulated to a “best view” in plane with the circumference of the canal. The entire bony otic capsule including the superior semicircular canals were then evaluated with 2 different 3D rendering modes. The first is a gray‐scaled “MinIP” or minimum intensity projection mode at 1 mm thickness. The second was a color 3D volume rendering mode, also at 1 mm thickness. The character and size of the dehiscence was measured using the “best view in plane” images on the workstation. The bone overlying the superior semicircular canal of each side and with each 3D rendering mode was characterized as: normal; thin; SCD ≤ 2 mm (small), > 2 mm < 4 mm (medium), ≥ 4 mm (large); or a channel, single or number of channels. For reporting purposes, we are reporting the images as “normal” if no dehiscence could be seen in any of the 3 semicircular canals, or anywhere else in the bony otic capsule, e.g., carotid‐cochlea dehiscence, cochlea‐facial nerve dehiscence, wide vestibular aqueduct or jugular bulb‐posterior semicircular canal dehiscence.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed in outlier participants 2 through 12 (Table 2, n = 11) and control participants (n = 4) who subsequently developed a delayed CT‐ TWS and recurrence of their SSCDS/TWS symptoms to determine if their superior semicircular canal remained plugged. MRI Scanner used was a Siemens Tim Trio 3.0 T MRI machine. The semicircular canal sequence used to detect patent versus plugged semicircular canal was CISS (constructive interference in steady state) 0.6 mm axial acquisitions, which were then evaluated in both 2D and 3D volume rendering on a Tera Aquarius Net viewer. The 3D volumes were then evaluated with Maximum Intensity Projection (MIP) slabs varying in the 10 mm to 20 mm range. These high‐resolution sequences were used to determine if there was fluid within the superior semicircular canals “patent” versus no fluid “plugged.”
Superior Canal Dehiscence Surgical Techniques
The same surgical technique was used for the 11 outlier participants and the 7 control participants with CT+ TWS SSCD and one neurotologist performed all of the surgical procedures (P.A.W.). A traditional MCF approach with the craniotomy centered on the zygomatic root and craniectomy to the skull base was used after intravenous administration of 10 mg of dexamethasone and 0.5 gm/kg of mannitol. The dura was elevated with an Adson periosteal elevator and a Fisch MCF retractor was placed with the retractor tip just past the petrous ridge. Using microsurgical techniques, the superior canal was inspected. If the dehiscence was not seen on the superior aspect of the canal, further dural elevation and subsequent use of a Buckingham mirror or endoscope to identify a dehiscence was completed. The canal was plugged using temporalis fascia or periosteum. Gelfoam (Pfizer, New York, NY) was then used to fill the middle ear if the ossicles were in contact with the herniated temporal lobe and dura. Likewise, Gelfoam was used to fill all of the remaining temporal bone defects. The superior semicircular canal and temporal bone was resurfaced with Cranios Reinforced Fast Set Putty (DePuy Synthes, West Chester, PA). DuraGen Dural Regeneration Matrix (Integra, Inc., Plainsboro, New Jersey) was then trimmed to fit the exposed dura after removing the Fisch retractor. If there were any dura defects present, the dura was repaired with either a fascia graft or a medial graft fashioned from DuraGen Dural Regeneration Matrix (Integra, Inc.). A single piece of Gelfoam was used to cover all of the exposed dura at the craniotomy/craniectomy site before titanium mesh was secured to the skull. Cranios Reinforced Fast Set Putty (DePuy Synthes) was then used to complete the cranioplasty prior to wound closure.
Round Window Reinforcement Surgical Techniques
Round Window Reinforcement with Loose Areolar Tissue Graft Technique
For the outlier and control patients who exclusively had a CT‐ TWS or developed a delayed CT‐ TWS after SSCD plugging (Table 2), the loose areolar tissue graft technique described previously for RWR was initially performed in 3 of the 12 outlier participants.2, 3 One neurotologist performed all of the surgical procedures (P.A.W.). The basic techniques are similar to those described a quarter century ago and recently republished.64, 65 Loose areolar tissue was harvested, and then minced into 0.25 mm pieces using a No. 10 Beaver blade. TISEEL, a 2‐component fibrin sealant, (Baxter Healthcare Corporation, Westlake Village, CA) was used for coating the pieces. One component is a sealer protein solution that contains human fibrinogen and a synthetic fibrinolysis inhibitor, aprotinin, which helps prevent premature degradation of the fibrin clot. The other component is a human thrombin solution and calcium chloride. Each of these solutions was prepared and kept isolated into petri dishes into which the minced tissue was divided. A Lumenis Spectra II (Lumenis Inc., San Jose, CA) laser was used with a Lumenis Acculite EndoOto hand held laser probe (Horn, 24 ga 20° angled, SubMiniature Type A [SMA] 906 connector, 200 µm). The Selecta II has a red 635nm (<5 mW) He NE aiming beam; and a Q‐switched frequency doubled 1064 nm Nd:YAG, (532 nm [green wavelength]) diode‐pumped solid state laser as its treatment beam. The specific treatment settings used were: power 1000 mW; pulse duration of 0.3 seconds; and pulse interval of 0.3 seconds. The laser was used to denude all of the mucosa around the RW niche and also around the anterior portion of bone surrounding the OW annular ligament. After placement of the reinforcement materials, the defocused laser was also used to further coagulate and denature these materials at the periphery so that greater adherence to the temporal bone could be achieved. The RW was reinforced using the loose areolar tissue coated with the thrombin and fibrinogen solutions. The OW reinforcement was accomplished by draped grafts around the anterior crus and packing them in place with Gelfoam. Too much tissue was intentionally placed in the RW niche and also around the stapes knowing that some would be resorbed during the healing and connective tissue remodeling phases. Following reinforcement, the middle ear was filled with Gelfoam and tympanomeatal flap returned to the anatomic position. Strips of dry Gelfoam were placed across the intact skin and the skin of the tympanomeatal flap and a small amount of antibiotic ointment is placed over this. Ofloxacin 0.3% otic solution was then placed into the external auditory canal. No additional dressing materials were required.
Round Window Reinforcement with the Perichondrial and Cartilage Graft Technique
For the outlier and control patients who exclusively had a CT‐ TWS or developed a delayed CT‐ TWS after SSCD plugging (Table 2), the perichondrial and cartilage graft technique described previously for RWR was ultimately performed in 11 of the 12 outlier participants.2, 3 The bone was drilled off of the RW niche overhang using a 0.8 mm diamond bur. A Lumenis Spectra II (Lumenis Inc., San Jose, CA) laser was used with a Lumenis Acculite EndoOto hand held laser probe (Horn, 24 ga 20° angled, SubMiniature Type A [SMA] 906 connector, 200 µm). The Selecta II has a red 635 nm (<5 mW) He NE aiming beam; and a Q‐switched frequency doubled 1064 nm Nd:YAG, (532 nm [green wavelength]) diode‐pumped solid state laser as its treatment beam. The specific treatment settings used were: power 1000 mW; pulse duration of 0.3 seconds; and pulse interval of 0.3 seconds. The laser was used to denude all of the mucosa around the RW niche and also around the anterior portion of bone surrounding the OW annular ligament. The perichondrium graft was thinned using a fascia press and was placed directly on the surface of the RW membrane and extended onto the denuded otic capsule. A 2‐mm conchal cartilage graft was harvested using a 2‐mm biopsy punch (Miltex, Inc., York, PA) and then split in half and placed into the perichondrial graft overlying the RW. Loose areolar tissue was minced into 0.25 mm pieces separated into 2 petri dishes. TISEEL, a 2‐component fibrin sealant, (Baxter Healthcare Corporation, Westlake Village, CA) was used for coating the pieces. One component was a sealer protein solution that contained human fibrinogen and a synthetic fibrinolysis inhibitor, aprotinin, which helps prevent premature degradation of the fibrin clot. The other component was a human thrombin solution and calcium chloride. Each of these solutions was prepared and kept isolated into petri dishes into which the minced tissue is divided. The latter was then circumferentially placed in a manner of a gasket around the cartilage and onto the perichondrium. Too much tissue was intentionally placed in the RW niche and also around the stapes knowing that some would be resorbed during the healing and connective tissue remodeling phases. Following reinforcement, the middle ear was filled with Gelfoam and tympanomeatal flap returned to the anatomic position. Strips of dry Gelfoam were placed across the intact skin and the skin of the tympanomeatal flap and a small amount of antibiotic ointment was placed over this. Ofloxacin 0.3% otic solution was then placed into the external auditory canal. No additional dressing materials were required.
Round Window Reinforcement with the Fuse Glass Ionomeric Cement Technique
For participants 2, 3, and 10, because of multiple revision RWR surgeries for each patient, a decision was made to use Fuse glass ionomer cement (Grace Medical, Inc., Memphis, TN) to close the round window (Table 2). Perichondrium was used to protect the round window membrane and inner ear and the cement was allowed to fuse to the denuded surrounding bone. For participant 2, this finally yielded resolution of her TWS symptoms.
Comprehensive Neurotologic, Neurologic and Psychologic Evaluations
In addition to one of the neurotologists (P.A.W.), 2 neurologists, one specializing in migraine (D.M.C.) and the other a neuro‐ophthalmologist (M.S.G.), and a psychologist (H.T.M‐P.) completed comprehensive evaluations.
Measurement of Cerebrospinal Fluid Opening Pressure
Fluoroscopic lumbar puncture to determine the cerebrospinal fluid (CSF) opening pressure in all outlier participants, except for participant 12. The opening CSF pressure was considered normal if the value fell within the accepted normal range 10–20 cm H2O.
Neuropsychology Testing
The neuropsychology test battery used is summarized in Table 3. These included: Millon Behavioral Medicine Diagnostic (MBMD) with Coping Styles (one subtest), Stress Moderators (3 subtests) and Management Guidelines (2 subtests); the Patient Heath Questionnaire‐9 (PHQ‐9); Generalized Anxiety Disorder‐7 (GAD‐7); Adverse Childhood Experiences Rating Scale (ACEs); the Wide Range Assessment of Memory and Learning 2 (WRAML2), including 3 of the 4 domains of Verbal Memory (2 subtests), Visual Index (2 subtests), and Attention/Concentration Index (2 subtests). The Working Memory domain was not included because this was accessed using the WAIS IV Working Memory Index. The WAIS IV, including the domains of Verbal Comprehension Index, Perceptual Reasoning Index, Processing Speed Index, and Working Memory Index (the composite WAIS IV intelligence score [Full Scale Intelligence Quotient] was also calculated and compared to the control cohort who underwent the Wide Range Intelligence Test); and the Delis‐Kaplan Executive Function System (DKEFS), Trail Making Tests, Conditions 4 (Number‐Letter Switching) and Condition 5 (Motor Speed). The entire test battery required 4 hours to complete and was divided between 2 sessions.
Table 3.
Comparison of Neuropsychology Assessment Results: Cohort Composed of Postoperative Outliers and Control Participants Composed of Postoperative and Previously Studied Third Window Syndrome Surgical Patients.
| Feature and Instrument | Outlier Cohort (n = 12) | Control Cohort (n = 17) | p‐Value |
|---|---|---|---|
| Female gender (n, %) | 8 (66.7%) | 14 (82.4%) | 0.595 |
| Mean Years of Age (range) | 49.8 (22.6–62.4) | 38.2 (16.1–64.0) | 0.054 |
| Millon Behavioral Medicine Diagnostic (MBMD) | n = 12 | n = 10 | |
| Coping Styles | |||
| Dejected | 65.8 (10–111) | 44.9 (10–94) | 0.127 |
| Stress Moderators | |||
|
Functional Deficits vs Functional Competence |
89.2 (73–110) | 43.8 (10–100) | 0.001 |
| Pain Sensitivity vs Pain Tolerance | 94.3 (65–110) | 47.5 (10–100) | 0.002 |
| Future Pessimism vs Future Optimism | 77.3 (35–94) | 41.0 (5–93) | 0.016 |
| Management Guidelines | |||
| Adjustment Difficulties | 92.3 (64–115) | 59.2 (15–90) | 0.003 |
| Psych Referral | 84.8 (45–115) | 50.4 (15–100) | 0.017 |
| Patient Health Questionnaire‐9 (PHQ‐9) | 14.7 (2–26) | 3.5 (0–18) n = 15 | <0.001 |
| General Anxiety Disorder‐7 (GAD‐7) | 8.3 (2–19) | 3.7 (0–19) n = 13 | 0.009 |
| Adverse Childhood Experiences Rating Scale (ACEs) | 2.9 (1–8) | 3.2 (0–7) n = 12 | 0.861 |
| Wide Range Assessment of Memory and Learning, 2nd Edition (WRAML2) | |||
| Verbal Memory | |||
| Story Memory | 10.2 (6–16) | 14.9 (11–19) n = 15 | 0.002 |
| Verbal Learning | 99.7 (82–126) | 120.9 (100–143) n = 16 | 0.002 |
| Visual Index | |||
| Design Memory | 11.5 (7–17) | NA n = 2 | |
| Picture Memory | 10.6 (5–14) | NA n = 2 | |
| Attention/Concentration Index | n = 12 | n = 15 | |
| Finger Windows | 6.8 (3–10) | 12.5 (3–19) | <0.001 |
| Number Letter | 10.3 (6–14) | 11.3 (8–15) | 0.491 |
| Wechsler Adult Intelligence Scale, 4th Edition (WAIS IV) | |||
| Verbal Comprehension Index | 109.1 (89–130) | 114.4 (92–135) n = 15 | 0.305 |
| Perceptual Reasoning Index | 103.5 (79–127) | 109.9 (88–129) n = 15 | 0.240 |
| Processing Speed Index | 93.2 (74–122) | NA n = 2 | |
| Working Memory Index | 100.1 (83–119) | 115.3 (108–128) n = 16 | 0.001 |
| FSIQ (WAIS IV [outliers]) vs WRIT (controls) | 102.3 (88–123) (n = 12) | 110.6 (81–136) (n = 16) | 0.056 |
| Delis–Kaplan Executive Function System (DKEFS) | |||
| Trail Making Tests, Conditions 4 & 5 (TMT) | n = 12 | n = 16 | |
| Number–Letter Switching (Condition 4) | 8.6 (1–13) | 10.9 (7–14) | 0.110 |
| Motor Speed (Condition 5) | 10.7 (9–12) | 12.2 (10–14) | 0.005 |
FSIQ = Full Scale Intelligence Quotient (composite WAIS IV intelligence score); WRIT = Wide Range Intelligence Test.
Statistical Analysis
The between group (outlier cohort versus control cohort) differences in neuropsychology test scores were evaluated using a 2‐tailed non‐parametric Wilcoxon signed‐rank test. This test was performed because of the non‐normally distributed nature of the data. All analyses were performed using the R statistical package (R: A language and environment for statistical computing).66 A criterion of p < 0.05 was regarded as significant.
RESULTS
The patient demographics, clinical features, diagnostic studies and surgical histories for each participant are summarized in Tables 1 and 2. The statistical comparison results are summarized in Table 3 and the comorbidities associated with each of the outlier participants are summarized in Table 4. Females were more commonly encountered in both the outlier group (n = 8 [66.7%]) and the control group (n = 14 [82.4%]); however, there was no statistically significant difference in sex between the groups. Likewise, there was no statistically significant difference in age between the 2 groups (p = 0.054).
Table 4.
Outlier Participant Number, Age and Comorbidities.
| Participant | Age | Comorbidities |
|---|---|---|
| 1 | 22.6 | TBI; factitious disorder; MDD; PTSD; suicidal ideation |
| 2 | 30.3 | TBI; elevated CSF pressure and subsequent VP shunt; suicidal ideation |
| 3 | 39.8 | TBI; atypical migraine |
| 4 | 48.2 | TBI |
| 5 | 52.9 | TBI; functional neurologic disorder, dissociative motor disorder variant; hemiplegic migraine; suicidal ideation |
| 6 | 54.2 | EtOH abuse (1.75 liters of vodka per day); tremor; 3 years later contralateral SSCD found |
| 7 | 55.3 | TBI; MDD; somatic symptom disorder; vestibular migraine; suicidal ideation |
| 8 | 56.7 | TBI |
| 9 | 57.1 | TBI; MDD |
| 10 | 57.5 | TBI; unilateral blindness; MDD; suicidal ideation; somatic symptom disorder |
| 11 | 60.1 | TBI; ADHD; history of DID, MDD, suicide ideation and attempts |
| 12 | 62.4 | Drug‐induced Parkinson‐like symptoms; idiopathic neurologic deterioration |
ADHD = attention deficit hyperactivity disorder; CSF = cerebrospinal fluid; DID = dissociative identity disorder; EtOH = ethanol; MDD = major depressive disorder; PTSD = post‐traumatic stress disorder; SSCD = superior semicircular canal dehiscence; TBI = traumatic brain injury; VP = ventriculoperitoneal shunt.
While not the focus of the present study, for the control cohort, once each patient completed their final surgical procedure and medical management resolved any of the factors complicating their postoperative recovery, their presenting symptoms and signs were returned near their baseline before developing SSCDS/TWS.6, 7, 8, 9 This was not the case for the outlier cohort, as summarized in the descriptions of each participant below. The clinical course of each outlier is summarized below. For all 12 of these individuals, their outcomes were complicated by comorbid factors summarized in Table 4. Videos of 4 outlier participants have been published.10, 11, 12, 13
The focus of this study was in comparing the neuropsychology test results and identifying comorbidities between the outlier and control cohorts. Therefore, we are not reporting hearing and balance function outcomes as we plan to do so along with the extensive neuroimaging studies and cerebrospinal fluid proteomic analyses. Reporting the outcomes of surgical intervention for CT+ SSCD TWS itself is complicated and without a standardized approach.40, 41, 67, 68 To further complicate the reporting of outcomes, our present 2 participant cohorts included patients with CT‐ TWS alone and patients who developed CT‐ TWS after plugging of their SSCD. These latter 2 groups had RWR and therefore additional soft tissue placed in their middle ears which would produce a conductive hearing loss and associated decrease in cVEMP amplitude for air‐conduction cVEMP and oVEMP studies independent of resolving the TWS. Reporting hearing outcomes in SSCD plugging only patients is also not a straightforward and there remains no standardized methodology.40, 41, 67, 68 Again, the focus of this study was in the use of neuropsychology testing and clinician analysis to better understand comorbid diseases that can confound surgical outcomes for patients with third window syndrome.
Moving Platform Pressure Test
As shown in Table 1 and Figure 1A, for the CT+ SSCD patients, the response was absent or very small in response to ±500 daPa of pressure applied to the left ear and then the right ear. Figure 1A shows a negative moving platform pressure test prior to a left MCF craniotomy and plugging of the SSCD. This patient initially had resolution of his TWS symptoms, but later developed a CT‐ TWS in the ipsilateral ear. Figure 1B shows the robust response to the moving platform pressure test seen with CT‐ TWS prior to his undergoing left RWR.62, 63
Figure 1.
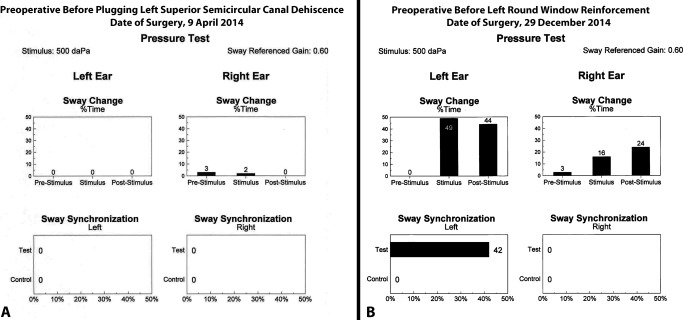
(A) Note the negative moving platform pressure test in response to ±500 daPa of pressure applied to the left ear and then the right ear prior to a left middle cranial fossa craniotomy and plugging of the CT positive (CT+) superior semicircular canal dehiscence. This patient initially had resolution of his third window syndrome (TWS) symptoms, but later developed a CT negative (CT‐) TWS in the ipsilateral ear. (B) There is a robust response to the moving platform pressure test in response to ±500 daPa of pressure applied to the left ear prior to his undergoing left round window reinforcement (RWR). For sway change as a percentage of time his left ear responses were 0% pre‐stimulus, 49% during the stimulus and 44% post‐stimulus. For sway synchronization, his left ear response was 42% during the test condition compared to 0% response during the control condition. While he also had a positive response on the contralateral side in sway change as a percentage of time, the sway synchronization was no different than the control condition. Clinically, he had no right‐sided TWS symptoms.
Computed Tomography of the Temporal Bone
All 12 of the outlier cohort and all 17 of the control cohort completed the high‐resolution temporal bone CT scans and additional postprocessing analysis. For the outlier cohort, as shown in Table 1, only participant 1 had a normal CT scan. The remaining 11 participants had CT+ radiographic evidence of SSCD and/or near‐SSCD. For the control cohort, all 17 completed the high‐resolution temporal bone CT scans and additional postprocessing. Seven control participants had CT+ evidence of SSCD TWS.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) was performed in outlier participants 2 through 12 (Table 2, n = 11) and control participants (n = 4) who developed a delayed CT‐ TWS and recurrence of their SSCDS/TWS symptoms to determine if their superior semicircular canal remained plugged. The MRI with CISS sequences demonstrated plugging of the superior semicircular canal in the 11 outlier participants and the 4 control participants.
Measurement of Cerebrospinal Fluid Opening Pressure
The opening CSF pressure for the outlier participants was within normal limits (normal range 10–20 cm H2O), except for outlier participant 2 whose opening pressure was abnormally high at 30 cm H2O.
Comprehensive Neurotologic, Neurologic and Psychologic Evaluations
In addition to one of the neurotologists authors (P.A.W.), two neurologists, one specializing in migraine (D.M.C.) and the other a neuro‐ophthalmologist (M.S.G.), and a psychologist (H.T.M‐P.) completed comprehensive evaluations. Summaries of the 12 participants in the outlier cohort are summarized below.
Participant 1
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. Since she was the only primary CT‐ TWS participant in the outlier cohort, demonstration of the decreased threshold of her cVEMP is shown in Figure 2. She did quite well for the first 7 weeks after her RWR surgery; however, she subsequently became disoriented and described having extremely poor memory. She was unable to walk more than 50 feet and began using a rolling walker and an untrained “service dog.” At that time, she described an experience of falling onto a car and had to be pulled away from the car. She felt like there was a magnetic attraction to the car and was pulled back onto the car by this force. She also gave an example of falling in a feed store. She described walking on her knees in a park and falling over. She fatigued easily and was having panic attacks, motion sickness, and inability to stand without a rolling walker. She also described having intermittent “deafness” in both ears. She did not have TWS symptoms postoperatively. At one postoperative visit her audiogram showed pure‐tone levels that were not consistent with her speech reception thresholds. She had 100% speech discrimination ability in the right ear and in the left ear, both with presentation levels of 30 dB. In contrast, her air‐conduction thresholds on the right unoperated ear were 65 dB at 250 Hz and the best air‐conduction threshold was 15 dB at 4000 Hz, she then fell to 60 dB at 6000 Hz. In the left her auditory thresholds by air‐conduction were 65 dB at 125 Hz and her best auditory threshold was 25 dB at 3000 Hz. Her computerized dynamic posturography also showed exaggeration. She had abnormally low equilibrium scores for somatosensory, visual, and vestibular, and a posterior malalignment of her center of gravity. She fell 7 times during the 18 trials of the Sensory Organization Test conditions and her falls were exaggerated. Auditory brainstem response (ABR) testing confirmed that she had normal auditory thresholds. As summarized in Table 4 she was found to have multiple comorbidities including a history of TBI, major depressive disorder (MDD), post‐traumatic stress disorder (PTSD), suicidal ideation, and after her neuropsychology testing and psychologic evaluation she was found to have factitious disorder. After sharing these findings with her she was referred to a neuropsychologist experienced in treating factitious disorder.
Figure 2.
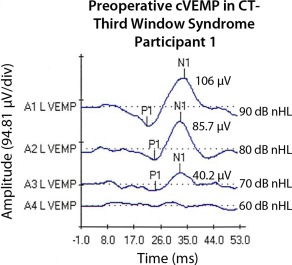
Preoperative cervical vestibular evoked potential (cVEMP) responses in outlier participant 1. This patient was the only primary CT negative third window syndrome (TWS) patient in the outlier cohort. Note the decreased threshold of 70 dB nHL (decibels normal hearing level) also typically, but not always, seen with CT positive TWS resulting from pathologies such as superior semicircular canal dehiscence.
Participant 2
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. Of the 12 outliers, it should be noted that she required an unusually high number of surgical procedures before her bilateral TWS symptoms resolved. In aggregate, she underwent 13 surgical procedures by the 2 surgeons (P.A.W. and G.J.G.) in 2 states. Two years after her superior canal plugging for bilateral CT+ SSCD TWS and confirmation of superior semicircular canal plugging by MRI with CISS sequences, she had a lumbar puncture (LP) to measure her opening pressure, which was found to be abnormally high at 30 cm H2O (normal range 10–20 cm H2O). She subsequently had a ventriculoperitoneal (VP) shunt placed to treat her diagnosis of pseudotumor cerebri. At CSF pressures that would limit her inner ear dysfunction she would experience debilitating headaches. With CSF pressures that minimized her headaches, she would repeatedly experience recurrence of her CT‐ TWS in both her left and right ear at variable intervals. Due to the unusually high number of repeated RWR surgery on the right, ultimately a decision was made to seal her right round window with Fuse glass ionomer cement as described in the Methods section. Her TWS symptoms are finally controlled and she is now pregnant with her first child. She plans to be delivered via caesarian section to avoid labor, bearing down, and risking the development of a recurrent CT‐ TWS. As summarized in Table 4 she was found to have a history of TBI with multiple concussions including a fall from a horse that resulted in loss of consciousness, as well as suicidal ideation. She is undergoing cognitive therapy treatment of her TBI.
Participant 3
His initial clinical and diagnostic findings are summarized in Table 1 and his surgical history is summarized in Table 2. He initially did well after his superior canal plugging for right CT+ SSCD TWS and had a marked reduction of his TWS symptoms. He also would have intervals where his clinical symptoms were consistent with endolymphatic hydrops (ELH). He continues with his medical management of ELH. He had recurrence of his TWS symptoms. Confirmation of his right superior semicircular canal plugging by MRI with CISS sequences was completed. After 4 RWR surgeries for CT‐ TWS a decision was made to seal his right round window with Fuse glass ionomer cement as described in the Methods section. His right‐sided TWS symptoms are somewhat controlled but he is developing mild left‐sided CT‐ TWS symptoms. His descriptions of his symptoms as well as his response to tuning fork placement on his knee and elbow, together with his perceived otolithic dysfunction with positive and negative pressure delivered to his right inner ear prior to another revision RWR surgery have been published.10 As summarized in Table 4 he was found to have the comorbidities of multiple concussions with resulting TBI and atypical migraine.
Participant 4
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. She initially did well after her superior semicircular canal plugging for bilateral CT+ SSCD TWS and was without TWS symptoms. However, 10 weeks after her second side, left SSCD plugging surgery, she contracted an influenza infection and following severe vomiting had recurrence of her left TWS symptoms. Plugging of both superior semicircular canals was confirmed by MRI with CISS sequences. She has had 3 RWR surgeries without resolution of her TWS symptoms. She also experienced progressive cognitive decline and ultimately was unable to return to work. As summarized in Table 4 she was found to have the comorbidity of multiple concussions with resulting TBI. She is undergoing cognitive therapy treatment of her TBI. Retrospective review of her preoperative video describing her symptoms highlights many clinical features consistent with TBI underscoring the overlap of TBI symptoms patients with otolithic dysfunction can have.11
Participant 5
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. As summarized in Table 4 she was found to have multiple comorbidities including a history of TBI, hemiplegic migraine, and suicidal ideation. She was found to have functional neurological symptom disorder, which in the past has been known as conversion disorder. Her presentation was interesting because in addition to her right CT+ SSCD TWS she had a history of migraine headaches and hemiplegic migraine. With surgical plugging of her SSCD, her symptoms resolved only to be manifest as a CT‐ TWS accompanied by her recurrence of her migraine headaches and hemiplegic migraines. Confirmation of her right superior semicircular canal plugging by MRI with CISS sequences was completed. As shown in her longitudinal video chronicle she developed left hemiplegic migraine with the application of ± 500 daPa of pressure applied to her right ear as well as with the acoustic presentation of the cVEMP stimuli.12 Interestingly, after her right RWR surgery she initially did well but returned with left‐sided CT‐ TWS symptoms. Her history, physical findings and diagnostic studies suggested that she had developed a left CT‐ TWS and therefore a RWR surgery was performed. She initially did well; however, her left‐sided symptoms recurred. After placement of Frenzel goggles one of the neurotologist authors (P.A.W.) placed a pneumatic otoscope into her left external auditory canal; however, no seal with the external canal was formed and while the patient could feel and hear the air movement, no positive or negative pressure was delivered to the ear, thereby creating a placebo stimulus.12 Despite this, the participant reported the onset of left‐sided motor weakness, migraine and slurred speech.12 There were no eye movements observed. These findings were consistent with the neuropsychology test findings of functional neurological symptom disorder (conversion disorder), including the variant of dissociative motor disorder, as will be described later. This diagnosis was also assigned based upon the neuropsychology clinical history intake interview (brief, unstructured) and the MBMD results. After sharing these findings with her she was referred to a neuropsychologist experienced in treating functional neurological symptom disorder (conversion disorder). She also had a history of hemiplegic migraine; however, after observing the onset of a hemiplegic migraine after a placebo delivery of positive and negative pressure to her ear this comorbidity diagnosis is suspect.12
Participant 6
His initial clinical and diagnostic findings are summarized in Table 1 and his surgical history is summarized in Table 2. He initially did well after his superior canal plugging for right CT+ SSCD TWS and was without TWS symptoms. However, 6 months after his right SSCD plugging surgery he had recurrence of his right TWS symptoms. Plugging of his right superior semicircular canal was confirmed by MRI with CISS sequences. He then underwent right RWR surgery 7.5 months after his initial surgery. He would have intervals where his clinical symptoms were more consistent with ELH. He continues with his medical management of ELH. By 15 months he was using a ladder without difficulty, but described a witnessed fall episode associated with “blacking out” for 5 hours followed by the onset of tremor. It was at this time that alcohol (EtOH) abuse was discovered with a daily consumption of 1.5 liters of vodka per day. He had recurrence of his right TWS symptoms 11 months after his right RWR surgery. At that time, he began experiencing sound‐induced nausea, dizziness, and exacerbation of his tremor. He also had recurrence of his autophony and described an experience with his dentist who was drilling a tooth in preparation of filling a cavity. He described the experience as the “vibration went directly into my right ear and I became nauseated and dizzy.” He elected conservative management with bedrest and his TWS symptoms resolved. He continued to have balance problems, tremor and cognitive dysfunction as well as his rate of alcohol consumption. As summarized in Table 4 he was found to have the comorbidities of EtOH abuse (1.75 liters of vodka per day), tremor, and 3 years after his initial surgery he developed left TWS symptoms and was recently found to have developed a left (contralateral) SSCD.
Participant 7
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. She had a left CT+ SSCD TWS treated with MCF approach and plugging of her SSCD. Early in her postoperative recovery she had 3 family members pass away unexpectedly, including her mother, and following air travel and intense crying she had recurrence of her TWS symptoms. After MRI with CISS sequences confirmed that the SSCD was plugged, and other diagnostic studies, she was assigned a diagnosis of CT‐ TWS and underwent left RWR surgery. As documented in her video chronicle her symptoms were markedly improved; however, by 3 months postoperatively her ipsilateral symptoms recurred. She was managed medically with bedrest and a low sodium diet but was returned to the operating room for revision surgery 4 months after the recurrence of her symptoms.13 She did well for 3 months and then while walking her 76‐pound dog while on a leash on a sandy beach had her dog suddenly jerk her to the side producing a whiplash‐like injury. She then had recurrence of her TWS symptoms. Her diagnostic studies were not consistent with a recurrent CT‐ TWS. Five months after her revision surgery she requested physician‐assisted suicide, which was denied. After the neuropsychology clinical history intake interview (brief, unstructured) and the MBMD results the diagnosis of somatic symptom disorder was assigned. Just as was performed for participant 5, one of the neurotologist authors (P.A.W.) used Frenzel goggles and then placed a pneumatic otoscope into her external auditory canals; however, no seal with the external canal was formed and while the participant could feel and hear the air movement, no positive or negative pressure was delivered to the ear, thereby creating a placebo stimulus.13 Despite this, the participant reported progressive dizziness and tilting was reported as the degree of pressure was “increased” in her “bad ear.”13 There were no eye movements observed. While the instructions and explanation provided by the senior author were leading and suggestive, as documented in the video, the participant nonetheless perceived these symptoms.13 In contrast to functional neurological symptom disorder (conversion disorder), there were no motor or prolonged sensory changes. As summarized in Table 4 she was found to have multiple comorbidities including a history of TBI, MDD, suicidal ideation, and vestibular migraine. After her neuropsychology testing and psychologic evaluation, she was found to have somatic symptom disorder. After sharing these findings with her she was referred to a neuropsychologist experienced in treating somatic symptom disorder.
Participant 8
His initial clinical and diagnostic findings are summarized in Table 1 and his surgical history is summarized in Table 2. He had a left superior semicircular canal plugging for a CT+ SSCD TWS. He initially had resolution of his TWS symptoms; however, he had recurrence of his left TWS symptoms 8 months after his initial surgery. After MRI with CISS sequences confirmed that the SSCD was plugged, and other diagnostic studies (Fig. 1), he was assigned a diagnosis of CT‐ TWS and underwent left RWR surgery. He remains without TWS symptoms. However, he has had progressive neurologic deterioration including: cognitive dysfunction; visual and auditory hallucinations; spatial disorientation with a sense of floating, detachment and out of body experiences; fatigue and left‐sided migraine headaches. MRI of his brain revealed atrophy more advanced than expected for his age. He remains unable to work. As summarized in Tables 1 and 4 he was found to have a history of TBI with multiple concussions and a recent motor vehicle accident. He is undergoing cognitive therapy treatment of his TBI.
Participant 9
His initial clinical and diagnostic findings are summarized in Table 1 and his surgical history is summarized in Table 2. He was initially treated for left ELH with medical management. Three years later he was treated with intratympanic dexamethasone metered perfusion. He was also experiencing progressive cognitive decline, fatigue and chronic headaches. He had a history of multiple concussions. In addition to these symptoms he subsequently developed left TWS symptoms. He was then found to have a CT+ near‐SSCD TWS and an asymptomatic right near‐SSCD. After undergoing left superior semicircular canal plugging. He had resolution of his left TWS symptoms; however, he developed right TWS symptoms 9 months after his initial surgery. After MRI with CISS sequences confirmed that the left SSCD was plugged, and other diagnostic studies, he was assigned a diagnosis of right CT+ near‐SSCD TWS and underwent plugging of his right superior semicircular canal. His TWS symptoms resolved, but he continued to have progressive cognitive decline, fatigue and chronic migraine headaches. He was without TWS symptoms for 5 months, but developed right‐sided symptoms again. After MRI with CISS sequences confirmed that both the left and right superior semicircular canals were plugged, and other diagnostic studies completed, he was assigned a diagnosis of right CT‐ TWS and underwent right RWR surgery. He remains without TWS symptoms. One year ago, he had a major motor vehicle accident with airbag deployment and complete loss of the value of his car. His gait worsened afterward and he continues to experience progressive cognitive decline and fatigue. He remains unable to work. As summarized in Tables 1 and 4 he was found to comorbidities of TBI and MDD with a history of multiple concussions and a recent motor vehicle accident. He is undergoing cognitive therapy treatment of his TBI.
Participant 10
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. She had bilateral CT+ SSCD TWS treated with MCF approach and plugging of her SSCDs. She had recurrence of her left TWS symptoms 4 months postoperatively. After MRI with CISS sequences confirmed that both the left and right superior semicircular canals were plugged, and other diagnostic studies completed, she was assigned a diagnosis of left CT‐ TWS and underwent left RWR surgery. She did well for 8 months on her right side without TWS symptoms but then developed recurrent right TWS symptoms with no redisposing event noted. She then underwent right revision RWR surgery. She was without left‐sided TWS symptoms for 6 months after her left revision RWR surgery. Because of the soft tissue in her middle ear, acoustic cVEMP findings were not helpful diagnostically. Pressure videonystagmography (VNG) yielded a subjective response on the left and coupled with her symptom description and how clinically dysfunctional she was–walking stick, unable to work, unable to contribute to distributed housework or be functional around her family. She also was experiencing MDD symptoms and suicidal ideation. She also had been communicating via social media with other patients who had undergone RWR using various cements and was intent on undergoing a similar procedure, regardless of where she had to travel to receive the procedure. One of the neurotologists (P.A.W.) ultimately agreed to perform the procedure with Fuse glass ionomer cement as described in the Methods section. She did not benefit from the procedure. Nineteen months after her right MCF SSCD plugging she developed an epidural abscess requiring removal of her cranioplasty material and 6 weeks of home intravenous antibiotics to resolve the P. acnes infection. As summarized in Table 4 she was found to have multiple comorbidities including a history of TBI, unilateral blindness, MDD, and suicidal ideation. After her neuropsychology testing and psychologic evaluation, she was found to have somatic symptom disorder. After sharing these findings with her she was at peace with this diagnosis and finally understood that her perceived symptoms were not otologic in nature. She was referred to a neuropsychologist experienced in treating somatic symptom disorder.
Participant 11
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. She had a right CT+ SSCD TWS diagnosed and followed medically for 5 months before she decided to undergo a right MCF approach and plugging of her SSCD. Initially she lost the sound‐induced nausea and dizziness but felt that her autophony was much worse after her surgery. By 3 months postoperatively she had right TWS symptoms again. For the next 13 months, her cognitive dysfunction worsened as did her light sensitivity. Her migraine headaches worsened and she developed coital cephalalgia. She was managed medically as a vestibular migraine patient. Her TBI symptoms worsened and she also suffered several additional falls with blows to her head over the 13 months postoperatively. By 12 months postoperatively she was spending 3 to 4 days per week in bed in addition to her normal nightly time in bed. After MRI with CISS sequences confirmed that the right superior semicircular canal was plugged, and other diagnostic studies were completed, she was assigned a diagnosis of right CT‐ TWS and underwent right RWR surgery. She no longer had right TWS symptoms but early after her right RWR surgery she reported left TWS symptoms. Physical examination and diagnostic studies showed no evidence of CT+ or CT‐ TWS. She continued having worsening of her memory, balance and fatigue without rotational receptor dysfunctional type of vertigo or otolithic dysfunction. She also slipped on a tarp and struck her head on concrete requiring hospitalization for management of her concussion. As summarized in Tables 1 and 4 she was found to have comorbidities of TBI, a history of multiple concussions and motor vehicle accidents, attention deficit hyperactivity disorder (ADHD), history of dissociative identity disorder (DID), MDD, as well as suicide ideation and attempts. She is undergoing cognitive therapy treatment of her TBI and psychologic and psychiatric care for her ADHD, DID, and MDD.
Participant 12
Her initial clinical and diagnostic findings are summarized in Table 1 and her surgical history is summarized in Table 2. She had a left CT+ SSCD TWS and a right near‐SSCD. After her left MCF approach and plugging of her SSCD she resolved her TWS symptoms on the left and remained without left TWS symptoms for 19 months. Four months after her left SSCD surgery the contribution of her right near‐SSCD became more evident to her. By 2 months after her right MCF approach and plugging of her superior semicircular canal she had resolution of her TWS symptoms, but now had a marked increase of her chronic migraine headaches. She also had symptoms consistent with ELH and was managed medically. By history she had 2 episodes of benign paroxysmal positional vertigo (BPPV), although this was never confirmed in the office. She had no TWS symptoms referable to her right ear until 4 months postoperatively. Plugging of both superior semicircular canals was confirmed by MRI with CISS sequences. After right RWR without perichondrium and cartilage for a CT‐ TWS, she had resolution of her TWS symptoms for 5.5 months, when they were noted to have recurred. At that time, she also had the onset of ocular migraines. She had another right RWR; however, with the revision surgery the perichondrium and cartilage technique was used for her RWR. She remains without right TWS symptoms. Nineteen months after her left plugging of the SSCD she had recurrence of her left TWS symptoms. Plugging of both superior semicircular canals was again confirmed by MRI with CISS sequences. After left RWR with perichondrium and cartilage for a CT‐ TWS, she had resolution of her TWS symptoms. By 2 years after her initial surgery she began having progressive neurologic deterioration associated with gait disturbance, cognitive decline and frequent falls. This continued to evolve and she experienced illusions of rolling, more frequent falls and impaired sleep. Her gait continued to deteriorate and walking became difficult and unsafe for her. As summarized in Table 4 she was found to have the comorbidites of drug‐induced Parkinson‐like symptoms and idiopathic neurologic deterioration. She was having extrapyramidal reactions secondary to the prochloperazine and doxepine prescribed for her by her primary physician. Eliminating these medications led to improvement of her Parkinson‐like symptoms; however, her idiopathic neurologic deterioration continues. She remains without TWS symptoms.
Neuropsychology Testing
Million Behavioral Medicine Diagnostic (MBMD)
Table 3 shows the mean, range, number of participants undergoing each test and the statistical comparison between groups completing the MBMD. For the Coping Styles, there was 1 subtest (Dejected). As shown in Table 3, there was no statistically significant difference between the outlier and control groups. For the Stress Moderators, there were 3 subtests (Functional Deficits versus Functional Competence, Pain Sensitivity versus Pain Tolerance, and Future Pessimism versus Future Optimism). All 3 of these subtests were highly statistically significantly worse in the outlier cohort; p = 0.001, p = 0.002, and 0.016, respectively. For the Management Guidelines, there were 2 subtests (Adjustment Difficulties and Psych Referral). For these 2 subtests, the outlier cohort scored statistically worse than the control cohort; p = 0.003 and p = 0.017, respectively. Completion of the MBMD required 30 minutes.
Patient Health Questionnaire‐9 (PHQ‐9)
Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the PQH‐9. The outlier cohort had worse scores than the control cohort and this difference was highly statistically different (p < 0.001). Completion of the PHQ‐9 required 10 minutes.
Generalized Anxiety Disorder‐7 (GAD‐7)
Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the GAD‐7. The outlier cohort had worse scores than the control cohort and this difference was highly statistically different (p = 0.009). Completion of the GAD‐7 required 10 minutes to complete.
Adverse Childhood Experiences Rating Scale (ACEs)
Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the ACEs. The outlier cohort was no different than the control group in regard to adverse childhood experiences scores, suggesting that this is not a contributing factor in why the outlier cohort had worse surgical outcomes (p = 0.861).
Wide Range Assessment of Memory and Learning‐2 (WRAML2)
For the WRAML2 3 of the 4 domains of Verbal Memory (2 subtests [Story Memory and Verbal Learning]), Visual Index (2 subtests [Design Memory and Picture Memory]), and Attention/Concentration Index (2 subtests [Finger Windows and Number Letter]) were completed. The fourth domain, Working Memory, was not included because this was accessed using the WAIS IV Working Memory Index. No statistical comparison could be made between the outlier cohort and the control cohort as only 2 control participants completed the subtests in the Visual Index domain (Table 3).
For the Verbal Memory domain, Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the Story Memory and Verbal Learning subtests. The outlier cohort had worse scores than the control cohort and the difference was highly statistically different (p = 0.002 and p = 0.002, respectively).
For the Attention/Concentration domain, Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the Finger Windows and Number Letter. The outlier cohort had worse scores than the control cohort and the difference was highly statistically different (p < 0.001); however, there was no difference for the Number Letter scores (p = 0.491).
Wechsler Adult Intellegence Scale IV
Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the Verbal Comprehension Index, Perceptual Reasoning Index, Processing Speed Index, and the Working Memory Index. No statistical comparison could be made between the outlier cohort and the control cohort as only 2 control participants completed the subtests in the Verbal Comprehension Index, Perceptual Reasoning Index, Processing Speed Index subtests (Table 3). For the Working Memory Index, the outlier cohort had worse scores than the control cohort and the difference was highly statistically different (p = 0.001). Since the composite WAIS IV intelligence score (FSIQ [Full Scale Intelligence Score]) can be compared to the Wide Range Intelligence Test (WRIT), this was completed.53, 54, 55, 56 There was no difference in the intelligence of the outlier and control cohort (n = 0.056); however, the difference was approaching statistical significance with the intelligence of the outlier cohort lower than the control cohort. Likewise, there was no difference in the Verbal Comprehension Index (p = 0.305) and Perceptual Reasoning Index (p = 0.240) between the 2 cohorts (Table 3).
Delis‐Kaplan Executive Function System (DKEFS)
Table 3 shows the mean, range, number of participants undergoing the Trial Making Test (Condition 4 [Number‐Letter Switching] and Condition 5 [Motor Speed]) and the statistical comparison between the groups completing these components of the DKEFS. As shown in Table 3, there was no statistically significant difference between the outlier and control groups for the Number‐Letter Switching (p = 0.110). As shown in Table 3, for Motor Speed there was a statistically significantly worse performance in the outlier cohort than in the control cohort (p = 0.005).
DISCUSSION
Based upon the comparison of this cohort of outliers to a cohort of participants who underwent surgical management of TWS, there were statistically significant worse outcomes in the neuropsychology scores in the outlier cohort (Table 3). While the current study greatly expanded the breadth of neuropsychology tests performed, most of the control cohort with the typical outcomes associated with the surgical management of TWS have been reported in a longitudinal study of neurocognitive recovery postoperatively3, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17 and were retested with the full neuropsychology test battery used in the present study. In the control cohort, they all had resolution of their TWS symptoms, marked improvement of their Dizziness Handicap Inventory scores, recovery of cognitive function and control or resolution of their migraine headaches, if present preoperatively.
The PHQ‐9, which is a self‐report measure used for diagnosis, screening, monitoring and measuring the severity of depression showed that there was a highly statistically significant higher (worse) scores in the outlier cohort as compared to the control cohort (p < 0.001). There was also a statistically significant elevation in GAD‐7 scores in the outlier cohort (p = 0.009) reflecting higher symptoms of generalized anxiety disorder.50 While GAD‐7 anxiety symptoms and depression symptoms frequently co‐occur, factor analysis confirm them to be distinct dimensions.50 Together with the domains of the MBMD analyzed statistically in this study, the PHQ‐9 and GAD‐7 were the most useful to differentiate the outliers from the control group and identify neuropsychiatric disorders.
Confounding Comorbidities: Present Preoperatively or Unmasked Postoperatively?
There were 11 outliers who initially had SSCD or near‐SSCD identified by high‐resolution temporal bone CT and confirmed at operation. One outlier (participant 1) was a primary CT‐ TWS patient (Tables 1 and 2; Fig. 2). While it is possible that the comorbidities developed after surgery to treat their third window syndrome(s), it is more likely that they were present before they developed their TWS. It is probable that the comorbidities were present before surgery, were not initially recognized, and then became exacerbated after their surgery. While there is no way to know, it should be noted is that one of the neurotologist authors (P.A.W.) did not recognize these comorbidities preoperatively, and the presence of these comorbidities confounded his assessment of their postoperative symptoms and decision for surgery. Based upon the data presented, it is hoped that other neurotologists will become aware of these issues and factors that could confound their patients' care; and that there will be an understanding that there are specific neuropsychology instruments that can be used to screen for these psychiatric/neuropsychological comorbidities.
Another limitation of this study was our challenge of how to present these data over time as this was a longitudinal study, with the greatest time from initial surgery to the last surgery being 4 years–plus additional follow up intervals. We attempted to convey this information in the individual case summaries included in the Results section.
Third Window Syndrome: A Spectrum of Various Locations of Perilymph Fistulae
The phenotype of SSCDS has been recognized in both CT+ TWS and CT‐ TWS. There have been reports of CT+ TWS with symptoms that are the same as SSCDS, but the patients do not have a SSCD. These include: posterior canal dehiscence, cochlea‐carotid dehiscence, cochlea‐facial nerve dehiscence, wide vestibular aqueduct and posterior semicircular canal‐jugular bulb dehiscence. In addition, we have reported CT‐ TWS with symptoms, physical examination, pseudoconductive hearing loss and cVEMPs that are the same as SSCDS–but without evidence of a bony dehiscence.2, 3, 5, 6, 7 Figures 1 and 2 and Table 1 summarize and illustrate the diagnostic and clinical features of TWS in this current patient cohort before their initial surgery; in particular participant 1 had a CT‐ TWS who had the classic cVEMP reduced threshold associated with SSCD yet had a normal CT scan (CT‐ TWS) (Fig. 2). In addition, Dennis Poe's group, in 2007, reported 4 patients who had all the same findings of SSCDS, but had no CT evidence of a dehiscence.31 For these reasons, SSCDS is an inaccurate and incorrect terminology for non‐SSCD and which is why we have introduced the more inclusive term “third window syndrome.” We encourage adoption of this nomenclature.
The moving platform pressure test was originally developed and FDA cleared for use in diagnosing “perilymph fistula” and utilizes the computerized dynamic posturography (CDP) platform (NeuroCom, Natus Medical Incorporated, Pleasanton, CA).62, 63 This of course, was before SSCD was recognized and all the other currently known CT+ third window syndrome bony dehiscence locations were discovered. By definition any hole or communication in the otic capsule is a fistula, which is in communication with perilymph. Yet, when the term “perilymph fistula” is used, most neurotologists do not think about a CT+ third window syndrome patient having a “perilymph fistula.” Table 1 qualitatively describes the magnitude of the moving platform pressure test in their initial presentation. Figure 1 illustrates how the moving platform pressure test results differ in CT+ TWS and CT‐ TWS in a patient with a CT+ SSCD confirmed at operation and who later developed a CT‐ TWS.
The moving platform pressure test was developed to detect abnormal postural sway associated with positive or negative pressure application to the external auditory canal. While the moving platform pressure test's utility was initially to detect an oval or round window perilymphatic fistula, we have employed the moving platform pressure test as a means to objectively identify physiologic responses in patients with third window syndrome (Table 1).
Audiometric and Cervical Vestibular Evoked Potential Findings in Third Window Syndrome
As summarized in Table 1, several of the TWS outlier participants had diagnostic findings on audiometry and with cVEMP testing that might be considered “discrepancies.” Table 1 reflects only the initial presentation and diagnostic testing results. While some of the results may appear to be “discrepancies,” the dogma that all SSCD patients have reduced cVEMP thresholds and a pseudoconductive hearing loss simply is not borne out in the literature. The cVEMP has been reported to be absent or without a reduced threshold, despite surgical confirmation of the SSCD.2, 3, 30, 31 Certainly most experienced surgeons who have cared for a large number of SSCD patients have shared these observations when discussing these phenomena with the 2 neurotologist authors. In Minor's 2005 series of 65 SSCD patients,30 the mean reduced threshold for the cVEMP was 81 ± 9 dB nHL–which means there would likely be an unknown, but certain percentage of his patients with SSCD who would not meet the 70 dB nHL threshold standard that we used in the present study and would be found to be “negative.” Likewise, the pseudoconductive hearing loss is not always present and in Poe's series of 65 patients published in 2007,31 86% had a pseudoconductive hearing loss, while 14% did not. In Minor's 2005 series only 70% had a pseudoconductive hearing loss of 10 dB or greater while 30% did not.30 Thus, what might appear to be a “discrepancy” is well‐described in the literature and should be factored into the decision‐making when managing patients with TWS.
Traumatic Brain Injury
TBI was the most common comorbidity observed in the outlier outcomes cohort with 10 of 12 (83.3%) having major and/or repeated TBI histories (Tables 1 and 4, subjects 1–5, and 7–11).11 While TBI was also common in the control cohort (8 of 17 [47.1%]), for all of these control participants there was only a single TBI episode.3 The Neurobehavioral Symptom Inventory (NSI) is widely used in the Department of Defense (DoD) for the evaluation of post‐concussive symptoms in service members.45 In addition, the Department of Veterans Affairs (VA) uses the NSI in its comprehensive TBI evaluation.45 The NSI has been selected by stakeholders in the DoD and VA as one of the core outcome measures for concussion health care.45, 69 Unfortunately, the NSI was not incorporated in the prospective study design of this research study, particularly since 10 of the 12 outliers were found to have repeated TBI incidents.
Research with the MBMD and acquired brain injury has revealed a pattern of clinical elevations in these Coping Scales: Introversive, Dejected, and Oppositional.70, 71 We focused our research with the MBMD on these scales plus the scales of Stress Moderators and Management Guidelines (Table 3), and clinically made note of clinical elevations in our participant groups in the other 26 scales comprising the battery during the formal clinical psychology evaluations of the outlier participants. Scales typically included in analyses reveal prevalence scores equal to or greater than 60; however, for the purposes of this study, we used a cut‐off score of 75 in order to maintain data manageability, and to focus our attention on clinical elevations indicative of decreased ability in both vocational and avocational activities of daily living. Additionally, differences in affective experience and regulation prevalent in the outlier participants can also be seen in higher reported levels of depression and anxiety; PHQ‐9 and GAD‐7, respectively. The affective dysregulation suggested by these self‐report measures, possibly exacerbated the symptoms and perception of functional deficits, pain sensitivity, and adjustment difficulties expressed by participants on the MBMD.
The WRAML2 was utilized as studies have shown that “diffuse closed head injuries, even of a mild nature, may affect memory and related attentional processes more so than other cognitive processes.”72, 73 Eighteen of our participants indicated a history of concussion and/or traumatic brain injury, although the frequency and severity of these TBI events was worse in the outlier cohort. As many as 65% of moderate to severe TBI patients report long‐term problems with cognitive functioning,74 and cognitive and behavioral changes are more closely associated with long‐term disability.75 Working Memory and Attention/Concentration are executive processes that play an integral role in memory functioning. Attention is critical to all areas of cognitive functioning, including language. The WAIS‐IV was chosen to provide a baseline postoperative IQ, and correlation with the test of premorbid function. Additionally, it was used to correlate Attention/Concentration scores with those obtained on the WRAML2.
The neuropsychology test findings associated with measurement of executive function will be discussed later; however, as discussed above, it should be noted that TBI is an important contributor to impaired executive function.76
Elevated Cerebrospinal Fluid Pressure
In 1987, Artistides Sismanis published his thesis for membership in the American Otological, Rhinological and Laryngological Society.77 This work focused on the otologic manifestations of benign intracranial hypertension (BIH) with increased intracranial pressure (ICP) without focal signs of neurological dysfunction.77 While the classic presenting symptoms of BIH are headache and/or visual disturbances, the otologic manifestations of this syndrome have been reported to include objective pulsatile tinnitus and low frequency hearing loss. The diagnosis is established by LP with measurement of opening pressure and elimination of other causes of ICP. Eleven of the 12 participants in the outlier cohort underwent fluoroscopic guided LP and measurement of opening pressure. Only participant 2 was found to have an elevated opening pressure. Elevated ICP was suspected when she began repeatedly having recurrence of her CT‐ TWS symptoms that would respond for intervals after RWR (Tables 1 and 2). She was ultimately treated with a VP shunt to treat her diagnosis of pseudotumor cerebri. After VP shunt placement, CSF pressures that would limit her inner ear dysfunction would result in debilitating headaches. With CSF pressures that minimized her headaches, she would repeatedly experience recurrence of her CT‐ TWS in both her left and right ear at variable intervals. Due to the unusually high number of repeated RWR surgery on the right, ultimately a decision was made to seal her right round window with Fuse glass ionomer cement and she is now 23 months without TWS symptoms. These observations suggest that the elevated ICP was transmitted to her inner ear and repeatedly created a third window with associated TWS symptoms.
Schutt and coworkers explored the relationship between obstructive sleep apnea (OSA) and SSCD.78 They found increased rates of OSA in those with SSCD suggesting a possible link between increased ICP and middle cranial fossa erosion (SSCD average 29.03% versus no SSCD 7.0%, P = 0.001).78 They also pointed out that OSA has been found to increase ICP, independent of obesity.78, 79, 80 In discussing the underlying mechanisms, they cited Sugita et al., who reported that transient hypercapnia and hypoxia during episodes of apnea lead to reflex increases in intracranial vascular volume and secondary increases in intracranial pressure.81 In that study, the ICP was monitored through a lumbar drain during sleep. CSF pressure increased between 50 and 750 mm H2O above baseline during apneic events. It was also found that ICP increases correlated to the duration of the apneic episode and decreases of O2 saturation.81 It is unknown if any of the participants from the outlier cohort or the control cohort have OSA, but if so, this could also be a contributing factor to the high recurrence of CT‐ TWS in the outlier cohort (Table 2).
Memory, Attention and Executive Function
Diffuse closed head injuries, or diffuse axonal injury, even of a seemingly mild nature, may affect memory and related attentional processes more than other cognitive processes.72, 82 “Therefore, while in one sense, there may be discrete memory centers, (e.g., hippocampus, cerebellum) there is also a non‐localized dimension of memory function83, 84, 85 as well, very much as Lashley86 concluded three‐quarter's of a century years ago when searching the ‘engram’ in his ablation studies with animals.”87 Memory itself is a complex and “constructive process through which we actively organize and shape information.”88 It is a 3‐step process comprising: 1) encoding: the processing of information into the memory system; 2) storage: the retention of encoded material over time; whenever people have access to information they no longer sense, memory is involved, and lastly; 3) retrieval: the process of getting the information out of memory storage. The brain encodes information in order to process it.
Encoding requires attention. According William James, “attention is the taking possession of the mind, in clear and vivid form, of one out of what may seem several simultaneously possible objects or trains of thoughts… It implies withdrawal from some things in order to deal effectively with others.”89 It is a basic component of our biology, and is present at birth… “[attention] help[s] us determine which events in our environment need to be attended to, [and as such it is] a process that aids in our ability to survive.”90 “Divided attention at encoding has been shown to significantly disrupt later memory for the studied information.” If one's attention is divided during this process, by internal or external distractors that mandate an equivalent level of processing, performance on other memory tasks will likely be compromised.91 This is especially evident during the retrieval process.92, 93, 94, 95 “It is reasonable to assume that without encoding and storage of information there can be no retrieval. But the converse is truer–without retrieval there is no evidence that either encoding or storage ever occurred… In essence, retrieval then is the measure of memory.”96 It is within this psychological construct that assessments of learning and memory are designed.
“Working memory involves the temporary storage and manipulation of information that is assumed to be necessary for a wide range of complex cognitive activities.”97 Working memory is an integral aspect of thinking, and its sub‐process, reasoning.92, 93, 94, 95 These processes allow one to figure out the meaning of what has just been said in conversation; to comprehend what one has just read; and to problem solve. The ability to do these tasks quickly and accurately depend on selective attention and vigilance, speed of processing ability, as well as the reserve of a priori and a posteriori knowledge. From these collective elements: attention; knowledge; working memory, processing speed; as well as perceptual (non‐verbal) reasoning abilities, arises what is known as intelligence, or intellectual ability; and in the paradigm of neuropsychology and cognitive neuroscience literature, cognitive efficiency.98
In an earlier study of patients undergoing surgery for CT+ SSCD TWS, CT‐ TWS as well as patients who had surgery for CT+ SSCD TWS and had surgery for a subsequent CT‐ TWS, we found impaired executive function in these patients preoperatively.3 We also performed neuropsychology testing longitudinally to better understand the rate and degree of recovery these patients experience postoperatively.3 In that study we also used the WRAML2; however, we included all 4 subtests of verbal memory, visual memory, attention/concentration and working memory to explore the impairment of executive function and recovery after surgery. We found that all the groups showed statistically significant improvement in the verbal subtest by the most recent neuropsychology test battery assessment and there was a statistically significant improvement in the visual subtest scores for the CT‐ TWS (RWR) group and the both CT+ SSCD TWS (plugging) and subsequently developed CT‐ TWS (RWR) group at both the initial postoperative assessment as well as at the most recent assessment.3 These patients were included in the control cohort for the present study. Preoperatively, the CT‐ TWS only (RWR) group showed abnormally low scores on the WRAML2 attention/concentration; however, the performance normalized after surgery. There were significant test time effects overall (improvement in all groups), initially (preoperative) worse in the CT+ SSCD TWS only (plugging) than the CT‐ TWS only (RWR) patients (p < 0.02, Fisher's Least Significant Difference test), but the same afterwards.3 Interestingly, analysis of WRAML2 of the Working Memory subtest revealed no significant differences preoperatively compared to the first and the most recent neuropsychology test battery assessments across all 3 groups.3 For this reason, we did not perform the WRAML2 Working Memory subtest in the present study.
In the present study, for the WRAML2, 3 of the 4 domains of Verbal Memory (2 subtests [Story Memory and Verbal Learning]), Visual Index (2 subtests [Design Memory and Picture Memory]), and Attention/Concentration Index (2 subtests [Finger Windows and Number Letter]) were completed. No statistical comparison could be made between the outlier cohort and the control cohort as only 2 control participants completed the subtests in the Visual Index domain (Table 3). The fourth domain, Working Memory, was not included because this was accessed using the WAIS IV Working Memory Index; as will be discussed later, there was a statistically worse performance in the outlier cohort as compared to the control cohort (p = 0.001).
For the Verbal Memory Domain, Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the Story Memory and Verbal Learning subtests. The outlier cohort had worse scores than the control cohort and the difference was highly statistically different (p = 0.002 and p = 0.002, respectively). Since multiple TBIs injuries occurred in the outlier cohort (n = 10 [Tables 1 and 4]), this would be one explanation for the differences observed in this aspect of executive function.
For the Attention/Concentration domain, Table 3 shows the mean score, range, number of participants undergoing each test and the statistical comparison between groups completing the Finger Windows and Number Letter. The outlier cohort had worse scores than the control cohort and the difference was highly statistically different (p < 0.001); however, there was no difference for the Number Letter scores (p = 0.491). The latter suggests that this subtest would not be useful to predict a poor outcome following TWS surgery. For the former, since multiple TBIs injuries occurred in the outlier cohort (n = 10 [Tables 1 and 4]), this would be one explanation for the differences observed in this aspect of executive function.
In our longitudinal cognitive dysfunction and recovery study described above, we also used the DKEFS and found that analysis of variance showed that there was significant postoperative improvement in both the Delis‐Kaplan Executive Function System (DKEFS) Motor Speed score (F(2,28) = 10.31, p < 0.01) and the Number–Letter Switching score (F(2,28) = 6.04, p < 0.05).3, 5 In the present study, there were differences found in the Motor Speed between the outlier cohort and the control cohort. As shown in Table 3, there was no statistically significant difference between the outlier and control groups for the Number‐Letter Switching (p = 0.110); however, for Motor Speed there was a statistically significantly worse performance in the outlier cohort than in the control cohort (p = 0.005). For the latter, since multiple TBIs injuries occurred in the outlier cohort (n = 10 [Tables 1 and 4]), this would be one explanation for the differences observed in the Motor Speed aspect of executive function.
Most will concede that men lack the ability to listen to more than one person speaking at a time, while women are able to listen to more than one person speaking at a time. This is more evident to women because of their experience in speaking to men. There is a surprising lack of literature exploring and defining this phenomenon (Judy Dubno, PhD personal communication September 18, 2015). One of the neurotologist authors (P.A.W.) has observed that patients with TWS who had the ability to listen to more than one person at a time in the past lose this ability. This ability typically recovers postoperatively. As summarized in Table 1, while this aspect of the study was qualitative, the women reported losing this ability and the men reported never having this ability. This is likely an executive function processing of auditory information.
Psychological Disorders
As summarized in Table 4, there was a high rate of psychological comorbidity (n = 6 [50%]) in the outlier cohort. The MBMD and the clinical psychology examinations were the most useful in identifying these comorbidities. Factitious disorder, functional neurologic symptom disorder (formerly conversion disorder) dissociative motor disorder variant, somatic symptom disorder, ADHD, DID, MDD, and PTSD were represented in 6 participants in the outlier cohort. Suicidal ideation was also common (Table 4 [n = 6]).
The psychological disorders, e.g., somatization, functional neurologic symptom disorder, somatic symptom disorder, general anxiety disorder, panic attacks and panic disorder, PTSD, represented by the outliers in our study all have anxiety as the basis. Anxiety, attentional and vestibular cortical and subcortical pathways have some overlap; however, all 3 converge together in the insular cortex and the anterior cingulate cortex.99 Congruent with both the James–Lange theory of emotions and Damasio's somatic marker hypothesis, the insular cortex is believed to be the brain site where the representations of bodily states are created in response to emotional stimuli and which mediates interoceptive awareness and the subjective experience of feelings.100 Insular dysfunction or hypofunction has also been associated with common neuropsychiatric disorders such as schizophrenia, autism, eating disorders, addiction, depression, and anxiety.101, 102 Subcortical structures known to be involved in anxiety are the amygdala and the hippocampus. The hippocampus is an important structure in the vestibular system as it is a key component in spatial sequencing and memory; of knowing where you are and where you are going.99
A study conducted at the University of Wisconsin, Madison looked at the way that those with a spider phobia reacted to the belief that they were going to encounter a spider. They found that those with the phobia had their dorsal anterior cingulate cortex, insula, and thalamus become more active than those without a phobia.103 Another study from the University of Wisconsin, Madison found that those with generalized anxiety disorder appeared to have a weaker connection between the white matter area of the brain and the prefrontal and anterior cortex. This was compared to those without generalized anxiety disorder and the results appeared to be significant.104 Another interesting relationship between anxiety and the brain is that long‐term anxiety may damage the brain in a way that could cause further anxiety. Researchers have found that when you leave your anxiety disorder untreated, the dorsomedial prefrontal cortex, anterior cingulate, hippocampus, dorsolateral prefrontal cortex, and orbitofrontal cortex all appear to decrease in size. The longer the anxiety goes untreated, the smaller and weaker they appear to be.105 While we will never know how much, or if, the TWS exacerbated or created the anxiety‐based neuropsychiatric disorders, we do know that: 1) for several of the outlier participants some of their anxiety‐based neuropsychiatric conditions predated their TWS symptoms; and 2) their comorbidities of neuropsychiatric disorders clinically confounded their surgical outcomes.
Factitious Disorder
In addition to her CT‐ TWS which resolved with RWR surgery, participant 1 was found to have factitious disorder, MDD, and PTSD. Factitious disorder (300.19 [DSM‐V], F68.1 [ICD‐10‐CM]) is a psychological disorder in which someone deceives others by appearing ill, by purposely getting sick, or by self‐injury. The “diagnosis of factitious disorder requires that the deception occur even in the absence of an external incentive. This suggests that individuals with factitious disorder are motivated by an internal incentive, where deceptive behaviors might serve the purpose of gaining nurturance, attention, or sympathy from family, friends, or medical providers.”106 While a preexisting medical condition may be present, the deceptive behavior or induction of injury associated with deceptions causes others to view such individuals as more ill or impaired, which can lead to excessive clinical intervention. Factitious disorder symptoms can range from mild (slight exaggeration of symptoms) to severe (reporting episodes of neurological symptoms: seizures, dizziness, or blacking out; or manipulating a laboratory test by adding blood to urine to falsely indicate an abnormality).107 This participant reported postoperative hearing loss in her contralateral, unoperated ear. While her behavioral audiometric testing suggested that her air‐conduction thresholds were consistent with this, her normal speech reception threshold and normal ABR thresholds suggested that her hearing remained normal. Factitious disorders have similarities to substance use disorders, eating disorders, impulse‐control disorders, and other established disorders related to both the persistence of the behavior and the intentional efforts to conceal the disordered behavior through deception. Although people with factitious disorder know they are causing their symptoms or illness, they may not understand the reasons for their behavior.
Functional Neurologic Symptom Disorder
As shown in Table 4, participant 5 was found to have functional neurological symptom disorder, which in the past has been known as conversion disorder (300.11 [DSM‐V, F44.4 to F44.7 [ICD‐10‐CM]). Her presentation was interesting because in addition to her right CT+ SSCD TWS she had a history of migraine headaches and hemiplegic migraine. With surgical plugging of her right SSCD, her TWS symptoms as well as her migraine headaches and hemiplegic migraines resolved only to be manifest later as a CT‐ TWS. This was also accompanied by her recurrence of her migraine headaches and hemiplegic migraines. As shown in her longitudinal video chronicle she developed left hemiplegic migraine with the application of ± 500 daPa of pressure applied to her right ear as well as with the acoustic presentation of the cVEMP stimuli.12 Interestingly, after her right RWR surgery she initially did well but returned with left‐sided CT‐ TWS symptoms. Her history, physical findings and diagnostic studies suggested that she had developed a left CT‐ TWS and therefore a RWR surgery was performed. She initially did well; however, her left‐sided symptoms recurred. After placement of Frenzel goggles one of the neurotologist authors (P.A.W.) placed a pneumatic otoscope into her left external auditory canal; however, no seal with the external canal was formed and while the patient could feel and hear the air movement, no positive or negative pressure was delivered to the ear, thereby creating a placebo stimulus.12 Despite this, the participant reported the onset of left‐sided motor weakness, migraine and slurred speech.12 There were no eye movements observed. These findings were interpreted as consistent with the neuropsychology test findings of functional neurological symptom disorder (formerly conversion disorder), including the variant of dissociative motor disorder, as will be described later. This diagnosis was also assigned based upon the neuropsychology clinical history intake interview (brief, unstructured) and the MBMD results.
Functional neurological symptom disorder (formerly known as conversion disorder) is a somatic disorder characterized by a persistent change in motor or sensory function with no medical or neurological cause. The symptoms are not “faked” or “made up” by the patient. Specific symptoms vary and can be manifested in a variety of ways. Some patients experience muscle weakness, numbness, or paralysis in one area of the whole body. Other patients experience abnormal movement, such as tremors, involuntary movements, seizures, or trouble walking. Functional neurological symptom disorder (conversion disorder) can also present as an inability or impairment in swallowing or difficulty speaking, e.g., slurred speech in participant 5. In some cases, patients experience specific sensory disturbances such as problems seeing or hearing. In many cases, the patient experiences a combination of these symptoms. Some patients experience an acute version of functional neurological symptom disorder (conversion disorder) that lasts only a few days or less. For some, symptoms can persist for weeks or months. According to the DSM‐V, functional neurological symptom disorder (conversion disorder) is most common after a stressful life event or period of stress and is 2 to 3 times more common in women than men.107
The DSM‐V is clear that although they are not required for diagnosis, patients often experience dissociative symptoms that begin around the onset of the episode. The DSM‐V further explains that many patients who present with functional neurological symptom disorder (conversion disorder) often have physical conditions unrelated to the current symptoms, and often have mental health issues such as anxiety or depression. Such was the case with participant 5 who had unilateral CT+ TWS followed by sequential bilateral CT‐ TWS. Many patients have a history of childhood sexual abuse.107 These are divided into 4 discrete categories: 1) dissociative motor disorders (F44.4 [ICD‐10‐CM]); 2) dissociative convulsions (F44.5 [ICD‐10‐CM]); 3) dissociative anesthesia and sensory loss (F44.6 [ICD‐10‐CM]); and 4) mixed dissociative disorders (F44.7 [ICD‐10‐CM]). With dissociative motor disorders, such as in participant 5, the most common varieties have a loss of ability to move the whole or a part of a limb or limbs. There may be close resemblance to almost any variety of ataxia, apraxia, akinesia, aphonia, dysarthria, dyskinesia, seizures, or paralysis. Dissociative convulsions may very closely and mimic epileptic seizures in terms of movements, but tongue‐biting, bruising due to falling, and incontinence of urine are rare, and consciousness is maintained or replaced by a state of stupor or trance. Anesthetic areas of skin often have boundaries that make it clear that they are associated with the patient's ideas about bodily functions, rather than medical knowledge. There may be differential loss between the sensory modalities which cannot be due to a neurological lesion. Sensory loss may be accompanied by complaints of paresthesia. Loss of vision and hearing are rarely complete in dissociative disorders; however, this is not always the case (e.g., psychogenic deafness). Finally, the mixed dissociative disorders include combinations of the disorders specified in ICD‐10‐CM, F44.0 through F44.6.
Somatic Symptom Disorder
Participants 7 and 10 were found to have somatic symptom disorder (300.82 [DSM‐V], F45.1 [ICD‐10‐CM]) as their major comorbidity, although both also had major depressive episodes and TBI history. As shown in Tables 1 and 2, participant 7 had a left CT+ TWS treated with MCF approach and plugging of her SSCD. Early in her postoperative recovery she had 2 family members pass away unexpectedly and following air travel and intense crying she had recurrence of her left TWS symptoms. After MRI with CISS sequences confirmed that the SSCD was plugged, and other diagnostic studies, she was assigned a diagnosis of CT‐ TWS and underwent left RWR surgery. As documented in her video chronicle her symptoms were markedly improved; however, her ipsilateral symptoms recurred and she was returned to the operating room for revision surgery.13 After the neuropsychology clinical history intake interview (brief, unstructured) and the MBMD results the diagnosis of somatic symptom disorder was assigned. Just as was performed for participant 5, one of the neurotologist authors (P.A.W.) used Frenzel goggles and then placed a pneumatic otoscope into her left and right external auditory canals; however, no seal with the external canal was formed and while the participant could feel and hear the air movement, no positive or negative pressure was delivered to the ear, thereby creating a placebo stimulus.13 Despite this, the participant reported progressive dizziness and tilting was reported as the degree of pressure was “increased.”13 There were no eye movements observed. While the instructions and explanation provided by one of the neurotologist authors (P.A.W.) were leading and suggestive, as documented in the video, the participant none‐the‐less perceived these symptoms.13 In contrast to functional neurological symptom disorder (conversion disorder) there were no motor or prolonged sensory changes.
Participant 10's management began before participant 7's did and her clinical course was more complicated. Unfortunately, one of the neurotologist authors (P.A.W.) repeatedly was convinced that her TWS had recurred despite soft evidence of this and regrettably returned her to the operating room multiple times, each time with short‐lived improvement. Experience with participant 10 increased the index of suspicion and thereby resulted in the surgeon avoiding the pattern of repeated surgery in participant 10 also had the same placebo “pressure stimulus” as participant 7 while wearing Frenzel lenses and reported progressive dizziness/tilting as the degree of pressure was “increased;” however, she did not consent to have her response video recorded. Both participant 7 and 10 had TBI, major depressive disorder, and suicidal ideation as additional comorbidities. In addition, participant 10 had PTSD and while it was suspected that participant 7 also had PTSD there was not enough information obtained to substantiate PTSD and this additional comorbidity for her.
The common feature of somatic symptom disorder is the prominence of somatic symptoms associated with significant distress and impairment. There may or may not be another diagnosed medical condition associated with these symptoms; however, as shown in Tables 1 and 4, TWS is one medical condition continuum that can be seen in somatic symptom disorder. This disorder is commonly encountered in primary care and other medical setting, but are less frequently encountered in in psychiatric and other mental health settings. Somatic symptom disorder involves having a significant focus on physical symptoms–such as pain, fatigue, neurological problems, gastrointestinal complaints, sexual symptoms–to the point that it can cause: 1) major emotional distress and problems functioning; 2) excessive thoughts, feelings and behaviors in response to physical symptoms may lead to frequent physician visits; and 3) even when other serious conditions have been excluded, these symptoms continue for 6 months or longer. For somatic symptom disorder, more important than the specific physical symptoms patients experience is the way they interpret and react to the symptoms and how they impact their daily life. These maladaptive thoughts, feelings and behaviors can include: 1) considering normal physical sensations as a sign of severe physical illness; 2) fearing the medical seriousness of symptoms, even when there is no evidence to support that concern; 3) feeling that medical evaluation and treatment have not been adequate; 4) being unresponsive to medical treatment or unusually sensitive to medication side effects; 5) having a more severe impairment than would usually be expected related to a medical condition. These were certainly all true for participants 7 and 10.
Unspecified Trauma and Stressor‐Related Disorder
Finally, another group of comorbidities found commonly in this cohort of outliers fall into the category of unspecified trauma‐ and stressor‐related disorder (309.9 [DSM‐V], F43.9 [ICD‐10‐CM]). Participants 1, 6, 7, 10, and 11 (Table 4). This category applies to presentations in which symptoms characteristic of a trauma‐ and stressor‐related disorder that cause clinically significant distress or impairment in social, occupational, or other important areas of functioning predominate but do not meet the full criteria for any of the disorders in the trauma‐ and stressor‐related disorders diagnostic class. In this case, it used as a diagnostic code for symptoms of multiple interpersonal traumas, e.g., neglect, emotional, physical, and sexual abuse, witnessing violence, familial substance abuse, familial mental illness and suicidal behaviors, separation, and loss. Individuals with a history of interpersonal trauma are more likely to seek treatment for other disorders or problematic symptoms (84%).107 Prominent characteristics include: 1) affect dysregulation (extreme reactions to mild stimuli, easily overwhelmed, difficulty calming or self‐soothing, suicidal ideation); 2) disturbances in consciousness or attention (dissociative episodes–depersonalization, derealization, intrusive images, increased internal arousal); 3) altered self‐perception (shame, sense of being “damaged,” sense of complete difference from others–no one can understand me, I am alone, I am special); 4) interpersonal difficulties (difficulty trusting others, isolation, anger outbursts); 5) somatization (persistent medical complaints that seem to defy medical explanation; overresponsive nervous system); 6) alterations in systems of meaning (hopelessness and despair, lack of purpose); and 7) if abuse history, faulty perceptions of the perpetrator, e.g., attribution of total power to abuser.108
Additional Comorbidities
An important goal of this work was to identify one or more screening instruments that would be helpful in predicting if the expected surgical outcome was likely. The Millon Behavioral Medicine Diagnostic assessment was designed to sharpen the accuracy in providing the psychosocial factors that can influence the course of treatment of medically ill individuals. It was originally developed from the Millon Behavioral Health Inventory by Theodore Millon and his colleagues.109 The MBMD developed normative data from 700 individuals with a variety of distinct medical conditions: heart problems, cancer, diabetes, gynecological problems, chronic pain (pain patient norms are based on 1,200 patients), accident/injury, migraines/headaches, neurological problems, gastrointestinal problems, organ transplants, and HIV/AIDS.110 Appropriate reference norms were obtained to ensure precision in clinical assessment of a wide range of medically‐based populations.110, 111 Scales were created for the MBMD to aid in identification of psychiatric syndromes, as well as to make treatment recommendations. Its validity is limited in the sense that it is a self‐report measure; however, it was developed in consonance with the most up‐to‐date knowledge of medical and psychological diagnostic and treatment protocols.
The MBMD was chosen for use in this study, in contrast to the Minnesota Multiphasic Personality Inventory112 and the Personality Assessment Inventory,113 due to the type and breadth of its normative medical population; ease of online administration, scoring and reporting; and short administration time of 30 minutes. Most importantly, the MBMD provides interpretation of psychological states and traits within a dimensional model, similar to that of the DSM‐V.114 Interpretation within this model adds complexity to the already, inherent complexity of psychological states and traits; on the flip side, this complexity engenders the possibility of a holistic and humane view of our study participants. After a qualitative review of the results of the entire MBMD, the largest differences between the 2 study groups for the 3 domains (and their subtests) Stress Moderators, Management Guidelines and Coping Styles, the statistical analysis was limited to these domains. This was only because of the magnitude of statistical comparisons that would have been needed and the magnitude of the reporting and discussing the findings.
As shown in Table 3, the outlier cohort was found to have statistically significant higher (worse) scores for the 3 subtests of Stress Moderators (Functional Deficits versus Functional Competence [p = 0.001], Pain Sensitivity versus Pain Tolerance [p = 0.002], and Future Pessimism versus Future Optimism [p = 0.016]). The Functional Deficits versus Functional Competence scale assesses the degree to which patients perceive that they are unable to carry out the vocational and avocational activities, roles, and responsibilities of daily life.46, 110 Indeed, the majority of the outlier cohort remain unable to work. The Pain Sensitivity versus Pain Tolerance scale addresses the tendency to be overly sensitized and reactive to mild/moderate bodily sensation and the degree to which symptoms are likely to dominate the clinical picture and potentially affect adjustment and recovery following treatment.46 Finally, the Future Pessimism versus Future Optimism scale assesses patients' perceptions of future health status. This patient characteristic is hypothesized to influence a number of medical outcomes including adherence to and confidence in medical regimens, emotional reactions to diagnostic test results, and possibly the actual physical course of disease.46 Patients with high scores on the Future Pessimism Scale probably do not anticipate a productive life, which is an accurate assessment of the outlier cohort.
As shown in Table 3, the outlier cohort was found to have statistically significant higher (worse) scores for the 2 subtests of Management Guidelines (Adjustment Difficulties [p = 0.003], and Psych Referral [p = 0.017]). The Adjustment Difficulties scale assesses the risk of treatment complications due to the patient's coping styles, current psychological issues operating in the patient's life, their available resources for managing stress, and their risk of engaging in unhealthy behavior. In general, this scale assesses problems that may call for the services of physicians, nurses, health psychologists, and other counseling and behavioral medicine specialists.46 This was true for the outlier cohort. Not only did they require more resources in patient telephone calls, email exchanges and office visits, they were concurrently engaged with multiple specialists, therapists and alternative health care providers. The Psych Referral scale indicates whether the patient might benefit from psychosocial intervention and the likelihood that they would respond well to a specific type of intervention.46 All 12 participants in the outlier cohort ultimately were referred for psychologic and/or psychiatric care.
As shown in Table 3, the outlier cohort was found to be no different than the control cohort for the one subtest of Coping Styles (Dejected [p = 0.127]). For those who record a high score on the Dejected scale are inclined to be persistently and characteristically disheartened, unable to experience the pleasures of joys of life. Notably disconsolate and with a somewhat hopeless orientation, they are easily disposed to give up trying to work through their emotional or physical problems. This pessimistic inclination will call for greater effort than usual from health care staff.46 There was no difference in the scores in the 2 cohorts; however, there was a wide range in scores in both groups (10–111 [outlier cohort] versus 10–94 [control cohort]). This suggests that there are individuals in each cohort who scored highly in this dimension, but this scale was not predictive of identifying a patient who may experience an outcome worse than expected following TWS surgery.
Based upon these findings, it is our recommendation that the MBMD, coupled with the PHQ‐9 and GAD‐7, be completed preoperatively before surgery for TWS and used to screen for patients who may ultimately have confounded outcomes than expected with resolution of their TWS symptoms following their surgical procedure. Fifty minutes was the total time needed for completion of the MBMD (30 minutes), PHQ‐9 (10 minutes) and the GAD‐7 (10 minutes).
Intelligence
In our study of the longitudinal cognitive dysfunction and recovery study of patients before and after surgery to treat CT+ TWS, CT‐ TWS and both, we found that there was no change in intelligence as measured by the WRIT.3 We concluded that the lack of change in intelligence was not surprising and suggested that it served as an internal control for these subjects; since it would not be expected that these chronic, uncompensated gravitational receptor asymmetries would alter inherent intelligence. In the present study, we compared the WRIT in the control cohort to the WAIS IV global FSIQ score in the outlier group and there was no difference between the cohorts (p = 0.056). Likewise, there was no difference in the Verbal Comprehension Index (p = 0.305) and Perceptual Reasoning Index (p = 0.240) between the 2 cohorts (Table 3).
The Verbal Comprehension Index primarily measures verbal concept formation, abstract reasoning, and associative and categorical thinking; as well as measuring crystallized intelligence, which is the information that a person has stored in memory about people, places, and things (this fund of stored memories, or knowledge, increases with education); and degree of language development.54 Again, there was no difference between the 2 cohorts.
The Perceptual Reasoning Index measures the ability to analyze and synthesize abstract visual information, nonverbal concept formation and reasoning, and the ability to separate figure–ground in visual stimuli; as well as measuring perceptual organization (knowledge of part–whole relationships, classification, and spatial ability) and measuring the ability to analyze and synthesize abstract visual stimuli and to anticipate relationships among parts of a whole. There are subtests that are designed to measure rote learning, attention, encoding, auditory processing, visuo–spatial imaging, and mental manipulation; and measure concentration, short‐ and long‐term memory, numerical reasoning ability, and mental manipulation.54 Again, there was no difference between the 2 cohorts.
There were enough participants who completed the Working Memory Index of the WAIS IV across both study cohorts to directly compare this component of the test. The working memory was highly significantly worse in the outlier cohort than in the control cohort (n = 0.001) (Table 3). All 4 subtests of the Working Memory Index are measures of fluid intelligence–the ability to form concepts, reason, and identify similarities; it is intuitive and embodies the activity involved when forming new mental constructs, seeing complex relationships, and solving problems.54 Therefore, the executive function of the outlier cohort was found to be worse than in the control cohort.
CONCLUSIONS
These data represent the first demonstration that comorbid conditions can confound the surgical outcomes of CT+ SSCD TWS and CT‐ TWS patients. The MBMD, PHQ‐9 and GAD‐7 results suggest that these instruments would useful as a screening tool preoperatively to identify psychological comorbidities that could affect outcomes. The identification of these comorbid psychological as well as other neurological degenerative disease processes can lead to alternate clinical management pathways for these patients.
ACKNOWLEDGMENTS
The authors thank Detlev Boison, PhD for his review and discussion of these findings.
Funding: The Legacy Good Samaritan Foundation and the Ear and Skull Base Center provided funding to help support this work.
The authors have no conflicts of interest to disclose.
IRB Approval Number: LRI‐685
BIBLIOGRAPHY
- 1. Bilgrei R. The psychology of vestibular disorders part I: cognitive aspects of vestibular disorders. Available at: https://vestibular.org/sites/default/files/page_files/Documents/Cognitive Aspects of Vestibular Disorders.pdf Accessed June 10, 2017.
- 2. Wackym PA, Wood SJ, Siker DA, Carter DM. Otic capsule dehiscence syndrome: superior canal dehiscence syndrome with no radiographically visible dehiscence. Ear Nose Throat J 2015;94:E8–E24. [DOI] [PubMed] [Google Scholar]
- 3. Wackym PA, Balaban CD, Mackay HT, et al. Longitudinal cognitive and neurobehavioral functional outcomes after repairing otic capsule dehiscence. Otol Neurotol 2016;37:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black FO, Pesznecker S, Norton T, et al. Surgical management of perilymphatic fistulas: a Portland experience. Am J Otol 1992;13:254–262. [PubMed] [Google Scholar]
- 5. Wackym PA. Traumatic otic capsule dehiscence syndrome after snowboarding accident. Control participant 1 describing his symptoms before and after round window reinforcement surgery. Available at: https://www.youtube.com/watch?v=7azu9sszZSk Accessed June 10, 2017.
- 6. Wackym PA. Right computed tomography negative third window syndrome. Right perilymph fistula: dizziness, migraine headaches and cognitive dysfunction. Control participant 2 describing her symptoms before and after round window reinforcement surgery. Available at: https://www.youtube.com/watch?v=ETjsJocMBYk Accessed June 10, 2017.
- 7. Wackym PA. Left computed tomography negative third window syndrome (otic capsule dehiscence syndrome) with hemiplegic migraine. Control participant 5 describing her symptoms before and after round window reinforcement surgery. Available at: https://www.youtube.com/watch?v=9xVNxNGys1w Accessed June 10, 2017.
- 8. Wackym PA. Cognitive dysfunction due to third window syndrome (otic capsule dehiscence syndrome). The second patient, who had a computed tomography positive superior semicircular canal dehiscence third window syndrome, describing her cognitive dysfunction and recovery is control participant 14. Available at: https://www.youtube.com/watch?v=1pNZUX1a04g. Accessed June 10, 2017.
- 9. Wackym PA. Computed tomography positive right superior semicircular canal dehiscence third window syndrome. Control participant 17 describes her migraine headaches, light sensitivity and third window syndrome symptoms before and after surgery. Available at: https://youtu.be/er4k8NZrG2I. Accessed June 10, 2017.
- 10. Wackym PA. Tuning fork testing in third window syndrome (otic capsule dehiscence syndrome). The first video segment demonstrates the elicited response after applying a 128 Hz tuning fork to the elbow and knee while the second video segment demonstrates the response elicited after application of positive and negative pressure to the ear producing a third window syndrome. These two video segments are of outlier participant 3. Available at: https://www.youtube.com/watch?v=Szp_kO8oVos. Accessed June 10, 2017.
- 11. Wackym PA. Computed tomography positive superior left semicircular canal dehiscence and right near‐superior semicircular canal dehiscence third window syndrome. The first video segment is preoperative, while the second video segment is after her left‐sided superior semicircular canal plugging and before her right‐sided surgery. This patient is outlier participant 4. Available at: https://youtu.be/d‐‐Wk5‐C8xk. Accessed June 10, 2017.
- 12. Wackym PA. Recurrent Third Window Syndrome Co‐Morbidities: Functional Neurological Symptom Disorder. Computed tomography positive superior right semicircular canal dehiscence and subsequent bilateral computed tomography negative third window syndrome. This patient was found to have functional neurologic disorder, dissociative motor disorder variant, manifest as left hemiplegic migraine. The last video segment demonstrates the response elicited after application of a placebo “positive and negative pressure” stimulus to the left ear producing third window syndrome symptoms and the onset of “hemiplegic migraine.” This patient is outlier participant 5. Available at: https://youtu.be/AgUy07QxTxo. Accessed June 10, 2017.
- 13. Wackym PA. Computed tomography positive superior left semicircular canal dehiscence and subsequent left computed tomography negative third window syndrome. This patient was found to have somatic symptom disorder manifest as left third window syndrome. The last video segment demonstrates the response elicited after application of a placebo “positive and negative pressure” stimulus to the “bad ear” (left) producing third window syndrome symptoms and no response when the placebo stimulus was applied to the “good ear.” This patient is outlier participant 7. Available at: https://youtu.be/dW‐rCbrkHSE. Accessed June 10, 2017.
- 14. Wackym PA. Computed tomography negative third window syndrome (otic capsule dehiscence syndrome) in one ear after a bicycle accident. Available at: https://www.youtube.com/watch?v=fkdFozzQBEc. Accessed June 10, 2017.
- 15. Wackym PA. Computed tomography negative traumatic third window syndrome (otic capsule dehiscence syndrome) after a skiing accident. Available at: https://www.youtube.com/watch?v=2‐kD59ygKrE. Accessed June 10, 2017.
- 16. Wackym PA. Computed tomography negative third window syndrome (otic capsule dehiscence syndrome) in one ear after a car accident. Available at: https://www.youtube.com/watch?v=1Nl9T6etxqM. Accessed June 10, 2017.
- 17. Wackym PA. Vestibular migraine. Patient video describing symptoms before and after treatment with Topamax. Available at: https://www.youtube.com/watch?v=Zy7YjCDnLYM. Accessed June 10, 2017.
- 18.Video stories of patients with third window syndrome (otic capsule dehiscence syndrome) describing their symptoms before and after surgical repair. Available at: https://www.youtube.com/user/EarInformation. Accessed June 10, 2017.
- 19. Goto F, Naoki Oishi N, Tsutsumi T, Ogawa K. A case of intractable suspected perilymph fistula with severe depression. Psychiatry Investig 2014;11:499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agrawal Y, Carey JP, Della Santina CC, Schubert MC, Minor LB. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med 2009;169:938–944. [DOI] [PubMed] [Google Scholar]
- 21. Balaban C, Hoffer ME, Szczupak M, et al. Oculomotor, vestibular, and reaction time tests in mild traumatic brain injury. PLoS One 2016;11:e0162168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bigelow RT, Agrawal Y. Vestibular involvement in cognition: Visuospatial ability, attention, executive function, and memory. J Vestib Res 2015;25:73–89. [DOI] [PubMed] [Google Scholar]
- 23. Bigelow RT, Semenov YR, Hoffman HJ, Agrawal Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: Data from the 2008 National Health Interview Survey. J Neurol Neurosurg Psychiatry 2016;87:367–372. [DOI] [PubMed] [Google Scholar]
- 24. Bigelow RT, Semenov YR, Trevino CG, et al. Association between visuospatial ability and vestibular function in the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc 2015;63:1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association between vestibular and cognitive function in US adults: data from the National Health and Nutrition Examination Survey. J Gerontol A Biol Sci Med Sci 2016;71:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhai, F , Wang J, Zhang Y, Dai C‐F. Quantitative analysis of psychiatric disorders in intractable peripheral vertiginous patients: A prospective study. Otol Neurotol 2016;37:539–544. [DOI] [PubMed] [Google Scholar]
- 27. Grimm RJ, Hemenway WG, Lebray PR, Black FO. The perilymph fistula syndrome defined in mild head trauma. Acta Otolaryngol Suppl 1989;464:1–40. [DOI] [PubMed] [Google Scholar]
- 28. Gizzi M, Zlotnick M, Cicerone K, Riley E. Vestibular disease and cognitive dysfunction: no evidence for a causal connection. J Head Trauma Rehabil 2003;18:398–407. [DOI] [PubMed] [Google Scholar]
- 29. Minor LB, Solomon D, Zinreich JS, Zee DS. Sound‐ and/or pressure‐induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg 1998;124:249–258. [DOI] [PubMed] [Google Scholar]
- 30. Minor LB. Clinical manifestations of superior semicircular canal dehiscence. Laryngoscope 2005;115:1717–1727. [DOI] [PubMed] [Google Scholar]
- 31. Zhou G, Gopen Q, Poe DS. Clinical and diagnostic characterization of canal dehiscence syndrome: a great otologic mimicker. Otol Neurotol 2007;28:920–6. [PubMed] [Google Scholar]
- 32. Young L, Isaacson B. Cochlear and petrous carotid canal erosion secondary to cholesteatoma. Otol Neurotol 2010;31:697–698. [DOI] [PubMed] [Google Scholar]
- 33. Meiklejohn DA, Corrales CE, Boldt BM, et al. Pediatric semicircular canal dehiscence: radiographic and histologic prevalence, with clinical correlations. Otol Neurotol 2015;36:1383–1389. [DOI] [PubMed] [Google Scholar]
- 34. Park JJ, Shen A, Loberg C, Westhofen M. The relationship between jugular bulb position and jugular bulb related inner ear dehiscence: a retrospective analysis. Am J Otolaryngol 2015;36:347–351. [DOI] [PubMed] [Google Scholar]
- 35. Gopen Q, Zhou G, Poe D, Kenna M, Jones D. Posterior semicircular canal dehiscence: first reported case series. Otol Neurotol 2010;31:339–344. [DOI] [PubMed] [Google Scholar]
- 36. Bear ZW, McEvoy TP, Mikulec AA. Quantification of hearing loss in patients with posterior semicircular canal dehiscence. Acta Otolaryngol 2015;135:974–977. [DOI] [PubMed] [Google Scholar]
- 37. Elmali M, Poltat AV, Kucuk H, Atmaca S, Aksoy A. Semicircular canal dehiscence: frequency and distribution on temporal bone CT and its relationship with the clinical outcomes. Eur J Radiol 2013;82:e606–e609. [DOI] [PubMed] [Google Scholar]
- 38. Blake DM, Tomovic S, Vazquez A, Lee HJ, Jyung RW. Cochlear‐facial dehiscence–a newly described entity. Laryngoscope 2014;124:283–289. [DOI] [PubMed] [Google Scholar]
- 39. Fang CH, Chung SY, Blake DM, et al. Prevalence of cochlear‐facial dehiscence in a study of 1,020 temporal bone specimens. Otol Neurotol 2016;37:967–972. [DOI] [PubMed] [Google Scholar]
- 40. Ward BK, Wenzel A, Ritzl EK, et al. Near‐dehiscence: clinical findings in patients with thin bone over the superior semicircular canal. Otol Neurotol 2013;34:1421–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sharon JD, Pross SE, Ward BK, Carey JP. Revision surgery for superior canal dehiscence syndrome. Otol Neurotol 2016;37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 42. Manzari L, Scagnelli P. Large bilateral internal auditory meatus associated with bilateral superior semicircular canal dehiscence. Ear Nose Throat J 2013;92:25–33. [DOI] [PubMed] [Google Scholar]
- 43. Hoffer ME, Szczupak M, Balaban C. Clinical trials in mild traumatic brain injury. J Neurosci Methods 2016;272:77–81. [DOI] [PubMed] [Google Scholar]
- 44. Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B Amelioration of acute sequelae of blast induced mild traumatic brain injury by N‐acetyl cysteine: A double‐blind, placebo controlled study. PLoS ONE 2013;8:e54163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DoD Worldwide Numbers for TBI. Defense and Veterans Brain Injury Center. Available at: http://dvbic.dcoe.mil/dod‐worldwide‐numbers‐tbi. Accessed June 10, 2017.
- 46. Millon T, Antoni M, Millon C, Minor S, Grossman S. Millon Behavioral Medicine Diagnostic Manual, 2nd Edition Bloomington, MN: PsychCorp; 2006. [Google Scholar]
- 47. Cronbach LJ, Warrington WG. Time‐limit tests: estimating their reliability and degree of speeding. Psychometrika 1951;16:167–188. [DOI] [PubMed] [Google Scholar]
- 48. Kroenke K, Spitzer RL, Williams JBW. The PHQ‐9 Validity of a Brief Depression Severity Measure. J Gen Intern Med 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moran M. Patient Health Questionnaire, 2013. Available at: http://www.statisticssolutions.com/. Accessed June 10, 2017.
- 50. Spitzer R, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: The GAD‐7. Arch Internal Med 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 51. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998;14:245–258. [DOI] [PubMed] [Google Scholar]
- 52. Larkin H, Felitti VJ, Anda RF. Social work and adverse childhood experiences research: implications for practice and health policy. Soc Work Public Health 2014;29:1–16. [DOI] [PubMed] [Google Scholar]
- 53. Sheslow D, Adams W. Wide Range Assessment of Memory and Learning Administration and Technical Manual, 2nd Edition Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 54. Coalson D, Raiford S. Wechsler Adult Intelligence Scale Technical and Interpretive Manual, 4th Edition San Antonio, TX: Pearson; 2008. [Google Scholar]
- 55. Weiss L, Saklofske D, Coalson D, Raiford S. (Eds.). WAIS‐IV Clinical Use and Interpretation. London, UK: Academic Press; 2010. [Google Scholar]
- 56. Glutting J, Adams W, Sheslow D. Wide Range Intelligence Test: WRIT. Wilmington, DE: Wide Range; 2000. [Google Scholar]
- 57. Delis D, Kaplan E, Kramer J. Delis‐Kaplan Executive Function System Examiner's Manual. Bloomington, MN: PsychCorp; 2001. [Google Scholar]
- 58. Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis‐Kaplan Executive Function System: an update. J Int Neuropsychol Soc 2004;10:301–303. [DOI] [PubMed] [Google Scholar]
- 59. Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 60. Margolis RH, Rieks D, Fournier EM, et al. Tympanic electrocochleography for diagnosis of Meniere's disease. Arch Otolaryngol Head Neck Surg 1995;121:44–55. [DOI] [PubMed] [Google Scholar]
- 61. Wackym PA, Ratigan JA, Birck JD, et al. Rapid cVEMP and oVEMP responses elicited by a novel head striker and recording device. Otol Neurotol 2012;33:1392–1400. [DOI] [PubMed] [Google Scholar]
- 62. Black FO, Lilly DJ, Peterka RJ, et al. The dynamic posturographic pressure test for the presumptive diagnosis of perilymph fistulas. Neurol Clin 1990;8:361–374. [PubMed] [Google Scholar]
- 63. Black FO, Lilly DJ, Nashner LM, Peterka RJ, Pesznecker SC. Quantitative diagnostic test for perilymph fistulas. Otolaryngol Head Neck Surg 1987;96:125–134. [DOI] [PubMed] [Google Scholar]
- 64. Black FO, Pesznecker S, Norton T, et al. Surgical management of perilymph fistulas: a new technique. Arch Otolaryngol 1991;117:641–648. [DOI] [PubMed] [Google Scholar]
- 65. Silverstein H, Kartush JM, Parnes LS, et al. Round window reinforcement for superior semicircular canal dehiscence: a retrospective multi‐center case series. Am J Otolaryngol 2014;35:286–293. [DOI] [PubMed] [Google Scholar]
- 66.R: A language and environment for statistical computing. Available at: http://www.gbif.org/resource/81287. Accessed June 10, 2017.
- 67. Vlastarakos PV, Proikas K, Tavoulari E, Kikidis D, Maragoudakis P, Nikolopoulous TP. Efficacy assessment and complications of surgical management for superior semicircular canal dehiscence: a meta‐analysis of published interventional studies. Eur Arch Otorhinolaryngol 2009;266:177–186. [DOI] [PubMed] [Google Scholar]
- 68. Ziylan F, Kinaci A, Beynon AJ, Kunst HPM. A comparison of surgical treatments for superior semicircular canal dehiscence: A systematic review. Otol Neurotol 2017;38:1–10. [DOI] [PubMed] [Google Scholar]
- 69. Meterko M, Baker E, Stolzmann KL, Hendricks AM, Cicerone KD, Lew HL. Psychometric assessment of the Neurobehavioral Symptom Inventory‐22: the structure of persistent post‐concussive symptoms following deployment‐related mild traumatic brain injury among veterans. J Head Trauma Rehab 2012;27:55–62. [DOI] [PubMed] [Google Scholar]
- 70. Beck KD. Personality and the Prediction of Outcome Following Rehabilitation in Persons with Acquired Brain Injuries: The Millon Behavioral Medicine Diagnostic (MBMD). Ann Arbor, MI: ProQuest; 2008. [Google Scholar]
- 71. Beck KD, Franks SF, Hall JR. Postinjury personality and outcome in acquired brain injury: the Millon Behavioral Medicine Diagnostic . Physical Med Rehab 2010;2:195–201. [DOI] [PubMed] [Google Scholar]
- 72. Levin H, Mattis S, Ronald R, et al. Neuro‐behavioral outcome following minor head injury: a three‐center study. J Neurosurg 1987;66:234–243. [DOI] [PubMed] [Google Scholar]
- 73. Rabinowitz AR, Levin HS. Cognitive sequelae of traumatic brain injury. Psychiatr Clin North Am 2014;37:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Whiteneck G, Gerhart K, Cusick C. Identifying environmental factors that influence the outcomes of people with traumatic brain injury. J Head Trauma Rehab 2004;19:191–204. [DOI] [PubMed] [Google Scholar]
- 75. NIH Consensus Development Panel on Rehabilitation of Persons with Traumatic Brain Injury . Rehabilitation of persons with traumatic brain injury. JAMA 1999;282:974–983. [PubMed] [Google Scholar]
- 76. Spitz G, Ponsford J, Rudzki D, Maller JJ. Association between cognitive performance and functional outcome following traumatic brain injury: a longitudinal multilevel examination. Neuropsychology 2012;26:604–612. [DOI] [PubMed] [Google Scholar]
- 77. Sismanis A. Otologic manifestations of benign intracranial hypertension syndrome: diagnosis and management. Laryngoscope 1987;97(8 Pt 2 Suppl 42):1–17. [DOI] [PubMed] [Google Scholar]
- 78. Schutt CA, Neubauer P, Samy RN, et al. The correlation between obesity, obstructive sleep apnea, and superior semicircular canal dehiscence: a new explanation for an increasingly common problem. Otol Neurotol 2015;36:551–554. [DOI] [PubMed] [Google Scholar]
- 79. Jennum P, Børgesen SE. Intracranial pressure and obstructive sleep apnea. Chest 1989;95:279–283. [DOI] [PubMed] [Google Scholar]
- 80. Lee AG, Golnik K, Kardon R, Wall M, Eggenberger E, Yedavally S. Sleep apnea and intracranial hypertension in men. Ophthalmology 2002;109:482–485. [DOI] [PubMed] [Google Scholar]
- 81. Sugita Y, Iijima S, Teshima Y, et al. Marked episodic elevation of cerebrospinal fluid pressure during nocturnal sleep in patients with sleep apnea hypersomnia syndrome. Electroencephalogr Clin Neurophysiol 1985;60:214–219. [DOI] [PubMed] [Google Scholar]
- 82. Smits M, Dippel DW, Houston GC, et al. Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Human Brain Mapping 2009;30:2789–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gu L, Li J, Feng DF, Cheng ET, Li DC, Yang XQ, Wang BC. Detection of white matter lesions in the acute stage of diffuse axonal injury predicts long‐term cognitive impairments: a clinical diffusion tensor imaging study. J Trauma Acute Care Surg 2013;74:242–247. [DOI] [PubMed] [Google Scholar]
- 84. Kondo K, Maruishi M, Ueno H, et al. The pathophysiology of prospective memory failure after diffuse axonal injury‐lesion‐symptom analysis using diffusion tensor imaging. Bio Med Central Neurosci 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sugiyama K, Kondo T, Oouchida Y, et al. Clinical utility of diffusion tensor imaging for evaluating patients with diffuse axonal injury and cognitive disorders in the chronic stage. J Neurotrauma 2009;26:1879–1890. [DOI] [PubMed] [Google Scholar]
- 86. Lashley KS. Brain Mechanisms and Intelligence: A Quantitative Study of the Brain. Chicago: Chicago University Press; 1929. [Google Scholar]
- 87. Sheslow D, Adams W. Wide Range Assessment of Memory and Learning Administration and Technical Manual, 2nd Edition Lutz, FL: Psychological Assessment Resources, Inc; 2003. [Google Scholar]
- 88. Jennings H. Memory. Available at: http://webcache.googleusercontent.com/search?q=cache:qDruZecFFmUJ:slideplayer.com/slide/4700624/+&cd=2&hl=en&ct=clnk&gl=us. Accessed June 10, 2017.
- 89. James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- 90. Revlin R. Cognition: Theory and Practice. New York: Worth Publishers; 2013. [Google Scholar]
- 91. Naveh‐Benjamin M, Guez J, Hara Y, Brubaker MS, Lowenschuss‐Erlich I. The effects of divided attention on encoding processes under incidental and intentional learning instructions: underlying mechanisms? Quart J Exper Psychol 2014;67:1682–1696. [DOI] [PubMed] [Google Scholar]
- 92. Barber SJ, Mather M. Forgetting in context: the effects of age, emotion, and social factors on retrieval‐induced forgetting. Memory Cognit 2012;40:874–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barber SJ, Rajaram S, Fox EB. Learning and remembering with others: the key role of retrieval in shaping group recall and collective memory. Social Cognit 2012;30:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Barber SJ, Rajaram S. Exploring the relationship between retrieval disruption from collaboration and recall. Memory 2011;19:462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Barber SJ, Rajaram S. Collaborative memory and part‐set cueing impairments: The role of executive depletion in modulating retrieval disruption. Memory 2011;19:378–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tulving E, Pearlstone Z. Availability versus accessibility of information in memory for words. J Verbal Learn Verbal Behav 1966;5:381–391. [Google Scholar]
- 97. Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci 2003;4:829–839. [DOI] [PubMed] [Google Scholar]
- 98. Poldrack RA. Is “efficiency” a useful concept in cognitive neuroscience? Devel Cognitive Neurosci 2015;11:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behavior 2002;77:469–475. [DOI] [PubMed] [Google Scholar]
- 100. Craig A. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci 2002;3:655–666. [DOI] [PubMed] [Google Scholar]
- 101. Rotge J, Guehl D, Dilharreguy B, et al. Provocation of obsessive‐compulsive symptoms: a quantitative voxel‐based meta‐analysis of functional neuroimaging studies. J Psychiatr Neurosci 2008;33:405–12. [PMC free article] [PubMed] [Google Scholar]
- 102. Paulus M, Stein M. An insular view of anxiety. Biol Psychiatr 2006;60:383–387. [DOI] [PubMed] [Google Scholar]
- 103. Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatr 2002;51:68–80. [DOI] [PubMed] [Google Scholar]
- 104. Tromp DP, Grupe DW, Oathes DJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatr 2012;69:925–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci 2004;1032:1–7. [DOI] [PubMed] [Google Scholar]
- 106. Martin PK, Schroeder RW. Challenges in assessing and managing malingering, factitious disorder, and related somatic disorders. Psychiatric Times 2015;32(10):1–4. [Google Scholar]
- 107. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (5th Ed). Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 108. Van der Hart O, Nijenhuis ER, Steele K. The Haunted Self: Structural Dissociation and the Treatment of Chronic Traumatization. WW Norton & Company; 2006. [Google Scholar]
- 109. Millon T, Green CJ, Meagher RB. The MBHI: A new inventory for the psychodiagnostician in medical settings. Professional Psychol 1979;10:529. [Google Scholar]
- 110. Millon Behavioral Medicine Diagnostic (MBMD) . Pearson Clinical Assessment. Available at: http://www.pearsonclinical.com/psychology/products/100000231/millon‐behavioral‐medicine‐diagnostic‐mbmd.html. Accessed June 10, 2017.
- 111. Millon T. The logic and methodology of the Millon inventories. Cross‐cultural personality assessment In Boyle G.J., Matthews G., and Saklofske D.H., eds. Sage Handbook of Personality Theory and Assessment. Vol. 2: Personality Theory and Assessment. Los Angeles, CA: Sage; 2008. [Google Scholar]
- 112. Hathaway SR, McKinley JC. A multiphasic personality schedule (Minnesota): I. Construction of the schedule. J Psychology 1940;10:249–254. [Google Scholar]
- 113. Morey LC. Personality Assessment Inventory. Odessa, FL: Routledge; 1991. [Google Scholar]
- 114. Trull TJ, Widiger TA. Dimensional models of personality: the five‐factor model and the DSM‐5. Dialogues Clin Neurosci 2013;15:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]


