Abstract
Substance abuse is a pressing problem with few therapeutic options. The identification of addiction resilience factors is a potential strategy to identify new mechanisms that can be targeted therapeutically. Heparan sulfate (HS) is a linear sulfated polysaccharide that is a component of the cell surface and extracellular matrix. Heparan sulfate modulates the activity and distribution of a set of negatively charged signaling peptides and proteins — known as the HS interactome — by acting as a co-receptor or alternative receptor for growth factors and other signaling peptides and sequestering and localizing them, among other actions. Here, we show that stimulants like cocaine and methamphetamine greatly increase HS content and sulfation levels in the lateral hypothalamus and that HS contributes to the regulation of cocaine seeking and taking. The ability of the HS-binding neuropeptide glial-cell-line-derived neurotrophic factor (GDNF) to increase cocaine intake was potentiated by a deletion that abolished its HS binding. The delivery of heparanase, the endo-β-D-glucuronidase that degrades HS, accelerated the acquisition of cocaine self-administration and promoted persistent responding during extinction. Altogether, these results indicate that HS is a resilience factor for cocaine abuse and a novel therapeutic target for the treatment of cocaine addiction.
Introduction
Substance abuse is a pressing problem with few therapeutic options. The identification of resilience factors for addiction is a potential strategy to identify new mechanisms that can be targeted therapeutically. Heparan sulfate (HS) is a linear sulfated polysaccharide that is a ubiquitous component of the cell surface and extracellular matrix1. Heparan sulfate is structurally similar to heparin, but heparin is more sulfated and more charged than HS and has a cell- and tissue-specific distribution2,3. Heparan sulfate binding modulates the activity and distribution of a set of negatively charged signaling peptides and proteins that constitute the HS interactome1,2,4–7. Thus, the dynamic regulation of HS and its interacting partners can provide novel therapeutic targets to affect various biological processes.
Through gene expression profiling, we previously found that rats with a history of excessive cocaine self-administration exhibited higher expression of the HS proteoglycan syndecan-3 in the lateral hypothalamus (LH)8, a brain region that is involved in the motivation for both natural rewards and drugs of abuse9–12. Syndecan-3-null mice self-administered more cocaine than wildtype mice8. Additionally, glial-cell-line-derived neurotrophic factor (GDNF), which binds syndecan-3 and has high affinity for GDNF family receptor α1 (GFR-α1), increased cocaine intake in syndecan-3-null mice significantly more than in wildtype mice8. One unresolved issue that was raised by these results is whether HS plays a general role in the motivation for cocaine. To test the hypothesis that HS contributes to the regulation of drug intake, we explored the regulation of HS by stimulants like cocaine and tested the effects of (i) a recombinant GDNF mutant that is defective in HS binding and (ii) overexpression of the HS degrading enzyme heparanase13 on the motivation for cocaine. The results showed that the HS content was increased in the LH by exposure to cocaine and exerted a considerable modulatory effect on cocaine intake that was distinct from and additive to the role that is played by syndecan-3 signaling. Altogether, these results indicate that HS is a resilience factor for cocaine abuse and a novel therapeutic target for the treatment of cocaine addiction.
Methods
Mice
Male C57BL/6 J and syndecan-3 knockout mice on a C57BL/6 J background were housed in a climate-controlled vivarium on a 12 h/12 h reverse light/dark cycle (lights on 9:00 AM) with food and water available ad libitum throughout the study. The experimental protocols were performed in accordance with US National Institutes of Health guidelines on animal care and were approved by The Scripps Research Institute Animal Care and Use Committee.
Materials
Heparin lyase I, II, and III were purchased from New England Biolabs (Andover, MA). Heparan sulfate sodium salt from bovine kidney (HSBK), Trizma base, sodium chloride, Triton X-100, and calcium chloride were purchased from Sigma-Aldrich. Ethylenediaminetetraacetic acid (EDTA) was purchased from Fluka. Ammonium acetate and C-18 zip tips (100 µl, Thermo Scientific) and LCMS-grade acetonitrile and water were purchased from Fisher Scientific. Complete mini protease inhibitor cocktail tablet (EDTA-free) was purchased from Roche Diagnostics.
Tissue lysis of mouse lateral hypothalamus
Fresh frozen mouse lateral hypothalamus tissue was lysed with buffer that contained 200 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, protease inhibitor cocktail tablet, and 0.5% Triton X-100. Tissues were homogenized and incubated for 30 min on ice, followed by centrifugation (Microfuge 22 R centrifuge, Beckmann Coulter) at 12,000 rotations per minute at 4 °C for 30 min. The supernatant was collected into separate tubes, and the protein concentration of each sample was determined using the BCA assay. The samples were stored at −20 °C until further use.
Enzyme digestion for heparan sulfate and size exclusion chromatography (SEC)
An equal amount of protein for each sample was taken. Five times acetone was added and further incubated at −20 °C overnight. The next day, the samples were centrifuged (Microfuge 22 R centrifuge, Beckmann Coulter) at 20,000 × g at 4 °C for 30 min. This was further washed with equal volumes of acetone:water (6:1) and centrifuged again. The supernatant was discarded, and the pellet was air dried. The protein pellet from each sample and three replicates of 10 µg each HS from bovine kidney as apositive control were resuspended in a final volume of 50 μl of 20 mM Tris-HCl buffer (pH 7.4) in the presence of 5 mM CaCl2 and 5 mU each of heparin lyase I, heparin lyase II, and heparin lyase III. The digestion was incubated at 37 °C overnight. The samples were then dried by vacuum centrifugation (SPD1010 Speedvac system, Thermo Savant) and resuspended in 2% ACN/water-0.1% TFA and passed through a C-18 zip tip (100 µl). This step was repeated three times. The released glycosaminoglycans were collected as flow-through for each sample and dried by vacuum centrifugation (SPD1010 Speedvac system, Thermo Savant). The HS disaccharide samples were further desalted using an SEC column (Superdex™ peptide PC 3.2/30, GE Healthcare) using 25 mM ammonium acetate in 5% ACN (pH 4.4) as the mobile phase at an isocratic flow of 0.04 ml/min for 60 min. The HS disaccharides were eluted between 35 and 45 min at an ultraviolet absorbance of 232 nm. The clean HS disaccharide samples were then dried by vacuum centrifugation (SPD1010 Speedvac system, Thermo Savant) and stored at −20 °C until further use.
Liquid chromatography-mass spectrometry analysis
Heparan sulfate disaccharides were analyzed using negative ionization mode electrospray liquid chromatography-mass spectrometry (LC-MS). Disaccharides were separated using 1.9 µm, 0.3 × 150 mm GlycanPac AXH-1 (ThermoScientific) packing material mounted on an Agilent 1200 LC system (Agilent Technologies, Santa Clara, CA). A 20 min isocratic method was used with 85% B and a flow rate of 7 µl/min. Solvent A was 50 mM ammonium formate (pH 4.4) in 10% ACN, and solvent B was 95% ACN and 5% water. Mass spectrometry was performed using an Agilent 6520 Q-TOF spectrometer (Agilent Technologies, Santa Clara, CA) with dual electrospray ionization. A 1 pmol quantity of ΔHexA2S-GlcNCoEt(6 S) (Iduron) was added to all of the samples as an internal standard before LC-MS analyses. Different concentrations (500 fmol, 1 pmol, 2 pmol, 5 pmol, and 10 pmol) of eight HS standard disaccharides (D0A0, D2A0, D0A6, D2A6, D0S0, D2SO, D0S6, and D2S6; Iduron) were also run on LC-MS as triplicates to plot a standard curve.
Mouse jugular vein catheterization
Catheterization surgery and the subsequent intravenous self-administration procedures were conducted as previously described8. Briefly, mice were implanted with intravenous jugular catheters under anesthesia with ketamine (Ketaset, 80 mg/kg, i.p., Fort Dodge Animal Health) and xylazine (16 mg/kg, i.p., Butler Animal Health). During surgery, the catheter tubing was threaded subcutaneously with the external port exiting the midscapular region, and a 1 cm length of the tip was inserted into the right external jugular vein. The catheter was filled with 25 µl of heparinized saline solution (30 USP U/ml, APP Pharmaceuticals) that contained cefazolin (100 mg/ml, Apotex) and flushed daily with the same solution for 3 additional days and thereafter with the heparinized saline solution without antibiotic. The mice were allowed at least 1 week to recover from surgery.
Intravenous cocaine self-administration in the mouse
Following the recovery period, the mice were trained to press a lever in an operant chamber (Med Associates) for intravenous injections of cocaine (0.6 mg/kg per injection) under a fixed-ratio 1 timeout 20-s schedule (FR1TO20) during 1 h daily sessions, 5 days per week. The criterion for the acquisition of cocaine self-administration was a stable level of cocaine self-administration behavior: (i) a minimum of 10 reinforcers earned per session and (ii) at least 70% of the total responses on the active lever (iii) over two consecutive sessions (±20%). After the successful acquisition of cocaine self-administration, a full dose-effect study was performed using an FR5 schedule, in which the response requirement was gradually increased to FR5 at the training dose (0.6 mg/kg per injection). Once stabilized self-administration behavior was reached, each of the unit doses (1.2, 0.6, 0.3, 0.15, 0.075, and 0.038 mg/kg per injection) was tested in descending order in 1-h daily sessions with at least two sessions for each dose. A progressive-ratio (PR) schedule of reinforcement was subsequently used to assess reinforcement strength with the training dose of 0.6 mg/kg/injection. The PR formula was the following: 5 × exp(reinforcer number × 0.2) − 5, which yields values of 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 62, 77, 95, 118, 145, etc. Sessions were terminated after 30 min elapsed without a reinforcer earned or 6 h, whichever came first. The breakpoint was defined as the step value associated with the last completed ratio prior to terminating the sessions. The number of reinforcers earned, total cocaine intake, and breakpoints from the mean of consecutive sessions at each dose were analyzed by two-way repeated-measures analysis of variance (ANOVA). At the end of experiment, catheter patency was verified with a solution of 0.25 mg ketamine and 0.0125 midazolam (in 50 µl). Mice with a defective catheter were excluded from the statistical analysis. All of the criteria were pre-established.
Adeno-associated virus (AAV) vectors
GDNF-AAV and the mutant ΔN2-GDNF-AAV were engineered as previously described8 in a self-complementary AAV viral backbone, scaav2-cmv-gfp, obtained from the National Gene Vector Biorepository (NGVB, Indiana University). These recombinant AAV viral vectors contain the expression cassette of either mouse GDNF or ΔN2-GDNF, which were synthesized by polymerase chain reaction (PCR) based on accession no. BC119031 with amino acids 24–29 (ENSRGKGRRGQRGKNR) deleted in the ΔN2-GDNF. Recombinant AAV-heparanase in a conventional AAV backbone was constructed based on mouse heparanase accession no. BC138784.
Microinfusion of AAV
The animals were anesthetized as described previously for intra-LH virus injections8. Briefly, the mice were immobilized in a stereotaxic frame in the flat-skull position (Kopf Instruments), and a bilateral stainless steel injector (33 gauge, extending 1 cm below the tip of 26-gauge sleeve tubing) was slowly lowered into the LH (coordinates: −0.94 mm anterior to bregma, ±1.2 mm lateral, and −5.4 mm ventral to skull surface at bregma). The AAV solution (0.5 µl) was delivered slowly through the injector by a syringe pump (KD Scientific) at a flow rate of 0.05 µl/min. The injector remained in place for at least 10 min and then was withdrawn slowly to avoid backflow of the virus. AAV expression could be detected, and the locations of injection sites were verified at the end of the experiments. The animals were randomly assigned to AAV treatment groups.
Statistical analysis
The data were analyzed using GraphPad Prism 7 (La Jolla, CA, USA). Variances were compared using the Bartlett test. The LC-MS/MS data were analyzed using one-way ANOVA followed by Fisher’s least significant difference (LSD) test. All of the behavioral data were analyzed using two-way repeated-measures ANOVA, with group as the between-subjects factor and dose and test session as the within-subjects factors, followed by Fisher’s LSD test.
Data Availability
All data generated or analyzed during this study are included in this published article.
Results
Stimulants like cocaine and methamphetamine increase brain heparan sulfate abundance and sulfation level
The action of the HS proteoglycan syndecan-3 in the LH as a resilience factor for cocaine abuse8 raises the fundamental issue of whether HS plays a general role in the motivation for drugs of abuse. To test this hypothesis, we initially explored whether brain HS content is altered by drugs of abuse, such as cocaine and methamphetamine. To this end, we exposed mice to either cocaine (20 mg/kg once per day) or methamphetamine (2.5 mg/kg twice per day) intraperitoneally for 13 days. The HS content in the LH was then analyzed using a novel ultra high-performance LC-MS/MS approach14. As shown in Fig. 1, both the HS content and its level of sulfation were significantly increased in the LH by a history of cocaine or methamphetamine exposure. Heparan sulfate regulates biological processes through interactions between sulfate groups and negatively charged polypeptides that have motivational significance, such as GDNF8. These results support the hypothesis that HS plays a general role in the effects of drugs of abuse, such as cocaine and methamphetamine. To further test this hypothesis, we investigated the effects of modulating the ability of GDNF to interact with HS and the effects of HS content on voluntary cocaine intake.
Figure 1.
Stimulants increase heparan sulfate abundance and sulfation. (a) Cocaine and methamphetamine increase HS content. Total HS abundance was significantly increased by cocaine (Coc, n = 6) and methamphetamine (Meth, n = 5) in the LH, a brain region where the HS proteoglycan syndecan-3 was previously found to regulate cocaine intake8. (b) Cocaine and methamphetamine significantly increase HS sulfation. *P < 0.05, ****P < 0.0001, significant difference from saline (Sal) control (n = 6; one-way ANOVA followed by Fisher’s LSD test). The data are expressed as mean + SEM.
Effects of GDNF on cocaine intake depend on GDNF interaction with heparan sulfate
To test the hypothesis that HS regulates cocaine intake, we first investigated the effects of GDNF binding to HS on cocaine self-administration. Evidence suggests that GDNF binding to HS requires a stretch of basic amino acids in the N-terminal region that encompasses residues 24–39 in the mature peptide15. Importantly, this HS-binding region of GDNF is distinct from the binding site for the high-affinity GDNF receptor GFR-α1; thus, GDNF binds both HS and GFR-α1 simultaneously15. In fact, the removal of residues 24–39 (ΔN2 GDNF mutant) substantially abolishes HS binding without affecting the binding or activation of its conventional receptor GFR-α115.
We constructed an AAV vector that expresses the ΔN2 GDNF mutant to interfere with GDNF HS to probe the role of HS in the actions of GDNF on the motivation for cocaine self-administration. The delivery of an AAV vector that expressed unmodified GDNF in the LH in syndecan-3-null mice increased the motivation for cocaine self-administration, reflected by a vertical shift of the unit dose-response curve16 (Fig. 2). This effect was not observed in wildtype mice, in which syndecan-3 acts as a resilience factor that reduces cocaine intake, as previously reported8. Interestingly, the AAV delivery of HS binding-deficient ΔN2 GDNF produced an even greater increase in cocaine self-administration in syndecan-3 knockout mice than the unmodified GDNF (Fig. 2c,d). This result indicates that, in addition to syndecan-3, other HS proteoglycans in the LH contribute to the reduction of cocaine intake through binding to the HS-interacting domain of GDNF, and their actions are unmasked by the removal of endogenous syndecan-3 in syndecan-3 knockout mice. These results support the hypothesis that overall HS tissue content regulates cocaine intake.
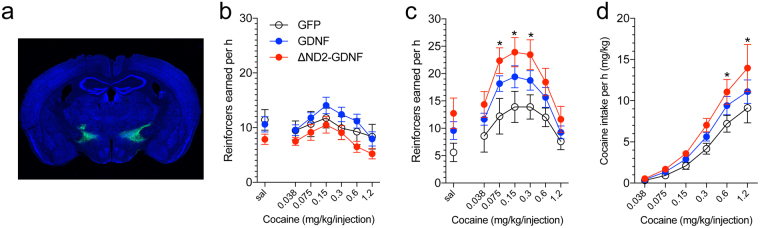
Figure 2.
Effect of lateral hypothalamus delivery of GDNF-AAV and ΔN2-GDNF-AAV on cocaine self-administration in wildtype and syndecan-3 knockout mice. A unit dose-response paradigm was used to probe the effects of GDNF heparin binding in syndecan-3 knockout and wildtype mice under an FR5 schedule of reinforcement8. (a) GDNF-AAV or ΔN2-GDNF-AAV was injected in the LH in wildtype and syndecan-3 KO mice. (b) No significant effect of GDNF-AAV or ΔN2-GDNF viruses was observed in wildtype (WT) mice compared with the GFP control group (F 2,228 = 1.48, P > 0.05, two-way repeated-measures ANOVA). GFP, n = 12; GDNF, n = 15; ΔND2-GDNF, n = 14. (c) In syndecan-3 knockout mice, both the GDNF-AAV and ΔN2-GDNF-AAV viruses caused an upward shift of the cocaine unit dose-response curve compared with the GFP control group. The two-way repeated-measures ANOVA revealed a significant main effect of virus infusion (F 2,180 = 4.26, P = 0.024). GFP, n = 10; GDNF, n = 12; ΔND2-GDNF, n = 11. The post hoc test revealed a significant group difference between the ΔN2-GDNF-AAV group and GFP group at the doses of 0.075, 0.15, and 0.3 mg/kg/injection. (d) ΔN2-GDNF-AAV delivery increased cocaine intake compared with the GFP control group. The two-way repeated-measures ANOVA revealed a significant main effect of viral infusion (F 2,30 = 2.828, P = 0.075). GFP, n = 10; GDNF, n = 12; ΔND2-GDNF, n = 11. The post hoc test revealed a significant group difference between the ΔN2-GDNF-AAV group and GFP group at the doses of 0.6 and 1.2 mg/kg/injection. The data are expressed as mean ± SEM.
Heparan sulfate content regulates cocaine taking and seeking
To test the hypothesis that overall HS tissue content regulates cocaine intake, we evaluated the effects of reducing HS content in the LH through the AAV-mediated delivery of heparanase, the endoglycosidase that is responsible for degrading HS at glucuronidic bonds17 at the cell-surface and in the extracellular matrix, resulting in remodeling of the extracellular matrix and the modulation of HS-dependent signaling13. As shown in Fig. 3, the AAV-mediated delivery of heparanase in the LH accelerated the acquisition of cocaine self-administration in both syndecan-3 knockout and wildtype mice (Fig. 3a). Notably, the effects of heparanase were distinct from the effects of syndecan-3 deficiency, in which heparanase accelerated the acquisition of cocaine self-administration behavior but did not significantly increase cocaine intake after acquisition (Fig. 3b,c). Heparanase-AAV delivery also prevented the extinction of cocaine-seeking behavior in syndecan-3 knockout mice (Fig. 3d).
Figure 3.
Delivery of recombinant AAV-heparanase in the LH facilitates the acquisition of cocaine self-administration in syndecan-3 knockout (KO) and wildtype (WT) mice and induces greater resistance to extinction in syndecan-3 KO mice. (a) Intra-LH heparanase-AAV virus facilitated the acquisition of cocaine self-administration in both wildtype (left) and syndecan-3 knockout (right) mice. The two-way repeated-measures ANOVA revealed significant main effects of repeated training (F 16,288 = 26.51, p < 0.0001) and heparanase-AAV administration (F 1,288 = 4.613, p = 0.046) and a significant interaction between these two factors (F 16,288 = 2.316, p = 0.003) in wildtype mice. Similarly, in knockout mice, there were significant main effects of repeated training (F 16,336 = 15.43, p < 0.0001) and heparanase-AAV administration (F 1,336 = 4.982, p = 0.037). The post hoc tests revealed significant differences on several training days between the corresponding groups (*P < 0.05, WT-GFP, n = 10; WT-Heparanase, n = 10; KO-GFP, n = 12; KO-Heparanase, n = 11). (b,c) The maintenance of cocaine self-administration was tested under a fixed-ratio 5 schedule of reinforcement (b), followed by a progressive-ratio schedule (c). Intra-LH heparanase-AAV did not significantly alter cocaine self-administration under either the FR or PR schedule. No significant main effects of heparanase-AAV administration were observed in either wildtype or knockout mice (P > 0.05). (d) The mice were then tested in daily extinction training. There was no significant main effect of heparanase-AAV in wildtype mice (F 1,450 = 0.398, p > 0.05). However, heparanase-AAV increased resistance to the extinction of cocaine seeking in syndecan-3 knockout mice, reflected by a significant main effect of heparanase-AAV treatment (F 1,450 = 5.33, p = 0.033; two-way repeated-measures ANOVA). The data are expressed as mean ± SEM.
Discussion
Heparan sulfate affects various biological processes through diverse mechanisms, including acting as a co-receptor or alternative receptor for growth factors and other signaling peptides, as well as sequestering and localizing them, among other actions1–7. The present study demonstrated that drugs of abuse like cocaine and methamphetamine alter HS abundance and sulfate content in the LH, and HS affects cocaine seeking and taking in mice.
We first analyzed the effect of chronic stimulant administration on HS abundance and sulfation in the LH, a region that is involved in the motivation for both natural rewards and drugs of abuse9–12 and where the proteoglycan syndecan-3 was previously shown to act as a cocaine resilience factor8. The results indicated that stimulants like cocaine and methamphetamine increase both HS abundance and sulfate content, and this effect was more pronounced for cocaine than for methamphetamine. Sulfate groups confer to HS a negatively charged character and provide docking sites for numerous protein ligands that are involved in diverse biological processes1,2,4–7. As we previously found that GDNF modulates cocaine intake in a syndecan-3-dependent manner8, we tested the role of HS in this action. Using an AAV vector that expressed a GDNF mutant that was defective in HS binding (ΔN2 GDNF), we observed that the ability of GDNF to increase cocaine intake is generally modulated by HS. The results indicated that, in addition to syndecan-3, other HS proteoglycans in the LH contribute to resilience to cocaine abuse. To further explore this finding, we tested the effect of overexpressing heparanase, the endo-β-D-glucuronidase that catalyzes the depolymerization of HS chains and plays a central role in the intracellular and extracellular degradation of HS13. Heparanase has been implicated in several normal and pathological processes, including cancer metastasis and angiogenesis, the regulation of food intake, and metabolism13,18–20. The effects of HS disruption by exogenous heparanase expression and deletion of syndecan-3 were distinguishable and additive. In particular, we found that heparanase overexpression in the mouse LH accelerated the acquisition of cocaine self-administration in both syndecan-3 knockout and wildtype mice. Additionally, the persistence of cocaine seeking during extinction was increased by the simultaneous delivery of heparanase and syndecan-3 deficiency.
Mounting evidence indicates that complex carbohydrates play key roles in the regulation of specific biological functions. The extensive functional repertoire of heparin and HS and their ability to interact with a large number of proteins suggest that novel therapeutic targets may be identified to affect pathological processes through the modulation of sulfated glycosaminoglycan compositions and interactions. Carbohydrate-related therapeutic targets are being pursued in infectious diseases, cancer, and inflammation21–26. Evidence also suggests that HS may play significant roles in neurological disease. For example, HS has been reported to be involved in amyloid-β peptide and tau fibrillization in Alzheimer’s disease27 and the pathogenesis of autism28,29. However, glycosaminoglycans remain an area of untapped potential for the identification of novel therapeutics for drug addiction. The present study begins to unravel the effects of stimulants like cocaine and methamphetamine on HS abundance and sulfate content and the potential to target the biochemical properties of HS for the treatment of drug abuse.
The present findings also underscore the pivotal role for the hypothalamus in motivation and addictive behaviors. The LH has substantial connections with the extended amygdala and mesocorticolimbic regions30. The LH supports intracranial self-stimulation31–33, which is acutely augmented by such drugs as cocaine and is decreased during withdrawal34–39. Connections between the LH, nucleus accumbens, and ventral tegmental area are key for both drug and alcohol seeking40,41. Together with the paraventricular nucleus of the hypothalamus, the LH contributes to emotional arousal and interfacing the central stress and autonomic systems42. Gene expression profiling provided evidence that transition to escalated cocaine intake is characterized by substantial structural plasticity of the LH12, which was found to involve genes that are related to proteoglycan signaling8. Lastly, we showed herein that cocaine and methamphetamine increased HS content and sulfation in the LH.
Altogether, the present results demonstrate that HS proteoglycans, including syndecan-3 and others, contribute to the regulation of cocaine seeking and taking and indicate that therapeutic targets that modulate cocaine taking and seeking can be identified through the manipulation of HS synthesis, degradation, and composition and the modulation of HS interactions with peptides whose actions are regulated by HS.
Acknowledgements
We are grateful to Drs George Koob (National Institute on Alcohol Abuse and Alcoholism) and James Paulson (The Scripps Research Institute) for helpful discussions and Michael Arends for editing the manuscript. Supported by NIH grants DA036241 and DA041750.
Author Contributions
P.P.S. designed the study. V.R.C. designed and produced the viral vectors. J.C. and T.K. performed the in vivo aspects of the work. J.Z. and M.S. performed the chemical analyses. T.K. and M.S. performed the statistical analyses. All of authors interpreted and discussed the results and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Meneghetti MC, et al. Heparan sulfate and heparin interactions with proteins. J R Soc Interface. 2015;12:0589. doi: 10.1098/rsif.2015.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oduah, E. I., Linhardt, R. J. & Sharfstein, S. T. Heparin: Past, Present, and Future. Pharmaceuticals (Basel)9, 10.3390/ph9030038 (2016). [DOI] [PMC free article] [PubMed]
- 4.Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem J. 2009;418:491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- 5.Bespalov, M. M. et al. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. J Cell Biol192, 153–169, 10.1083/jcb.201009136 (2011). [DOI] [PMC free article] [PubMed]
- 6.Diamantopoulou Z, Kitsou P, Menashi S, Courty J, Katsoris P. Loss of receptor protein tyrosine phosphatase beta/zeta (RPTPbeta/zeta) promotes prostate cancer metastasis. J Biol Chem. 2012;287:40339–40349. doi: 10.1074/jbc.M112.405852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu D, Esko JD. Demystifying heparan sulfate-protein interactions. Annu Rev Biochem. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, et al. Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nat Commun. 2013;4:1955. doi: 10.1038/ncomms2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cason, A. M. et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav100, 419-428, doi:S0031-9384(10)00125-3[pii]10.1016/j.physbeh.2010.03.009 (2010). [DOI] [PMC free article] [PubMed]
- 10.Winsky-Sommerer R, et al. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson EB, Wissman AM, Loriaux AL, Kourrich S, Self DW. Optogenetic stimulation of accumbens shell or shell projections to lateral hypothalamus produce differential effects on the motivation for cocaine. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:3537–3543. doi: 10.1523/JNEUROSCI.1524-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed SH, et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci USA. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vlodavsky I, et al. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. doi: 10.1016/j.drup.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill VL, Aich U, Rao S, Pohl C, Zaia J. Disaccharide analysis of glycosaminoglycans using hydrophilic interaction chromatography and mass spectrometry. Anal Chem. 2013;85:1138–1145. doi: 10.1021/ac3030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfano I, Vora P, Mummery RS, Mulloy B, Rider CC. The major determinant of the heparin binding of glial cell-line-derived neurotrophic factor is near the N-terminus and is dispensable for receptor binding. Biochem J. 2007;404:131–140. doi: 10.1042/BJ20061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J Neurosci. 2000;20:4226–4232. doi: 10.1523/JNEUROSCI.20-11-04226.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogren S, Lindahl U. Cleavage of macromolecular heparin by an enzyme from mouse mastocytoma. The Journal of biological chemistry. 1975;250:2690–2697. [PubMed] [Google Scholar]
- 18.Karlsson-Lindahl L, et al. Heparanase affects food intake and regulates energy balance in mice. PLoS One. 2012;7:e34313. doi: 10.1371/journal.pone.0034313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batool T, et al. Overexpression of heparanase attenuated TGF-beta-stimulated signaling in tumor cells. FEBS Open Bio. 2017;7:405–413. doi: 10.1002/2211-5463.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg R, et al. Heparanase augments insulin receptor signaling in breast carcinoma. Oncotarget. 2017;8:19403–19412. doi: 10.18632/oncotarget.14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, H. Y. et al. Sulfatase-1 overexpression indicates poor prognosis in urothelial carcinoma of the urinary bladder and upper tract. Oncotarget, 10.18632/oncotarget.17590 (2017). [DOI] [PMC free article] [PubMed]
- 22.Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64. doi: 10.1053/j.gastro.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Vanheule V, et al. CXCL9-Derived Peptides Differentially Inhibit Neutrophil Migration In Vivo through Interference with Glycosaminoglycan Interactions. Front Immunol. 2017;8:530. doi: 10.3389/fimmu.2017.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baburajeev CP, et al. Identification of Novel Class of Triazolo-Thiadiazoles as Potent Inhibitors of Human Heparanase and their Anticancer Activity. BMC Cancer. 2017;17:235. doi: 10.1186/s12885-017-3214-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyman B, Yang Y. Mechanisms of heparanase inhibitors in cancer therapy. Exp Hematol. 2016;44:1002–1012. doi: 10.1016/j.exphem.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nadir Y, Brenner B. Heparanase procoagulant activity in cancer progression. Thromb Res. 2016;140(Suppl 1):S44–48. doi: 10.1016/S0049-3848(16)30097-4. [DOI] [PubMed] [Google Scholar]
- 27.Liu CC, et al. Neuronal heparan sulfates promote amyloid pathology by modulating brain amyloid-beta clearance and aggregation in Alzheimer’s disease. Sci Transl Med. 2016;8:332ra344. doi: 10.1126/scitranslmed.aad3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie F, Badie-Mahdavi H, Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5052–5056. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson BL, Corley MJ, Vasconcellos A, Blanchard DC, Blanchard RJ. Heparan sulfate deficiency in autistic postmortem brain tissue from the subventricular zone of the lateral ventricles. Behavioural brain research. 2013;243:138–145. doi: 10.1016/j.bbr.2012.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hand TH, Franklin KB. 6-OHDA lesions of the ventral tegmental area block morphine-induced but not amphetamine-induced facilitation of self-stimulation. Brain Res. 1985;328:233–241. doi: 10.1016/0006-8993(85)91034-0. [DOI] [PubMed] [Google Scholar]
- 32.Miliaressis E, Emond C, Merali Z. Re-evaluation of the role of dopamine in intracranial self-stimulation using in vivo microdialysis. Behav Brain Res. 1991;46:43–48. doi: 10.1016/S0166-4328(05)80095-6. [DOI] [PubMed] [Google Scholar]
- 33.Garris PA, et al. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- 34.Markou A, Koob GF. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology. 1991;4:17–26. [PubMed] [Google Scholar]
- 35.Schulteis G, Markou A, Cole M, Koob GF. Decreased brain reward produced by ethanol withdrawal. Proc. Natl. Acad. Sci. 1995;92:5880–5884. doi: 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology (Berl) 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- 37.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 38.Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- 39.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 40.Millan EZ, Furlong TM, McNally GP. Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci. 2010;30:4626–4635. doi: 10.1523/JNEUROSCI.4933-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aston-Jones G, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LeDoux JE. Emotional memory: in search of systems and synapses. Ann N Y Acad Sci. 1993;702:149–157. doi: 10.1111/j.1749-6632.1993.tb17246.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.