ABSTRACT
Cefiderocol (S-649266) is a novel siderophore cephalosporin with potent in vitro activity against clinically encountered multidrug-resistant (MDR) Gram-negative isolates; however, its spectrum of antibacterial activity against these difficult-to-treat isolates remains to be fully explored in vivo. Here, we evaluated the efficacy of cefiderocol humanized exposures in a neutropenic murine thigh model to support a suitable MIC breakpoint. Furthermore, we compared cefiderocol's efficacy with humanized exposures of meropenem and cefepime against a subset of these phenotypically diverse isolates. Ninety-five Gram-negative isolates were studied. Efficacy was determined as the change in log10 CFU at 24 h compared with 0-h controls. Bacterial stasis or ≥1 log reduction in 67 isolates with MICs of ≤4 μg/ml was noted in 77, 88, and 85% of Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa, respectively. For isolates with MICs of ≥8 μg/ml, bacterial stasis or ≥1 log10 reduction was observed in only 2 of 28 (8 Enterobacteriaceae, 19 A. baumannii, and 1 P. aeruginosa) strains. Against highly resistant meropenem and cefepime organisms, cefiderocol maintained its in vivo efficacy. Overall, humanized exposures of cefiderocol produced similar reductions in bacterial density for organisms with MICs of ≤4 μg/ml, whereas isolates with MICs of ≥8 μg/ml generally displayed bacterial growth in the presence of the compound. Data derived in the current study will assist with the delineation of MIC susceptibility breakpoints for cefiderocol against these important nosocomial Gram-negative pathogens; however, additional clinical data are required to substantiate these observations.
KEYWORDS: cefiderocol, Gram-negative bacteria, siderophore, Acinetobacter, Escherichia coli, Klebsiella, Pseudomonas aeruginosa
INTRODUCTION
The field of infectious diseases is constantly challenged by the continuous rise of antimicrobial resistance in Gram-negative bacteria (1). Innovative approaches for the treatment of these pathogenic organisms are warranted, as effective antimicrobials remain scarce. Enterobacteriaceae, especially Escherichia coli and Klebsiella pneumoniae, are notorious for their resistance to third-generation cephalosporins, often attributed to the production of extended-spectrum β-lactamases (ESBLs). Carbapenems, usually reserved for difficult-to-treat pathogens, are not immune to these problematic enzymes, as exemplified by Acinetobacter baumannii producing carbapenemases, such as OXA-type β-lactamases, and Pseudomonas aeruginosa producing metallo-β-lactamases (MBLs) (2, 3). Furthermore, other resistance mechanisms, such as porin channel mutations, efflux pumps, and, rarely, target site mutations, add to the complexity of antimicrobial resistance.
As new resistance pathways emerge, novel therapies demonstrating unique mechanisms to overcome resistance are needed. Historically, β-lactam–β-lactamase inhibitors and carbapenems have been relied upon for combating β-lactamase-producing organisms. However, a β-lactam–β-lactamase inhibitor or carbapenem that overcomes ESBLs and carbapenemases, specifically MBLs, has yet to be developed, emphasizing the need for antimicrobials that act in a manner unknown to these resistant isolates. Cefiderocol (S-649266), a siderophore cephalosporin, is differentiated from other cephalosporins via a catechol moiety at the third-position side chain. This unique structural characteristic allows the agent to form a chelating complex with free iron, which is then actively transported across the outer membrane into the cell via specialized iron transporters (4).
Cefiderocol has demonstrated in vitro and in vivo activity against multidrug-resistant (MDR) Gram-negative organisms, including those that produce MBLs, which are classified as class B β-lactamases (5–9). However, cefiderocol's spectrum of antibacterial activity against a multitude of clinically encountered MDR Gram-negative isolates remains to be fully explored. Thus, the purpose of our study was to establish the efficacy of simulated human exposures of cefiderocol against a wide variety of P. aeruginosa, A. baumannii, and Enterobacteriaceae isolates displaying MICs that span the current distribution of clinical isolates to identify a potential MIC breakpoint. Furthermore, we sought to define cefiderocol's potential clinical role by comparing the efficacies of humanized regimens of cefiderocol, cefepime, and meropenem against a subset of these Gram-negative isolates.
RESULTS
In vitro susceptibility.
Cefiderocol MICs for the 95 isolates with adequate growth in the in vivo bacterial-density studies ranged from 0.12 to >256 μg/ml (see the supplemental material). The cefiderocol, cefepime, and meropenem MICs for the subgroup of 15 isolates used in the comparator studies are shown in Table 1. Three of the 15 isolates were susceptible to both meropenem and cefepime. All the remaining isolates demonstrated in vitro resistance to one or both comparator agents.
TABLE 1.
MICs of cefiderocol and comparators (meropenem and cefepime) against a collection of 15 P. aeruginosa, A. baumannii, and Enterobacteriaceae isolates
| Isolate | MIC (μg/ml) |
||
|---|---|---|---|
| Meropenem | Cefepime | Cefiderocol | |
| A. baumannii 154 | 64 | 64 | 0.12 |
| E. coli 465 | ≤0.06 | ≤0.5 | 0.25 |
| E. coli 463 | ≤0.06 | ≤0.5 | 0.5 |
| K. pneumoniae 528 | 0.125 | ≤0.5 | 0.5 |
| P. aeruginosa 1559 | 32 | 128 | 0.5 |
| P. aeruginosa 1560 | 8 | 32 | 0.5 |
| P. aeruginosa 1567 | 16 | 128 | 0.5 |
| A. baumannii 139 | 512 | 64 | 1 |
| A. baumannii 141 | 64 | 64 | 1 |
| E. coli 458 | ≤0.5 | 512 | 1 |
| A. baumannii 146 | 512 | >512 | 2 |
| K. pneumoniae 522 | >512 | >512 | 4 |
| K. pneumoniae 539 | ≤0.5 | >512 | 4 |
| K. pneumoniae 541 | 256 | >512 | 4 |
| K. pneumoniae 543 | 512 | >512 | 8 |
Pharmacokinetics of cefiderocol, meropenem, and cefepime.
The confirmatory pharmacokinetic (PK) study of cefiderocol revealed that the delivered dosing regimen provided a free-drug profile similar to that of the human regimen that was used in prior studies (10, 11). Additional studies confirmed the adequacy of cefepime and meropenem in producing free-drug exposure profiles similar to that of humans. Comparisons of %fT>MIC values achieved with cefiderocol, cefepime, and meropenem at MICs ranging between 4 and 256 μg/ml in both humans and mice receiving the humanized regimens are presented in Table 2.
TABLE 2.
Comparison of %fT>MIC values achieved with cefiderocol, meropenem, and cefepime at each MIC in humans and in mice receiving humanized regimens
| Drug | Species | %fT>MIC for a MIC (μg/ml) of: |
||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 16 | 32 | 64 | 128 | 256 | ||
| Cefiderocol | Mouse | 96 | 80 | 45 | 9 | 0 | 0 | 0 |
| Humana | 99 | 76 | 48 | 11 | 0 | 0 | 0 | |
| Meropenem | Mouse | 100 | 78 | 50 | 19 | 0 | 0 | 0 |
| Humana,b | 94 | 75 | 55 | 29 | 5 | 0 | 0 | |
| Cefepime | Mouse | 100 | 100 | 93 | 56 | 0 | 0 | 0 |
| Humana,b | 100 | 92 | 78 | 50 | 0 | 0 | 0 | |
Human exposures modeled from 2-g q8h (3-h infusion) in healthy volunteers.
Human exposures modeled based on pharmacokinetics of ventilator-associated and hospital-acquired pneumonia patients.
Bacterial-density studies.
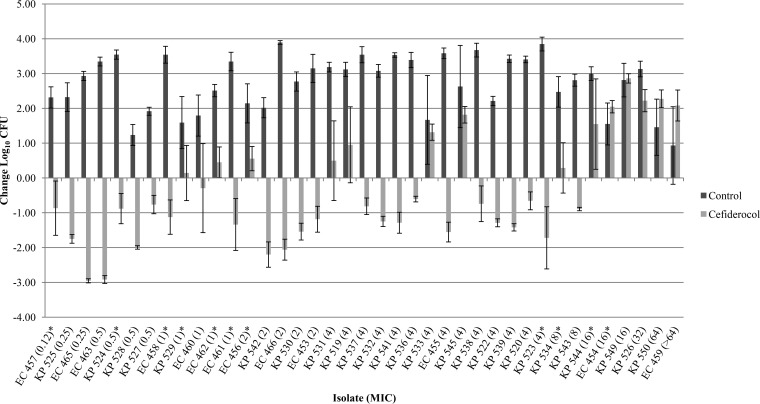
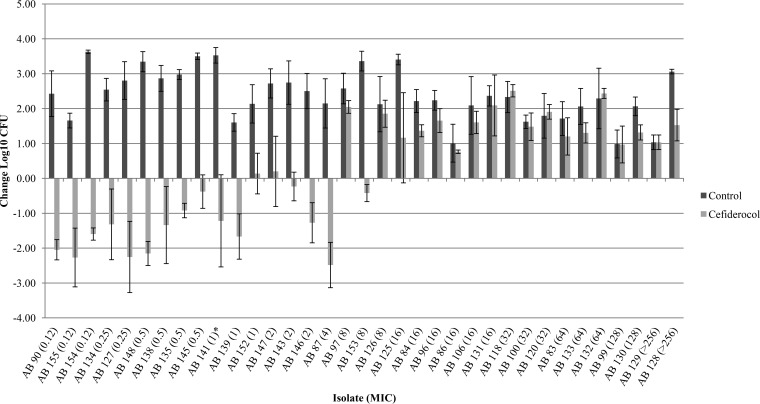
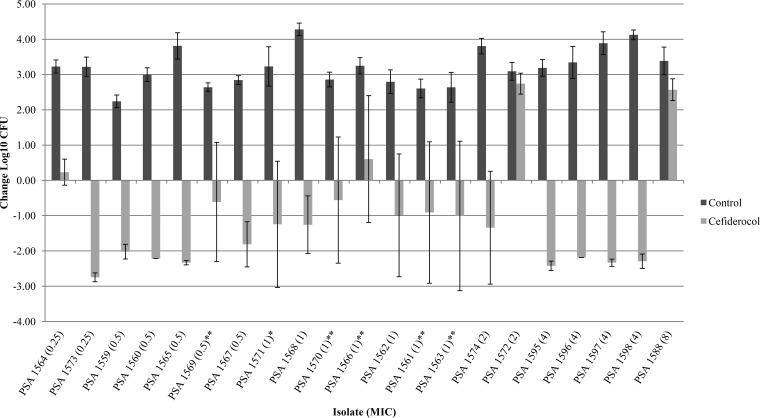
Of the 104 isolates studied, 95 isolates (21 P. aeruginosa, 35 A. baumannii, and 39 Enterobacteriaceae) demonstrated adequate growth (≥1 log10 CFU at 24 h) in the neutropenic thigh infection model. P. aeruginosa JJ 5-35, responded as previously observed, producing similar bacterial reductions of 2.56 ± 0.40 log10 CFU at 24 h with humanized cefiderocol exposures, confirming internal stability across all 10 in vivo runs. The results of the cefiderocol bacterial-density studies for each of the isolates grouped by organism are presented in Fig. 1 to 3. Initial thigh bacterial densities (0 h) ranged between 4.77 and 6.72 log10 CFU. Bacterial stasis or ≥1-log-unit reduction in 48 isolates with MICs of ≤2 μg/ml was achieved in 82, 87, and 81% of Enterobacteriaceae (17 strains), A. baumannii (15 strains), and P. aeruginosa (16 strains), respectively. Bacterial stasis or ≥1 log10-unit reduction in 67 isolates with MICs of ≤4 μg/ml was noted in 77, 88, and 85% of Enterobacteriaceae (31 strains), A. baumannii (16 strains), and P. aeruginosa (20 strains), respectively. For isolates with MICs of ≥8 μg/ml, bacterial stasis or ≥1-log-unit reduction was observed in only 2 of 28 strains (8 Enterobacteriaceae, 19 A. baumannii, and 1 P. aeruginosa). Notably, 59% (13 of 22) of isolates with cefiderocol MICs of >8 μg/ml displayed suppression of bacterial growth compared with the control. Among the species, P. aeruginosa demonstrated the greatest intragroup variability. Studies of several of these isolates were repeated to confirm our findings, as indicated in Fig. 3.
FIG 1.
Mean absolute growth or reduction from the starting inoculum (log10 CFU ± standard deviation [SD]) at 24 h by cefiderocol against Enterobacteriaceae in the neutropenic murine thigh infection model. Each asterisk represents a repeat test of the isolate. All repeat data were averaged together. EC, E. coli; KP, K. pneumoniae.
FIG 2.
Mean absolute growth or reduction from the starting inoculum (log10 CFU ± SD) at 24 h by cefiderocol against A. baumannii in the neutropenic murine thigh infection model. Each asterisk represents a repeat test of the isolate. All repeat data were averaged together. AB, A. baumannii.
FIG 3.
Mean absolute growth or reduction from the starting inoculum (log10 CFU ± SD) at 24 h by cefiderocol against P. aeruginosa in the neutropenic murine thigh infection model. Each asterisk represents a repeat test of the isolate. All repeat data were averaged together. PSA, P. aeruginosa.
Bacterial-density studies were repeated to reassess the efficacy of isolates with MICs of ≤8 μg/ml that initially demonstrated growth in the presence of cefiderocol. The results of these repeat bacterial density studies of 15 isolates were similar to the initial findings, irrespective of bacterial growth or kill (data not shown). The postexposure MICs were similar among these isolates, with the exception of A. baumannii 147, A. baumannii 97, and K. pneumoniae 545, which demonstrated >2-fold dilution differences in MICs (postexposure MICs ≥ 64 μg/ml) compared with preexposure values.
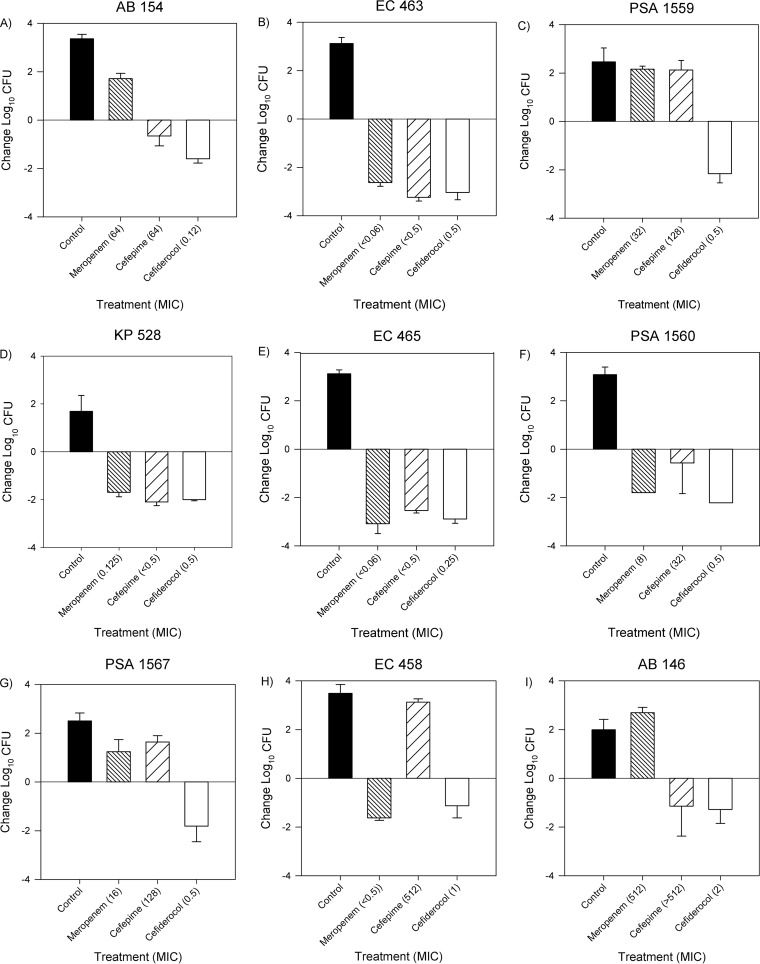
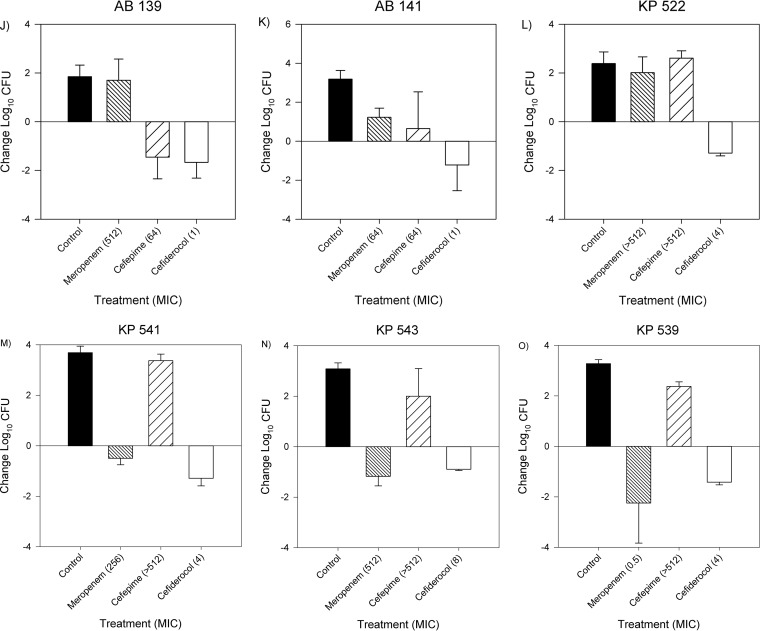
The bacterial-density studies using a subset of 15 isolates comparing cefiderocol, meropenem, and cefepime activities are depicted in Fig. 4A to O. Cefiderocol was efficacious against all 15 isolates, regardless of cefepime or meropenem resistance, producing bacterial reductions from 0 h between 0.89 and 3.04 log10 CFU. Against cefepime- and meropenem-susceptible isolates, cefiderocol treatment resulted in bacterial kills of 2.6 ± 0.5 and 2.1 ± 0.9 log10 CFU, respectively, similar to those of cefepime (2.6 ± 0.5 log10 CFU) and meropenem (2.2 ± 0.6 log10 CFU). Against cefepime- and meropenem-resistant isolates, cefiderocol produced a mean bacterial reduction of 1.5 ± 0.4 log10 CFU at 24 h. Unexpectedly, cefepime displayed efficacy against cefepime-resistant A. baumannii 146 (MIC > 512 μg/ml). Similarly, meropenem was efficacious against meropenem-resistant isolates K. pneumoniae 541 (MIC = 256 μg/ml) and K. pneumoniae 543 (MIC = 512 μg/ml). Importantly, the other 2 antimicrobials behaved as expected based on their respective phenotypic profiles against these 3 isolates. Further investigation using Xpert Carba-R (Cepheid, Sunnyvale, CA) revealed that K. pneumoniae 541 and K. pneumoniae 543 produced New Delhi metallo-beta-lactamases (NDM).
FIG 4.
Mean absolute growth or reduction from the starting inoculum (log10 CFU + SD) at 24 h by cefiderocol, meropenem, and cefepime against Enterobacteriaceae, A. baumannii, and P. aeruginosa.
DISCUSSION
These studies were performed to evaluate the efficacy of humanized exposures of cefiderocol over 24 h within the neutropenic murine thigh infection model against Enterobacteriaceae, A. baumannii, and P. aeruginosa isolates with diverse phenotypic profiles, as well as to further compare cefiderocol efficacy to those of meropenem and cefepime in a select group of 15 isolates.
The cefiderocol MIC distribution for each species was selected to determine the efficacy of cefiderocol against a broad range of exposures, with MICs higher than those identified from previous surveillance studies of Gram-negative pathogens (6). While isolates with cefiderocol MICs of >256 μg/ml were utilized in these studies, the MIC90s of cefiderocol against nonfermentative Gram-negative and K. pneumoniae isolates have been shown to be 0.5 μg/ml and 1 μg/ml, respectively (12). Importantly, the majority of these isolates were MDR, similar to our study, including isolates expressing resistance to agents that have become clinically available within the past several years (ceftolozane/tazobactam and ceftazidime/avibactam) (12, 13).
The pharmacodynamic (PD) driver of efficacy for cefiderocol is %fT>MIC (8). Previous cefiderocol pharmacokinetic/pharmacodynamic studies have suggested a target of approximately 75 to 90% %fT>MIC to achieve a 1-log10-unit reduction in bacterial density (8, 14, 15). Our humanized pharmacokinetic data predict an %fT>MIC of 96.2% in isolates with MICs of ≤4 μg/ml, suggesting that isolates with MICs of ≤4 μg/ml would achieve adequate bacterial reduction in the presence of humanized cefiderocol exposures. Of the 6 strains with MICs of 8 μg/ml, efficacy was observed in 50% of strains. We were not able to do a more robust pharmacodynamic analysis of MICs of ≥8 μg/ml because the organisms do not exist at present despite interrogation of the global surveillance programs under way.
Overall, the humanized 24-h exposures of cefiderocol produced similar reductions in bacterial density for organisms with MICs of ≤4 μg/ml, whereas isolates with MICs of ≥8 μg/ml displayed mixed results in the presence of the compound. Importantly, for the isolates with cefiderocol MICs of ≤4 μg/ml lacking bacterial kill, bacterial growth was suppressed compared with the 24-h control, although 2 strains of A. baumannii and 1 of K. pneumoniae demonstrated significant changes from preexposure MICs. Importantly, this change in the MIC was investigated in only 1 of 6 thighs. Recognizing that adaptive resistance was observed with previous siderophore antibacterial agents, investigations are under way to assess potential MIC changes over a 72-h period (16).
Against highly meropenem- and cefepime-resistant organisms, cefiderocol maintained its in vivo efficacy, while meropenem and cefepime treatment effects were reflective of the corresponding MICs, with the exception of three isolates (K. pneumoniae 541, K. pneumoniae 543, and A. baumannii 146). The efficacy of meropenem against 2 resistant isolates, K. pneumoniae 541 and K. pneumoniae 543, can be explained by the presence of NDM, which has been previously associated with this in vitro-in vivo discordance (17). While A. baumannii 146 was not demonstrated to produce IMP, VIM, NDM, KPC, or OXA-48 enzymes, further genotypic profiling may assist in identifying the cause of this discordance. Importantly, cefiderocol was effective in vivo against these NDM-producing organisms, confirming previous in vitro findings (9). Overall, humanized cefiderocol exposures produced antibacterial efficacies similar to those of cefepime and meropenem for susceptible pathogens while also displaying activity against Enterobacteriaceae, A. baumannii, and P. aeruginosa with phenotypic resistance to cefepime or meropenem.
Cefiderocol displayed consistent in vivo activity against P. aeruginosa, A. baumannii, and Enterobacteriaceae with MICs of ≤4 μg/ml, suggestive of a potential breakpoint. Breakpoint determinations are regulated by the FDA and EUCAST. In particular, the former relies on clinical trial experience where clinical and microbiological outcomes are assessed for each species of pathogen identified. However, for new antibiotics that address a high unmet medical need, streamlined clinical development programs have limited clinical trial data, usually only a few hundred isolates. Approval for these “limited-use” antibiotics relies more heavily on preclinical efficacy models and PK/PD evaluations from patient PK data and modeling. One result of this limited data package is the absence of interpretive criteria or breakpoints for highly drug-resistant organisms that are not captured in clinical trials but for which both in vitro and in vivo preclinical data provide evidence of efficacy. One question that needs to be addressed is whether the MIC of the organism or the specific species can be integrated with other clinical efficacy data to provide interpretive criteria for less common but highly resistant pathogens.
In conclusion, we discovered that an MIC of ≤4 μg/ml for Gram-negative isolates may be predictive of cefiderocol clinical efficacy, although human microbiological and clinical outcomes are still necessary to support these findings. As the rate of multidrug resistance continues to rise, our studies demonstrate the potential clinical utility of cefiderocol.
MATERIALS AND METHODS
Antimicrobial test agents.
Cefiderocol vials (lot 12M01; n = 30) were supplied by Shionogi & Co,. Ltd., for in vivo testing. The vials were stored frozen at −80°C and were protected from light during storage and throughout solution preparation and administration. The cefiderocol vials were reconstituted to a 49.9-mg/ml concentration with 10 ml of sterile 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL). Subsequent dilutions in sterile 0.9% normal saline solution were made to attain final concentrations. Cefiderocol was administered by subcutaneous (s.c.) injections of 0.2 ml at specific dosing intervals to create exposures equivalent to the 2-g every 8 h (q8h) (3-h infusion) dose being evaluated clinically in humans (10, 11).
Commercially available cefepime (1 g; lot 106014C; Sagent Pharmaceuticals) and meropenem (500 mg; lot 0039D55; Fresenius Kabi USA LLC) vials were acquired from Cardinal Health, Inc. The cefepime vials were reconstituted and diluted with 0.9% normal saline solution and administered as 0.2-ml s.c. injections at a simulated human dose of 2 g q8h (3-h infusion) (18, 19). The meropenem vials were reconstituted and diluted with 0.9% normal saline solution (Hospira, Inc., Lake Forest, IL) and administered as 0.2-ml s.c. injections at a simulated human dose of 2 g q8h (3-h infusion) (20, 21).
Bacterial isolates.
A total of 140 Gram-negative isolates comprising P. aeruginosa (23 isolates), A. baumannii (74 isolates), and Enterobacteriaceae (43 isolates) with cefiderocol MICs ranging from low to high (i.e., 0.12, 0.25, 0.5, 1, 2, 4, 8, 16, 32, 64, 128, and >256 μg/ml) were provided by the Shionogi & Co., Ltd., or IHMA, Inc., collection (13). The isolates were screened for MICs of cefiderocol in triplicate using iron-depleted cation-adjusted Mueller-Hinton broth (ID-CAMHB) as recommended by CLSI for this class of compounds (22). The model MIC was reported. From this collection, 15 isolates were additionally screened for cefepime and meropenem MICs in triplicate using CLSI broth microdilution methods (22).
As an early initial assessment, postexposure MICs were determined by the same method described above for isolates with preexposure cefiderocol MICs of ≤8 μg/ml that demonstrated bacterial growth after cefiderocol exposure in the bacterial-density studies. Bacteria isolated from one thigh from each group underwent MIC testing. A postexposure MIC value with >2-fold dilution difference from the preexposure MIC value was considered a meaningful change. Selected isolates demonstrating in vitro-in vivo discordance underwent further genotypic profiling via Xpert Carba-R to identify the presence of carbapenemases (17).
Animal infection model. (i) Laboratory animals.
Specific pathogen-free female ICR mice weighing 20 to 22 g were obtained from Envigo RMS, Inc. (Indianapolis, IN). The animals were allowed to acclimate for a minimum of 48 h before commencement of experimentation and were provided food and water ad libitum. The protocol was reviewed and approved by the Institutional Animal Care and Use Committee at Hartford Hospital. The mice were rendered transiently neutropenic by injecting cyclophosphamide intraperitoneally (i.p.) at a dose of 150 mg/kg of body weight 4 days before inoculation and 100 mg/kg 1 day before inoculation. In addition, uranyl nitrate at 5 mg/kg was administered 3 days prior to inoculation to produce a controlled degree of renal impairment.
(ii) Neutropenic thigh infection model.
All isolates were previously frozen at −80°C in skim milk (BD BioSciences, Sparks, MD). Prior to mouse thigh inoculation, two transfers of the organism onto trypticase soy agar plates with 5% sheep blood (TSA II; Becton, Dickinson & Co., Sparks, MD) were performed, and the plates were incubated at 37°C for approximately 24 h. After an 18- to 24-h incubation of the second bacterial transfer, a bacterial suspension of approximately 107 CFU/ml was made for inoculation. Final inoculum concentrations were confirmed by serial-dilution and plating techniques. The thigh infection was produced by intramuscular injection of 0.1 ml of the inoculum into each thigh of the mouse 2 h prior to the initiation of antimicrobial therapy.
(iii) Pharmacokinetic studies.
Confirmatory pharmacokinetic studies for cefiderocol, cefepime, and meropenem were carried out to ensure that the regimens utilized provided humanized exposures similar to 2 g q8h (3-h infusion). Humanized regimens for cefepime and meropenem were simulated (Phoenix version 6.3; Pharsight Corp., Mountain View, CA) to target %fT>MICs similar to those observed in patients (19, 21). The humanized regimen for cefiderocol was simulated to have %fT>MICs similar to those observed when 2 g q8h (3-h infusion) was administered to healthy volunteers (10, 11). For murine regimens, correction for free-drug concentrations were incorporated into the calculation of simulated regimens using protein binding values for cefepime, meropenem, and cefiderocol of 0%, 8%, and 31.6%, respectively (10, 11, 18, 20). Infected animals received 0.2-ml s.c. injections as required based on the dosing regimens. Groups of 6 mice were euthanized at predefined time points. Terminal blood samples from CO2-asphyxiated mice were collected via cardiac puncture and placed in sodium heparin Vacutainer tubes (BD, Franklin Lakes, NJ). Plasma was separated by centrifugation for 10 min at 4°C at 10,000 relative centrifugal force before transfer into polypropylene tubes. The tubes were stored at −80°C until they were analyzed. The plasma concentrations of cefiderocol were determined by Shionogi & Co. via a validated liquid chromatography-tandem mass spectrometry (LC–MS-MS) method as follows. Samples were deproteinated with a cefiderocol-d12 internal-standard working solution in 0.1% trifluoroacetic acid (TFA) in methanol. For plasma calibration standards, appropriate concentrations of cefiderocol were spiked into rat plasma to give eight standards from 0.1 to 200 μg/ml. Quality control (QC) samples were prepared by spiking rat plasma with cefiderocol to achieve final concentrations of 0.3 (low QC), 4 (middle QC), and 160 (high QC) μg/ml. The LC–MS-MS system was comprised of an LC-20A Shimadzu Corporation high-performance liquid chromatography (HPLC) system in tandem with an A. baumannii Sciex API 5000 triple-quadrupole mass spectrometer in electrospray ionization mode. Chromatographic separation was performed using Cadenza CD-C18 HT columns (3 μm; 2.0-mm inside diameter [i.d.] by 50 mm; Imtakt Corporation) with a gradient using mobile phases of water/heptafluorobutyric acid (1,000:1 [vol/vol]) and acetonitrile/heptafluorobutyric acid (1,000:1 [vol/vol]) at a flow rate of 0.8 ml/min. Cefiderocol concentrations were obtained using LC–MS-MS and by monitoring the product ion transitions of m/z 752.0 and m/z 285.0. The analysis run time was approximately 1.3 min. The assay was linear over a range from 0.1 to 200 μg/ml (r2 > 0.999). The accuracy of variation for cefiderocol QC samples ranged from 97.4 to 103.6%. The lower limit of quantification was 0.1 μg/ml. The concentrations of cefepime (23) and meropenem (24) were analyzed by the Center for Anti-Infective Research and Development, Hartford, CT.
(iv) Bacterial-density studies.
The purpose of the bacterial-density studies was to assess the in vivo activity of a simulated human regimen of cefiderocol against a collection of Gram-negative organisms having a wide range of susceptibilities to cefiderocol. In total, 104 isolates (23 P. aeruginosa [MIC range, 0.25 to 8 μg/ml], 39 A. baumannii [MIC range, 0.12 to >256 μg/ml], and 42 Enterobacteriaceae [MIC range, 0.12 to >64 μg/ml]) were tested in the neutropenic model.
Mice were prepared and inoculated as previously described, and treatment was initiated 2 h later. The humanized regimens were studied in groups of 3 mice over a 24-h treatment period. Control animals received the diluent vehicle at the same volume, by the same route, and on the same schedule as the most frequently dosed drug regimen. For each isolate tested, 3 untreated mice were used as 0-h controls and 3 additional mice (receiving normal saline) as 24-h controls, and 3 treatment mice were utilized for the cefiderocol dosing regimen, for a total of 9 mice needed for each isolate. A subgroup of isolates demonstrating various resistance phenotypes underwent additional testing with meropenem and cefepime (3 for cefepime and 3 for meropenem); therefore, 15 mice were needed for each of the subgroup isolates. After the 24-h treatment period, all the animals were euthanized by CO2 asphyxiation, followed by cervical dislocation. After sacrifice, the thighs were removed and individually homogenized in normal saline. Serial dilutions were plated on an appropriate agar medium for CFU enumeration. Reductions in CFU numbers at 24 h were evaluated for each isolate relative to the 0-h starting inoculum to define the total reduction in log10 CFU per thigh achieved. P. aeruginosa JJ 5-35 (MIC = 0.25 μg/ml), an isolate used in previous studies that defined cefiderocol activity with bacterial reductions of 2.71 ± 0.10 log10 CFU at 24 h, was included in each run to internally validate the model (11). Experiments with certain isolates were repeated in order to confirm questionable observations. Data from the repeat experiments were combined with data from the original experiments, and log10 CFU values were averaged. Isolates that failed to establish infection at 0 h or failed to grow over 24 h (<1 log10 CFU growth) were excluded from the study. Antibacterial efficacy was defined as bacterial stasis or ≥1 log10 CFU reduction at 24 h compared with the 0-h control.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jennifer Tabor-Rennie, Sara Giovagnoli, Debora Santini, Elizabeth Cyr, Christina Sutherland, Kimelyn Greenwood, Alissa Padgett, Sean Stainton, Safa Abuhussain, Kamilia Abdelraouf, and Mordechai Grupper from the Center for Anti-Infective Research and Development, Hartford, CT, for their assistance with the conduct of the study.
The study was conducted using funding from Shionogi & Co., Ltd., Osaka, Japan. D.P.N. received research support from Shionogi & Co. M.T., Y.Y., and R.E. are employees of Shionogi & Co.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01022-17.
REFERENCES
- 1.Doi Y, Bonomo RA, Hooper DC, Kaye KS, Johnson JR, Clancy CJ, Thaden JT, Stryjewski ME, van Duin D, Gram-Negative Committee of the Antibacterial Resistance Leadership Group. 2017. Gram-negative bacterial infections: research priorities, accomplishments, and future directions of the Antibacterial Resistance Leadership Group. Clin Infect Dis 64(Suppl 1):S30–S35. doi: 10.1093/cid/ciw829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meletis G, Exindari M, Vavatsi N, Sofianou D, Diza E. 2012. Mechanisms responsible for the emergence of carbapenem resistance in Pseudomonas aeruginosa. Hippokratia 16:303–307. [PMC free article] [PubMed] [Google Scholar]
- 3.Poirel L, Nordmann P. 2006. Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836. doi: 10.1111/j.1469-0691.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 4.Ito A, Nishikawa T, Matsumoto S, Yoshizawa H, Sato T, Nakamura R, Tsuji M, Yamano Y. 2016. Siderophore cephalosporin cefiderocol utilizes ferric iron transporter systems for antibacterial activity against Pseudomonas aeruginosa. Antimicrob Agents Chemother 60:7396–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito A, Kohira N, Bouchillon SK, West J, Rittenhouse S, Sader HS, Rhomberg PR, Jones RN, Yoshizawa H, Nakamura R, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of S-649266, a catechol substituted siderophore cephalosporin, when tested against non-fermenting Gram negative bacteria. J Antimicrob Chemother 71:670–677. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 6.Kohira N, West J, Ito A, Ito-Horiyama T, Nakamura R, Sato T, Rittenhouse S, Tsuji M, Yamano Y. 2016. In vitro antimicrobial activity of siderophore cephalosporin S-649266 against Enterobacteriaceae clinical isolates including carbapenem-resistant strains. Antimicrob Agents Chemother 60:729–734. doi: 10.1128/AAC.01695-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura R, Toba S, Tsuji M, Yamano Y, Shimada J. 2014. S-649266, a novel siderophore cephalosporin. IV. In vivo efficacy in various murine infection models, abstr F1-1558. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 8.Nakamura R, Toba S, Ito A, Tsuji M, Yamano Y, Shimada J. 2014. S-649266, a novel siderophore cephalosporin. V. Pharmacodynamic assessment in murine thigh infection models, abstr F1-1559, Abstr 54th Intersci Conf Antimicrob Agents Chemother, American Society for Microbiology, Washington, DC. [Google Scholar]
- 9.Ito-Horiyama T, Ishii Y, Ito A, Sato T, Nakamura R, Fukuhara N, Tsuji M, Yamano Y, Yamaguchi K, Tateda K. 2016. Stability of novel siderophore cephalosporin S-649266 against clinically relevant carbapenemases. Antimicrob Agents Chemother 60:4384–4386. doi: 10.1128/AAC.03098-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsube T, Echols R, Ferreira JC, Krenz HK, Berg JK, Galloway C. 2017. Cefiderocol, a Siderophore cephalosporin for Gram-negative bacterial infections: pharmacokinetics and safety in subjects with renal impairment. J Clin Pharmacol 57:584–591. doi: 10.1002/jcph.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghazi IM, Tsuji M, Nicolau DP. 2016. Activity of S-649266 siderophore cephalosporin and comparators against Pseudomonas aeruginosa in murine thigh infection model, abstr Monday-516. Abstr American Society for Microbiology Microbe 2016, Boston, MA. [Google Scholar]
- 12.Falagas ME, Skalidis T, Vardakas KZ, Legakis NJ, Hellenic Cefiderocol Study Group. 2017. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J Antimicrob Chemother 72:1704–1708. doi: 10.1093/jac/dkx049. [DOI] [PubMed] [Google Scholar]
- 13.Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. 2017. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant Gram-negative bacilli from North America and Europe, including carbapenem nonsusceptible isolates (SIDERO-WT-2014 study). Antimicrob Agents Chemother 61:e00093-17. doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horiyama T, Toba S, Nakamura R. 2014. S-649266, a novel siderophore cephalosporin. VI. Magnitude of PK/PD required for efficacy in murine lung infection model, poster F1560. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 15.Horiyama T, Toba S, Nakamura R. 2014. S-649266, a novel siderophore cephalosporin. VII. Magnitude of PK/PD required for efficacy in murine thigh infection model, poster F1561. Abstr 54th Intersci Conf Antimicrob Agents Chemother. American Society for Microbiology, Washington, DC. [Google Scholar]
- 16.Kim A, Kutschke A, Ehmann DE, Patey SA, Crandon JL, Gorseth E, Miller AA, McLaughlin RE, Blinn CM, Chen A, Nayar AS, Dangel B, Tsai AS, Rooney MT, Murphy-Benenato KE, Eakin AE, Nicolau DP. 2015. Pharmacodynamic profiling of a siderophore-conjugated monocarbam in Pseudomonas aeruginosa: assessing the risk for resistance and attenuated efficacy. Antimicrob Agents Chemother 59:7743–7752. doi: 10.1128/AAC.00831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacVane SH, Crandon JL, Nichols WW, Nicolau DP. 2014. Unexpected in vivo activity of ceftazidime alone and in combination with avibactam against New Delhi metallo-β-lactamase-producing Enterobacteriaceae in a murine thigh infection model. Antimicrob Agents Chemother 58:7007–7009. doi: 10.1128/AAC.02662-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crandon JL, Nicolau DP. 2015. In vivo activity of humanized cefepime and cefepime/AAI101 against multidrug-resistant Gram-negative Enterobacteriaceae. Antimicrob Agents Chemother 59:2688–2694. doi: 10.1128/AAC.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, Nicolau DP. 2009. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother 53:1476–1481. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeRyke CA, Banevicius MA, Fan HW, Nicolau DP. 2007. Bactericidal activities of meropenem and ertapenem against extended-spectrum-beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in a neutropenic mouse thigh model. Antimicrob Agents Chemother 51:1481–1486. doi: 10.1128/AAC.00752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim A, Kuti JL, Nicolau DP. 2009. Probability of pharmacodynamic target attainment with standard and prolonged-infusion antibiotic regimens for empiric therapy in adults with hospital-acquired pneumonia. Clin Ther 31:2765–2778. doi: 10.1016/j.clinthera.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. CLSI document M100-S17. CLSI, Wayne, PA. [Google Scholar]
- 23.Barbhaiya RH, Forgue ST, Shyu WC, Papp EA, Pittman KA. 1987. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother 31:55–59. doi: 10.1128/AAC.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkhaïli H, Niedergang S, Pompei D, Linger L, Leveque D, Jehl F. 1996. High-performance liquid chromatographic assay for meropenem in serum. J Chromatogr B Biomed Appl 686:19–26. doi: 10.1016/S0378-4347(96)00205-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.