ABSTRACT
Antibiotics such as vancomycin are empirically dosed on the basis of body weight, which may not be optimal across the expanding adult body size distribution. Our aim was to compare the relationships between morphomic parameters generated from computed tomography images to conventional body size metrics as predictors of vancomycin pharmacokinetics (PK). This single-center retrospective study included 300 patients with 1,622 vancomycin concentration (52% trough) measurements. Bayesian estimation was used to compute individual vancomycin volume of distribution of the central compartment (Vc) and clearance (CL). Approximately 45% of patients were obese with an overall median (5th, 95th percentile) weight and body mass index of 87.2 (54.7, 123) kg and 28.8 (18.9, 43.7) kg/m2, respectively. Morphomic parameters of body size such as body depth, total body area, and torso volume of the twelfth thoracic through fourth lumbar vertebrae (T12 to L4) correlated with Vc. The relationship of vancomycin Vc was poorly predicted by body size but was stronger with T12-to-L4 torso volume (coefficient of determination [R2] = 0.11) than weight (R2 = 0.04). No relationships between vancomycin CL and traditional body size metrics could be discerned; however, relationships with skeletal muscle volume and total psoas area were found. Vancomycin CL independently correlated with total psoas area and inversely correlated with age. Thus, vancomycin CL was significantly related to total psoas area over age (R2 = 0.23, P < 0.0001). This proof-of-concept study suggests a potential role for translation of radiographic information into parameters predictive of drug pharmacokinetics. Prediction of individual antimicrobial pharmacokinetic parameters using analytic morphomics has the potential to improve antimicrobial dose selection and outcomes of obese patients.
KEYWORDS: morphomics, precision medicine, drug dosing, safety, muscle, psoas, antimicrobial safety
INTRODUCTION
Empirical antimicrobial drug dosing is often selected based on a traditional body-size-dependent approach (1). This dosing paradigm most commonly relies on body weight to define the drug dose. Body weight is a relatively simple and useful measurement for drug dosing but cannot accurately reflect body composition even when coupled with height (2). This limitation is relevant because body composition is expected to impact drug distribution and clearance (3). Overcoming this limitation is essential in this period of human history, when overweight and obese individuals outnumber those that are underweight (4). Linear translation of drug doses on body weight across a 5-fold adult body size distribution (40 kg – 200 kg) can increase the risk of overexposure and toxicity (1). Several alternative body size scalars, such as ideal body weight (IBW), adjusted body weight (ABW), lean body weight (LBW), and body surface area (BSA), are used to overcome this dose overestimation problem (5). However, these scalars are simply mathematical transformations of height and weight by sex, and also fail to capture body composition (1, 3, 5). They also do not incorporate well known ethnicity-dependent factors that affect individual body composition in an age-dependent manner (6). An individual measurement of body composition may support optimized dose selection and improve drug safety and efficacy by better capturing interindividual variability in pharmacokinetic parameters.
Several techniques exist to quantify body composition, but they are difficult to implement in clinical practice, especially among acutely ill patients (2). Radiological assessments such as computed tomography (CT) are routinely performed in patients for diagnostic purposes and could be used to gain individual body composition information. The Morphomic Analysis Group has developed algorithms that translate CT examination radiographic imaging into body composition metrics, called analytic morphomics (7–9). This approach may have major clinical translational utility because radiographic data generated as a part of standard diagnostic care could be repurposed to define individual body composition. This information may be especially helpful for the refinement of empirical antibiotic regimens that are currently dosed on the basis of body weight.
Vancomycin is a highly prescribed antibiotic that is dosed according to body weight with specific guidance on dose modification based on therapeutic drug monitoring (TDM) (10). The volume of distribution (V) of this agent is scaled to body weight (0.7 L/kg), and the clearance (CL) is scaled to body weight based on kidney function (11). Unnecessarily high doses of vancomycin could increase the risk of kidney injury among obese patients (12). Hence, improving empirical dose selection of vancomycin can have a major impact on improving the safety and efficacy of this antibiotic. We hypothesized that CT-derived body composition metrics (morphomic parameters) could reduce the interindividual variability of vancomycin V and CL estimates to a greater extent than body weight could. The results of this study support this hypothesis and create an avenue for future refinement of drug dosing in patients with obesity.
RESULTS
Subject demographics.
A total of 10,468 unique patient encounters in which vancomycin treatment was used were identified over the 3-year study period. A flow chart detailing the rationale behind the exclusion of subjects for this analysis is provided in the supplemental material (Fig. S1). A majority of encounters were excluded because of missed or undocumented dose administrations, use of oral vancomycin doses, measurement of concentrations with no corresponding dose information, or fewer than three concentration measurements. A total of 317 unique patients were identified after exclusion of subjects by age, body weight measurement discrepancies, lack of an informative CT scan, and informative vancomycin sampling data. An additional 17 patients were excluded due to computation of extreme values of volume of distribution of the central compartment (Vc) (>137 liters) and CL (>10 liters/h), associated with aberrant concentration measurements and dosing information. The 300 patients (59.3% male) included in this analysis had median (5th percentile, 95th percentile) ages, heights, weights, and body mass indexes of 59 (26, 81) years, 173 (152, 188) cm, 87.2 (54.7, 123) kg, and 28.8 (18.9, 43.7) kg/m2, respectively. The median (5th percentile, 95th percentile) values of IBW, ABW, LBW, and BSA were 66.1 (45.5, 82.2) kg, 74.7 (53.1, 95.6) kg, 57.6 (37.3, 77.3) kg, and 2.02 (1.56, 2.47) m2. The distribution of subjects by body mass index (BMI) classification was 4.33% underweight, 22.3% normal weight, 28% overweight, 21.3% obese class 1, 14% obese class 2, and 10% obese class 3 (see Materials and Methods for World Health Organization BMI classification parameters).
Subject morphomic parameters.
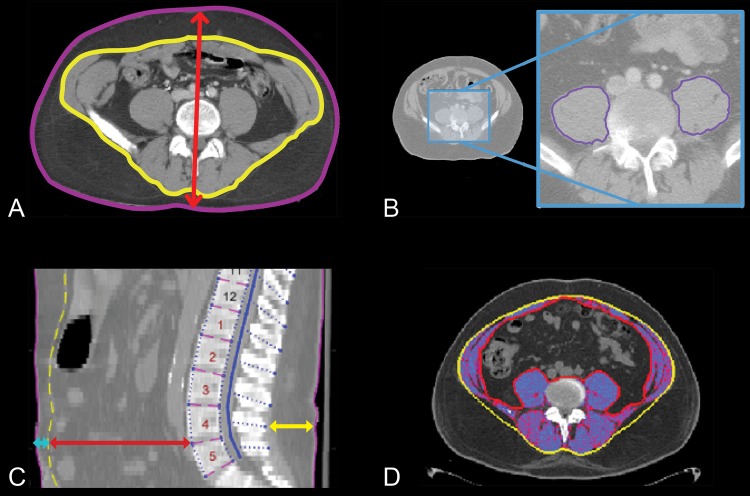
The majority (85.3%) of CT data were acquired using GE Medical System Discovery CT750 HD (42.8%) or LightSpeed VCT (56.5%) scanners. The peak kilovoltage (kVp) was 120 in 97.3% of cases, and a “standard” convolution kernel was applied to the images from 84.7% of subjects. Approximately 48% of CT scans were acquired during the period of vancomycin sample measurement, and 100% were acquired within 2 weeks of sample measurement. Figure 1 includes CT scan images to illustrate regions probed to quantify key morphomic parameters. Table 1 summarizes the median (5th, 95th percentile) morphomic parameters derived from the CT scan imaging data acquired at the transverse level of the fourth lumbar vertebral body (L4) for area/depth measures and the twelfth thoracic vertebral body (T12) to L4 for volume measures. Additional details about these morphomic parameters can be accessed through the Morphomics Data Dictionary (http://www.med.umich.edu/surgery/morphomics/data_dictionary). The L4 body and subcutaneous area and T12-to-L4 subcutaneous and torso volumes were underestimated in 57.7% of subjects, because larger individuals were less likely to fit within the scan field of view. An example of a “cutoff” field of view is included in the supplemental material (Fig. S2) to illustrate this limitation. As a consequence, data from these four morphomic parameters were limited to 127 subjects with a complete field of view. This subgroup was similar by age but significantly smaller in size (as expected) relative to the overall population. The median (5th percentile, 95th percentile) age, height, weight, and body mass index for this group of 127 subjects are 60 (26, 81) years, 175 (155, 191) cm, 78 (49.9, 117) kg, and 26.2 (17.8, 36.0) kg/m2, respectively. This scan field view limitation did not impact other morphomic parameters estimates such as total psoas area. Given this potential loss of information, body depth was investigated as a potential surrogate parameter of body area and volume measurements as it was not impacted by the cutoff field view. Body depth was strongly correlated to L4 body area (R = 0.96), subcutaneous area (R = 0.75), T12-to-L4 subcutaneous volume (R = 0.74), and torso volumes (R = 0.91) in the 127 subjects. This strong correlation of body depth, L4 body area, and torso volume suggests that it is a reasonable surrogate parameter for these measurements.
FIG 1.
Representative images of key morphomic parameters that include (A) body depth (red arrow), fascia area (yellow border), total body area (purple border), and subcutaneous fat area (area between purple and yellow borders); (B) total psoas area (sum of left and right areas within purple border); (C) spinous process to back skin (yellow line), vertebral body to fascia (red line), and fascia to front skin (turquoise line); (D) skeletal muscle area (shaded region represents muscle tissue within the fascial boundary).
TABLE 1.
Summary of median (5th, 95th percentile) morphomic parameters by WHO BMI classification group
| Parametera | Underweight | Normal Weight | Overweight | Obese class |
||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Number (%) | 13 (4.33) | 67 (22.3) | 84 (28.0) | 64 (21.3) | 42 (14.0) | 30 (10.0) |
| Weight (kg) | 56.7 (33.6, 63.5) | 66.6 (48.0, 81.5) | 79.5 (63.6, 96.6) | 98.9 (79.1, 118) | 104 (82.2, 127) | 122 (99.8, 163) |
| Body depth (mm) | 212 (175, 278) | 234 (190, 281) | 270 (232, 313) | 301 (249, 348) | 313 (274, 374) | 344 (310, 417) |
| Fascia area (cm2) | 400 (267, 682) | 448 (298, 561) | 503 (356, 678) | 574 (366, 776) | 561 (412, 892) | 673 (490, 999) |
| VB2 fascia (mm) | 84 (61, 169) | 101 (62, 156) | 121 (74, 169) | 134 (75, 182) | 136 (101, 209) | 158 (102, 253) |
| SP2B skin (mm) | 11.5 (3.3, 30.6) | 26.4 (6.4, 46.2) | 36.1 (14.9, 60.8) | 49.3 (30.7, 68.7) | 57.5 (38, 75.2) | 67.4 (51.3, 90) |
| F2F skin (mm) | 7.9 (1.2, 15.4) | 18 (4.3, 30.8) | 17.3 (8.3, 39.9) | 27.4 (7.1, 43.2) | 28.1 (7.9, 50.5) | 30.8 (13.3, 52.5) |
| Total psoas area (cm2) | 18 (9, 32) | 19 (9, 33) | 20 (11, 35) | 24 (15, 34) | 18 (12, 40) | 21 (15, 37) |
| Subcutaneous area (cm2)b | 90 (39, 155) | 159 (62, 325) | 230 (136, 395) | 376 (243, 514) | 442 (386, 542) | 460 (423, 497) |
| Total body area (cm2)b | 519 (371, 727) | 599 (413, 768) | 739 (552, 999) | 1021 (745, 1177) | 1209 (999, 1385) | 1218 (1178, 1258) |
| Fascia volume (liters) | 6.85 (3.63, 9.38) | 6.92 (4.46, 9.45) | 7.62 (5.45, 10.8) | 9.01 (5.38, 12.6) | 8.02 (5.01, 13.6) | 8.98 (6.37, 14.6) |
| Subcutaneous fat volume (liters)b | 0.72 (0.41, 1.58) | 1.32 (0.42, 3.14) | 2.08 (1.01, 3.49) | 3.10 (1.91, 4.95) | 4.32 (3.19, 5.65) | 4.72 (4.62, 4.81) |
| Skeletal muscle volume (liters) | 0.91 (0.45, 1.39) | 1.00 (0.63, 1.61) | 1.17 (0.73, 1.77) | 1.44 (0.80, 1.99) | 1.07 (0.79, 2.04) | 1.15 (0.76, 2.26) |
| T12-to-L4 torso vol (liters)b | 7.18 (4.97, 9.88) | 8.13 (5.66, 10.2) | 9.89 (6.92, 14.7) | 13.1 (9.06, 17.0) | 15.5 (13.3, 19.0) | 15.6 (15.5, 15.7) |
VB2 fascia, vertebral body to fascia; SP2B skin, spinous process to back skin; F2F skin: fascia to front skin.
Data based on 127 subjects with only 2 subjects in obese class 3.
Vancomycin concentration-time profiles.
A total of 1,622 vancomycin concentrations were analyzed that included a median (5th percentile, 95th percentile) of 4 (3, 12) samples per subject. Figure 2 illustrates the distribution of concentration measurements relative to the time since last dose. A majority of the data set included trough concentrations (n = 830), followed by mid-dosing interval concentrations (n = 615), and peak concentrations (n = 177).
FIG 2.
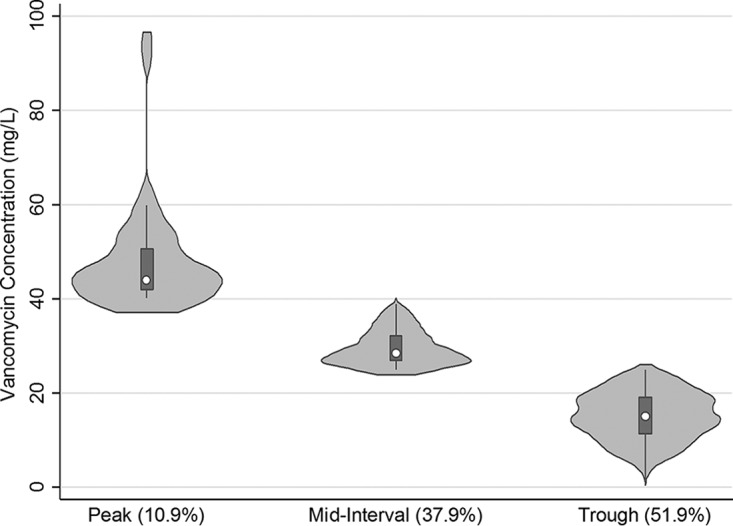
Violin plot of the distribution of concentrations obtained from subjects to inform the pharmacokinetic parameter estimates based on measurement type (percentage of total samples).
Vancomycin pharmacokinetic parameters.
Both the 2-compartment and 1-compartment models provided a good fit to the data. The individual Bayesian fits for the subjects had a median (5th percentile, 95th percentile) coefficient of determination (R2) of 0.83 (0.51, 0.98). The median (5th percentile, 95th percentile) estimates of Vc and CL were 69.1 (49.1, 102) liters and 2.65 (0.93, 6.13) liters/h for the 1-compartment model. The 2-compartment parameter estimates for Vc, CL, Vp (volume of the peripheral compartment), and CLd (intercompartmental clearance) were 38.1 (24.7, 48.3) liters, 2.14 (0.71, 5.52) liters/h, 35.0 (19.4, 103) liters, and 6.12 (4.21, 7.46) liters/h, respectively. Given the sparseness of concentration data immediately postinfusion, individual pharmacokinetic parameter estimates from the 1-compartment model were used to compare against the traditional body size and newer morphomic parameters.
Relationships of vancomycin pharmacokinetic to conventional body size parameters.
Vancomycin Vc was poorly correlated to weight (R = 0.19) and height (R = 0.16). Alternative body size scalars, ABW and BSA, had an improved but overall poor correlation (R = 0.22) with Vc. The correlation of CL to body size was weaker (R < 0.11) for all parameters tested and was not significant for any parameter by linear regression (P > 0.05). Regression of Vc to weight was significant with a very poor coefficient of determination (R2 = 0.04). Figure 3 illustrates this poor relationship with a mean (95% confidence interval) slope and intercept of 0.132 (0.057, 0.208) liters/kg and 60.1 (53.2, 66.9) liters, respectively. This is in contrast to the regression without constant (intercept = 0), mean (95% confidence interval) slope of 0.775 (0.748, 0.802) liters/kg that matches most population estimates but poorly represents the actual data. Regression of Vc to ABW or BSA also resulted in significant (P < 0.001) but poor (R2 = 0.05) relationships.
FIG 3.
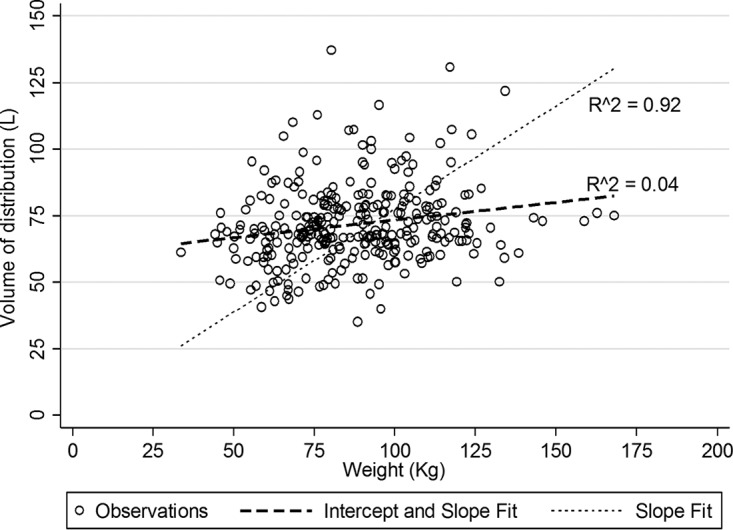
Scatter and linear regression fits of vancomycin volume of distribution (vertical axis) over weight.
Relationship of vancomycin pharmacokinetic parameters to morphomic parameters.
Relative to traditional body size relationships to vancomycin Vc, this parameter had a higher correlation to T12-to-L4 torso volume (R = 0.33), total body depth (R = 0.32), and total body area (R = 0.31). Regression of Vc to T12-to-L4 torso volume was significant with a poor coefficient of determination (R2 = 0.11). The relationship of Vc to body depth was similar (R2 = 0.10) to that of Vc to torso volume (n = 127 subjects). Vancomycin CL correlated with total psoas area (R = 0.33) and skeletal muscle volume (R = 0.27). Stepwise regression of CL to morphomic parameters identified total psoas area as an independent predictor of CL (R2 = 0.10), and this relationship improved when combined with age (R2 = 0.20). Scatter and linear fit plots of CL to total psoas area, CL to age, and CL to total psoas area by age illustrate a positive correlation, negative correlation, and no correlation, respectively (Fig. S3). As a consequence, regression of vancomycin CL as a function of total psoas muscle area/age (cm2/year) improved the relationship to an R2 of 0.23. The mean coefficient (95% confidence interval) for total psoas muscle area/age and constant values are 2.81 (2.22, 3.40) and 1.68 (1.38, 1.99), respectively.
DISCUSSION
Drug dosing based on body weight is often used to select empirical doses of antimicrobial agents such as vancomycin (10). This dosing strategy was designed in an age when the prevalence of obesity was much lower (13). The prevalence of obesity among adults in the United States over the age of 20 years almost tripled from 13.4% to 35.7% between 1962 and 2010 (14). Our study sample included a large proportion of obese subjects (45.3%), reflecting our hospital inpatient sample. The median weight was 87.2 kg, which is 25% higher than the classically held view of a reference individual of 70 kg (1). Higher body weight measurements can lead to computation of higher drug doses and systemic exposures that may not be appropriate in an individual patient. This may be relevant for an agent such as vancomycin, given that weight ≥101 kg has been identified as one of several risk factors for the occurrence of acute kidney injury (15). Overcoming the present limitations of body weight for drug dosing requires consideration of alternative metrics such as those generated by analytic morphomics. We show that morphomic parameters corresponding to body volume correlated with vancomycin Vc, and those corresponding to muscle mass correlated with vancomycin CL. Although these correlations may be regarded as weak (R2 < 0.3), these physiologically relevant morphomic parameters outperformed our current standard.
Parameters associated with T12-to-L4 torso volume, such as total body area and body depth, correlated with vancomycin Vc. Importantly, T12-to-L4 torso volume had a stronger relationship with vancomycin Vc than did body weight. Demonstration that this relationship remained with body depth is relevant given that this parameter was measurable in the full data set. However, it should be noted that the Vc of vancomycin was not significantly (P = 0.274) different between BMI categories. The mean (SD) Vc of vancomycin was 69.8 (16.9) liters in normal weight subjects compared with 74.7 (15.7) liters in obese subjects. This finding is consistent with studies that suggest improved prediction of vancomycin Vc with use of a traditional body-size-independent model (16, 17). This classical error is also illustrated in Fig. 3, which shows the potential miscalculation of vancomycin Vc when a slope-based estimate such as 0.7 liters/kg is used to compute this parameter.
Vancomycin CL estimation is often based on creatinine clearance estimates using the Cockcroft-Gault equation (11). This equation provides a point estimate of kidney function and relies on body weight that is substituted with alternative body size descriptors when used in obese subjects (18). The underlying mathematical basis of that equation is to generate a surrogate estimate of lean body mass or skeletal mass as the primary input function of creatinine production because it is not easy to measure (18). Our observation of correlations between total psoas muscle area and skeletal muscle volume and vancomycin CL is consistent with this physiological expectation. Within the general population, total psoas area varies by age and gender; it grows steadily and quickly to a peak at age 20 before slowly decreasing with advancing age. Preliminary data suggest that there may also be racial differences. Additional details about this metric can be retrieved through the Reference Analytic Morphomics Population (RAMP) database (http://www.med.umich.edu/surgery/morphomics/ramp). The focus of this study was on traditional body size and newer morphomic parameters as predictors of vancomycin Vc and CL. Our ability to explain 23% of the interindividual variability of vancomycin CL with total psoas area and age is encouraging given that the data inputs were derived retrospectively in a diverse inpatient clinical sample. This finding is relevant because the total psoas area has been shown to be a reliable surrogate of cardiovascular fitness and body composition (19). Also, total psoas area was not easily predicted by body size and was numerically lower in obese class 2 and obese class 3 patients than in obese class 1 patients. This implies that radiographic acquisition of this information could serve as an important physiological surrogate of drug clearance, especially for agents like vancomycin that are eliminated unchanged by the kidneys. This morphomic parameter may be especially relevant to explore in paraplegic and quadriplegic patients who have significantly reduced total psoas areas to see if dosing can be improved in this population.
Although important new information was gained from this proof-of-concept study, limitations exist that require additional studies to qualify the generalizability of the knowledge gained. We included subjects from a single institution and relied on a retrospective data set. We excluded a large number of subjects because we used a conservative approach to selection of data for pharmacokinetic analyses. This is both a strength (quality) and a limitation, given that we may have inadvertently excluded relevant subjects. An unintentional finding was that a large number of subjects may have incorrect height and weight information in their medical record. Although this was not an aim of this study, this finding suggests the potential for dosing errors with incorrectly recorded traditional body size measures and the need for quality improvement to ensure accurate entry of biometric information. We were also not able to capture race and ethnicity information as this was not reliably input into the medical record. The concentration data used to calculate vancomycin pharmacokinetic parameters were primarily trough-based measurements, and only 10.9% of samples were peak measurements. Although midpoint concentrations permitted PK parameter estimation, this sampling limitation weakens the strength of Vc estimates gained from some subjects in our study. An important limitation of our analyses is its applicability to obese class 3 subjects, whose larger size prevented accurate estimation of body area and volume measurements. Even with these limitations, our ability to identify physiologically relevant morphomic parameters as potential predictors of vancomycin pharmacokinetics is intriguing. Given that physiology-based pharmacokinetic (PBPK) models of vancomycin are sparse in the literature, these findings will support development of new PBPK models for similar agents.
In conclusion, we sought to compare CT-scan-derived morphomic parameters to body weight and other traditional body size metrics as predictors of vancomycin PK parameters. We demonstrated that morphomic parameters associated with volume were predictive of vancomycin volume of distribution. Stronger relationships were observed between vancomycin CL and morphomic parameters of lean muscle mass by age. Further investigation of this approach through a prospective PK study design and with other antimicrobial agents is necessary to validate these findings. The proposed pharmacomorphomic approach may help to improve the dosing and clinical outcomes of obese patients, who are currently being dosed on the basis of body weight.
MATERIALS AND METHODS
Regulatory review.
The current study was approved as an expedited protocol (no more than minimal risk) by the University of Michigan Institutional Review Board (IRB) under protocol HUM00041441, “Review of Computed Tomography Scans,” prior to the retrospective acquisition of data for analysis.
Inclusion criteria.
Subject records fulfilling the following criteria were eligible: (i) male or female ≥21 years of age, (ii) at least three vancomycin concentrations measured, (iii) vancomycin administered by intravenous route, (iv) CT scan within 14 days prior to the first vancomycin concentration collection date, and (v) admission to the University of Michigan Hospital over a 3-year period (1 January 2014 to 31 December 2016).
Exclusion criteria.
Subject records fulfilling the following criteria were excluded: (i) measurement of concentrations with no dosing information prior to measurement, (ii) inconsistent body weight or height measurements during the admission period, such as an extreme value or coefficient of variation ≥4% during the admission, (iii) no midpoint or peak concentration measurement, (iv) CT scan imaging data with an insufficient display file of view to capture the necessary anatomy for computing morphomic parameters, (v) subject medication administration records with dosing data suggestive of missed doses, and (vi) subject on a renal replacement modality.
Data query and management.
A self-service tool developed by the University of Michigan Health System known as “DATADIRECT” was used to generate the query. The information queried included patient demographics, encounters, medications ordered (restricted to vancomycin), medication administration (restricted to vancomycin), and laboratory information (restricted to vancomycin measurements). The generated query was merged with a radiological information set to identify subjects with CT scan data generated during the vancomycin measurement period. The filtered data set was password protected and stored on a secure, HIPAA (Health Insurance Portability and Accountability Act of 1996)-compliant, cloud-based platform (U-M Box). The data set was organized and filtered using the R statistical programming language, including the dplyr package. Data visualization was accomplished using the ggplot2 package for R. Specifically, each individual subject dose and concentration details were visualized from time zero (first dose) to time last (last concentration measurement) and reviewed by the study investigator for accuracy. Individual final dosing and concentration-time information files were created for pharmacokinetic analyses.
Pharmacokinetic data analysis.
Our previously published vancomycin pharmacokinetic model and covariance matrix was used as the Bayesian prior to compute 1-compartment and 2-compartment parameter estimates (17). The Bayesian analyses were accomplished using the maximum a posteriori (MAP) function in ADAPT 5 (BMSR, University of Southern California, Los Angeles, CA) (20). The individual analyses were automated using batch scripts and the data outputs were extracted using R.
Analytic morphomics.
Details regarding the approach used to compute individual morphomic parameters are provided in our previous publications (7–9, 21–23). In brief, a custom-built MATLAB-based program (The Mathworks Inc., Natick MA) is used to postprocess Digital Imaging and Communications in Medicine (DICOM) files of CT scans. The algorithm is designed to identify the vertebral elements in a semiautomated manner to generate an anatomic index. Measurements at the inferior aspect of the L4 vertebral body provide consistent estimates of abdominal fat, muscle, and fascial area. Volumetric assessments are made by multiplying the height of each vertebral body by the cross-sectional area and summing across each vertebral body from T12 to L4. Data about the specific metrics are outlined in the supplementary file.
Statistical analyses.
Subject demographics and morphomic parameters were summarized descriptively. Subjects were grouped based on World Health Organization body mass index classification as underweight (<18.50 kg/m2), normal weight (18.50 to 24.99 kg/m2), overweight (25.00 to 29.99 kg/m2), obese class 1 (30.00 to 34.99 kg/m2), obese class 2 (35.00 to 39.99 kg/m2), or obese class 3 (≥40.00 kg/m2). Alternative body size descriptor values (IBW, ABW, LBW, BSA) were computed using equations as previously described (18). Vancomycin concentrations were categorized as a peak if collected within 2 h of the end of infusion, a trough if collected within 1 h prior to the next dose, and midinterval if collected between peak and trough measurement definitions. Vancomycin pharmacokinetic parameters were compared to each body size and morphomic variable by Pearson's correlation coefficient. Scatter plots of the vancomycin pharmacokinetic parameter to variables identified to have the highest Pearson's correlation coefficient were visualized. Stepwise backward selection with a significance level of 0.25 was used for removal from the model to identify potential variables predictive of vancomycin pharmacokinetic parameters. Ordinary least-squares regression and nonlinear regression methods (power function, logistic function, and exponential function) were also tested to identify composite variables predictive of vancomycin pharmacokinetic parameters. All statistical analyses were performed using Stata/IC 14.2 (StataCorp LLC, College Station, TX). Significance was defined as a P value < 0.05.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part through start-up funds from the University of Michigan, College of Pharmacy, to M.P.P.
We acknowledge the support of Sven Holcombe, Binu Enchakalody, and Nick Wang for writing the MATLAB code necessary to extract morphomic data from CT scans.
M.P.P. and S.C.W. conceived of the study; B.D. constructed the queries, extracted morphomics data from the database, and reviewed morphomic measurements for accuracy; M.L. supported the data analyses and pharmacokinetic batch scripting; B.E.R. performed scan selection and processing; J.A.S. supported the regulatory approval and coordination of this study; M.P.P. performed the pharmacokinetic and statistical analyses; all authors contributed to the design, writing, and editing of the final manuscript for submission.
S.C.W. and J.A.S. are inventors of the Analytic Morphomics: High Speed Medical Image Automated Analysis Method (US patent 14/014,485). All other authors have no conflicts of interest to disclose regarding this work.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01402-17.
REFERENCES
- 1.Pai MP. 2012. Drug dosing based on weight and body surface area: mathematical assumptions and limitations in obese adults. Pharmacotherapy 32:856–868. doi: 10.1002/j.1875-9114.2012.01108.x. [DOI] [PubMed] [Google Scholar]
- 2.Wells JC, Fewtrell MS. 2006. Measuring body composition. Arch Dis Child 91:612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanley MJ, Abernethy DR, Greenblatt DJ. 2010. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 49:71–87. doi: 10.2165/11318100-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M, Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) 2011. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green B, Duffull SB. 2004. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol 58:119–133. doi: 10.1111/j.1365-2125.2004.02157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wulan SN, Westerterp KR, Plasqui G. 2010. Ethnic differences in body composition and the associated metabolic profile: a comparative study between Asians and Caucasians. Maturitas 65:315–319. doi: 10.1016/j.maturitas.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Lee JS, He K, Harbaugh CM, Schaubel DE, Sonnenday CJ, Wang SC, Englesbe MJ, Eliason JL, Michigan Analytic Morphomics Group (MAMG) 2011. Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg 53:912–917. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Terjimanian MN, Tishberg LM, Alawieh AZ, Harbaugh CM, Sheetz KH, Holcombe SA, Wang SC, Sonnenday CJ, Englesbe MJ. 2011. Surgical site infection and analytic morphometric assessment of body composition in patients undergoing midline laparotomy. J Am Coll Surg 213:236–244. doi: 10.1016/j.jamcollsurg.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Englesbe MJ, Lee JS, He K, Fan L, Schaubel DE, Sheetz KH, Harbaugh CM, Holcombe SA, Campbell DA Jr, Sonnenday CJ, Wang SC. 2012. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg 256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 10.Rybak M, Lomaestro B, Rotschafer JC, Moellering R Jr, Craig W, Billeter M, Dalovisio JR, Levine DP. 2009. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 66:82–98. doi: 10.2146/ajhp080434. [DOI] [PubMed] [Google Scholar]
- 11.Matzke GR, McGory RW, Halstenson CE, Keane WF. 1984. Pharmacokinetics of vancomycin in patients with various degrees of renal function. Antimicrob Agents Chemother 25:433–437. doi: 10.1128/AAC.25.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, Biyani M, Khaira A. 2011. Vancomycin nephrotoxicity: myths and facts. Neth J Med 69:379–383. [PubMed] [Google Scholar]
- 13.Moellering RC Jr, Krogstad DJ, Greenblatt DJ. 1981. Vancomycin therapy in patients with impaired renal function: a nomogram for dosage. Ann Intern Med 94:343–346. doi: 10.7326/0003-4819-94-3-343. [DOI] [PubMed] [Google Scholar]
- 14.Ogden CL, Carroll MD. 2010. Prevalence of overweight, obesity, and extreme obesity among adults: United States, trends 1960–1962 through 2007–2008. National Center for Health Statistics, Atlanta, GA: https://www.cdc.gov/NCHS/data/hestat/obesity_adult_07_08/obesity_adult_07_08.pdf. Accessed July 7, 2017. [Google Scholar]
- 15.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 16.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai MP, Hong J, Krop L. 2017. Peak measurement for vancomycin AUC estimation in obese adults improves precision and lowers bias. Antimicrob Agents Chemother 61:e02490-16. doi: 10.1128/AAC.02490-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pai MP. 2010. Estimating the glomerular filtration rate in obese adult patients for drug dosing. Adv Chronic Kidney Dis 17:e53-62. doi: 10.1053/j.ackd.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Fitzpatrick J, Chambers ES, Parkinson JRC, Frost G, Bell JD, Thomas EL. 2017. Psoas major cross-sectional area: a potential marker of cardiorespiratory fitness. Int J Clin Exp Physiol 4:15–20. doi: 10.4103/ijcep.ijcep_6_17. [DOI] [Google Scholar]
- 20.D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 User's Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software, Los Angeles, California. [Google Scholar]
- 21.Krishnamurthy V, Zhang P, Ethiraj S, Enchakalody B, Waljee AK, Wang L, Wang SC, Su GL. 2015. Use of analytic morphomics of liver, spleen, and body composition to identify patients at risk for cirrhosis. Clin Gastroenterol Hepatol 13:360–368.e5. doi: 10.1016/j.cgh.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parenteau CS, Wang NC, Zhang P, Caird MS, Wang SC. 2014. Quantification of pediatric and adult cervical vertebra—anatomical characteristics by age and gender for automotive application. Traffic Inj Prev 15:572–582. doi: 10.1080/15389588.2013.843774. [DOI] [PubMed] [Google Scholar]
- 23.Parenteau CS, Zhang P, Holcombe S, Kohoyda-Inglis C, Wang SC. 2014. Can anatomical morphomic variables help predict abdominal injury rates in frontal vehicle crashes? Traffic Inj Prev 15:619–626. doi: 10.1080/15389588.2013.852665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.