ABSTRACT
We have previously reported that an erg11 mutation affecting ergosterol synthesis and a hem13 mutation in the heme synthesis pathway significantly sensitize the fission yeast Schizosaccharomyces pombe to hydroxyurea (HU) (1, 2). Here we show that treatment with inhibitors of Erg11 and heme biosynthesis phenocopies the two mutations in sensitizing wild-type cells to HU. Importantly, HU synergistically interacts with the heme biosynthesis inhibitor sampangine and several Erg11 inhibitors, the antifungal azoles, in causing cell lethality. Since the synergistic drug interactions are also observed in the phylogenetically divergent Saccharomyces cerevisiae and the opportunistic fungal pathogen Candida albicans, the synergism is likely conserved in eukaryotes. Interestingly, our genetic data for S. pombe has also led to the discovery of a robust synergism between sampangine and the azoles in C. albicans. Thus, combinations of HU, sampangine, and the azoles can be further studied as a new method for the treatment of fungal infections.
KEYWORDS: hydroxyurea, antifungal agents, cytokinesis arrest, oxidative stress, sampangine, azoles, terbinafine, combination therapy
INTRODUCTION
It is estimated that there are at least 1.5 million and probably 3 to 6 million species of fungi existing on Earth (3, 4). They play indispensable roles in evolution, ecosystems, and human progress such as production of food, vitamins, antibiotics, breads, and wines, etc. However, there are about 300 fungal species that are known to cause diseases in humans (5), which affect approximately 1.2 billion people worldwide (6, 7). Although most of the fungal infections are superficial, affecting the skin, nails, and mucosae, a large number of the infections are invasive or chronic and very difficult to treat. It is estimated that about 1.5 million people die of fungal infection each year (6). Most of the mortality is caused in individuals with a compromised immune system, such as those living with HIV, recipients of organ or stem cell transplants, cancer patients, or hospitalized individuals (8–10).
Species in four fungal genera Candida, Aspergillus, Cryptococcus, and Pneumocystis are responsible for majority of the mortality caused by fungal infections (6). Although effective drugs such as the antifungal azoles (7), echinocandins (11), and polyenes (12) are available, several factors hamper the effective treatment of fungal infections. One of the major factors is drug resistance. While some fungal species are intrinsically resistant to antifungal agents, other pathogens acquire the resistance over time (6, 7). Other factors affecting the treatment include drug toxicity and limited availability of novel antifungals. Thus, it is necessary to discover more effective and safer drugs to tackle the increasing challenge of fungal diseases, particularly the invasive infections.
Hydroxyurea (HU) is an inhibitor of ribonucleotide reductase. Since its discovery over 160 years ago, HU has been used for the treatment of cancers, sickle cell anemia, psoriasis, and infections caused by bacterial pathogens or HIV (13, 14). It is a water-soluble, well-tolerated, and well-absorbed small-molecule drug that can be used either intravenously or orally. We have previously shown in the fission yeast Schizosaccharomyces pombe that hypomorphic mutations in the heme or ergosterol biosynthesis pathway significantly sensitize the cells to HU (1, 2). In this study, we further investigated the cell-killing effect of HU in various combinations with inhibitors of heme and ergosterol biosynthesis in S. pombe, the baker's yeast Saccharomyces cerevisiae, and the opportunistic pathogen Candida albicans. Our results clearly showed that in vitro, the drug combinations worked synergistically in inducing cell death. Based on the genetic screening data for S. pombe, we also found that combinations of the inhibitor of heme synthesis sampangine (SMP) (15) with various antifungal azoles, the Erg11 inhibitors, showed an even more robust synergism in C. albicans. Together, we show that combination therapies using HU, SMP, and the azoles have a great potential to be further developed as a safer and more effective method for the treatment of fungal diseases. The same strategy may also be developed for managing other pathological conditions such as cancer. Furthermore, S. pombe may serve as a reliable model for antifungal research.
RESULTS
SMP, terbinafine, and antifungal azoles significantly enhance the cell-killing effect of HU in S. pombe.
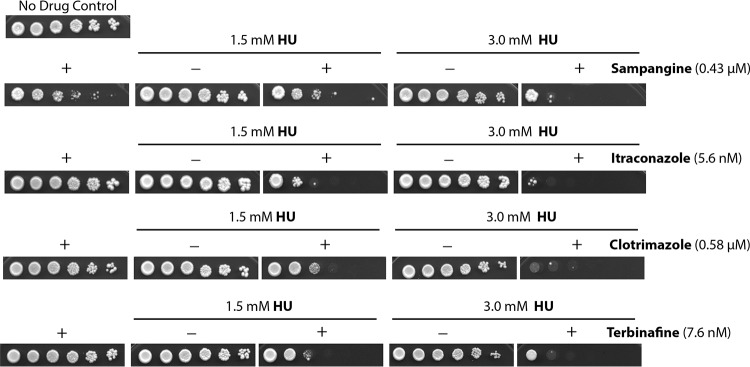
We have recently reported two mutants, the hem13-1 and erg11-1 mutants, of the fission yeast S. pombe that are highly sensitive to HU (1, 2). Since HU is a relatively safe drug and has been used for the treatment of various pathological conditions but not fungal infections, we were interested in testing the enhancement of its cytotoxicity with antifungal azoles that inhibit Erg11 and the heme synthesis inhibitor sampangine (SMP). We first assessed this in wild-type S. pombe by using the standard spot assay (Fig. 1). S. pombe is not a pathogen. It belongs to the phylum Ascomycota, the largest and most diverse group of fungi, and has been used as an established model for studying eukaryotic cellular mechanisms (16). We performed the spot assay on plates containing HU in the presence (+) or absence (−) of SMP (Fig. 1, top panels), the two antifungal azoles itraconazole and clotrimazole, and another Erg11 inhibitor and antifungal agent, terbinafine, at the indicated concentrations (Fig. 1, bottom panels). All doses of the drugs, when used alone, had a minimal to moderate effect on cell growth. When used in combinations, however, SMP, itraconazole, clotrimazole, and terbinafine could significantly enhance the effect of HU on suppressing the cell growth. We also tested two other azoles, fluconazole and ketoconazole, and similar results were observed (data not shown).
FIG 1.
The cytotoxic effects of hydroxyurea (HU), sampangine, itraconazole, clotrimazole, and terbinafine used alone or in combinations were assessed in S. pombe by standard spot assay. Serial 5-fold dilutions of logarithmically growing wild-type S. pombe were spotted on plates of the rich medium YE6S containing 1.5 mM or 3.0 mM HU in the presence (+) or absence (−) of the heme synthesis inhibitor sampangine or the ergosterol synthesis inhibitors itraconazole, clotrimazole, and terbinafine at the indicated concentrations. The plate on the upper left does not contain any drug. All plates were incubated at 30°C for 3 days and then photographed.
Synergistic drug interactions between HU and SMP or azoles in S. pombe.
The results described above strongly suggest that HU may work synergistically with SMP, antifungal azoles, or terbinafine in suppressing yeast cell growth. To analyze the drug interactions quantitatively, we employed the 96-well plate assay as described in Materials and Methods. The dose-response curve for each drug was first examined in order to determine the 50% inhibitory concentrations (IC50s) (see Fig. S1A in the supplemental material). The drug concentrations showing minimal to ≤15% cell killing effects were then chosen for various drug combinations. The synergistic, additive, and antagonistic drug interactions were determined using the Chou-Talalay method (17).
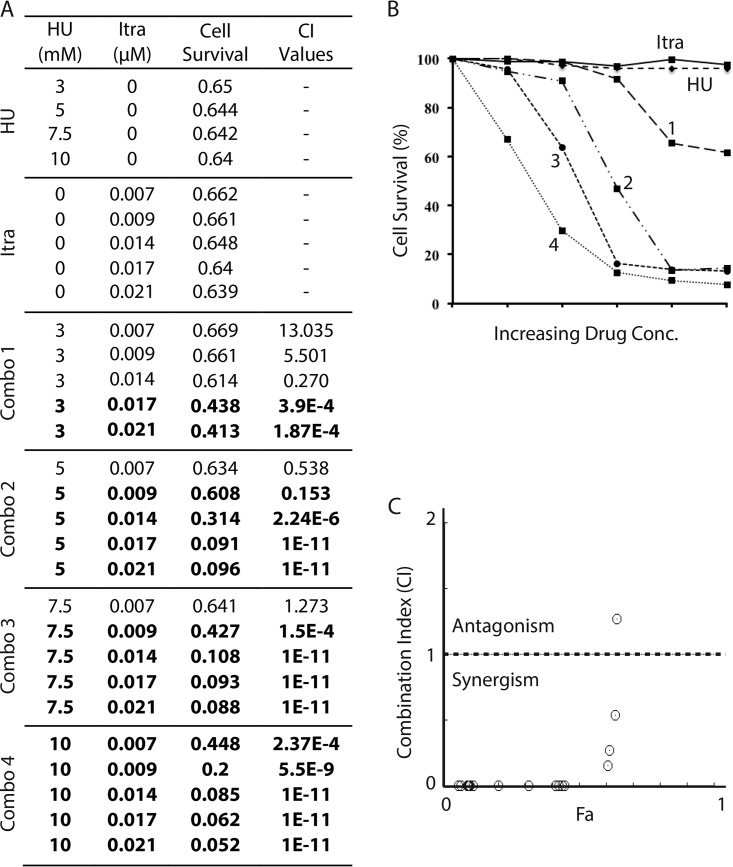
As shown in Fig. 2A, when used alone, both HU and SMP showed a minimal (≤10%) effect on cell survival at the indicated concentrations. We then measured the cell growth in the presence of both agents (combinations [combos] 1 to 4 in Fig. 2A). The A600 readings (Fig. 2A, cell survival column) were then used for calculating the cell survival rate and are presented as percentages of the values for untreated controls (Fig. 2B). Values of the drug combination index (CI) were calculated using CompuSyn (Fig. 2A, CI value column) and schematically shown in a CI-Fa plot (Fig. 2C) (where Fa is cell growth inhibition at various CI values). We found that although three specific combinations of HU and SMP were additive (CI values of ≈1.0) or antagonistic (CI values of >1.0), more than half of the combinations showed a very strong synergism (CI values of <0.1) (Fig. 2A, numbers in bold), which clearly showed that HU works synergistically with SMP in suppressing S. pombe cell growth.
FIG 2.
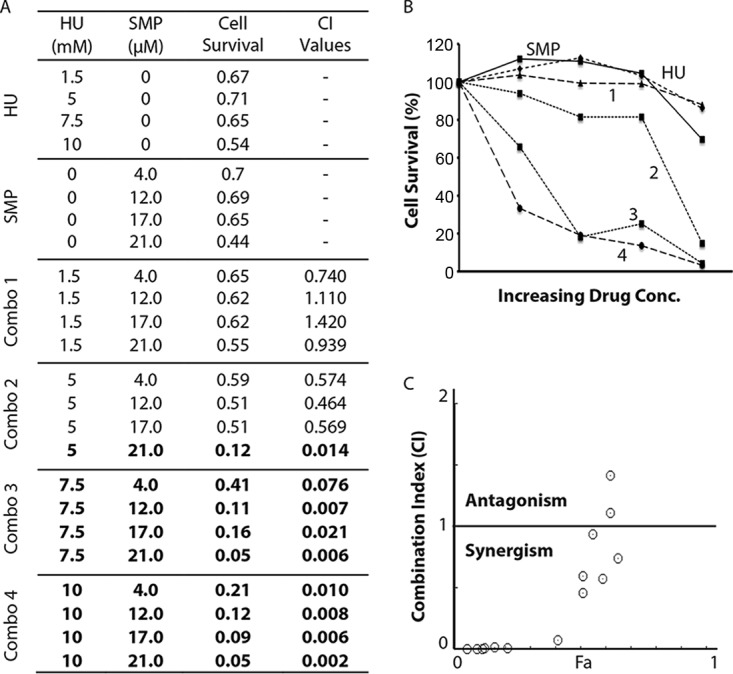
Combinations of HU with sampangine (SMP) show a highly synergistic cytotoxic effect in S. pombe. (A) The cell survival of wild-type S. pombe in the presence of increasing concentrations of HU or SMP alone or in various combinations of the two drugs (combos 1 to 4) was assessed by the 96-well plate assay as described in Materials and Methods. The A600 values are shown to present cell survival. The combination index (CI) values shown on the right of the table were calculated by using the Chou-Talalay method (17). The values marked in bold indicate the drug combinations showing very strong synergism (CI values of <0.1). (B) Graphical representation of the cell-killing effects of HU, SMP, and the four combinations of HU and SMP. The cell survival rate was calculated using the A600 values from panel A relative to the no-drug control and shown as percentages. (C) Combination index plot. Fa on the x axis is the cell growth inhibition value at various CI values. The horizontal line indicates the additive drug interaction (CI values are between 0.9 to 1.1). The CI values above and below the horizontal line represent antagonistic and synergistic drug effects, respectively.
We next assessed the effect of HU in the presence of the four antifungal azoles itraconazole (Fig. 3), clotrimazole (see Fig. S2 in the supplemental material), ketoconazole (see Fig. S3 in the supplemental material), and fluconazole (see Fig. S4 in the supplemental material) and the allylamine antifungal agent terbinafine (see Fig. S5 in the supplemental material). As shown in Fig. 3A and B, when itraconazole was used alone at 0.007 to 0.021 μM, it minimally suppressed the cell growth. In the presence of HU, however, the drug effect was significantly enhanced, and the majority of the combinations showed a very strong synergism (Fig. 3C).
FIG 3.
Combinations of HU with the antifungal azole itraconazole show a highly synergistic cell-killing effect in S. pombe. (A) The cell survival of wild-type S. pombe in the presence of increasing concentrations of HU or itraconazole alone or in various combinations of the two drugs (combos 1 to 4) was assessed by the 96-well plate assay. The cell survival rate and the CI values were calculated as described for Fig. 2. The numbers in bold indicate the drug combinations with very strong synergism. (B) Graphical representation of the cytotoxic effects of HU, itraconazole, and the four combinations of the two drugs shown in panel A. (C) CI-Fa plot.
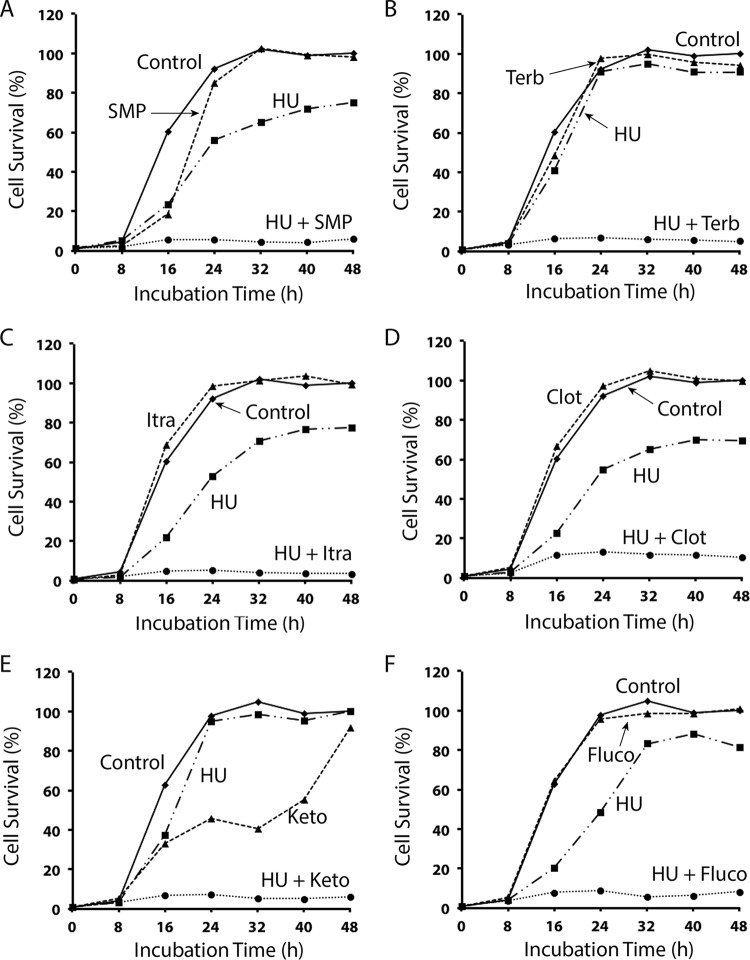
To confirm the results, we carried out a time course analysis of the combinations of HU with SMP (Fig. 4A), HU with terbinafine (Fig. 4B), and HU with the four azoles (Fig. 4C to F) over a period of 48 h. In the presence of SMP, terbinafine, and all tested azoles, the cell growth was minimally suppressed, although ketoconazole showed slightly higher growth suppression (Fig. 4E). When combined with HU, however, the cell growth was almost completely suppressed by all tested drugs, particularly the combination of HU with terbinafine (Fig. 4B). HU and azoles are generally considered to be cytostatic. However, since most of the cells could not survive even after a further incubation for two more days or when the drug-treated cells were spread on YE6S plates (data not shown), the drug combinations may cause irreversible cell damage and are therefore cytotoxic.
FIG 4.
Time course analysis of the synergistic cytotoxic effect of HU in combination with SMP, terbinafine, itraconazole, clotrimazole, ketoconazole, or fluconazole in S. pombe. Logarithmically growing wild-type S. pombe was inoculated in YE6S medium on 96-well plates at 3,000 cells/well, and then 10 mM HU and 21 μM SMP (A), 5 mM HU and 0.091 μM terbinafine (B), 10 mM HU and 0.021 μM itraconazole (C), 10 mM HU and 0.043 μM clotrimazole (D), 5 mM HU and 9.4 μM ketoconazole (E), or 10 mM HU and 22.8 μM fluconazole (F) were added to the cell cultures either alone or in combinations. The same amounts of carriers were added for the control. The plates were incubated at 30°C and scanned at the indicated time points to monitor cell survival. All data points are the averages of the readings from three separate wells.
The synergistic cell-killing mechanisms are highly conserved.
To further investigate the synergism, we then examined the combinations in S. cerevisiae, another established yeast model that is phylogenetically distinct from S. pombe (18). The dose-response curve of each single drug was assessed in a manner similar to that for S. pombe (Fig. S1B). We found that although a higher dose of HU was required to suppress the cell growth in S. cerevisiae, a number of combinations of HU with SMP (see Fig. S6 in the supplemental material), HU with terbinafine (see Fig. S7 in the supplemental material), and HU with the four azoles showed very strong synergistic cell-killing effects (see Fig. S8 to S11 in the supplemental material). We also performed time course analysis of the combinations in S. cerevisiae (see Fig. S10D, S11D, and S12 in the supplemental material). The results clearly showed that in the presence of SMP, ketoconazole, and itraconazole, the cytotoxicity of HU was significantly increased, although a lesser effect was observed for the combinations of HU with clotrimazole (Fig. S10) and with fluconazole (Fig. S11).
Synergistic cell-killing effect in Candida albicans.
The strong synergistic effect observed in both S. pombe and S. cerevisiae suggest that the cell-killing mechanisms are likely conserved in other eukaryotes. We are particularly interested in pathogenic fungi because the four tested azoles are antifungals and SMP has also been reported to have antifungal activity (15, 19, 20). We investigated this hypothesis in the opportunistic pathogen C. albicans because it causes 50 to 90% of all cases of candidiasis, the most common opportunistic yeast infection in humans (21). Systemic infection of C. albicans has also emerged as an important cause of morbidity and mortality in immunocompromised patients, and fluconazole-resistant Candida has become an increasingly serious threat to human health (22).
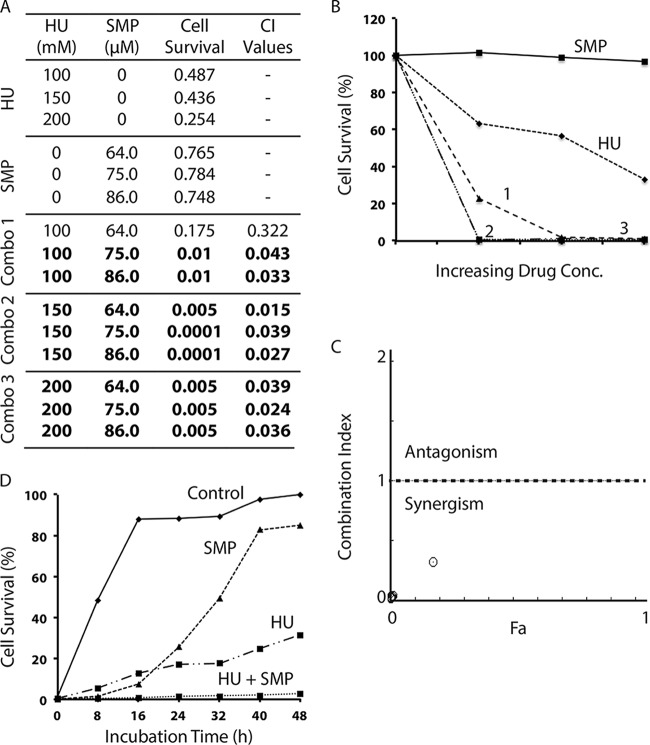
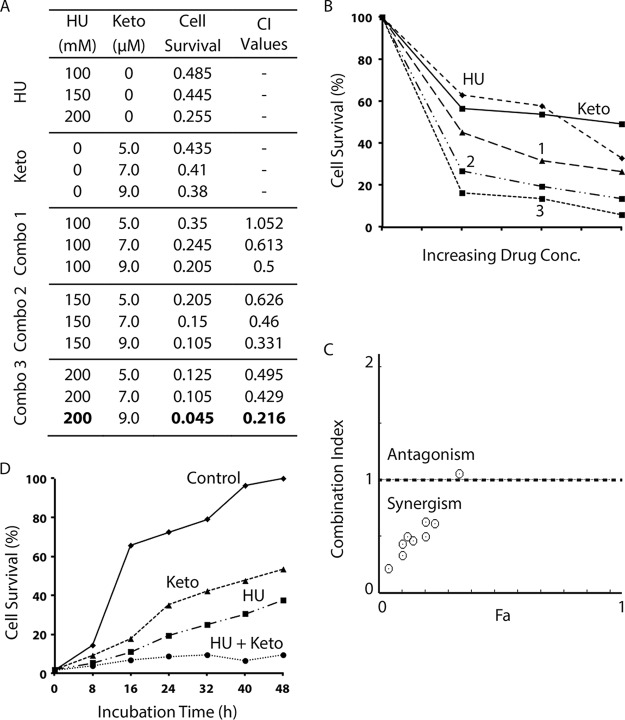
Unlike S. pombe, C. albicans was found to be less sensitive to HU and showed a dose-response curve similar to that in S. cerevisiae (Fig. S1C). It was also less sensitive to SMP but highly sensitive to all tested azoles and terbinafine (Fig. S1C). We then examined the combinations of HU with SMP and found that most of the combinations were strongly synergistic (Fig. 5). A subsequent time course study confirmed the synergism (Fig. 5D). The synergism was also observed in most of the combinations of HU with ketoconazole (Fig. 6), itraconazole (see Fig. S13 in the supplemental material), clotrimazole (see Fig. S14 in the supplemental material), and terbinafine (see Fig. S15 in the supplemental material), although a small number of the combinations were slightly antagonistic.
FIG 5.
Synergistic drug interaction between HU and SMP in the pathogenic fungus C. albicans. (A) The cell survival of the wild-type C. albicans strain SC5314 in the presence of increasing concentrations of HU or SMP alone or in drug combinations (combos 1 to 3) was monitored by the 96-well plate assay. The cell survival rate and the CI values were calculated as described for Fig. 2. The values in bold indicate the combinations with very strong synergism. (B) Graphical representation of the cell survival rate in the presence of HU, SMP, and the three combinations of the two drugs shown in panel A. (C) Combination index plot. (D) Time course study of the synergistic drug effect of 200 mM HU and 86 μM SMP in C. albicans.
FIG 6.
Synergistic drug interaction between HU and the ergosterol synthesis inhibitor ketoconazole in C. albicans. (A) The cell survival of wild-type C. albicans in the presence of HU or ketoconazole alone or in combinations was monitored. (B) Graphical representation of the cell survival rate in the presence of HU, ketoconazole, and the three combinations of the two drugs shown in panel A. (C) Combination index plot. (D) Time course study of the synergistic drug effect of 200 mM HU and 9 μM ketoconazole.
Identification of erg11 as a multicopy suppressor of the hem13-1 mutation in S. pombe.
The results described above clearly showed that combinations of HU with SMP, HU with terbinafine, and HU with several azoles work synergistically in inducing cell death in S. pombe, S. cerevisiae, and C. albicans. To identify new therapeutic targets with related cell-killing mechanisms, we carried out a genetic screen in S. pombe, looking for multiple-copy suppressors of the hem13-1 mutation. This screen identified erg11.
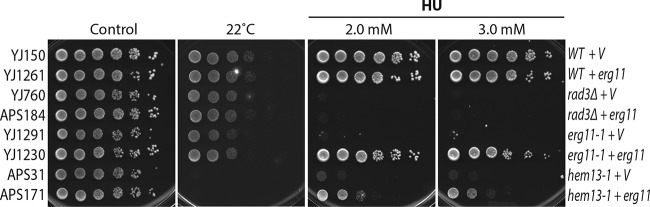
To investigate the suppressor, we cloned erg11 into an expression vector and expressed the protein in wild-type and erg11-1 and hem13-1 mutant cells and assessed the HU sensitivity by standard spot assay. We also included the rad3 checkpoint mutant (ortholog of human ATR and S. cerevisiae Mec1) as a control because it is highly sensitive to the replication stress induced by HU. Since the hem13-1 mutant is sensitive to low temperature (2), the cells were also incubated at the restrictive temperature 22°C. As shown in Fig. 7, while the suppressor had little effect on the wild-type and rad3 cells, it significantly suppressed the HU sensitivity of the erg11-1 and hem13-1 mutants. Because erg11 did not suppress the cold sensitivity of the hem13-1 mutant (Fig. 7, lower part), it cannot rescue the compromised physiological function of the mutant protein. We have previously shown that HU treatment generates oxidative stress in the hem13-1 mutant (2). This result clearly showed that erg11 specifically mitigates the oxidative stress induced by HU in the hem13-1 mutant.
FIG 7.
Overexpression of Erg11 can partially suppress the HU sensitivity but not the cold sensitivity of the hem13-1 mutant. Erg11 was expressed on an S. pombe expression vector in wild-type, erg11-1 mutant, and hem13-1 mutant cells under the control of its own promoter (marked on the right). The rad3Δ checkpoint mutant was used as a control. Serial dilutions of the S. pombe strains, which are labeled on the left (see strain list in Table S1 in the supplemental material), were spotted on YE6S plates or YE6S plates containing 2 or 3 mM HU. The plates were incubated for 3 days at 30°C and then photographed. Since the hem13-1 mutation causes cold sensitivity, one of the YE6S plates was also incubated at 22°C for 3 days (second from the left). V, empty vector control.
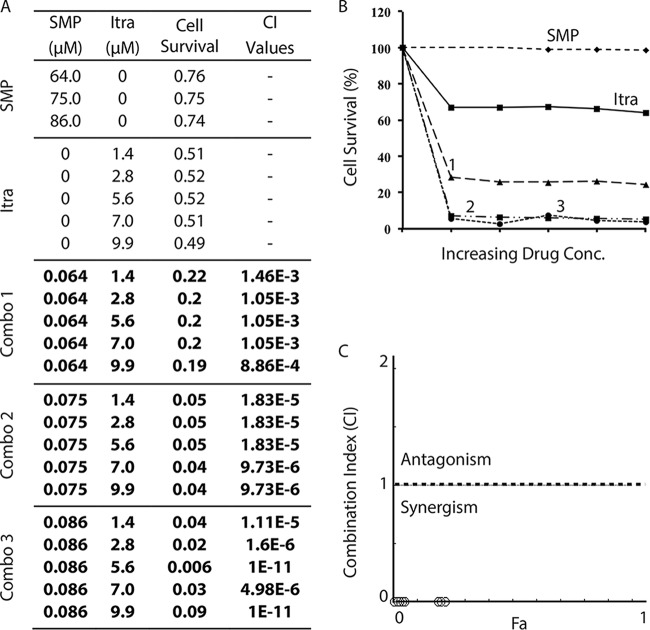
Based on the screening data for S. pombe, we hypothesized that overexpression of erg11 might be a cellular defense mechanism against HU-induced oxidative stress. Earlier studies have shown that treatment with SMP also generates oxidative stress and thus induces cell death in S. cerevisiae and human HL-60 leukemia cells (19, 23). If our hypothesis is true, inhibition of Erg11 may also enhance the cytotoxicity of SMP. To investigate this, we tested the combinations of SMP with itraconazole (Fig. 8), clotrimazole (see Fig. S16 in the supplemental material), and ketoconazole (see Fig. S17 in the supplemental material). The results clearly showed that almost all of the tested combinations were very robust and highly synergistic in inducing cell death in C. albicans.
FIG 8.
The robust and highly synergistic cell-killing effect of SMP and itraconazole in C. albicans. (A) The cell growth of wild-type C. albicans in the presence of SMP and itraconazole alone or in three combinations was examined as described for Fig. 2. Numbers in bold indicate very strong synergistic drug interactions. (B) Graphical representation of the cell survival rate in the presence of SMP, itraconazole, and three combinations of the two drugs. (C) Combination index plot.
DISCUSSION
Combination therapy using multiple drugs is a therapeutic strategy that can address various issues associated with single-drug therapy, such as toxicity, lower therapeutic effect, a narrower spectrum of activity, and, more importantly, the development of drug resistance. Since eukaryotic genomes are interconnected, a proper drug combination may exploit additional therapeutic targets (24). Combination therapy has been successfully used for the treatment of various infectious diseases caused by viruses (25), bacteria (26, 27), and parasites (28, 29). However, only a small number of drug combinations are available for the treatment of fungal infections. Due to the limited numbers of antifungal agents that are currently used in clinics, synergistic combinations, particularly those with nonantifungal drugs, are of great interest.
HU is a nonalkylating antiproliferative and antiviral agent that has been used in the past decades for the management of a variety of neoplastic and nonneoplastic conditions. It is water soluble, readily absorbed, and widely distributed throughout the body (13, 14). It is listed as an essential medicine by WHO (30). HU is very tolerable for patients, especially when applied to the skin. An earlier study showed that creams containing 10% to 15% (∼1.3 M to 2.0 M) HU were tolerable and generated a good clinical response in patients with psoriasis (31). Based on our preliminary data, the immediate implication is that it may significantly improve the therapeutic effect of various antifungal azoles and terbinafine for superficial fungal infections. In support of this, combination of HU with terbinafine, which is effective against various dermatophyte isolates (32, 33), showed the most significant synergism in S. pombe (see Fig. S5 in the supplemental material). Since S. pombe is highly sensitive to HU, it is likely that similar sensitivity can be found in certain fungal pathogens, which may further expand its use for other fungal diseases. Importantly, although we have not examined the combinations of SMP and the azoles with other fungal pathogens in this study, the robust synergism strongly suggests that they may be highly effective for the treatment of various fungal diseases, particularly invasive and/or chronic infections (34). Further in vivo studies with animal models are needed to investigate these potentials.
It is known that SMP generates oxidative stress and induce apoptosis in human HL-60 leukemia cells (23). Since HU has been used as an anticancer drug and the S. pombe cellular mechanisms are conserved in humans (35–37), combinations of HU with SMP or with azoles may also be used for the treatment of cancer, particularly those with mutations that affect cholesterol or heme synthesis or cause oxidative stress. For example, oxidative stress is observed in hematopoietic malignancies (38), and itraconazole has been repurposed for the treatment of prostate cancer (39, 40). Although the focus of this study is on antifungal activity, it will be interesting to see whether the drug combinations have a synergistic effect on cancer cells.
Synergistic drug interaction can be explained by several mechanisms, although many drug combinations have multiple mechanisms and do not fall cleanly into one single category (24). The combined drugs may target different stages of the same biological pathway or parallel pathways that converge on an essential cellular function. Alternatively, one drug may increase the bioavailability of the second drug through enhancing the cell permeability, decreasing the drug's degradation, or affecting the pharmacokinetics of the drug. Terbinafine and azoles may enhance the cell-killing effect of HU through increased permeability of cell membrane and thus the bioavailability of HU, similar to that reported for azoles and flucytosine (41). The same mechanism may also explain the synergism between SMP and HU, because SMP inhibits heme biosynthesis (15, 19) and heme in turn is required for ergosterol synthesis. Although this is certainly a possibility, the novel cell-killing mechanisms of HU described in our previous studies (1, 2) strongly suggest that the synergism is more likely due to the suppression of the biosynthesis of heme and ergosterol.
The fission yeast S. pombe is a well-established model for studying the cellular mechanisms that are conserved in higher eukaryotes (16). Virtually any genetic and biochemical experiment or technique can be applied to this system. S. pombe is not pathogenic, is easily accessible, is easily grown and manipulated to make mutants, and is able to be maintained in either a haploid or a diploid state. Its genome has been sequenced, which makes knock-in and knockout of each specific gene fairly easy, and a collection of genome-wide deletion mutants is already available for large-scale screens of new drug targets (42, 43). The cell division cycle is readily observable, and a collection of mutants with defined growth defects has been extensively studied. Considering all these benefits, the limited repertoire of molecular and genetic techniques in pathogenic fungi such as C. albicans, and the data presented in this and the previous studies (1, 2, 43), S. pombe may serve, complementarily with S. cerevisiae and C. albicans (18, 44–46), as a good therapeutic model for antifungal research.
MATERIALS AND METHODS
Materials.
SMP was obtained from the natural products repository of the National Center for Natural Products Research at the University of Mississippi. Itraconazole was purchased from TCI America (Portland, OR), and HU, clotrimazole, fluconazole, ketoconazole, and terbinafine were all purchased from Sigma (St. Louis, MO). HU was dissolved in distilled water or medium at 1.0 M, filtered, and saved at −20°C as the stock solution. SMP, ketoconazole, and fluconazole were dissolved in DMSO and saved at −20°C. The cell culture media were made following the standard protocol of the Clinical and Laboratory Standards Institute (47, 48) using components that were mostly purchased from Sigma. All yeast strains used in this study are listed in Table S1 in the supplemental material.
Standard spot assay.
Logarithmically growing S. pombe cultures (2 × 107 cells/ml) were diluted in 5-fold steps and spotted onto YE6S (5 g/liter yeast extract, 30 g/liter dextrose, and 6 supplements) plates containing HU, SMP, or various azoles at the indicated drug concentrations and combinations. The plates were incubated at 30°C for 3 days and then photographed.
Ninety-six-well plate assay.
Logarithmically growing yeast cells were inoculated on 96-well plates at 3,000 cells/well in YE6S, YPD (10 g/liter yeast extract, 20 g/liter bacterial peptone, and 20 g/liter dextrose), and SDB (Sabouraud dextrose broth containing 10 g/liter mycological peptone and 40 g/liter dextrose) media for S. pombe, S. cerevisiae, and C. albicans, respectively, following the standard protocol with slight modifications (47). The drugs were added at the indicated concentrations to a final volume of 200 μl either alone or in combinations. The same amounts of carriers were added as the control. Cells were incubated at 30°C for 48 h. The plates were scanned in a plate reader for A600 measurements (Safire2; Tecan, Research Triangle Park, NC). The synergistic, additive, or antagonistic effect of the drug combinations as indicated by the combination index (CI) values was calculated by using CompuSyn (49), computer software developed by Chou and Talalay (17), where CI < 1, CI = 1, and CI > 1 indicate synergism, an additive effect, and antagonism, respectively. Very strong synergism is defined by CI values that are less than 0.1.
Screening for multiple-copy suppressors in S. pombe.
The hem13-1 mutant was transformed with S. pombe genomic DNA libraries that carry the ura4 marker. Colonies grown on plates lacking uracil were replicated on plates containing HU to screen for those that exhibited HU resistance. Plasmids were recovered from the HU-resistant yeast colonies for DNA sequencing and subsequent identification of the suppressor genes.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to Joseph Heitman for providing the wild-type Candida albicans SC5314 strain and to Michael Kemp, Eric J. Romer, and two anonymous reviewers for critical reading of the manuscript. We acknowledge other members of the Xu lab for their help and support.
This work was supported by NIH R01 grant GM110132 and the start-up fund provided by Wright State University.
We declare that we have no conflicts of interest with the contents of this article.
A.S. and Y.-J.X. designed the experiments. A.S. performed the experiments. A.S., A.A., and Y.-J.X. analyzed the data and wrote the paper.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00734-17.
REFERENCES
- 1.Xu YJ, Singh A, Alter GM. 2016. Hydroxyurea induces cytokinesis arrest in cells expressing a mutated sterol-14alpha-demethylase in the ergosterol biosynthesis pathway. Genetics 204:959–973. doi: 10.1534/genetics.116.191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Xu YJ. 2017. Heme deficiency sensitizes yeast cells to oxidative stress induced by hydroxyurea. J Biol Chem 292:9088–9103. doi: 10.1074/jbc.M117.781211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawksworth DL. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res 95:641–655. doi: 10.1016/S0953-7562(09)80810-1. [DOI] [Google Scholar]
- 4.Crous PW, Hawksworth DL, Wingfield MJ. 2015. Identifying and naming plant-pathogenic fungi: past, present, and future. Annu Rev Phytopathol 53:247–267. doi: 10.1146/annurev-phyto-080614-120245. [DOI] [PubMed] [Google Scholar]
- 5.CDC. Types of fungal diseases. 2017. CDC, Atlanta, GA. https://www.cdc.gov/fungal/diseases/index.html.
- 6.Denning DW, Bromley MJ. 2015. How to bolster the antifungal pipeline. Science 347:1414–1416. doi: 10.1126/science.aaa6097. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro RS, Robbins N, Cowen LE. 2011. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267. doi: 10.1128/MMBR.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoham S, Marr KA. 2012. Invasive fungal infections in solid organ transplant recipients. Future Microbiol 7:639–655. doi: 10.2217/fmb.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent JL, Anaissie E, Bruining H, Demajo W, el-Ebiary M, Haber J, Hiramatsu Y, Nitenberg G, Nystrom PO, Pittet D, Rogers T, Sandven P, Sganga G, Schaller MD, Solomkin J. 1998. Epidemiology, diagnosis and treatment of systemic Candida infection in surgical patients under intensive care. Intensive Care Med 24:206–216. doi: 10.1007/s001340050552. [DOI] [PubMed] [Google Scholar]
- 11.Denning DW. 2003. Echinocandin antifungal drugs. Lancet 362:1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 12.Anderson TM, Clay MC, Cioffi AG, Diaz KA, Hisao GS, Tuttle MD, Nieuwkoop AJ, Comellas G, Maryum N, Wang S, Uno BE, Wildeman EL, Gonen T, Rienstra CM, Burke MD. 2014. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat Chem Biol 10:400–406. doi: 10.1038/nchembio.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spivak JL, Hasselbalch H. 2011. Hydroxycarbamide: a user's guide for chronic myeloproliferative disorders. Expert Rev Anti-Cancer Ther 11:403–414. doi: 10.1586/era.11.10. [DOI] [PubMed] [Google Scholar]
- 14.Singh A, Xu YJ. 2016. The cell killing mechanisms of hydroxyurea. Genes (Basel) 7:99. doi: 10.3390/genes7110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Z, Chen K, Xu T, Zhang J, Li Y, Li W, Agarwal AK, Clark AM, Phillips JD, Pan X. 2011. Sampangine inhibits heme biosynthesis in both yeast and human. Eukaryot Cell 10:1536–1544. doi: 10.1128/EC.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fantes PA, Hoffman CS. 2016. A brief history of Schizosaccharomyces pombe research: a perspective over the past 70 years. Genetics 203:621–629. doi: 10.1534/genetics.116.189407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 18.Forsburg SL. 2005. The yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe: models for cell biology research. Gravit Space Biol Bull 18:3–9. [PubMed] [Google Scholar]
- 19.Agarwal AK, Xu T, Jacob MR, Feng Q, Lorenz MC, Walker LA, Clark AM. 2008. Role of heme in the antifungal activity of the azaoxoaporphine alkaloid sampangine. Eukaryot Cell 7:387–400. doi: 10.1128/EC.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strushkevich N, Usanov SA, Park HW. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol 397:1067–1078. doi: 10.1016/j.jmb.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 21.Martins N, Ferreira IC, Barros L, Silva S, Henriques M. 2014. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 177:223–240. doi: 10.1007/s11046-014-9749-1. [DOI] [PubMed] [Google Scholar]
- 22.CDC. 2013. Antibiotic resistance threats in the United States. CDC, Atlanta, GA. [Google Scholar]
- 23.Kluza J, Mazinghien R, Degardin K, Lansiaux A, Bailly C. 2005. Induction of apoptosis by the plant alkaloid sampangine in human HL-60 leukemia cells is mediated by reactive oxygen species. Eur J Pharmacol 525:32–40. doi: 10.1016/j.ejphar.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 24.Spitzer M, Robbins N, Wright GD. 2016. Combinatorial strategies for combating invasive fungal infections. Virulence 8:169–185. doi: 10.1080/21505594.2016.1196300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. 2001. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS 15:1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 26.Zumla A, Hafner R, Lienhardt C, Hoelscher M, Nunn A. 2012. Advancing the development of tuberculosis therapy. Nat Rev Drug Discov 11:171–172. doi: 10.1038/nrd3694. [DOI] [PubMed] [Google Scholar]
- 27.Bouza E, Munoz P. 2000. Monotherapy versus combination therapy for bacterial infections. Med Clin North Am 84:1357–1389. doi: 10.1016/S0025-7125(05)70293-5. [DOI] [PubMed] [Google Scholar]
- 28.Eastman RT, Fidock DA. 2009. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol 7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephenson I, Wiselka M. 2000. Drug treatment of tropical parasitic infections: recent achievements and developments. Drugs 60:985–995. doi: 10.2165/00003495-200060050-00002. [DOI] [PubMed] [Google Scholar]
- 30.WHO. 2015. 19th WHO model list of essential medicines. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FINAL_amended_NOV2015.pdf?ua=1.
- 31.Zackheim HS, Karasek MA, Cox AJ Jr. 1972. Topical hydroxyurea and psoriasis. J Investig Dermatol 58:24–27. doi: 10.1111/1523-1747.ep13077204. [DOI] [PubMed] [Google Scholar]
- 32.Tauber A, Muller-Goymann CC. 2014. Comparison of the antifungal efficacy of terbinafine hydrochloride and ciclopirox olamine containing formulations against the dermatophyte Trichophyton rubrum in an infected nail plate model. Mol Pharm 11:1991–1996. doi: 10.1021/mp400711q. [DOI] [PubMed] [Google Scholar]
- 33.Baudraz-Rosselet F, Ruffieux C, Lurati M, Bontems O, Monod M. 2010. Onychomycosis insensitive to systemic terbinafine and azole treatments reveals non-dermatophyte moulds as infectious agents. Dermatology 220:164–168. doi: 10.1159/000277762. [DOI] [PubMed] [Google Scholar]
- 34.Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. 2012. Hidden killers: human fungal infections. Sci Transl Med 4:165rv113. doi: 10.1126/scitranslmed.3004404. [DOI] [PubMed] [Google Scholar]
- 35.Hayles J, Wood V, Jeffery L, Hoe KL, Kim DU, Park HO, Salas-Pino S, Heichinger C, Nurse P. 2013. A genome-wide resource of cell cycle and cell shape genes of fission yeast. Open Biol 3:130053. doi: 10.1098/rsob.130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langan TA, Gautier J, Lohka M, Hollingsworth R, Moreno S, Nurse P, Maller J, Sclafani RA. 1989. Mammalian growth-associated H1 histone kinase: a homolog of cdc2+/CDC28 protein kinases controlling mitotic entry in yeast and frog cells. Mol Cell Biol 9:3860–3868. doi: 10.1128/MCB.9.9.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gould KL, Nurse P. 1989. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature 342:39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- 38.Hole PS, Darley RL, Tonks A. 2011. Do reactive oxygen species play a role in myeloid leukemias? Blood 117:5816–5826. doi: 10.1182/blood-2011-01-326025. [DOI] [PubMed] [Google Scholar]
- 39.Antonarakis ES, Heath EI, Smith DC, Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS, Zhao M, Mendonca J, Kachhap SK, Rudek MA, Carducci MA. 2013. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 18:163–173. doi: 10.1634/theoncologist.2012-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pace JR, DeBerardinis AM, Sail V, Tacheva-Grigorova SK, Chan KA, Tran R, Raccuia DS, Wechsler-Reya RJ, Hadden MK. 2016. Repurposing the clinically efficacious antifungal agent itraconazole as an anticancer chemotherapeutic. J Med Chem 59:3635–3649. doi: 10.1021/acs.jmedchem.5b01718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto Y, Maesaki S, Kakeya H, Yanagihara K, Ohno H, Ogawa K, Hirakata Y, Tomono K, Tashiro T, Kohno S. 1997. Combination therapy with fluconazole and flucytosine for pulmonary cryptococcosis. Chemotherapy 43:436–441. doi: 10.1159/000239603. [DOI] [PubMed] [Google Scholar]
- 42.Spirek M, Benko Z, Carnecka M, Rumpf C, Cipak L, Batova M, Marova I, Nam M, Kim DU, Park HO, Hayles J, Hoe KL, Nurse P, Gregan J. 2010. S. pombe genome deletion project: an update. Cell Cycle 9:2399–2402. doi: 10.4161/cc.9.12.11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fang Y, Hu L, Zhou X, Jaiseng W, Zhang B, Takami T, Kuno T. 2012. A genomewide screen in Schizosaccharomyces pombe for genes affecting the sensitivity of antifungal drugs that target ergosterol biosynthesis. Antimicrob Agents Chemother 56:1949–1959. doi: 10.1128/AAC.05126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sipiczki M. 2000. Where does fission yeast sit on the tree of life? Genome Biol 1:reviews1011. doi: 10.1186/gb-2000-1-2-reviews1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berbee ML, Carmean DA, Winka K. 2000. Ribosomal DNA and resolution of branching order among the ascomycota: how many nucleotides are enough? Mol Phylogenet Evol 17:337–344. doi: 10.1006/mpev.2000.0835. [DOI] [PubMed] [Google Scholar]
- 46.Heckman DS, Geiser DM, Eidell BR, Stauffer RL, Kardos NL, Hedges SB. 2001. Molecular evidence for the early colonization of land by fungi and plants. Science 293:1129–1133. doi: 10.1126/science.1061457. [DOI] [PubMed] [Google Scholar]
- 47.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, 2nd ed CLSI document M38-A2. CLSI, Wayne, PA. [Google Scholar]
- 48.Moreno S, Klar A, Nurse P. 1991. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194:795–823. doi: 10.1016/0076-6879(91)94059-L. [DOI] [PubMed] [Google Scholar]
- 49.Chou TC, Martin N. 2005. CompuSyn for drug combinations: PC software and user's guide: a computer program for quantitation of synergism and antagonism in drug combinations, and the determination of IC50 and ED50 and LD50 values. ComboSyn Inc., Paramus, NJ. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.