ABSTRACT
Vancomycin has been associated with acute kidney injury in preclinical and clinical settings; however, the precise exposure profiles associated with vancomycin-induced acute kidney injury have not been defined. We sought to determine pharmacokinetic/pharmacodynamics indices associated with the development of acute kidney injury using sensitive urinary biomarkers. Male Sprague-Dawley rats received clinical-grade vancomycin or normal saline as an intraperitoneal injection. Total daily doses between 0 and 400 mg/kg of body weight were administered as a single dose or 2 divided doses over a 24-h period. At least five rats were utilized for each dosing protocol. A maximum of 8 plasma samples per rat were obtained, and urine was collected over the 24-h period. Kidney injury molecule-1 (KIM-1), clusterin, osteopontin, cystatin C, and neutrophil gelatinase-associated lipocalin levels were determined using Milliplex multianalyte profiling rat kidney panels. Vancomycin plasma concentrations were determined via a validated high-performance liquid chromatography methodology. Pharmacokinetic analyses were conducted using the Pmetrics package for R. Bayesian maximal a posteriori concentrations were generated and utilized to calculate the 24-h area under the concentration-time curve (AUC), the maximum concentration (Cmax), and the minimum concentration. Spearman's rank correlation coefficient (rs) was used to assess the correlations between exposure parameters, biomarkers, and histopathological damage. Forty-seven rats contributed pharmacokinetic and toxicodynamic data. KIM-1 was the only urinary biomarker that correlated with both composite histopathological damage (rs = 0.348, P = 0.017) and proximal tubule damage (rs = 0.342, P = 0.019). The vancomycin AUC and Cmax were most predictive of increases in KIM-1 levels (rs = 0.438 and P = 0.002 for AUC and rs = 0.451 and P = 0.002 for Cmax). Novel urinary biomarkers demonstrate that kidney injury can occur within 24 h of vancomycin exposure as a function of either AUC or Cmax.
KEYWORDS: vancomycin, acute kidney injury, pharmacokinetics, biological markers, pharmacodynamics
INTRODUCTION
Receipt of intravenous vancomycin is a risk factor for the development of acute kidney injury (AKI) among hospitalized patients. Even mild cases of AKI are associated with higher rates of morbidity and mortality (1–3). The clinical attribution of AKI causality is difficult, as multiple factors, such as the severity of illness, the presence of comorbid conditions, and the receipt of concomitant nephrotoxic medications, compound the damage. Notably, vancomycin-induced kidney injury (VIKI) is a potentially preventable cause of clinical AKI. We previously demonstrated that VIKI can be detected as preclinical injury with novel urinary biomarkers in a rat model (4). As vancomycin is one of the most frequently prescribed antibiotics in U.S. hospitals (5, 6), elucidation of the vancomycin exposures that cause AKI is key to designing strategies to reduce the frequency of VIKI while maintaining vancomycin efficacy.
Rates of VIKI ranging widely from 5 to 43% have been reported in the clinical literature (7–9). A single prospective randomized trial identified that 18.8% of vancomycin-treated patients with good baseline renal function experienced renal toxicity, whereas 5.6% of linezolid-treated patients with good baseline renal function experienced renal toxicity (10). Additionally, a vancomycin exposure-toxicity gradient was observed, in that day 3 vancomycin trough levels (minimum concentrations [Cmin]) of ≥20 μg/ml resulted in renal toxicity in 37% of patients, whereas trough concentrations of 15 to 20 μg/ml resulted in renal toxicity in 22% of patients and trough concentrations of ≤15 μg/ml resulted in renal toxicity in 18% of patients. It is unclear, however, whether trough concentrations became elevated in those that were already in renal failure by day 3. That is, the causality of the elevated trough concentrations causing VIKI was not discernible from the prospective study. Retrospective studies and meta-analyses have identified several risk factors for VIKI. From these studies, other exposure proxies, such as higher-dose vancomycin regimens, elevated trough concentrations, and an increasing number of vancomycin doses, have been associated with VIKI (9, 11–13). However, vancomycin exposures have been highly variable in those with kidney injury diagnosed as acute tubular necrosis (14).
While these proxies for vancomycin exposure have been associated with VIKI, the vancomycin exposure profile that drives VIKI has not been elucidated. Nonmodifiable patient factors, such as severity of illness, obesity, and concomitant nephrotoxic medications, compound VIKI and are notoriously difficult to adjust/control in retrospective studies (15). As such, animal models provide a means to understand the exposure-response relationship in VIKI. Novel urinary biomarkers provide an interrogation window into early damage in the kidney and are more sensitive and specific than traditional biomarkers, such as serum creatinine (SCr) and blood urea nitrogen (BUN) concentrations (16–18). Our group recently demonstrated that sensitive urinary biomarkers for kidney injury, such as kidney injury molecule-1 (KIM-1), osteopontin (OPN), and clusterin, were more highly correlated with vancomycin exposures, as defined by the area under the concentration-time curve (AUC) from 0 to 24 h postdosing (AUC0–24) and the maximum concentration (Cmax) from 0 to 24 h postdosing (Cmax 0–24), than the traditionally measured trough concentration (Cmin from 0 to 24 h postdosing [Cmin 0–24]) in rats receiving vancomycin at doses of between 0 and 200 mg/kg of body weight/day for 24 or 72 h of therapy (4). This study was limited by the use of low doses of vancomycin. No correlation between biomarkers and histopathology was observed, presumably as a function of the low doses given. In that study, we further observed that the kidney injury occurred early. Assessment of the first 24-h exposure window was required to explore pharmacokinetic (PK) variables before they became part of the causal pathway (e.g., Cmin rises with kidney injury but does not drive the event). This study expands the previous work by increasing the range of doses studied and focuses on a 24-h exposure window.
RESULTS
Characteristics of animal cohort.
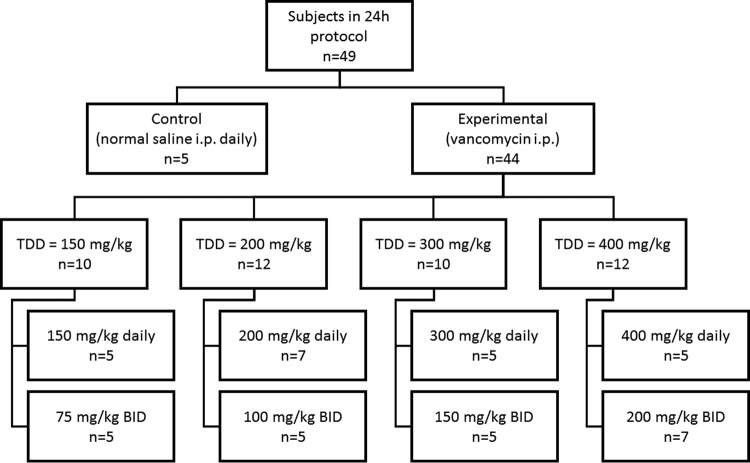
A total of 49 animals were analyzed, with the animal dosing group disposition being shown in Fig. 1. One animal was euthanized early according to IACUC protocols, and one animal provided only a terminal sample due to an occluded catheter. These two animals contributed only to the PK model; all 47 other animals contributed complete model data (except for 5 control animals that did not contribute vancomycin PK data). Mean weight loss was not different between the control and the vancomycin-treated animals (25.6 g versus 20.1 g; P = 0.143) (Table 1), nor were median composite histopathological scores statistically significantly different (1 versus 1; P = 0.63). Changes in urine output were not associated with changes in total daily dose (P = 0.10).
FIG 1.
Animal dosing group disposition. Abbreviations: i.p., intraperitoneally; TDD, total daily dose; BID, twice daily.
TABLE 1.
Summary of weight loss, urinary output, histopathology score, and urinary biomarkersa
| Characteristic | Value(s) |
P value | |
|---|---|---|---|
| Control | Vancomycin | ||
| No. of animals | 5 | 42 | |
| Mean ± SD wt loss (g) | 25.6 ± 0.40 | 20.1 ± 0.81 | 0.14 |
| Mean ± SD urine output (ml) | NR | 11.5 ± 4.69 | |
| Median (IQR) worst composite score | 1 (1–1) | 1 (0–1) | 0.61 |
| Median (IQR) concn (ng/ml) | |||
| KIM-1 | 0.927 (0.551–1.18) | 1.83 (1.01–3.92) | 0.027 |
| Clusterin (ng/ml) | 449.24 (409.08–486.24) | 515.61 (381.75–853.61) | 0.41 |
| Osteopontin | 0.062 (0.062–0.065) | 0.123 (0.094–0.277) | 0.001 |
| Cystatin C | 443.9 (271.1–765.5) | 516.6 (434.8–560.9) | 0.95 |
| NGAL | 1423.8 (762.4–2618.5) | 634.3 (399.6–1113.6) | 0.07 |
Data are for 47 animals. IQR, interquartile range; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin; NR, not reported.
Vancomycin PK models and exposure measure determination.
The best and most parsimonious model was a 3-compartment model standardizing the volume of distribution (V) in the central compartment to the animal weight (Akaike information criterion value = 2,132). Nineteen support points were identified for the 44 vancomycin-treated animals (i.e., 42 animals with complete protocol data and 2 animals with partial PK data). For the predictive performance of the model, the observed versus Bayesian predicted concentrations' bias, imprecision, and coefficient of determination were −0.173, 0.898, and 84.6%, respectively (see Fig. S1 in the supplemental material). Interparameter covariance is shown in Table S1.
Median Bayesian predicted concentrations for each treatment group are shown in Table S2. Because of intraperitoneal (i.p.) dosing, exposure heterogeneity within treatment groups was noted (Fig. S2). The estimated population mean absorption rate constant (Ka) was 1.63 h−1. Pharmacokinetic measures (i.e., AUC0–24, Cmax 0–24, Cmin 0–24) displayed a substantial intercorrelation by Spearman's rank correlation coefficient (rs) (for AUC and Cmax, rs = 0.86; for AUC and Cmin, rs = 0.71; for Cmax and Cmin, rs = 0.37; all P values were <0.01), and these data are graphically displayed in Fig. S3.
Correlation between urinary biomarkers of AKI and renal histopathology.
All 47 animals that contributed toxicodynamic (TD) data were used for analyses of the correlation between novel urinary biomarkers of AKI and the composite histopathology score (Table 2). Spearman's rank correlation coefficients for composite histopathological damage were the highest for KIM-1 (rs = 0.348, P = 0.017) and clusterin (rs = 0.405, P = 0.005); a negative correlation between neutrophil gelatinase-associated lipocalin (NGAL) and composite histopathological damage was observed (rs = −0.2880, P = 0.050). Similar results were observed for correlations between urinary biomarkers and proximal tubule damage (for KIM-1, rs = 0.342 and P = 0.019; for clusterin, rs = 0.223 and P = 0.132; for OPN, rs = 0.365 and P = 0.012); a negative correlation between cystatin C and proximal tubular damage was observed (rs = −0.3034, P = 0.038). SCr and BUN concentrations were available for 14 animals each. No correlation between SCr and BUN concentrations and renal histopathology was observed.
TABLE 2.
Spearman rank correlation coefficients for exposure metrics, histopathological damage, and urinary biomarkersa
| Biomarker | AUC0–24 |
Cmax 0–24 |
Cmin 0–24 |
Composite histopathologic score |
Proximal tubule damage score |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs | P value | rs | P value | rs | P value | rs | P value | rs | P value | |
| KIM-1 | 0.438 | 0.002 | 0.451 | 0.002 | 0.227 | 0.126 | 0.348 | 0.017 | 0.342 | 0.019 |
| Clusterin | 0.282 | 0.055 | 0.358 | 0.013 | 0.079 | 0.599 | 0.405 | 0.005 | 0.223 | 0.132 |
| Osteopontin | 0.397 | 0.006 | 0.447 | 0.002 | 0.305 | 0.037 | 0.224 | 0.131 | 0.365 | 0.012 |
| Cystatin C | −0.229 | 0.122 | −0.094 | 0.531 | −0.153 | 0.305 | −0.087 | 0.562 | −0.303 | 0.038 |
| NGAL | 0.063 | 0.672 | 0.025 | 0.868 | 0.240 | 0.105 | −0.288 | 0.050 | −0.177 | 0.233 |
| Composite histopathologic score | 0.249 | 0.084 | 0.252 | 0.081 | 0.055 | 0.707 | ||||
| Proximal tubule damage score | 0.319 | 0.026 | 0.270 | 0.061 | 0.231 | 0.110 | ||||
rs, Spearman's rank correlation coefficient; KIM-1, kidney injury molecule-1; NGAL, neutrophil gelatinase-associated lipocalin.
PK/TD correlations.
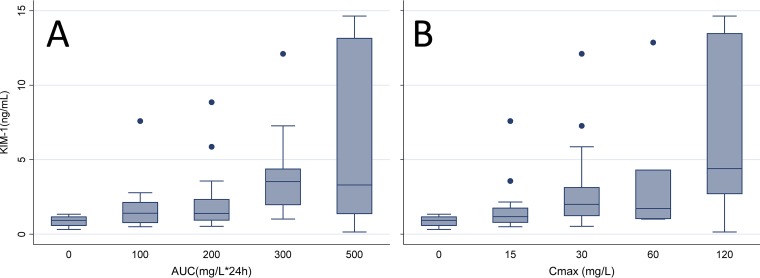
Urinary biomarkers of AKI were correlated with vancomycin exposure metrics (i.e., AUC0–24, Cmax 0–24, Cmin 0–24), as shown in Table 2. KIM-1 levels showed the highest correlation to AUC0–24 (rs = 0.438, P = 0.002) and Cmax 0–24 (rs = 0.451, P = 0.002) when the correlations were compared to those for the other biomarkers measured. A statistically significant relationship between KIM-1 levels and Cmin 0–24 was not observed (rs = 0.227, P = 0.126). The OPN level was also significantly correlated with AUC0–24 (rs = 0.397, P = 0.006), Cmax 0–24 (rs = 0.447, P = 0.002), and Cmin 0–24 (rs = 0.305, P = 0.037), and the clusterin level was significantly associated with Cmax 0–24 (rs = 0.358, P = 0.013). Only the OPN level was significantly correlated with an increase in Cmin 0–24 (rs = 0.305, P = 0.037). Both increased SCr levels (rs = 0.549, P = 0.042) and increased BUN levels (rs = 0.533, P = 0.050) were correlated with an increase in Cmax 0–24, but they were not correlated with other measures. The KIM-1 levels, in categorical fashion, rose with an increase in AUC and Cmax values (Fig. 2; Table S3 and S4). Additionally, borderline significant correlations between AUC0–24 and a worsening composite histopathological score (rs = 0.249 and P = 0.084) and between Cmax 0–24 and a worsening composite histopathological score (rs = 0.252 and P = 0.081) were identified. Only AUC0–24 showed a significant correlation with the proximal tubular damage score (rs = 0.319; P = 0.026). Cmin 0–24 was not correlated with the composite histopathological score (rs = 0.055, P = 0.707) or the proximal tubule damage score (rs = 0.231, P = 0.110).
FIG 2.
Box plot of urinary KIM-1 values in each exposure bin for AUC (A) and Cmax (B).
Sigmoidal models were fit to each pharmacokinetic parameter and the level of urinary KIM-1 expression (Fig. S4). The coefficients of determination for the sigmoidal models revealed reasonable fits for AUC0–24 (R2 = 0.29), Cmax 0–24 (R2 = 0.21), and Cmin 0–24 (R2 = 0.33). A single outlier appeared to account for the constrained fits. With the outlier removed, the fits for AUC0–24 (R2 = 0.49), Cmax 0–24 (R2 = 0.36), and Cmin 0–24 (R2 = 0.47) were improved.
DISCUSSION
This study expands upon previous knowledge regarding vancomycin exposures and AKI. Previously, we utilized lower study doses (i.e., 150 to 200 mg/kg given as either a single or a twice-daily injection). Our previous study similarly utilized rats and vancomycin treatment doses roughly equivalent to 30 mg/kg/day in humans for 1 to 3 days. There, we identified a correlation between the vancomycin AUC0–24 and increases in novel urinary biomarkers as well as Cmax 0–24 and these biomarkers; however, the correlations between histopathological damage and biomarkers were not identified (4). The present study included only animals treated for 24 h and increased the magnitude of the dose administered to mimic the higher end of the range of concentrations of drug given clinically. By doing so, we were further able to discern between the exposure parameters that correlated with elevated urinary biomarker concentrations indicative of kidney injury.
Overall, we found that AUC0–24 and Cmax 0–24 are more highly correlated with increased urinary concentrations of KIM-1, clusterin, and OPN than Cmin 0–24. Importantly, these results further substantiate that the vancomycin Cmin is not likely the parameter that mediates VIKI. Such an understanding is critical, as current vancomycin treatment guidelines (19) focus on the measurement of trough concentrations only and identical Cmin values can be associated with highly variable AUCs (20). Only the OPN concentration was correlated with an increase in Cmin 0–24, and this correlation was less pronounced than what was seen with the other two exposure metrics. The relationships of our PK exposure metric with novel urinary biomarkers are also supported by correlations between composite histopathological scores and exposure metrics. Both AUC0–24 and Cmax 0–24 showed borderline significant correlations with a worsening composite histopathological score, though only AUC0–24 showed a significant correlation with the proximal tubular damage score. It is notable that the biomarkers were able to detect any histopathologically confirmed damage and proximal tubular damage within 24 h (P = 0.017 and P = 0.019, respectively, for KIM-1). Clinically, this may translate into a highly useful tool, as early detection of vancomycin injury is possible using urine and does not require an invasive biopsy.
Histopathology scores are the old “gold standard” for toxicodynamic studies (21). A reliance on histopathology scores alone may be problematic, as the results are more variable and difficult to quantify well statistically. Histopathology scores provide less of a quantitative spread to analyze, and analysis of biomarker concentrations is likely able to detect damage that occurs prior to observation of gross histopathological changes. For instance, the KIM-1 concentration is known to rise within hours of anoxic injury mediated by renal pedicle clamping and remains elevated for 5 days (17). Similar to the findings of Vaidya et al. (17), control subjects in our study also exhibited concentrations that were approximately at the lower limit of quantitation of urinary KIM-1 concentrations, and the median concentrations in controls were significantly lower than the median concentration for all subjects treated with vancomycin (0.927 versus 1.83 ng/ml, P = 0.027). Thus, KIM-1 is highly specific. In summation, these data support a stronger association between increases in the exposure metrics Cmax 0–24 and AUC0–24 and worsening kidney damage than the association for Cmin 0–24, which has traditionally been used as a marker for efficacy and toxicity.
Our findings are consistent with those of the dose-toxicity works of others. While the findings are consistent, the KIM-1 concentrations that were seen in the 24 h of the study were lower than those that have been seen with longer durations of therapy (e.g., 3, 7, 14, or 28 days) (17, 22). However, these data indicate that vancomycin-induced kidney damage is detectable within the first 24 h of therapy. In the clinical setting, the median time of onset of vancomycin-induced AKI has been reported to be 6 to 7 days into therapy (10, 12, 23), but these studies have relied on traditional and insensitive markers of AKI, such as SCr and BUN levels. In our study, increases in the levels of both markers were associated with increases in Cmax 0–24; however, no correlation between SCr or BUN levels and the levels of the urinary biomarkers or histopathological changes was identified (data not shown), limiting the interpretation of this finding. Thirty to 50% parenchymal damage is required before an elevated SCr concentration is detectable (24). Thus, since kidney injury can be severe with even small changes in the SCr concentration, it is not surprising that small changes in the SCr concentration have been associated with increases in mortality (1–3). The use of sensitive biomarkers for the detection of early damage can provide an early indication of renal injury and allow clinicians to change therapy before more substantial damage occurs. Taken together, these results may also indicate merit in using prolonged-infusion vancomycin, the microbiological efficacy of which is similar to that of intermittent infusion vancomycin (25). Further study is needed.
Several limitations of our study should be noted. First, by nature of the fact that 24-h doses were studied and i.p. dosing was utilized, exposures were relatively constrained. Only six rats achieved an AUC0–24 of greater than 400 mg · h/liter, and only 10 rats achieved a Cmax 0–24 of greater than 40 mg/liter. i.p. dosing of vancomycin is thought to be limited by incomplete and variable absorption (26). The variability in the values of the PK parameters observed by dosing group in our study further supports that notion. In our study, the dosing groups displayed coefficients of variation (CV) exceeding 100% across exposure measurements. We did not investigate the effects of pH on the solubility of the vancomycin concentration used (100 mg/ml). Precipitation of drug within the peritoneal cavity is possible and may have contributed to the variability (i.e., there is not a linear dose-exposure relationship with i.p. dosing) (26). It is also possible that the absorption of vancomycin from the peritoneum is a saturable process, though nonlinear Ka models were not significantly better than the final model used (data not shown). This heterogeneous absorption allowed variable exposures and facilitated the assessments between AUC0–24 and Cmax 0–24 and increases in the concentrations of biomarkers, such as KIM-1. The depot effect provided by i.p. dosing also allowed a more humanized exposure due to the differences in clearance between rats and humans; unfortunately, many of the rats had exposures less than those seen with contemporary dosing in humans. Second, we studied only 24-h dosing, and the worst histopathological score in this cohort was mild damage. However, more comprehensive pathological studies have clearly shown strong quantitative relationships (i.e., high sensitivity and specificity) between the KIM-1 level and the histopathological grading (17). Our study is confirmatory, and the Hill-type curves fit to the exposure data and the levels of KIM-1 expression showed a clear relationship between an increase in the level of exposure (i.e., AUC and Cmax) and an increase in KIM-1 levels. Minor differences in R2 values existed on the basis of calculations by the use of the Spearman correlation or sigmoidal nonlinear regression. One potential interpretive difference is that Cmin is less predictive of biomarker concentrations by use of the Spearman correlation, but Cmin and KIM-1 had reasonable R2 values by nonlinear regression. Notably, several rats with low Cmin values had high KIM-1 levels; thus, the Spearman correlation may better capture the full relationship for Cmin. Third, our PK parameters remained highly correlated, and Cmax and AUC could not be separated (Fig. S3). Additional dosing scheme designs will be needed to further separate the metrics. Finally, we had only limited data on SCr and BUN levels because of a technical malfunction and the loss of sample. However, KIM-1, clusterin, and cystatin C have been qualified for use in preclinical toxicological evaluations by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMEA), and the Pharmaceutical and Medical Devices Agency (PMDA) of Japan (https://c-path.org/programs/pstc/pstc-tools/#tab-content).
In summary, these data demonstrate that VIKI correlates with and is caused by elevated peak plasma concentrations (Cmax 0–24) or total plasma exposures (AUC0–24) of vancomycin rather than with trough concentrations (Cmin 0–24). Further clarification of the most predictive exposure as well as the thresholds associated with an increased risk of kidney damage is needed to improve dosing regimens that maximize efficacy while minimizing toxicity.
MATERIALS AND METHODS
This pharmacokinetic (PK)/toxicodynamic (TD) study was conducted at Midwestern University in Downers Grove, IL. All study methods were approved by the Midwestern University Institutional Animal Care and Use Committee (IACUC; protocol number 2295).
Experimental design and animals.
The experimental methods are similar to those previously described, with the exception that the animals received only 24 h of therapy (with associated plasma sampling) (4). Control animals and those receiving vancomycin at doses of 150 to 200 mg/kg were carried forward from our previous study (4). In brief, animals were randomized into a treatment group and a nontreatment group, i.e., a vancomycin-treated group and a control group receiving a normal saline (NS) injection. All doses were administered via intraperitoneal (i.p.) injection. Vancomycin-treated rats received total daily doses of 150, 200, 300, or 400 mg/kg either as a single dose or as a twice-daily divided dose (e.g., 400 mg/kg was given as a single injection or as 200 mg/kg twice daily). Twice-daily doses were given approximately 12 h apart. This range of doses was chosen on the basis of previous data suggesting that kidney damage occurs with treatment with doses within this range (17, 22). Additionally, these doses encompass the allometric equivalent of the previously identified dosing kidney injury threshold of ≥4 g/day in a 70-kg patient (i.e., 57 mg/kg/day in humans is roughly the allometric equivalent of 350 mg/kg in rats) (11, 28).
Male Sprague-Dawley rats (n = 49; age, approximately 8 to 10 weeks; mean weight, 286 g) were housed in a light- and temperature-controlled room for the duration of the study and allowed free access to water and food, except that the latter was restricted during the metabolic cage period. Rats (n = 5 to 7 per dosing protocol) were administered i.p. injections of vancomycin in NS or of NS (control). All animals were placed in metabolic cages for urine collection following the 2-h sampling time point. Data for all animals that entered a protocol were analyzed. When animals contributed incomplete data (e.g., because of early protocol termination), urinary biomarkers and urine output were treated as missing data.
Blood and urine sampling.
All catheters were surgically implanted while the animals were under ketamine (100 mg/ml) and xylazine (10 mg/ml) anesthesia. Blood samples were drawn from a single right-sided internal jugular vein catheter in a sedation-free manner when possible. Isoflurane gas was used for temporary sedation when needed (5% initially, followed by 1 to 3% isoflurane gas for maintenance). Animals were allowed 24 h to recover following surgery before sampling began. A maximum target of 8 samples per animal was obtained during the 24-h sampling period, with a preference for sampling from the first dose exposure (i.e., usually 6 samples in the first 4 h). Each sample (a 0.25-ml aliquot) was replaced with an equivalent volume of NS to maintain euvolemia. Blood samples from vancomycin-treated animals were immediately transferred to a disodium EDTA-treated microcentrifuge tube and centrifuged at 3,000 rpm for 10 min. Plasma supernatant was collected and stored at −80°C for batch sample analysis.
Following collection of the 2-h blood sample, animals were placed in metabolic cages for urine collection (catalogue number 650-0350; Nalgene, Rochester, NY) for the remainder of the 24-h study (with the exception that they were briefly removed for additional blood sampling during this time). Urine was measured for volume at 24 h, centrifuged at 400 × g for 5 min, and then stored at −80°C until batch analysis.
Chemicals and reagents.
Animals were administered vancomycin hydrochloride for injection (lot number 447358E02) obtained commercially (Hospira, Lake Forrest, IL). High-performance liquid chromatography (HPLC) was utilized to quantify the vancomycin in plasma as described below. HPLC standard curves were generated using commercially obtained vancomycin hydrochloride, USP (Enzo Life Science, Farmingdale, NY), with a purity of 99.3%. Caffeine (Alfa Aesar, Ward Hill, MA) with a purity of 99.7%, acetonitrile, and methanol were purchased from VWR International (Radnor, PA). Formic acid was obtained from Fisher Scientific (Waltham, MA). All solvents were of HPLC or liquid chromatography-tandem mass spectrometry grade. Frozen, nonmedicated, nonimmunized, pooled Sprague-Dawley rat plasma (anticoagulated with disodium EDTA) was used for the calibration of standard curves (BioreclamationIVT, Westbury, NY).
Determination of vancomycin concentrations in plasma.
Plasma concentrations of vancomycin were quantified using HPLC with UV detection (29). The assay was linear between concentrations of 3 and 75 μg/ml (R2 = 0.999). Precision was <6.1% for all measurements, including intra- and interassay measurements. Greater than 96.3% of the analyte was recovered in all samples tested.
Determination of urinary biomarkers of AKI.
Urine samples were analyzed in batch to determine the concentrations of clusterin, cystatin C, KIM-1, neutrophil gelatinase-associated lipocalin-2 (NGAL), and OPN. The microsphere-based Luminex X-MAP technology was used for the determination of all biomarker concentrations, as previously described (30–32). Urine samples were aliquoted into 96-well plates supplied with Milliplex MAP rat kidney toxicity magnetic bead panels 1 and 2 (EMD Millipore Corporation, Charles, MO), prepared, and analyzed according to the manufacturer's recommendations. Serum creatinine (SCr) and blood urea nitrogen (BUN) concentrations were measured using a Stat Profile pHOx Ultra analyzer (Nova Biomedical, Waltham, MA) per the manufacturer's instructions.
Evaluation of histopathological evidence of renal cell damage.
Following terminal blood sampling, a bilateral nephrectomy was performed under anesthesia (i.e., ketamine [100 mg/ml] and xylazine [10 mg/ml]) for macroscopic postmortem examination. The kidneys were briefly washed in cold isotonic saline and preserved in 10% formalin solution for histologic examination. Histopathological analyses were conducted using light microscopy on hematoxylin- and eosin-stained, paraffin-embedded specimens by Charles Rivers Pathology Associates (Wilmington, MA). Pathologists received access only to a nominal dosing group assignment and not the urinary biomarker concentrations or PK exposures (i.e., AUC0–24, Cmax 0–24, Cmin 0–24). Scoring was conducted according to the Critical Path Institute's Predictive Safety Testing Consortium Nephrotoxicity Working Group's histologic injury lexicon, which utilizes a 0- to 5-point ordinal scale (33). This scoring system assigns higher scores to increasing levels of damage (0, no evidence of damage; 1, minimal damage; 2, mild damage; 3, moderate damage; 4, marked damage; 5, severe damage) and has been validated previously (17, 33). The composite score for each animal was calculated as the highest ordinal score for any histopathological process at any kidney site (33). Scores for proximal tubular cell damage were also collected and analyzed, as proximal tubular cells are the proposed site of the nephrotoxic action of vancomycin (34–36).
Vancomycin pharmacokinetic model and exposure determination.
Our previously published 3-compartment model (4) was compared to other compartmental structural models. Models were parameterized with the absorption rate constant (Ka) to describe drug absorption from the peritoneum, the volume of distribution (V), and intercompartmental transfer constants (e.g., k12 and k21). Linear and nonlinear absorption terms were tested to evaluate variable absorption from the peritoneum.
PK model fitting was completed utilizing the nonparametric adaptive grid algorithm within the Pmetrics (version 1.5.0) package (Los Angeles, CA) for R (37) as previously described (4). The initial estimate of parameter weighting was accomplished using the inverse of the assay variance. Final parameter weighting utilized gamma, a multiplicative observation error model to account for process noise [i.e., error = (SD · gamma)], where SD is the standard deviation, with an initial gamma value of 3 being used. Assay error (SD) was accounted for using an error polynomial as a function of the measured concentration, Y, [i.e., SD = (C0 + C1 · Y)] (where C0 is the initial concentration and C1 is the measured concentration) with inputs of 1.5 and 0.8, respectively. Bayesian posterior parameter value distributions were calculated for the final population model using each animal's measured vancomycin concentrations, exact dosing schedule, and body weight. Compartmental model performance was evaluated and compared with the observed data utilizing a regression of observed versus predicted concentrations, visual plots of parameter estimates, Akaike's information criterion, and the rule of parsimony.
Estimation of PK exposure profiles and statistical analysis.
The best-fit model (as determined above) was utilized to obtain median MAP Bayesian vancomycin plasma concentration estimates at 12-min intervals over the 24-h study period. These concentrations were used to determine exposures over that time period (i.e., AUC0–24, Cmax 0–24, Cmin 0–24). The highest estimated concentration was determined to be each individual animal's Cmax 0–24, and the lowest concentration in hours 11 to 24 following the initial vancomycin dose was determined to be that subject's Cmin 0–24. Twenty-four-hour exposure, as measured by AUC0–24, was calculated using the trapezoidal rule. PK exposure measure variability was calculated as the coefficient of variation (CV; in percent). All PK parameters were additionally categorically binned in categories, with a minimum of 5 animals being needed per created bin. For Cmax 0–24, bins of 0 (i.e., control) and 15, 30, 60, and 120 mg/liter were established. For AUC0–24, bins of 0, 100, 200, 300, and 500 mg · h/liter were established. Relationships between PK exposure parameters were evaluated using Spearman's rank correlation coefficient (rs) in intercooled Stata (version 14.0) software (StataCorp, College Station, TX). Three-way relationships and mesh fits were completed and visualized using the SigmaPlot (version 12.5) program (Systat Software, San Jose, CA).
Association of PK measures with urinary AKI biomarkers and renal histopathology.
Exposure parameters were assessed for relationships with urinary biomarkers as well as composite histopathological damage using Stata (version 14.0) software. PK/TD exposure-response relationships were evaluated using rs. In addition, each biomarker was assessed for relationships with the composite histopathological score using Spearman's rank correlation coefficient. Hill-type functions were fit to the most explanatory biomarker and exposure metric data using the 4-parameter sigmoidal nonlinear regression function in GraphPad Prism (version 7.02) software (GraphPad Software Inc., La Jolla, CA).
Statistical analysis for between-treatment-group comparisons.
Urine output, renal histopathology scores, body weight loss, and PK exposure measures were compared across the vancomycin total daily dose and dosing frequency groups. Log transformations were employed as needed to create parametric distributions. Differences were evaluated using either Student's t test or the Wilcoxon rank sum test, as appropriate. Regressions on biomarkers were completed with categories treated as the dependent variable and control animals set as the referent category. All tests were two-tailed, with an a priori level of statistical significance set at an alpha value of 0.05.
Supplementary Material
ACKNOWLEDGMENTS
The research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases under award number R15-AI105742.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00416-17.
REFERENCES
- 1.Ostermann M, Chang RW. 2007. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 35:1837–1843. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, Lapinsky S, Dial S, Arabi Y, Dodek P, Wood G, Ellis P, Guzman J, Marshall J, Parrillo JE, Skrobik Y, Kumar A. 2009. Acute kidney injury in septic shock: clinical outcomes and impact of duration of hypotension prior to initiation of antimicrobial therapy. Intensive Care Med 35:871–881. doi: 10.1007/s00134-008-1367-2. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, George C, Dinu I, Bellomo R. 2008. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 23:1203–1210. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes NJ, Prozialeck WC, Lodise TP, Venkatesan N, O'Donnell JN, Pais G, Cluff C, Lamar PC, Neely MN, Gulati A, Scheetz MH. 2016. Evaluation of vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury in vancomycin-treated rats. Antimicrob Agents Chemother 60:5742–5751. doi: 10.1128/AAC.00591-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelesidis T, Braykov N, Uslan DZ, Morgan DJ, Gandra S, Johannsson B, Schweizer ML, Weisenberg SA, Young H, Cantey J, Perencevich E, Septimus E, Srinivasan A, Laxminarayan R. 2016. Indications and types of antibiotic agents used in 6 acute care hospitals, 2009-2010: a pragmatic retrospective observational study. Infect Control Hosp Epidemiol 37:70–79. doi: 10.1017/ice.2015.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. 2016. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med 176:1639–1648. doi: 10.1001/jamainternmed.2016.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. 2012. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol 68:1243–1255. doi: 10.1007/s00228-012-1259-9. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand MS, Cleveland KO. 2013. Vancomycin-induced nephrotoxicity. Antimicrob Agents Chemother 57:2435. doi: 10.1128/AAC.00253-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Hal SJ, Paterson DL, Lodise TP. 2013. Systematic review and meta-analysis of vancomycin-induced nephrotoxicity associated with dosing schedules that maintain troughs between 15 and 20 milligrams per liter. Antimicrob Agents Chemother 57:734–744. doi: 10.1128/AAC.01568-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wunderink RG, Niederman MS, Kollef MH, Shorr AF, Kunkel MJ, Baruch A, McGee WT, Reisman A, Chastre J. 2012. Linezolid in methicillin-resistant Staphylococcus aureus nosocomial pneumonia: a randomized, controlled study. Clin Infect Dis 54:621–629. doi: 10.1093/cid/cir895. [DOI] [PubMed] [Google Scholar]
- 11.Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob Agents Chemother 52:1330–1336. doi: 10.1128/AAC.01602-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 49:507–514. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 13.Bosch K, McLaughlin MM, Esterly JS, Rhodes NJ, Postelnick MJ, Scheetz MH. 2014. Impact of vancomycin treatment duration and dose on kidney injury. Int J Antimicrob Agents 43:297–298. doi: 10.1016/j.ijantimicag.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.O'Donnell JN, Ghossein C, Rhodes NJ, Peng J, Lertharakul T, Pham CK, Scheetz MH. 2017. Eight unexpected cases of vancomycin associated acute kidney injury with contemporary dosing. J Infect Chemother 23:326–332. doi: 10.1016/j.jiac.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A, Biyani M, Khaira A. 2011. Vancomycin nephrotoxicity: myths and facts. Neth J Med 69:379–383. [PubMed] [Google Scholar]
- 16.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. 2010. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol 28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ, Bobadilla NA, Marrer E, Perentes E, Cordier A, Vonderscher J, Maurer G, Goering PL, Sistare FD, Bonventre JV. 2010. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol 28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. 2006. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 19.Rybak MJ, Lomaestro BM, Rotscahfer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. 2009. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the Infectious Diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 49:325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 20.Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, Lodise TP. 2014. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother 58:309–316. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blank M, De Felice A, Goodsaid F, Harlow P, Hausner E, Jacobson-Kram D, Taylor W, Thompson A, Throckmorton D, Xiao S. 2009. Review of qualification data for biomarkers of nephrotoxicity submitted by the Predictive Safety Testing Consortium. BQRT, Center for Drug Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD. [Google Scholar]
- 22.Fuchs TC, Frick K, Emde B, Czasch S, von Landenberg F, Hewitt P. 2012. Evaluation of novel acute urinary rat kidney toxicity biomarker for subacute toxicity studies in preclinical trials. Toxicol Pathol 40:1031–1048. doi: 10.1177/0192623312444618. [DOI] [PubMed] [Google Scholar]
- 23.Minejima E, Choi J, Beringer P, Lou M, Tse E, Wong-Beringer A. 2011. Applying new diagnostic criteria for acute kidney injury to facilitate early identification of nephrotoxicity in vancomycin-treated patients. Antimicrob Agents Chemother 55:3278–3283. doi: 10.1128/AAC.00173-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duarte CG, Preuss HG. 1993. Assessment of renal function—glomerular and tubular. Clin Lab Med 13:33–52. [PubMed] [Google Scholar]
- 25.Nicasio AM, Bulitta JB, Lodise TP, D'Hondt RE, Kulawy R, Louie A, Drusano GL. 2012. Evaluation of once-daily vancomycin against methicillin-resistant Staphylococcus aureus in a hollow-fiber infection model. Antimicrob Agents Chemother 56:682–686. doi: 10.1128/AAC.05664-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wold JS, Turnipseed SA. 1981. Toxicology of vancomycin in laboratory animals. Rev Infect Dis 3(Suppl):S224–S229. [PubMed] [Google Scholar]
- 27.Reference deleted.
- 28.U.S. Food and Drug Administration. 2005. Guidance for industry. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. Center for Drug Evaluation and Research, U.S. Food and Drug Administration, U.S. Department of Health and Human Services, Rockville, MD. [Google Scholar]
- 29.Rhodes NJ, Prozialeck WC, Lodise T, Venkatesan N, O'Donnell JN, Pais G, Lamar P, Gulati A, Kamilar JM, Scheetz M. 2015. Vancomycin exposures associated with elevations in novel urinary biomarkers of acute kidney injury, poster A-977. Abstr 55th Intersci Conf Antimicrob Agents Chemother, San Diego, CA. American Society for Microbiology, Washington, DC. [Google Scholar]
- 30.Prozialeck WC, Edwards JR, Lamar PC, Liu J, Vaidya VS, Bonventre JV. 2009. Expression of kidney injury molecule-1 (Kim-1) in relation to necrosis and apoptosis during the early stages of Cd-induced proximal tubule injury. Toxicol Appl Pharmacol 238:306–314. doi: 10.1016/j.taap.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prozialeck WC, Edwards JR, Vaidya VS, Bonventre JV. 2009. Preclinical evaluation of novel urinary biomarkers of cadmium nephrotoxicity. Toxicol Appl Pharmacol 238:301–305. doi: 10.1016/j.taap.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prozialeck WC, Vaidya VS, Liu J, Waalkes MP, Edwards JR, Lamar PC, Bernard AM, Dumont X, Bonventre JV. 2007. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int 72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dieterle F, Sistare F, Goodsaid F, Papaluca M, Ozer JS, Webb CP, Baer W, Senagore A, Schipper MJ, Vonderscher J, Sultana S, Gerhold DL, Phillips JA, Maurer G, Carl K, Laurie D, Harpur E, Sonee M, Ennulat D, Holder D, Andrews-Cleavenger D, Gu YZ, Thompson KL, Goering PL, Vidal JM, Abadie E, Maciulaitis R, Jacobson-Kram D, Defelice AF, Hausner EA, Blank M, Thompson A, Harlow P, Throckmorton D, Xiao S, Xu N, Taylor W, Vamvakas S, Flamion B, Lima BS, Kasper P, Pasanen M, Prasad K, Troth S, Bounous D, Robinson-Gravatt D, Betton G, Davis MA, Akunda J, McDuffie JE, et al. 2010. Renal biomarker qualification submission: a dialog between the FDA-EMEA and Predictive Safety Testing Consortium. Nat Biotechnol 28:455–462. doi: 10.1038/nbt.1625. [DOI] [PubMed] [Google Scholar]
- 34.Ahmida MH. 2012. Protective role of curcumin in nephrotoxic oxidative damage induced by vancomycin in rats. Exp Toxicol Pathol 64:149–153. doi: 10.1016/j.etp.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Oktem F, Arslan MK, Ozguner F, Candir O, Yilmaz HR, Ciris M, Uz E. 2005. In vivo evidences suggesting the role of oxidative stress in pathogenesis of vancomycin-induced nephrotoxicity: protection by erdosteine. Toxicology 215:227–233. doi: 10.1016/j.tox.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Nishino Y, Takemura S, Minamiyama Y, Hirohashi K, Tanaka H, Inoue M, Okada S, Kinoshita H. 2002. Inhibition of vancomycin-induced nephrotoxicity by targeting superoxide dismutase to renal proximal tubule cells in the rat. Redox Rep 7:317–319. doi: 10.1179/135100002125000884. [DOI] [PubMed] [Google Scholar]
- 37.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. 2012. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit 34:467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.