ABSTRACT
Hybrid therapy is a novel two-step treatment achieving a high eradication rate for Helicobacter pylori infection. Currently, whether this new therapy achieves a higher eradication rate than bismuth quadruple therapy remains an unanswered question. The aim of this prospective, randomized comparative study was to investigate the efficacies of 14-day hybrid therapy and bismuth quadruple therapy in the treatment of H. pylori infection. From July 2013 to June 2015, eligible H. pylori-infected subjects were randomly assigned to receive either 14-day bismuth quadruple therapy (pantoprazole, bismuth subcitrate, tetracycline, and metronidazole for 14 days) or 14-day hybrid therapy (a 7-day dual therapy with pantoprazole plus amoxicillin, followed by a 7-day quadruple therapy with pantoprazole plus amoxicillin, clarithromycin, and metronidazole). H. pylori status was examined 6 weeks after the end of treatment. Three hundred thirty H. pylori-infected participants were randomized to receive 14-day bismuth quadruple therapy (n = 164) or 14-day hybrid therapy (n = 166). The eradication rates by intention-to-treat analysis were similar: 93.9% versus 92.8%, respectively (95% confidence interval [CI], −4.3% to 5.4%; P = 0.68). Per-protocol analysis yielded similar results (96.7% versus 94.9%, respectively; P = 0.44). However, bismuth quadruple therapy had a higher frequency of adverse events than hybrid therapy (55.5% versus 15.7%, respectively; 95% CI, 30.4% to 49.2%; P < 0.001). The two treatments exhibited comparable drug adherence (93.9% versus 97%, respectively). The resistance rates of antibiotics were: clarithromycin, 16.7% of patients; amoxicillin, 1.3%; metronidazole, 25%; and tetracycline, 0%. In the bismuth quadruple therapy group, the eradication rate of metronidazole-resistant strains was lower than that of metronidazole-susceptible strains (70.0% versus 96.4%, respectively; P = 0.04). In the hybrid therapy group, no significant impact of clarithromycin or metronidazole resistance on eradication rates was identified. Both 14-day hybrid and bismuth quadruple therapies cure most patients with H. pylori infection in populations with moderate antibiotic resistance. However, the 14-day hybrid therapy has fewer adverse effects than the bismuth quadruple therapy. (This study has been registered at ClinicalTrials.gov under identifier NCT02541864.)
KEYWORDS: antibiotic resistance, bismuth quadruple therapy, hybrid therapy, Helicobacter pylori
INTRODUCTION
Helicobacter pylori infection is the principal cause of chronic gastritis, gastric ulcers, duodenal ulcers, gastric adenocarcinomas, and gastric mucosa-associated lymphoid tissue lymphomas (MALTomas) (1). Conventional strategies for eradicating H. pylori usually involve the application of antibiotic monotherapy to eradicate the bacteria, but the eradication rate is low (2). Dual therapy, including either bismuth compounds or proton-pump inhibitors (PPI) and one antibiotic, also has insufficient cure rates. Currently, standard triple therapy consisting of a PPI, clarithromycin, and amoxicillin (or metronidazole) for 7 to 14 days is the recommended therapy for H. pylori infection in most international guidelines (3–6), especially in areas of low clarithromycin resistance (<15%). Recently, the eradication rates of standard triple therapy have declined to less than 80% in many countries, largely owing to emerging bacterial resistance (7–10). Some European studies reported poor treatment outcomes following standard therapy, with failure rates of 25 to 60% (11–13). Several strategies, including bismuth-containing and non-bismuth-containing quadruple therapies (including sequential, concomitant, and hybrid therapies) have shown acceptable cure rates in the presence of clarithromycin resistance (14–16).
Bismuth-containing quadruple therapy is recommended as the treatment of choice for H. pylori infection in areas of either low or high clarithromycin resistance in the Maastricht IV/Florence consensus report in 2012 (5). Although the optimal treatment duration of bismuth-containing quadruple therapy remains unclear, a 10 to 14 day course is most commonly employed in clinical practice. A recent study by Malfertheiner et al. compared the efficacy of a 10-day bismuth-containing quadruple therapy (omeprazole, bismuth, metronidazole, and tetracycline) and a 7-day triple therapy (omeprazole, clarithromycin, and amoxicillin) (17). The data indicated that the 10-day quadruple therapy had a higher eradication rate than the 7-day triple therapy (80% versus 55%, respectively, by intention-to-treat [ITT] analysis).
The hybrid therapy reported by Hsu et al. in 2011 consists of a dual therapy with a PPI (standard dose, twice a day [b.i.d.]) and amoxicillin (1 g, b.i.d.) for 7 days followed by a quadruple therapy with a PPI (standard dose, b.i.d.), amoxicillin (1 g, b.i.d.), clarithromycin (500 mg, b.i.d.), and metronidazole (500 mg, b.i.d.) for 7 days (16). The hybrid idea is based on some important data. First, the novel therapy includes metronidazole, which increases eradication efficacy in clarithromycin-resistant strains. Clarithromycin resistance is the key factor that determines the eradication efficacy of standard triple therapy consisting of a PPI, amoxicillin, and clarithromycin (8, 10). Previous studies documented that 7-day quadruple therapy containing a PPI, amoxicillin, clarithromycin, and metronidazole was superior to 7-day standard triple therapy in the treatment of H. pylori infection (18). The data indicate that adding metronidazole to a clarithromycin-containing triple regimen may increase the eradication efficacy of standard triple therapy. Second, hybrid therapy increases the duration of amoxicillin treatment to 14 days to improve the eradication efficacy for clarithromycin and metronidazole dual-resistant strains, because the frequency of amoxicillin-resistant H. pylori strains is extremely low worldwide (0 to 2%) (18–20). The pilot study showed that 14-day hybrid therapy achieved excellent eradication rates of 99% and 97% according to per-protocol (PP) and ITT analyses, respectively (16). Its eradication rate for clarithromycin and metronidazole dual-resistant strains was high (16). Several randomized controlled trials subsequently demonstrated that hybrid regimens were comparable with or more effective than sequential regimens (21–25). A recent large multicenter randomized trial in areas with high clarithromycin and metronidazole resistance confirmed that both 14-day hybrid and concomitant therapies cured more than 90% of H. pylori infections (26).
Currently, the question of whether hybrid therapy can achieve a higher eradication rate than bismuth quadruple therapy for H. pylori infection remains unanswered. Therefore, we conducted a randomized controlled trial to compare the efficacies of 14-day hybrid therapy and 14-day bismuth quadruple therapy and to investigate the impacts of antibiotic resistances on the eradication rates of the two regimens.
RESULTS
Characteristics of study groups.
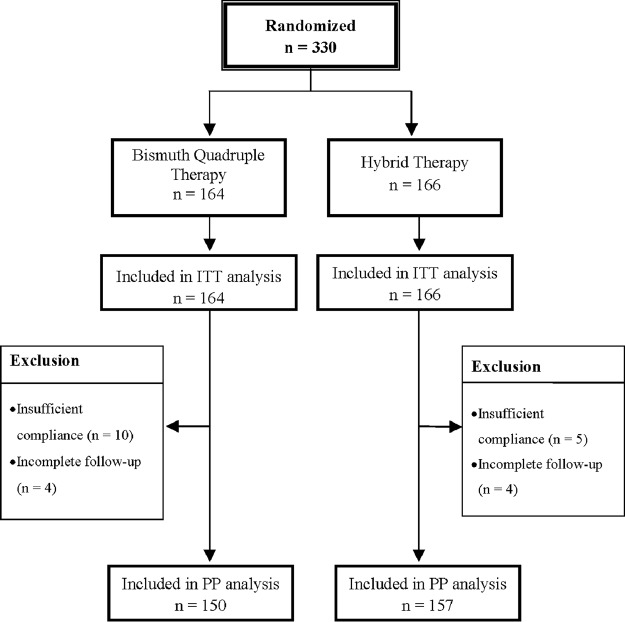
From July 2013 to June 2015, a total of 330 H. pylori-infected participants were randomized to two groups: 164 received 14-day bismuth quadruple therapy and 166 received 14-day hybrid therapy. Table 1 shows the demographic and clinical characteristics of the patients. The two patient groups were comparable with respect to age, sex, history of smoking, alcohol, coffee, and tea consumption, endoscopic findings, and antibiotic resistance of H. pylori. Among the recruited subjects, 14 patients in the bismuth group and 9 in the hybrid group were excluded from PP analysis due to incomplete follow-up and poor adherence. Figure 1 summarizes the patient disposition.
TABLE 1.
Demographic and clinical data from two patient groups
| Characteristic | Bismuth quadruple therapy (n = 164) | Hybrid therapy (n = 166) | P value |
|---|---|---|---|
| Age (yr) (mean ± SD) | 53.46 ± 12.28 | 54.48 ± 11.45 | 0.43 |
| Sex (no. male/no. female) | 94/70 | 79/87 | 0.07 |
| Smoking (n [%]) | 36 (22) | 39 (19.9) | 0.64 |
| Consumption (n [%]) | |||
| Alcohol | 8 (4.9) | 8 (4.8) | 0.98 |
| Coffee | 39 (23.8) | 40 (24.1) | 0.94 |
| Tea | 46 (28) | 45 (27.1) | 0.84 |
| NSAIDa use (n [%]) | 2 (1.2) | 2 (1.2) | 1.00 |
| Underlying disease (n [%]) | 38 (23.2) | 47 (28.3) | 0.28 |
| Endoscopic findings (n [%]) | |||
| Gastritis | 86 (52.4) | 72 (43.4) | 0.10 |
| Peptic ulcer | 78 (47.6) | 94 (56.6) | 0.10 |
| Antibiotic resistanceb (% [no./total]) | |||
| Clarithromycin | 15.8 (6/38) | 17.6 (6/34) | 0.83 |
| Amoxicillin | 2.6 (1/38) | 0 (0/34) | —c |
| Metronidazole | 26.3 (10/38) | 23.5 (8/34) | 0.78 |
| Tetracycline | 0 (0/38) | 0 (0/34) | — |
NSAID, nonsteroidal anti-inflammatory drug.
Seventy-two strains were isolated.
—, not applicable.
FIG 1.
Disposition of patients. We recruited 330 H. pylori-infected patients: 164 to the 14-day bismuth quadruple therapy group and 166 to the 14-day hybrid therapy group. In the bismuth group, 14 patients were excluded from the per-protocol analysis for poor adherence or incomplete follow-up. In the hybrid group, 9 patients were excluded from the per-protocol analysis.
Eradication of H. pylori.
Table 2 displays the major outcomes of eradication therapies. An ITT analysis showed that the eradication rates of bismuth quadruple therapy and hybrid therapy were 93.9% ([154/164] 95% confidence interval [CI], 90.3 to 97.5%) and 92.8% ([154/166] 95% CI, 88.9 to 96.7%), respectively. The two therapies had comparable eradication rates (95% CI, −4.3% to 5.4%; P = 0.68). A PP analysis yielded similar results (96.7% versus 94.9%; P = 0.44). We further stratified the data based on gastritis versus ulcer presentation with regard to eradication rates (Table 3). In patients with gastritis, bismuth quadruple therapy and hybrid therapy had ITT eradication rates of 95.3% and 94.4%, respectively. Eradication rates did not significantly differ between the two groups. In patients with peptic ulcer disease, the two therapies also displayed comparable eradication rates (92.3% versus 91.5%, P = 0.85). The PP analysis showed that bismuth quadruple therapy and hybrid therapy had similar eradication rates in patients with gastritis (96.2% versus 94.4%, respectively) or peptic ulcer disease (92.1% versus 91.1%, respectively).
TABLE 2.
Major outcomes of bismuth quadruple therapy and hybrid therapy
| Outcome | Bismuth quadruple therapy (n = 164) |
Hybrid therapy (n = 166) |
P value | ||
|---|---|---|---|---|---|
| % (no./total) | 95% CI | % (no./total) | 95% CI | ||
| Eradication rate | |||||
| Intention-to-treat | 93.9 (154/164) | 90.3–97.5 | 92.8 (154/166) | 88.9–96.7 | 0.68 |
| Per-protocol | 96.7 (145/150) | 93.9–99.5 | 94.9 (149/157) | 91.5–98.3 | 0.44 |
| Adverse events | 55.5 (91/164) | 47.9–63.1 | 15.7 (26/166) | 10.2–21.2 | 0.00a |
| Adherence | 93.9 (154/164) | 90.3–97.5 | 97.0 (161/166) | 94.5–99.5 | 0.17 |
P < 0.05.
TABLE 3.
Eradication rates and frequencies of adverse events in patients with gastritis or peptic ulcer disease receiving either bismuth quadruple or hybrid therapy
| Outcome | % (no./total): |
P value | |
|---|---|---|---|
| Bismuth quadruple therapy (n = 164) | Hybrid therapy (n = 166) | ||
| Patients with gastritis | |||
| Eradication rate | |||
| Intention-to-treat | 95.3 (82/86) | 94.4 (68/72) | 1.00 |
| Per-protocol | 96.2 (75/78) | 94.4 (67/71) | 0.71 |
| Adverse events | 60.5 (52/86) | 19.4 (14/72) | 0.00a |
| Patients with peptic ulcer | |||
| Eradication rate | |||
| Intention-to-treat | 92.3 (72/78) | 91.5 (86/94) | 0.85 |
| Per-protocol | 92.1 (70/76) | 91.1 (82/90) | 0.82 |
| Adverse events | 51.3 (40/78) | 12.8 (12/94) | 0.00a |
P < 0.05.
Adverse events and adherence.
Subjects who received at least one dose of eradication drugs were included in the adverse event analysis. The incidence of adverse events was 55.5% (95% CI, 47.9% to 63.1%) in the participants receiving bismuth quadruple therapy and 15.7% (95% CI, 10.2 to 21.2%) in those receiving hybrid therapy.
Table 4 lists the profiles of adverse events of the two eradication therapies. The bismuth quadruple group had higher frequencies of abdominal pain, dizziness, nausea, and fatigue than the hybrid group (P = 0.01, 0.03, <0.001, and 0.05, respectively). In the bismuth group, 10 patients discontinued treatment owing to adverse events (nausea, four patients; dizziness, two patients; skin rash, two patients, headache, one patient; diarrhea, one patient). Four patients in the hybrid group stopped the anti-H. pylori medication because of adverse events (dizziness, one patient; headache, one patient; diarrhea, one patient; skin rash, one patient). The two treatment groups displayed similar adherence rates (93.9% [95% CI, 90.3% to 97.5%] and 97.0%, [95% CI, 94.5% to 99.5%]) (Table 2).
TABLE 4.
Adverse events of bismuth quadruple therapy and hybrid therapy
| Adverse event | No. (%): |
P value | |
|---|---|---|---|
| Bismuth quadruple therapy (n = 164) | Hybrid therapy (n = 166) | ||
| Any | 91 (55.5) | 26 (15.7) | 0.00a |
| Abdominal pain | 8 (4.8) | 1 (0.6) | 0.01a |
| Constipation | 1 (0.6) | 1 (0.6) | 1.00 |
| Diarrhea | 3 (1.8) | 1 (0.6) | 0.37 |
| Dizziness | 17 (10.3) | 7 (4.2) | 0.03a |
| Bad taste | 7 (4.2) | 4 (2.4) | 0.37 |
| Headache | 3 (1.8) | 5 (3.0) | 0.72 |
| Nausea | 75 (45.7) | 12 (7.2) | 0.00a |
| Vomiting | 6 (3.6) | 2 (1.2) | 0.17 |
| Skin rash | 2 (1.2) | 4 (2.4) | 0.68 |
| Fatigue | 10 (6.0) | 3 (1.8) | 0.05 |
| Other | 5 (3.0) | 3 (1.8) | 0.50 |
P < 0.05.
Adverse events were then compared between patients with gastritis and patients with ulcers (Table 3). In patients with gastritis, bismuth quadruple therapy had a higher frequency of adverse events than hybrid therapy (60.5% versus 19.4%, respectively; P < 0.001). In patients with peptic ulcer disease, the bismuth quadruple therapy also resulted in more adverse events than the hybrid therapy (51.3% versus 12.8%, respectively; P < 0.001). An additional analysis showed that the frequencies of adverse events did not significantly differ between patients with gastritis and patients with peptic ulcers in the bismuth quadruple group (60.5% versus 51.3%, respectively) and in the hybrid group (19.4% versus 12.8%, respectively).
Impacts of antibiotic resistances on eradication rates.
The current study did not involve the routine culture of H. pylori, and only 90 patients received endoscopy with bacterial culture at enrollment. Among them, H. pylori strains were successfully isolated from 72 patients. The rates of strains resistant to clarithromycin, amoxicillin, metronidazole, and tetracycline were 16.7% (12/72), 1.3% (1/72), 25% (18/72), and 0% (0/72), respectively. The rate of clarithromycin and metronidazole dual-resistant strains was 6.9% (5/72).
Table 5 shows the impacts of antibiotic resistances on the eradication rates of therapies. In the bismuth quadruple therapy group, no tetracycline-resistant strains were isolated. An ITT analysis revealed that the eradication rate of metronidazole-resistant strains was lower than that of metronidazole-susceptible strains (70.0% versus 96.4%, respectively; P = 0.04). In the hybrid therapy group, no differences in eradication rates existed between clarithromycin-resistant and -sensitive stains (83.3% versus 100.0%, respectively; P = 0.17) and between metronidazole-resistant and -sensitive strains (100.0% versus 96.2%, respectively; P = 1.00). The eradication rate of clarithromycin and metronidazole dual-resistant strains was 100.0% (3/3).
TABLE 5.
Impact of antibiotic resistance on the eradication rate
| Resistance | Eradication rate |
|||
|---|---|---|---|---|
| ITT analysis |
PP analysis |
|||
| No./total (%) | P value | No./total (%) | P value | |
| Bismuth quadruple therapy (n = 38) | ||||
| Clarithromycin-resistant | 1.00 | 1.00 | ||
| − | 28/32 (87.5) | 27/31 (87.1) | ||
| + | 6/6 (100) | 6/6 (100) | ||
| Amoxicillin-resistant | 1.00 | 1.00 | ||
| − | 33/37 (89.2) | 32/36 (88.9) | ||
| + | 1/1 (100) | 1/1 (100) | ||
| Metronidazole-resistant | 0.04b | 0.05 | ||
| − | 27/28 (96.4) | 26/27 (96.3) | ||
| + | 7/10 (70) | 7/10 (70) | ||
| Tetracycline | —c | — | ||
| − | 34/38 (89.5) | 33/37 (89.2) | ||
| + | — | — | ||
| Dual-resistanta | 1.00 | 1.00 | ||
| − | 32/36 (88.9) | 31/35 (88.6) | ||
| + | 2/2 (100) | 2/2 (100) | ||
| Hybrid therapy (n = 34) | ||||
| Clarithromycin-resistant | 0.17 | 0.17 | ||
| − | 28/28 (100) | 28/28 (100) | ||
| + | 5/6 (83.3) | 5/6 (83.3) | ||
| Amoxicillin-resistant | — | — | ||
| − | 33/34 (97.1) | 33/34 (97.1) | ||
| + | — | — | ||
| Metronidazole-resistant | 1.00 | 1.00 | ||
| − | 25/26 (96.2) | 25/26 (96.2) | ||
| + | 8/8 (100) | 8/8 (100) | ||
| Tetracycline | — | — | ||
| − | 33/34 (97.1) | 33/34 (97.1) | ||
| + | — | — | ||
| Dual-resistanta | 1.00 | 1.00 | ||
| − | 30/31 (96.8) | 30/31 (96.8) | ||
| + | 3/3 (100) | 3/3 (100) | ||
Dual-resistant (resistances to both clarithromycin and metronidazole).
P < 0.05.
—, not applicable.
DISCUSSION
This study performed the first head-to-head, randomized controlled trial to test whether 14-day hybrid therapy can achieve a higher eradication rate than 14-day bismuth quadruple therapy for H. pylori infection. Both ITT and PP analyses showed that the two therapies cured most patients with H. pylori infection (92.8% versus 93.9% and 94.9% versus 96.7%, respectively). The experimental results demonstrated several new findings. First, the two therapies had comparable eradication rates. Second, patients treated with 14-day hybrid therapy reported fewer adverse effects than those receiving quadruple therapy (15.7% versus 55.5%, respectively; P < 0.001). Third, the two treatments had comparable drug adherences (97.0% versus 93.9%). This study also collected data for the eradication rates of the two therapies for antibiotic-resistant and -sensitive stains. The results indicate that both 14-day hybrid and 14-day bismuth quadruple therapies can be recommended for the first-line treatment of H. pylori infection in areas of moderate clarithromycin resistance.
Approximately half of the subjects in the bismuth quadruple therapy group suffered from at least one adverse event. The most common adverse event of the eradication therapy was nausea (45.7%), which might be due to bismuth, tetracycline, and metronidazole in this regimen. Among the patients receiving 14-day bismuth quadruple therapy, four patients (2.4%) experienced severe nausea and stopped the treatment early. In addition to nausea, the bismuth quadruple therapy group also had higher frequencies of abdominal pain, dizziness, and fatigue than the hybrid therapy group.
Currently, the optimum duration of bismuth quadruple therapy remains unclear. In this study, 14-day bismuth quadruple therapy cured most patients with H. pylori infection (93.9% and 96.7% by ITT and PP analyses, respectively). However, our previous study showed that the eradication of 7-day bismuth quadruple therapy was only 74% (27). A systemic review by Graham et al. also demonstrated that 14-day bismuth quadruple therapy achieved a higher eradication rate for metronidazole-resistant strains than 7-day bismuth quadruple therapy (28). The 14-day bismuth quadruple therapy is recommended as the treatment of choice for first-line therapy of H. pylori infection in areas of high clarithromycin resistance and as an alternative in areas of low clarithromycin resistance (29). However, extending the duration of bismuth quadruple therapy from 7 days to 14 days might potentially increase the frequency of adverse events.
Antibiotic resistance is a crucial determinant of the treatment outcome in bacterial eradication (8, 28). From this study, in the patients receiving bismuth quadruple therapy, those with metronidazole-resistant strains had a lower eradication rate than those with metronidazole-susceptible strains (70.0% versus 96.4%, respectively). Another independent, randomized controlled trial from Thailand also supported this finding and showed that eradication rate in patients with metronidazole-resistant strains undergoing 7-day bismuth quadruple therapy was lower than that in those with metronidazole-sensitive strains (72.7% versus 90.1%, respectively) (30). A systemic review of bismuth quadruple therapy in the treatment of H. pylori infection revealed that the effectiveness of bismuth quadruple therapy is affected by metronidazole resistance, the dose of metronidazole, and the duration of the eradication regimen (28). The eradication rates of 14-day bismuth quadruple therapy for metronidazole-sensitive and -resistant strains were 97% and 90%, respectively (28). In this study, 70% of the H. pylori strains with metronidazole resistance were eradicated by 14-day bismuth quadruple therapy. The moderate eradication efficacy of the treatment for metronidazole-resistant strains might have resulted from the combined use of tetracycline and bismuth and the long treatment duration. This study also showed that the eradication rates of 14-day hybrid therapy for clarithromycin-resistant and metronidazole-resistant strains were 83.3% and 100%, respectively. The data were consistent with our previous report in which 14-day hybrid therapy obtained 100% eradication in both clarithromycin-resistant and metronidazole-resistant strains (16).The high eradication efficacy of hybrid therapy for H. pylori strains with antibiotic resistance might be due to the combined use of other antibiotics and long treatment duration with amoxicillin.
The strengths of this study include the comparison with a randomized controlled trial and a large sample size (>150 in each group). Additionally, this study provided the impacts of antibiotic resistances on eradication results. One limitation of this study is that it was performed in a single center. Therefore, the results will need to be confirmed in other regions where different patterns of antibiotic resistance are present. Second, the number of eradication failures was too small in both therapeutic groups and precluded further regression analyses. Third, H. pylori-infected patients might have mixed isolates of H. pylori (coinfection with more than one strain) and harbor both susceptible and resistant strains. Kao et al. examined the antibiotic susceptibility of H. pylori isolated from 412 patients without H. pylori eradication (31). Their analytical results showed 19 (4.6%) patients harbored antibiotic-heteroresistant H. pylori. Our study did not investigate whether patients had mixed or isolated H. pylori; therefore, we could not exclude the possibility. Nonetheless, this study is the first randomized controlled trial comparing 14-day hybrid therapy and bismuth quadruple therapy in the treatment of H. pylori infection.
In summary, both 14-day hybrid and bismuth quadruple therapies cure most patients with H. pylori infection in populations with moderate antibiotic resistance, and the two therapies have comparable eradication rates. However, the 14-day hybrid therapy has fewer adverse effects.
MATERIALS AND METHODS
Participants.
The randomized trial was conducted at the Kaohsiung Veterans General Hospital in accordance with the principles of good clinical practice from the Declaration of Helsinki. Consecutive H. pylori-infected outpatients, at least 20 years of age with endoscopically proven peptic ulcer diseases or gastritis were recruited for the study. H. pylori infection was documented by at least two positive results of rapid urease test, histology, and culture (32). Exclusion criteria included (i) previous eradication therapy, (ii) an allergy to any antibiotic used in our study, (iii) a previous gastrectomy, (iv) the coexistence of severe concomitant illness, and (v) pregnancy or lactation in women.
The medical committee of the Kaohsiung Veterans General Hospital approved the trial (VGHKS12–CT11-08). All participants gave written informed consent before enrollment. This study has been registered at ClinicalTrials.gov under identifier NCT02541864.
Randomization and treatment.
Using a computer-generated number sequence, the eligible H. pylori-infected patients were randomly assigned to a 14-day bismuth quadruple therapy (pantoprazole, 40 mg, b.i.d.; bismuth subcitrate, 120 mg, four times a day [q.i.d.]; tetracycline, 500 mg, q.i.d.; and metronidazole, 250 mg, q.i.d. for 14 days) or a 14-day hybrid therapy (a dual therapy with pantoprazole, 40 mg, b.i.d. and amoxicillin, 1 g, b.i.d. for 7 days, followed by a quadruple therapy with pantoprazole, 40 mg, b.i.d.; amoxicillin, 1 g, b.i.d.; clarithromycin, 500 mg, b.i.d.; and metronidazole, 500 mg, b.i.d. for a further 7 days). The consumption of alcohol during treatment was prohibited to avoid the possible side effects of an interaction with metronidazole.
The patients were informed of the common adverse events from the study drugs before treatment and were asked to record these symptoms during treatment in provided diaries.
Procedures.
Before enrollment, the status of H. pylori infection was determined by rapid urease test, histology, and/or culture (32). Patients with positive results in at least two of these tests were eligible for enrollment. The eligible patients were requested to complete a standard questionnaire that contained questions regarding demographic data and histories of smoking, alcohol drinking, nonsteroidal anti-inflammatory drug use, and underlying diseases.
Patients were asked to return the second week to assess drug adherence and adverse effects. The adverse events were assessed by a research assistant according to defined criteria. Drug adherence was assessed via pill counts. Adherence was defined as good if the patient recorded taking equal to or more than 80% of the total medication or as poor (i.e., taking less) via pill counts (32, 33).
Because of the possibility that a gastric cancer might be missed in the initial endoscopy as a benign gastric ulcer, a repeated endoscopy with both rapid urease test and histological examination 6 weeks after the end of anti-H. pylori therapy was performed in gastric ulcer patients to assess eradication efficacy and the healing status of ulcer lesions. Since there was no concern about the malignant changes of duodenal ulcers or gastritis, a urea breath test was conducted to assess H. pylori status in participants with duodenal ulcer or gastritis. A staff member who was blind to the eradication arm performed the urea breath tests. The cutoff value was set at 4.8‰ of δ13CO2 (34). Eradication was defined as (i) negative results of both rapid urease test and histology or (ii) a negative result of urea breath test.
Culture and antimicrobial resistance.
An antral gastric biopsy specimen was obtained for H. pylori culture, using previously described methods (32). H. pylori subculturing was done by rubbing the specimens on the surface of a Campy-BAP agar plate (Brucella agar; Difco, Sparks, MD) plus IsoVitalex (Gibco, Grand Island, NY) plus 10% whole sheep blood followed by incubation at 37°C under microaerobic conditions (5% O2, 10% CO2, and 85% N2) for 4 to 5 days. H. pylori strains were tested for clarithromycin, amoxicillin, tetracycline, and metronidazole susceptibility using the Etest (AB Biodisk, Solna, Sweden). H. pylori strains with MIC values of >1 μg/ml, >0.5 μg/ml, >4 μg/ml, and >8 μg/ml were considered to be resistant to clarithromycin, amoxicillin, tetracycline and metronidazole, respectively (35).
Statistical analysis.
The primary endpoint was eradication rate, and secondary endpoints were adverse events and drug adherence. The eradication rates with 95% confidence intervals (CI) were used for ITT and PP analyses. The chi-square test for continuity and Fisher's exact test were used when appropriate to compare the major outcomes between two groups. A P value of less than 0.05 was considered statistically significant. According to our previous studies (16, 36), the eradication rate of hybrid therapy was 95%. When we started the study, there were no data available about the eradication rate of 14-day bismuth quadruple therapy in Taiwan. However, our previous study showed that the eradication rate with 7-day bismuth quadruple therapy was 74% in Taiwan (27). We proposed that extending the duration of bismuth quadruple therapy to 14 days would increase the eradication rate to 84% (10% higher eradication rate than that for the 7-day regimen). It was estimated that we required a minimum of 328 participants to achieve a statistical power of 90% with a type I error of 0.05, assuming a 3% loss to follow-up.
ACKNOWLEDGMENTS
We thank the study nurses, Yu-Shan Chen and Lee-Ya Wang, at the Kaohsiung Veterans General Hospital who assisted in the collection of the data on patients' characteristics and for explaining drug administration. All the authors disclose no conflict of interests.
This work was funded by the Research Fund of the Kaohsiung Veterans General Hospital (VGHKS102-084, VGHKS103-004, VGHNSU103-001, and VGHKS104-001).
REFERENCES
- 1.Hunt RH, Lam SK. 1998. Helicobacter pylori: from art to a science. J Gastroenterol Hepatol 13:21–28. doi: 10.1111/j.1440-1746.1998.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 2.Gisbert JP, Pajares R, Pajares JM. 2007. Evolution of Helicobacter pylori therapy from a meta-analytical perspective. Helicobacter 12(Suppl 2):S50–S58. doi: 10.1111/j.1523-5378.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- 3.Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY, Jung HC, Hoang BH, Kachintorn U, Goh KL, Chiba T, Rani AA, Second Asia-Pacific Conference. 2009. Second Asia-Pacific Consensus guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 24:1587–1600. doi: 10.1111/j.1440-1746.2009.05982.x. [DOI] [PubMed] [Google Scholar]
- 4.Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, Uemura N, Murakami K, Satoh K, Sugano K, Japanese Society for Helicobacter Research. 2010. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 15:1–20. doi: 10.1111/j.1523-5378.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, European Helicobacter Study Group. 2012. Management of Helicobacter pylori infection–the Maastricht IV/Florence consensus report. Gut 61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 6.Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. 2007. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 7.Graham DY, Shiotani A. 2008. New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clin Pract Gastroenterol Hepatol 5:321–331. doi: 10.1038/ncpgasthep1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Megraud F. 2004. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53:1374–1384. doi: 10.1136/gut.2003.022111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. 2010. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterol 105:65–73. doi: 10.1038/ajg.2009.508. [DOI] [PubMed] [Google Scholar]
- 10.De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani L, Burattini O, Stella F, Di Leo A, Russo F, Marangi S, Monno R, Stoppino V, Morini S, Panella C, Ierardi E. 2006. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med 144:94–100. doi: 10.7326/0003-4819-144-2-200601170-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gumurdulu Y, Serin E, Ozer B, Kayaselcuk F, Ozsahin K, Cosar AM, Gursoy M, Gur G, Yilmaz U, Boyacioglu S. 2004. Low eradication rate of Helicobacter pylori with triple 7–14 days and quadruple therapy in Turkey. World J Gastroenterol 10:668–671. doi: 10.3748/wjg.v10.i5.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bigard MA, Delchier JC, Riachi G, Thibault P, Barthelemy P. 1998. One-week triple therapy using omeprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment Pharmacol Ther 12:383–388. doi: 10.1046/j.1365-2036.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- 13.De Francesco V, Margiotta M, Zullo A, Hassan C, Giorgio F, Burattini O, Stoppino G, Cea U, Pace A, Zotti M, Morini S, Panella C, Ierardi E. 2007. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15 year period in Italy. J Antimicrob Chemother 59:783–785. doi: 10.1093/jac/dkm005. [DOI] [PubMed] [Google Scholar]
- 14.Zullo A, De Francesco V, Hassan C, Morini S, Vaira D. 2007. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 56:1353–1357. doi: 10.1136/gut.2007.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essa AS, Kramer JR, Graham DY, Treiber G. 2009. Meta-analysis: four-drug, three-antibiotic, non-bismuth-containing “concomitant therapy” versus triple therapy for Helicobacter pylori eradication. Helicobacter 14:109–118. doi: 10.1111/j.1523-5378.2009.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu PI, Wu DC, Wu JY, Graham DY. 2011. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 16:139–145. doi: 10.1111/j.1523-5378.2011.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F, Pylera Study Group. 2011. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 18.Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, Kao SS, Lai KH, Chen A, Tsay FW. 2014. Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for Helicobacter pylori infection. Antimicrob Agents Chemother 58:5936–5942. doi: 10.1128/AAC.02922-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duck WM, Sobel J, Pruckler JM, Song Q, Swerdlow D, Friedman C, Sulka A, Swaminathan B, Taylor T, Hoekstra M, Griffin P, Smoot D, Peek R, Metz DC, Bloom PB, Goldschmidt S, Parsonnet J, Triadafilopoulos G, Perez-Perez GI, Vakil N, Ernst P, Czinn S, Dunne D, Gold BD. 2004. Antimicrobial resistance incidence and risk factors among Helicobacter pylori-infected persons, United States. Emerg Infect Dis 10:1088–1094. doi: 10.3201/eid1006.030744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meyer JM, Silliman NP, Wang W, Siepman NY, Sugg JE, Morris D, Zhang J, Bhattacharyya H, King EC, Hopkins RJ. 2002. Risk factors for Helicobacter pylori resistance in the United States: the surveillance of H. pylori antimicrobial resistance partnership (SHARP) study, 1993–1999. Ann Intern Med 136:13–24. doi: 10.7326/0003-4819-136-1-200201010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. 2013. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 18:129–134. doi: 10.1111/hel.12017. [DOI] [PubMed] [Google Scholar]
- 22.Zullo A, Scaccianoce G, De Francesco V, Ruggiero V, D'Ambrosio P, Castorani L, Bonfrate L, Vannella L, Hassan C, Portincasa P. 2013. Concomitant, sequential, and hybrid therapy for H. pylori eradication: a pilot study. Clin Res Hepatol Gastroenterol 37:647–650. doi: 10.1016/j.clinre.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 23.De Francesco V, Hassan C, Ridola L, Giorgio F, Ierardi E, Zullo A. 2014. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol 63:748–752. doi: 10.1099/jmm.0.072322-0. [DOI] [PubMed] [Google Scholar]
- 24.Oh DH, Lee DH, Kang KK, Park YS, Shin CM, Kim N, Yoon H, Hwang JH, Jeoung SH, Kim JW, Jang ES, Jung HC. 2014. The efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol 29:1171–1176. doi: 10.1111/jgh.12518. [DOI] [PubMed] [Google Scholar]
- 25.Hsu PI, Lin PC, Graham DY. 2015. Hybrid therapy for Helicobacter pylori infection: a systemic review and meta-analysis. World J Gastroenterol 21:12954–12962. doi: 10.3748/wjg.v21.i45.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina-Infante J, Romano M, Fernandez-Bermejo M, Federico A, Gravina AG, Pozzati L, Garcia-Abadia E, Vinagre-Rodriguez G, Martinez-Alcala C, Hernandez-Alonso M, Miranda A, Iovene MR, Pazos-Pacheco C, Gisbert JP. 2013. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 145:121–128. doi: 10.1053/j.gastro.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Hsu PI, Lai KH, Lin CK, Lo GH, Tseng HH, Tsai CC, Cheng JS, Huang RL, Huang JS, Tseng WY, Yu CL, Chien EJ. 1998. One week quadruple therapy in the management of Helicobacter pylori infection in Chinese. J Intern Med Taiwan 9:82–88. [Google Scholar]
- 28.Graham DY, Lee SY. 2015. How to effectively use bismuth quadruple therapy: the good, the bad, and the ugly. Gastroenterol Clin North Am 44:537–563. doi: 10.1016/j.gtc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European Helicobacter and Microbiota Study Group and Consensus Panel. 2017. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 30.Vilaichone RK, Prapitpaiboon H, Gamnarai P, Namtanee J, Wongcha-um A, Chaithongrat S, Mahachai V. 2015. Seven-day bismuth-based quadruple therapy as an initial treatment for Helicobacter pylori infection in a high metronidazole resistant Area. Asian Pac J Cancer Prev 16:6089–6092. doi: 10.7314/APJCP.2015.16.14.6089. [DOI] [PubMed] [Google Scholar]
- 31.Kao CY, Lee AY, Huang AH, Song PY, Yang YJ, Sheu SM, Chang WL, Sheu BS, Wu JJ. 2014. Heteroresistance of Helicobacter pylori from the same patient prior to antibiotic treatment. Infect Genet Evol 23:196–202. doi: 10.1016/j.meegid.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Hsu PI, Lai KH, Lin CK, Chen WC, Yu HC, Cheng JS, Tsay FW, Wu CJ, Lo CC, Tseng HH, Yamaoka Y, Chen JL, Lo GH. 2005. A prospective randomized trial of esomeprazole- versus pantoprazole-based triple therapy for Helicobacter pylori eradication. Am J Gastroenterol 100:2387–2392. doi: 10.1111/j.1572-0241.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 33.Hsu PI, Wu DC, Wu JY, Graham DY. 2011. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter 16:146–152. doi: 10.1111/j.1523-5378.2011.00829.x. [DOI] [PubMed] [Google Scholar]
- 34.Hsu PI, Lai KH, Liu CP. 2011. Esomeprazole with clopidogrel reduces peptic ulcer recurrence, compared with clopidogrel alone, in patients with atherosclerosis. Gastroenterology 140:791–798. doi: 10.1053/j.gastro.2010.11.056. [DOI] [PubMed] [Google Scholar]
- 35.Wu DC, Kuo CH, Tsay FW, Hsu WH, Chen A, Hsu PI. 2016. A pilot randomized controlled study of dexlansoprazole MR-based triple therapy for Helicobacter pylori infection. Medicine (Baltimore) 95:e2698. doi: 10.1097/MD.0000000000002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu JY, Hsu PI, Wu DC, Graham DY, Wang WM. 2014. Feasibility of shortening 14-day hybrid therapy while maintaining an excellent Helicobacter pylori eradication rate. Helicobacter 19:207–213. doi: 10.1111/hel.12113. [DOI] [PubMed] [Google Scholar]