ABSTRACT
Colistin has been administered via nebulization for the treatment of respiratory tract infections. Recently, dry powder inhalation (DPI) has attracted increasing attention. The current study aimed to investigate the pharmacokinetics (PK) of colistin in epithelial lining fluid (ELF) and plasma following DPI and intravenous (i.v.) administration in healthy Sprague-Dawley rats. Rats were given colistin as DPI intratracheally (0.66 and 1.32 mg base/kg of body weight) or i.v. injection (0.66 mg base/kg). Histopathological examination of lung tissue was performed at 24 h. Colistin concentrations in both ELF and plasma were quantified, and a population PK model was developed and compared to a previously published PK model of nebulized colistin in rats. A two-compartment structural model was developed to describe the PK of colistin in both ELF and plasma following pulmonary or i.v. administration. The model-estimated clearance from the central plasma compartment was 0.271 liter/h/kg (standard error [SE] = 2.51%). The transfer of colistin from the ELF compartment to the plasma compartment was best described by a first-order rate constant (clearance of colistin from the ELF compartment to the plasma compartment = 4.03 × 10−4 liter/h/kg, SE = 15%). DPI appeared to have a higher rate of absorption (time to the maximum concentration in plasma after administration of colistin by DPI, ≤10 min) than nebulization (time to the maximum concentration in plasma after administration of colistin by nebulization, 20 to 30 min), but the systemic bioavailabilities by the two routes of administration were similar (∼46.5%, SE = 8.43%). Histopathological examination revealed no significant differences in inflammation in lung tissues between the two treatments. Our findings suggest that colistin DPI is a promising alternative to nebulization considering the similar PK and safety profiles of the two forms of administration. The PK and histopathological information obtained is critical for the development of optimal aerosolized colistin regimens with activity against lung infections caused by Gram-negative bacteria.
KEYWORDS: polymyxin, colistin, dry powder, pulmonary delivery, disposition
INTRODUCTION
Respiratory tract infections caused by multidrug-resistant (MDR) Gram-negative bacteria are a major public health burden globally (1, 2). With their increasing prevalence and a scarcity of effective antibiotics, respiratory tract infections caused by these superbugs are alarmingly dangerous, often resulting in high rates of morbidity and mortality (3). Colistin (i.e., polymyxin E), a polypeptide antibiotic, has been increasingly used as a last resort for the treatment of respiratory tract infections caused by MDR Gram-negative pathogens (4, 5). Traditional parenteral administration of colistin may not provide optimal efficacy for the treatment of respiratory tract infections, as recent animal studies demonstrated the very limited exposure of colistin in the epithelial lining fluid (ELF) (6–10). A recent pharmacokinetic/pharmacodynamic (PK/PD) study of parenteral colistin against Pseudomonas aeruginosa in mouse thigh and lung infection models was conducted; the ratio of the area under the unbound (free) plasma concentration-time curve over the MIC (fAUCplasma/MIC) required to achieve bacteriostasis in the lungs was approximately 2.5- to 5-fold higher than that required to achieve bacteriostasis in the thighs (11). Increasing the dose of parenteral colistin is not feasible due to the dose-limiting nephrotoxicity of colistin (12, 13), which highlights the need for alternative routes of colistin administration to treat respiratory tract infections.
Pulmonary administration of colistin can achieve a high drug exposure in the lungs while minimizing systemic exposure and nephrotoxicity. The advantage of the pulmonary delivery of colistin has been illustrated in several preclinical (6–10, 14) and clinical (15, 16) PK studies. Yapa et al. reported in a clinical study that pulmonary nebulization of colistin methanesulfonate (CMS) (2 million and 4 million international units once daily, equivalent to 60 and 120 mg colistin base activity [CBA], respectively) resulted in higher exposure of CMS and formed colistin in sputum (maximum sputum concentration [Cmax, sputum] = 2 to 21 mg/liter) than that achieved after intravenous (i.v.) administration (Cmax, sputum = 0.12 to 0.72 mg/liter) (15). In a PK/PD study of colistin against P. aeruginosa ATCC 27853 in the mouse lungs, the required plasma fAUCplasma/MIC following pulmonary administration (2.99) was 11-fold lower than that following subcutaneous administration (34.1) (11, 17). Nebulized antimicrobials have improved the life expectancy of patients with cystic fibrosis (CF) (18–20); however, they require expensive and complicated delivery devices and prolonged administration times (21–27), and the delivery efficiency is low (<15% of the administered dose reaches the lungs following jet nebulization) (28). Consequently, patient compliance with nebulized antibiotics is low (29, 30). Dry powder inhalation (DPI) provides a suitable alternative, as powder inhaler devices are portable, easy to use, and, most importantly, able to deliver drug efficiently (31–33) and improve efficacy and patient compliance (34, 35).
To date, the majority of in vivo studies of aerosolized CMS and colistin have focused on nebulization (6–10, 14, 15, 36–39), whereas only a few investigated DPI (23–26). The safety and efficacy of jet-milled CMS/colistin powder have been evaluated in healthy volunteers and patients with CF (23–26). However, these DPI formulations have a relatively low aerosolization efficiency (40% in the fine-particle fraction [FPF]) (23). In our earlier study, inhalable dry powder formulations of colistin with a high aerosolization efficiency were developed by spray-drying (total FPF, >83% via an Aerolizer device) (22). These highly dispersible colistin DPI formulations provide higher levels of drug deposition in the lungs and thus have a greater potential for the effective treatment of respiratory infections caused by MDR Gram-negative bacteria (40, 41). Although a CMS DPI product (Colobreathe) has been approved in Europe, the dosage regimens of DPI have not been appropriately optimized on the basis of PK/PD studies (32). The present study aimed to investigate the PK of our highly efficient colistin DPI formulation in healthy rats after pulmonary administration.
RESULTS
Following DPI, colistin was rapidly absorbed into the systemic circulation, with the maximum plasma concentration (Cmax, plasma) being detected within 10 min after administration, followed by a biexponential decline over the 12-h sampling period (Fig. 1 and Table 1). High levels of colistin exposure in the ELF were achieved following DPI (maximum ELF concentration [Cmax, ELF] = 400.1 ± 44.9 and 607.0 ± 58.9 mg/liter for doses of 0.66 and 1.32 mg colistin base/kg of body weight, respectively; Table 1) and was maintained for at least 12 h (Fig. 1). The concentrations of urea measured in the bronchoalveolar lavage fluid (BALF) and plasma were 1.01 ± 0.43 mg/dl and 48 ± 10 mg/dl, respectively.
FIG 1.
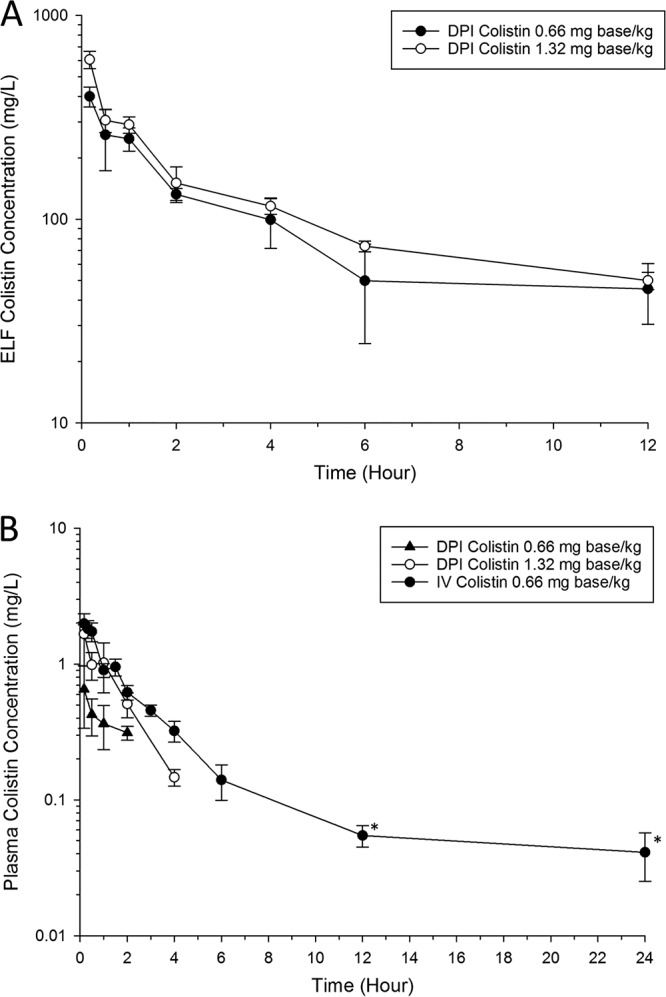
(A) Colistin ELF concentration-time profiles following DPI of 0.66 mg base/kg and 1.32 mg base/kg colistin. The colistin ELF concentration following i.v. bolus administration was below the LOQ. (B) Total plasma colistin concentration-time profiles following DPI and i.v. administration of colistin in healthy Sprague-Dawley rats. Each symbol represents the mean ± standard deviation (SD; n = 3 or more). *, the concentration is below the LOQ (0.10 mg/liter).
TABLE 1.
PK parameters for colistin in healthy rats following DPI administrationa
| Dose | Cmax, ELF (mg/liter) | Cmax, plasma (mg/liter) | Tmax (min) |
|---|---|---|---|
| Single pulmonary dose of: | |||
| 0.66 mg base/kg | 400.1 ± 44.9 | 0.65 ± 0.32 | 10 |
| 1.32 mg base/kg | 607.0 ± 58.9 | 1.66 ± 0.69 | 10 |
| Single i.v. dose of 0.66 mg base/kg | <LOQ | NA | NA |
Data are presented as means ± standard deviations. NA, not applicable.
The disposition of colistin in the ELF and plasma following DPI and i.v. administration was best described by two compartments (Fig. 2 to 4 ; see also Fig. S1 in the supplemental material). The PK parameters generated from the population PK model are listed in Table 2. The estimated apparent volumes of distribution in the ELF (VELF) in ELF compartments 1 and 2 (VE1 and VE2, respectively; manifested as ELF1 and ELF2 in Fig. 4, respectively) were 2.90 × 10−5 liter/kg (standard error [SE] = 35.7%) and 9.67 × 10−4 liter/kg (SE = 18.8%), respectively. The estimated total systemic body clearance (CLtotal) from the central plasma compartment for colistin was 0.271 liter/h/kg (SE = 2.51%). A first-order process was sufficient to describe the transfer of colistin from ELF to the central plasma compartment. The estimated systemic bioavailability of DPI (FDPI) was 46.5% (SE = 8.43%) (Table 2).
FIG 2.
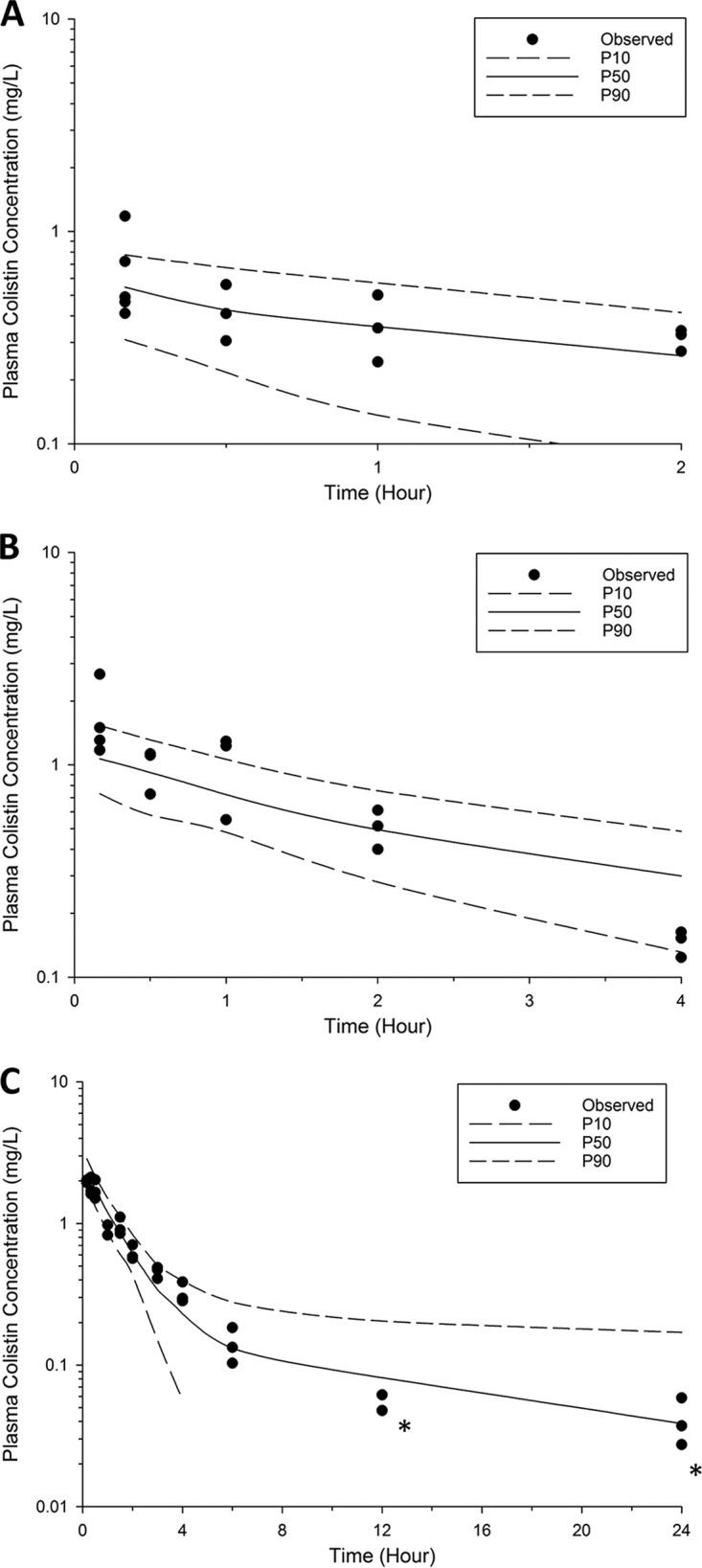
Visual predictive checks for colistin in plasma following DPI of 0.66 mg base/kg (A) and 1.32 mg base/kg (B) and i.v. administration of 0.66 mg base/kg (C). Solid line, the median model-predicted concentrations (P50); broken lines, model-predicted 10th percentile (P10) and 90th percentile (P90) concentrations; solid dots, observed concentrations. After 4 h, the 10th percentile concentration approaches zero. *, the concentration is below the LOQ (0.10 mg/liter).
FIG 3.
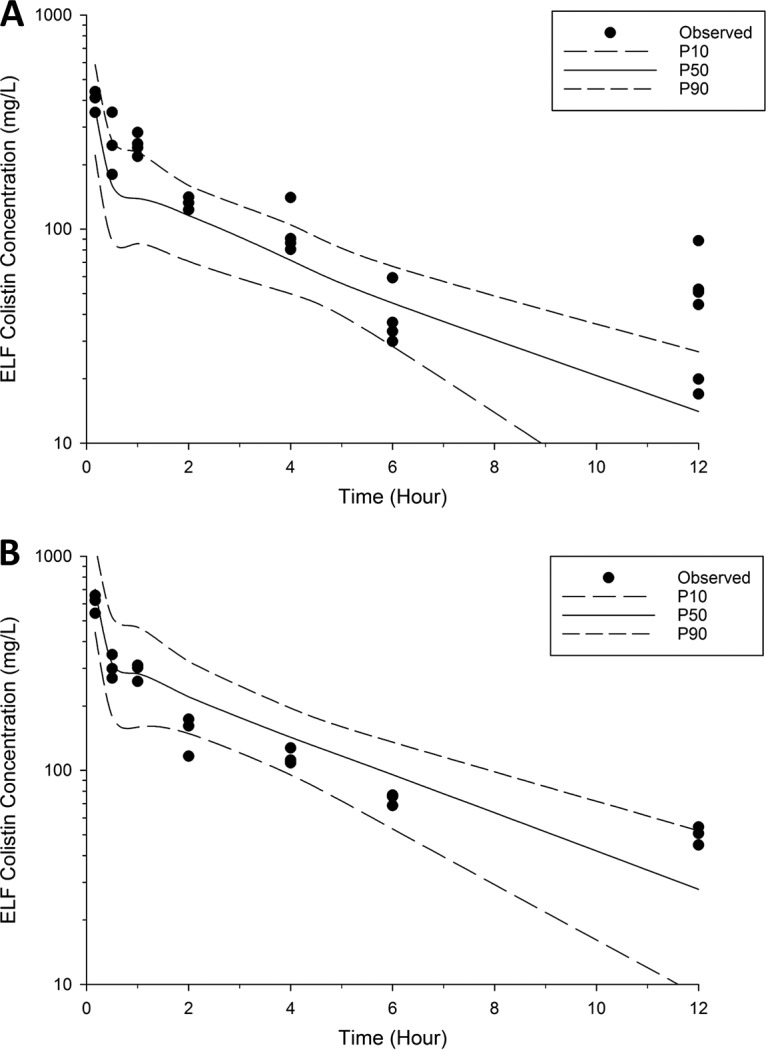
Visual predictive checks for colistin in ELF following DPI of 0.66 mg base/kg (A) and 1.32 mg base/kg colistin (B). Solid line, the median model-predicted concentrations (P50); broken lines, the model-predicted 10th percentile (P10), and 90th percentile (P90) concentrations; solid dots, observed concentrations.
FIG 4.
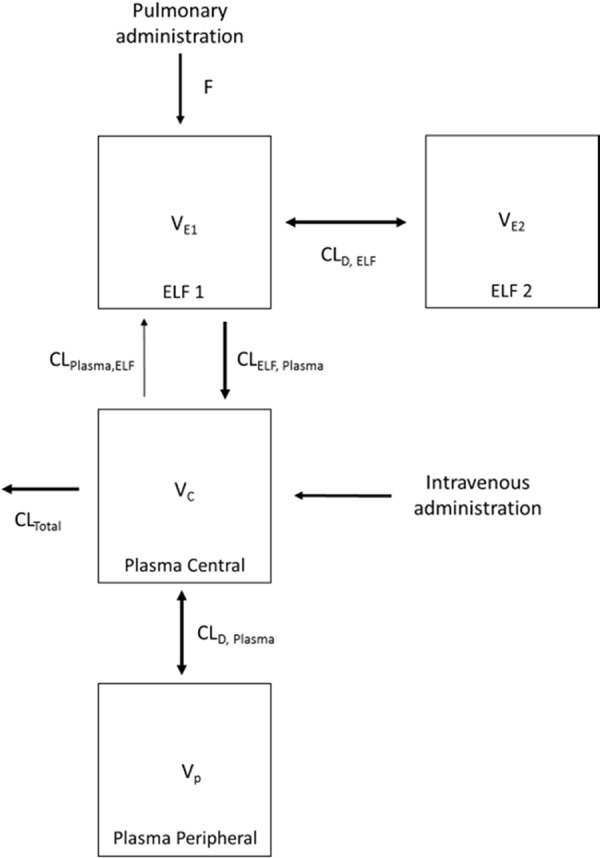
A population PK structural model describing the disposition of colistin in ELF and plasma following DPI or i.v. administration of colistin. The parameters are presented in Table 2.
TABLE 2.
Estimated values of population PK parameters for colistin in healthy rats following DPI or i.v. administration
| Parameter | Description | Unit | Estimated value | SE (%) |
|---|---|---|---|---|
| VE1 | Vol of distribution in ELF1 (ELF compartment) | liter/kg | 2.90 × 10−5 | 35.7 |
| VE2 | Vol of distribution in ELF2 (peripheral lung compartment) | liter/kg | 9.67 × 10−4 | 18.8 |
| Vc | Volume of distribution in central plasma compartment | liter/kg | 0.572 | 17.9 |
| Vp | Volume of distribution in peripheral plasma compartment | liter/kg | 1.47 | 35.3 |
| CLD, ELF | ELF intercompartmental clearance | liter/h/kg | 4.26 × 10−4 | 37.8 |
| CLD, plasma | Plasma intercompartmental clearance | liter/h/kg | 0.172 | 24.3 |
| CLtotal | Total systemic body clearance | liter/h/kg | 0.271 | 2.51 |
| CLELF, plasma | Intercompartmental clearance from ELF to plasma | liter/h/kg | 4.03 × 10−4 | 15.0 |
| CLplasma, ELF | Intercompartmental clearance from plasma to ELF | liter/h/kg | 9.93 × 10−7 | 46.0 |
| fu | Unbound fraction | 0.44 (fixed) | NAa | |
| FDPI | Bioavailability | % | 46.5 | 8.43 |
NA, not applicable.
Histopathological examination of the lung tissue at 24 h following DPI and wet nebulization of colistin showed mean pathological scores of about 1.0 to <2.0 (indicating mild damage), whereas the saline-treated group had a mean pathological score of 0.3 (indicating no damage; Table 3). Furthermore, no significant difference in the pathological scores was observed among the lung tissue samples obtained at 24 h from the nebulization (1.32 mg base/kg) and DPI (0.66 mg base/kg) groups (Table 3). Even with the highest dose (1.32 mg base/kg), DPI and wet nebulization resulted in mild damage in the lung tissue with similar pathological scores (1.9 and 1.5, respectively) (Table 3).
TABLE 3.
Histopathological examination of the lungs of healthy rats following DPI of colistin
| Dose (mg base/kg) | Formulation | Time (h) | No. of rats | Mean pathological score |
|---|---|---|---|---|
| Saline | Solution | 0.5 | 1.0 | |
| Saline | Solution | 24 | 0.3 | |
| 0.66 | Dry powder | 0.5 | 4 | 1.5 |
| 0.66 | Dry powder | 24 | 4 | 1.0 |
| 1.32 | Dry powder | 0.5 | 4 | 1.8 |
| 1.32 | Dry powder | 2 | 4 | 1.2 |
| 1.32 | Dry powder | 6 | 4 | 0.8 |
| 1.32 | Dry powder | 24 | 4 | 1.9 |
| 1.32 | Solution | 24 | 4 | 1.5 |
DISCUSSION
Aerosolized polymyxin therapy administered via nebulization has become a common complementary practice for the treatment of respiratory tract infections, such as those in CF patients (42, 43) and in critically ill patients with ventilator-associated pneumonia (44, 45). With recent advancements in particle engineering technology, we have successfully formulated a colistin dry powder with a markedly higher aerosolization efficiency (total FPF, >83% via an Aerolizer device) than those colistin dry powder formulations in the previous studies (22, 25, 26). DPI offers a potentially superior alternative inhalational delivery method that provides a better drug deposition in the lungs than nebulization and that results in a higher rate of patient compliance than nebulization (23–26, 33). This is the first preclinical study to investigate the PK of the spray-dried colistin powder aerosol in healthy rats.
In the present study, a population PK model was constructed to better understand the disposition of colistin in ELF and plasma following pulmonary and i.v. administration. Data were distributed around the line of identity (see Fig. S1 in the supplemental material), and the majority of the observed concentrations were scattered within the 10th and 90th percentiles (Fig. 2 and 3). Therefore, our population PK model well described colistin exposure in ELF (overall R2 = 0.87) and plasma (overall R2 = 0.91). The model revealed that the plasma concentrations of colistin rapidly decreased following i.v. and pulmonary administration (Fig. 1). Following administration of colistin, the model-predicted total systemic body clearance (CLtotal = 0.271 liter/h/kg, SE = 2.51%; Table 2) and volume of distribution of the central plasma compartment (Vc = 0.572 liter/kg, SE = 17.9%; Table 2) were comparable to those reported in the literature (Vc = 0.22 to 0.43 liter/kg and CLtotal = 0.22 to 0.43 liter/h/kg) (7, 10, 36, 46).
In the current study, the PK linearity observed with colistin following DPI administration in healthy Sprague-Dawley rats is consistent with the findings of Yapa et al. following wet nebulization in rats (7). In contrast, PK nonlinearity was reported by Gontijo et al. following wet nebulization of colistin in rats (10). A more complex PK model with Michaelis-Menten (nonlinear) kinetics was proposed by Gontijo et al. to describe the transfer of colistin across the lung alveolar epithelium after nebulization (10). However, we did not find a significant improvement in model fit upon incorporation of such a process, with a first-order rate constant being sufficient to describe our data. This is in agreement with the PK models developed for rats (6), sheep (14), baboon monkeys (9), and critically ill patients (16) after the nebulization of colistin. The mechanism of colistin transport across the lung epithelium remains unclear and is being investigated in our laboratory.
The systemic absorption of colistin was faster in rats when it was administered as DPI, with the time to reach the maximum plasma concentration (Tmax) being 20 to 30 min for the nebulization and <10 min for DPI (7). The slightly faster absorption rate following DPI is very likely due to the higher aerosolization efficiency of spray-dried colistin powder and the differential distribution of PEPT2 in different regions of the respiratory tract (47, 48). As opposed to wet nebulization using a MicroSprayer device, the large fine-particle fraction of the colistin dry powder upon pulmonary administration is able to reach deeper airways, where PEPT2 is abundantly expressed (47–49). Such a faster absorption rate with DPI than with nebulization was also observed in our earlier study, where the Kv1.3-blocking peptide HsTX1[R14A] was administered to healthy rats using the same Penn-Century device used in the present study (50). Despite the more rapid absorption of the powder formulation, the systemic bioavailability of colistin solution (Fsolution = 31 to 69%) (7, 10) and the spray-dried powder formulation (FDPI = 46.5%) (Table 2) was similar in healthy Sprague-Dawley rats. The translation of PK data from rats to humans after pulmonary administration has remained challenging, as the airway anatomy of rats differs from that of humans (51) and the Penn-Century device dispersed powder differently from the clinical dry powder inhaler (52). With the Penn-Century device, the colistin dry powder is dispersed passively into the lungs, which may not be comparable to the dispersal achieved with clinically used dry powder inhalers (i.e., Twincer), with which patients are required to actively inhale CMS/colistin dry powder (25–27). Importantly, the human PK of CMS administered via clinical dry powder inhalers are likely influenced by interindividual and interdevice variabilities (27). Clinical studies are thus needed to characterize the extent of potential patient- and device-specific differences in the PK of DPI of polymyxins.
Consistent with the observation following the nebulization of colistin, DPI treatment resulted in the prolonged and extensive exposure of colistin in the ELF (Fig. 1) (6, 7, 10, 36). The colistin exposure in the ELF achieved after DPI (0.35 mg base/kg colistin), reflected by an area under the concentration-time curve (AUC) from 0 to 4 postdosing for ELF (AUC0–4 h,ELF; ∼328 mg · h/liter, calculated using PK simulation), was comparable to that following wet nebulization of 0.35 mg base/kg colistin in healthy rats (AUC0–4 h,ELF = ∼369 mg · h/liter) (10). It is important to appreciate that ELF exposure can be influenced by VELF, which was variable in rat studies (7.6 ± 5.8 to 110 ± 23 μl) due to the different techniques used for the sampling of BALF and the urea quantification method utilized (6, 7, 10, 36).
A key finding of our current study is that colistin concentrations in ELF were much higher following DPI treatment (Cmax, ELF = 400.1 ± 44.9 and 607.0 ± 58.9 mg/liter for colistin at 0.66 and 1.32 mg base/kg, respectively) than following i.v. administration, with the concentrations dramatically exceeding the MIC90 (≤1 mg/liter) against P. aeruginosa for at least 12 h (Fig. 2) (53). Colistin ELF concentrations following i.v. administration were below the limit of quantitation (LOQ) of ELF (4.84 mg/liter, after considering the dilution factor for the interconversion of BALF and ELF concentrations). The population PK model developed in our study facilitated the prediction of colistin concentrations in ELF following i.v. administration. Our results show that the simulated colistin concentration in ELF after i.v. administration was well below the ELF LOQ (<4.84 mg/liter) at any time point. This was consistent with the observation in patients with CF, in which a low concentration of formed colistin (<1 mg/liter) was observed following i.v. administration of CMS (150 mg CBA) (15). Another important finding in our population PK model is that the model-estimated VE2 was larger than the model-estimated VE1 (Table 2). This potentially infers the binding of colistin to pulmonary substances, such as mucin (54) and pulmonary surfactant (55), or a significant distribution of colistin into lung epithelial cells in rats. This finding is consistent with the literature that colistin accumulates in the lung tissue (56) and binds to lung epithelium via an electrostatic interaction (57).
Collectively, the population PK model developed in the current study suggests that the spray-dried colistin DPI formulation has PK properties (e.g., ELF exposure and systemic bioavailability) comparable to those of the wet nebulized formulation. The majority of CF patients usually require long-term nebulized colistin therapy (as CMS) (58). Although nebulized colistin therapy has significantly improved patients' life expectancy, the complex nebulization system may have a negative impact on the quality of life and compliance in patients (27). On the basis of the findings of previous clinical studies (59, 60), the inhalation of colistin via dry powder inhalers is an attractive and effective alternative for the treatment of respiratory infections owing to the improved convenience, reduced duration of administration, and positive effects on quality of life, compliance, and, possibly, therapeutic outcomes in patients (61).
Aerosolized colistin in both solution and dry powder forms may cause pulmonary adverse effects, such as a cough or throat irritation (32). The mechanisms underlying such effects are unclear but may be related to mast cell degranulation (62, 63). The safety of pulmonary administration of colistin solution was previously examined, and no significant inflammation or damage to the lungs was observed after a single dose of CMS or colistin in rats (the doses were not provided) (6, 7). In the present study, the safety of colistin DPI at 0.66 and 1.32 mg base/kg was evaluated by histopathological examination and compared to the findings obtained following a similar dose of colistin solution (Table 3). The pulmonary administration of neither colistin solution nor DPI resulted in severe inflammation or damage to the lung epithelium (scores, 1.0 to <2.0, showing mild to moderate lesions). Our results indicate that DPI of colistin is as safe as nebulization in healthy rats. PK/PD/toxicodynamic optimization of aerosolized polymyxins is needed in patients to minimize any potential side effects (i.e., bronchospasm and cough).
To the best of our knowledge, the PK model described here is the first detailed preclinical PK model describing the disposition of colistin in ELF and plasma following DPI in rats. Our study has shown that spray-dried colistin DPI achieves drug exposure in ELF dramatically higher than that achieved by i.v. administration. Furthermore, histopathological examination of lung tissue demonstrates that DPI has safety comparable to that of wet nebulization. DPI is a promising alternative to nebulization, especially considering the convenience of the dry powder inhaler and its positive impacts on patient compliance. With the increasing incidence of life-threatening lung infections caused by MDR Gram-negative bacteria, the PK and safety information obtained here is critical to the optimization of DPI of polymyxin therapy in patients.
MATERIALS AND METHODS
Chemicals.
Colistin sulfate (lot number 08M1526V; ≥15,000 U/mg) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All organic solvents were of analytical grade unless stated otherwise.
Colistin dry powder.
Colistin dry powder was prepared by spray-drying as previously described (22). Briefly, colistin sulfate was dissolved in water and spray-drying was performed using a B-290 mini-spray dryer (Buchi Laboratories, Flawil, Switzerland). The spray-drying conditions were as follows: inlet temperature, 80°C; atomizer setting, 700 liter/h; aspirator, 40 m3/h; and feed rate, 2 ml/min. The spray-dried samples were stored in a desiccator at room temperature.
Animals.
All rat experiments (see Table S1 in the supplemental material) were approved by the Animal Ethics Committee of the Monash Institute of Pharmaceutical Sciences, Monash University, and conducted in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. Male Sprague-Dawley rats (weight, 300 to 350 g; Monash Animal Research Platform, Victoria, Australia) were housed with a 12-h light/12-h dark cycle at ambient temperature (21 ± 3°C) and a relative humidity of 40 to 70%. Food and water were available ad libitum.
Pharmacokinetic studies.
Prior to the PK studies, the right carotid artery and right jugular vein of rats were cannulated for blood collection and i.v. drug administration, respectively (6, 7). Following surgery, the rats were individually housed in metabolic cages and allowed to recover overnight.
Dosing of colistin.
A single-dose PK study was performed in healthy rats following administration of an i.v. bolus of colistin at 0.66 mg base/kg via the right jugular vein (n = 3). Blood samples were collected at 10, 20, and 30 min and 1, 1.5, 2, 3, 4, 6, 12, and 24 h postadministration. Extra rats (n = 3 per time point) received an i.v. bolus of colistin at 0.66 mg base/kg for BALF collection (6, 7) at 10 min and 2, 6, and 12 h postadministration.
DPI of colistin.
Two groups of rats were utilized to characterize the pulmonary and systemic PK of colistin following DPI. In group 1, rats were given a colistin powder formulation (0.66 and 1.32 mg base/kg) by pulmonary administration. Briefly, rats were anesthetized using gaseous isoflurane (Abbott Animal Health, Abbott Park, IL). The anesthetized rats were placed on a Perspex support in a vertical upright position. The pulmonary administration was performed using a dry powder insufflator (DP-4M; Penn-Century, Glenside, PA) (52). The chamber of the dry powder insufflator was loaded with colistin dry powder, and 6 puffs of 2 ml air were applied via an air pump (AP-1; Penn-Century, Glenside, PA) to disperse the powder. The total drug administration time was approximately 20 to 30 s per animal. The dry powder insufflator was weighed before and after administration to determine the amount of colistin delivered. On average, more than 90% of the colistin powder was dispersed and emitted from the device. The insufflator was cleaned with 10 puffs of 3 ml air before and after each experiment to remove excess powder in the chamber and cannula. Blood samples were collected via the right carotid artery at 10 and 30 min and 1, 2, and 4 h after pulmonary administration. In group 2, rats were given colistin dry powder (0.66 and 1.32 mg base/kg) by pulmonary administration, and BALF was collected at 10 and 30 min and 1, 2, 4, 6, and 12 h (6, 7). In group 1, at least 3 animals were included per dosage regimen, while in group 2, at least 3 animals were employed per time point.
The maximum dosage regimens for i.v. and pulmonary administration of the colistin dry powder aerosol were chosen on the basis of its tolerability in rats (6). The lowest dose selected was based upon the LOQ of the method used for the analysis of colistin in plasma.
Measurement of colistin concentrations in plasma, BALF, and ELF.
Colistin concentrations in plasma and BALF samples were determined by a validated liquid chromatography-mass spectrometry (LC-MS) method (64), with minor modifications. Briefly, BALF and plasma samples were deproteinized with 0.1% formic acid in acetonitrile (1:2 dilution) and centrifuged at 18,210 × g. For each analytical run, peak area ratios of the analyte (the sum of the peak areas for colistin A and colistin B) and the internal standard (the sum of the peak areas for polymyxin B1 and polymyxin B2) were plotted against the nominal concentrations of the calibration standards. Linear least-squares regression analysis without weighting was performed to determine the linearity, intercept, and slope. Calibration curves were prepared with blank BALF or plasma, and the concentrations ranged from 0.10 to 10.0 mg/liter. The LOQ was 0.10 mg/liter for both plasma and BALF samples. The inter- and intraday accuracy and precision of the assay for plasma and BALF samples are provided in Table S2. The VELF was determined using urea as an endogenous dilution marker (65). The urea concentrations in BALF and plasma were determined using a QuantiChrom urea assay kit (Bioassay Systems, CA, USA). The standard curve was prepared with urea in Milli-Q water, and the linear detection range was from 0.08 mg/dl (13 μM) to 100 mg/dl (17 mM) urea. The accuracy and precision of the QuantiChrom urea assay kit for the measurement of the concentration of urea in biological samples were examined previously (7). Quality control samples were prepared at 4 levels, and the accuracy and precision of the kit at the 4 levels (1, 10, 25, and 50 mg/dl) were within 10%.
VELF was calculated as follows: VELF = ([urea]BALF/[urea]plasma) · VBALF, where [urea]BALF and [urea]plasma are the urea concentrations (in milligrams per deciliter) in BALF and plasma, respectively, and VBALF is the volume of BALF recovered.
The colistin concentration in ELF was calculated as follows: [colistin]ELF = [colistin]BALF · ([urea]plasma/[urea]BALF), where [colistin]ELF and [colistin]BALF are the colistin concentrations (in milligrams per liter) in ELF and BALF, respectively.
Population pharmacokinetic model.
A nonlinear mixed-effects model was employed to characterize the disposition of colistin in ELF and plasma following DPI in health rats. During PK analysis, several structural models were tested and evaluated using the Monte Carlo parametric expectation maximization algorithm (importance sampling, pmethod = 4) in the S-ADAPT program (version 1.57), facilitated by the S-ADAPT TRAN set of programs (66). In the final composite model, two compartments were required to describe the kinetics of colistin in both ELF and plasma following pulmonary and i.v. administration. The residual error model was estimated using heteroscedastic error (additive and proportional) structures. The Beal M3 method was employed to handle concentrations below the LOQ (67). Graphical analysis of goodness-of-fit plots (e.g., observed concentration versus population predicted concentration) and objective function values (reported as −1 × log likelihood in S-ADAPT) was employed to evaluate the suitability of the model (68–70). A decrease in the objective function value of 1.92 units (chi-square test with 1 degree of freedom) was considered significant. The final model was evaluated using the visual predictive checks (71).
Histopathological examination of the lungs following treatment with aerosolized colistin.
Histopathological examination of rat lung tissue was performed to compare the effect of colistin on pulmonary epithelial cells after the pulmonary administration of colistin by DPI and by nebulization. Treatment groups received either 0.66 or 1.32 mg base/kg colistin dry powder or 1.32 mg base/kg colistin solution, and the control group received 50 μl saline. Rats were humanely killed at 0.5 and 24 h (0.66 mg base/kg) or at 0.5, 2, 6, and 24 h (1.32 mg base/kg) following dosing. To avoid any artificial damage, bronchoalveolar lavage and cardiac puncture were not performed prior to tissue harvesting. To quantify the extent of lung damage, a pathological grading system developed by the Australian Phenomics Network was adapted (Australian Phenomics Network Histopathology and Organ Pathology Service, Victoria, Australia). Briefly, the severity and the nature of the histopathological changes in lung epithelial cells were graded as follows: score of 0, no changes or mild changes considered insignificant; score of 1, mild damage; score of >2, mild to moderate damage; and score of >3, moderate to severe damage. Scoring and grading of the lungs were performed blind by histopathologists at the Australian Phenomics Network Histopathology and Organ Pathology Service.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the financial support from a National Health and Medical Research Council (NHMRC) project grant (APP1065046). J.L. and A.F. are supported by a research grant from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (R01 AI111965). Y.-W.L. is the recipient of an Australian postgraduate award. J.L. is an Australian NHMRC senior research fellow. This study utilized the Australian Phenomics Network Histopathology and Organ Pathology Service at the University of Melbourne.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We thank Tien Nguyen for her assistance.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00973-17.
REFERENCES
- 1.Arias CA, Murray BE. 2009. Antibiotic-resistant bugs in the 21st century—a clinical super-challenge N Engl J Med 360:439–443. doi: 10.1056/NEJMp0804651. [DOI] [PubMed] [Google Scholar]
- 2.Döring G, Conway S, Heijerman H, Hodson M, Høiby N, Smyth A, Touw D. 2000. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. Eur Respir J 16:749–767. doi: 10.1034/j.1399-3003.2000.16d30.x. [DOI] [PubMed] [Google Scholar]
- 3.Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, Bartlett JG. 2006. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- 4.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, Mouton JW, Paterson DL, Tam VH, Theuretzbacher U. 2015. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. 2005. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents 25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Yapa SW, Li J, Porter CJ, Nation RL, Patel K, McIntosh MP. 2013. Population pharmacokinetics of colistin methanesulfonate in rats: achieving sustained lung concentrations of colistin for targeting respiratory infections. Antimicrob Agents Chemother 57:5087–5095. doi: 10.1128/AAC.01127-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yapa SW. 2013. PhD thesis. Monash University, Melbourne, Victoria, Australia. [Google Scholar]
- 8.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin M-H, Le Naour G, Marquette C-H. 2010. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med 36:1147–1155. doi: 10.1007/s00134-010-1879-4. [DOI] [PubMed] [Google Scholar]
- 9.Marchand S, Bouchene S, de Monte M, Guilleminault L, Montharu J, Cabrera M, Grégoire N, Gobin P, Diot P, Couet W. 2015. Pharmacokinetics of colistin methansulphonate (CMS) and colistin after CMS nebulisation in baboon monkeys. Pharm Res 32:3403–3414. doi: 10.1007/s11095-015-1716-0. [DOI] [PubMed] [Google Scholar]
- 10.Gontijo AVL, Grégoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 2. Colistin. Antimicrob Agents Chemother 58:3950–3956. doi: 10.1128/AAC.02819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheah S-E, Wang J, Turnidge JD, Li J, Nation RL. 2015. New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 70:3291–3297. doi: 10.1093/jac/dkv267. [DOI] [PubMed] [Google Scholar]
- 12.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. 2009. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis 48:1724–1728. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- 14.Landersdorfer CB, Nguyen T-H, Lieu LT, Nguyen G, Bischof RJ, Meeusen EN, Li J, Nation RL, McIntosh MP. 2017. Substantial targeting advantage achieved by pulmonary administration of colistin methanesulfonate in a large-animal model. Antimicrob Agents Chemother 61:e01934-16. doi: 10.1128/AAC.01934-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob Agents Chemother 58:2570–2579. doi: 10.1128/AAC.01705-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boisson M, Jacobs M, Grégoire N, Gobin P, Marchand S, Couet W, Mimoz O. 2014. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother 58:7331–7339. doi: 10.1128/AAC.03510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin Y-W, Zhou QT, Cheah S-E, Zhao J, Chen K, Wang J, Chan H-K, Li J. 2017. Pharmacokinetics/pharmacodynamics of pulmonary delivery of colistin against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother 61:e02025-16. doi: 10.1128/AAC.02025-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Touw D, Brimicombe R, Hodson M, Heijerman H, Bakker W. 1995. Inhalation of antibiotics in cystic fibrosis. Eur Respir J 8:1594–1604. [PubMed] [Google Scholar]
- 19.Mukhopadhyay S, Singh M, Cater J, Ogston S, Franklin M, Olver R. 1996. Nebulised antipseudomonal antibiotic therapy in cystic fibrosis: a meta-analysis of benefits and risks. Thorax 51:364–368. doi: 10.1136/thx.51.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodson M, Gallagher C, Govan J. 2002. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. Eur Respir J 20:658–664. doi: 10.1183/09031936.02.00248102. [DOI] [PubMed] [Google Scholar]
- 21.Pilcer G, Amighi K. 2010. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm 392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Zhou QT, Morton DA, Yu HH, Jacob J, Wang J, Li J, Chan HK. 2013. Colistin powders with high aerosolisation efficiency for respiratory infection: preparation and in vitro evaluation. J Pharm Sci 102:3736–3747. doi: 10.1002/jps.23685. [DOI] [PubMed] [Google Scholar]
- 23.De Boer A, Le Brun P, Van der Woude H, Hagedoorn P, Heijerman H, Frijlink H. 2002. Dry powder inhalation of antibiotics in cystic fibrosis therapy, part 1: development of a powder formulation with colistin sulfate for a special test inhaler with an air classifier as de-agglomeration principle. Eur J Pharm Biopharm 54:17–24. doi: 10.1016/S0939-6411(02)00043-7. [DOI] [PubMed] [Google Scholar]
- 24.Le Brun P, De Boer A, Mannes G, de Fraîture DM, Brimicombe R, Touw D, Vinks A, Frijlink H, Heijerman H. 2002. Dry powder inhalation of antibiotics in cystic fibrosis therapy: part 2: inhalation of a novel colistin dry powder formulation: a feasibility study in healthy volunteers and patients. Eur J Pharm Biopharm 54:25–32. doi: 10.1016/S0939-6411(02)00044-9. [DOI] [PubMed] [Google Scholar]
- 25.Westerman EM, de Boer AH, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. 2007. Dry powder inhalation of colistin sulphomethate in healthy volunteers: a pilot study. Int J Pharm 335:41–45. doi: 10.1016/j.ijpharm.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Westerman EM, De Boer AH, Le Brun PP, Touw DJ, Roldaan AC, Frijlink HW, Heijerman HG. 2007. Dry powder inhalation of colistin in cystic fibrosis patients: a single dose pilot study. J Cyst Fibros 6:284–292. doi: 10.1016/j.jcf.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Westerman E, Heijerman H, Frijlink H. 2007. Dry powder inhalation versus wet nebulisation delivery of antibiotics in cystic fibrosis patients. Expert Opin Drug Deliv 4:91–94. doi: 10.1517/17425247.4.2.91. [DOI] [PubMed] [Google Scholar]
- 28.Reychler G, Keyeux A, Cremers C, Veriter C, Rodenstein DO, Liistro G. 2004. Comparison of lung deposition in two types of nebulization: intrapulmonary percussive ventilation vs jet nebulization. Chest J 125:502–508. doi: 10.1378/chest.125.2.502. [DOI] [PubMed] [Google Scholar]
- 29.Abbott J, Dodd M, Bilton D, Webb A. 1994. Treatment compliance in adults with cystic fibrosis. Thorax 49:115–120. doi: 10.1136/thx.49.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burrows JA, Bunting JP, Masel PJ, Bell SC. 2002. Nebulised dornase alpha: adherence in adults with cystic fibrosis. J Cyst Fibros 1:255–259. doi: 10.1016/S1569-1993(02)00095-4. [DOI] [PubMed] [Google Scholar]
- 31.de Boer AH, Hagedoorn P, Westerman EM, Le Brun PP, Heijerman HG, Frijlink HW. 2006. Design and in vitro performance testing of multiple air classifier technology in a new disposable inhaler concept (Twincer®) for high powder doses. Eur J Pharm Sci 28:171–178. doi: 10.1016/j.ejps.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Velkov T, Rahim NA, Zhou QT, Chan H-K, Li J. 2015. Inhaled anti-infective chemotherapy for respiratory tract infections: successes, challenges and the road ahead. Adv Drug Deliv Rev 85:65–82. doi: 10.1016/j.addr.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou QT, Tang P, Leung SSY, Chan JGY, Chan H-K. 2014. Emerging inhalation aerosol devices and strategies: Where are we headed? Adv Drug Deliv Rev 75:3–17. doi: 10.1016/j.addr.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 34.VanDevanter DR, Geller DE. 2011. Tobramycin administered by the TOBI® Podhaler® for persons with cystic fibrosis: a review. Med Devices (Auckl) 4:179–188. doi: 10.2147/MDER.S16360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuster A, Haliburn C, Döring G, Goldman MH, Freedom Study Group. 2013. Safety, efficacy and convenience of colistimethate sodium dry powder for inhalation (Colobreathe DPI) in patients with cystic fibrosis: a randomised study. Thorax 68:344–350. doi: 10.1136/thoraxjnl-2012-202059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier J-C, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob Agents Chemother 54:3702–3707. doi: 10.1128/AAC.00411-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, Van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J Antimicrob Chemother 57:306–311. doi: 10.1093/jac/dki461. [DOI] [PubMed] [Google Scholar]
- 38.Athanassa ZE, Markantonis SL, Fousteri M-ZF, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ. 2012. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med 38:1779–1786. doi: 10.1007/s00134-012-2628-7. [DOI] [PubMed] [Google Scholar]
- 39.Nakwan N, Lertpichaluk P, Chokephaibulkit K, Villani P, Regazzi M, Imberti R. 2015. Pulmonary and systemic pharmacokinetics of colistin following a single dose of nebulized colistimethate in mechanically ventilated neonates. Pediatr Infect Dis J 34:961–963. doi: 10.1097/INF.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 40.Zhou QT, Leung SS, Tang P, Parumasivam T, Loh ZH, Chan HK. 2015. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Adv Drug Deliv Rev 85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Lin YW, Wong J, Qu L, Chan HK, Zhou QT. 2015. Powder production and particle engineering for dry powder inhaler formulations. Curr Pharm Des 21:3902–3916. doi: 10.2174/1381612821666150820111134. [DOI] [PubMed] [Google Scholar]
- 42.Jensen T, Pedersen SS, Garne S, Heilmann C, Høiby N, Koch C. 1987. Colistin inhalation therapy in cystic fibrosis patients with chronic Pseudomonas aeruginosa lung infection. J Antimicrob Chemother 19:831–838. doi: 10.1093/jac/19.6.831. [DOI] [PubMed] [Google Scholar]
- 43.Hansen C, Pressler T, Høiby N. 2008. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros 7:523–530. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Chastre J, Fagon J-Y. 2002. Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
- 45.Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME. 2005. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit Care 9:R53–R59. doi: 10.1186/cc3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother 47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groneberg DA, Nickolaus M, Springer J, Döring F, Daniel H, Fischer A. 2001. Localization of the peptide transporter PEPT2 in the lung: implications for pulmonary oligopeptide uptake. Am J Pathol 158:707–714. doi: 10.1016/S0002-9440(10)64013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groneberg D, Eynott P, Doring F, Thai D, Oates T, Barnes P, Chung K, Daniel H, Fischer A. 2002. Distribution and function of the peptide transporter PEPT2 in normal and cystic fibrosis human lung. Thorax 57:55–60. doi: 10.1136/thorax.57.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamal MA, Keep RF, Smith DE. 2008. Role and relevance of PEPT2 in drug disposition, dynamics, and toxicity. Drug Metab Pharmacokinet 23:236–242. doi: 10.2133/dmpk.23.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin L, Zhou QT, Chan H-K, Larson IC, Pennington MW, Morales RA, Boyd BJ, Norton RS, Nicolazzo JA. 2016. Pulmonary delivery of the Kv1. 3-blocking peptide HsTX1 [R14A] for the treatment of autoimmune diseases. J Pharm Sci 105:650–656. doi: 10.1016/j.xphs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 51.Fernandes CA, Vanbever R. 2009. Preclinical models for pulmonary drug delivery. Expert Opin Drug Deliv 6:1231–1245. doi: 10.1517/17425240903241788. [DOI] [PubMed] [Google Scholar]
- 52.Morello M, Krone CL, Dickerson S, Howerth E, Germishuizen WA, Wong Y-L, Edwards D, Bloom BR, Hondalus MK. 2009. Dry-powder pulmonary insufflation in the mouse for application to vaccine or drug studies. Tuberculosis (Edinb) 89:371–377. doi: 10.1016/j.tube.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 53.Gales AC, Jones RN, Sader HS. 2011. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY antimicrobial surveillance program (2006-09). J Antimicrob Chemother 66:2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 54.Huang JX, Blaskovich MA, Pelingon R, Ramu S, Kavanagh A, Elliott AG, Butler MS, Montgomery AB, Cooper MA. 2015. Mucin binding reduces colistin antimicrobial activity. Antimicrob Agents Chemother 59:5925–5931. doi: 10.1128/AAC.00808-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwameis R, Erdogan-Yildirim Z, Manafi M, Zeitlinger M, Strommer S, Sauermann R. 2013. Effect of pulmonary surfactant on antimicrobial activity in vitro. Antimicrob Agents Chemother 57:5151–5154. doi: 10.1128/AAC.00778-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manchandani P, Zhou J, Ledesma KR, Truong LD, Chow DS-L, Eriksen JL, Tam VH. 2016. Characterization of polymyxin B biodistribution and disposition in an animal model. Antimicrob Agents Chemother 60:1029–1034. doi: 10.1128/AAC.02445-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kunin CM, Bugg A. 1971. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body. J Infect Dis 124:394–400. doi: 10.1093/infdis/124.4.394. [DOI] [PubMed] [Google Scholar]
- 58.Beringer P. 2001. The clinical use of colistin in patients with cystic fibrosis. Curr Opin Pulm Med 7:434–440. doi: 10.1097/00063198-200111000-00013. [DOI] [PubMed] [Google Scholar]
- 59.Kettler L, Sawyer S, Winefield H, Greville H. 2002. Determinants of adherence in adults with cystic fibrosis. Thorax 57:459–464. doi: 10.1136/thorax.57.5.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawicki GS, Sellers DE, Robinson WM. 2009. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 8:91–96. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conole D, Keating GM. 2014. Colistimethate sodium dry powder for inhalation: a review of its use in the treatment of chronic Pseudomonas aeruginosa infection in patients with cystic fibrosis. Drugs 74:377–387. doi: 10.1007/s40265-014-0181-0. [DOI] [PubMed] [Google Scholar]
- 62.Alothman GA, Ho B, Alsaadi MM, Ho SL, O'Drowsky L, Louca E, Coates AL. 2005. Bronchial constriction and inhaled colistin in cystic fibrosis. Chest J 127:522–529. doi: 10.1378/chest.127.2.522. [DOI] [PubMed] [Google Scholar]
- 63.Maddison J, Dodd M, Webb A. 1994. Nebulized colistin causes chest tightness in adults with cystic fibrosis. Respir Med 88:145–147. doi: 10.1016/0954-6111(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 64.Cheah S-E, Bulitta JB, Li J, Nation RL. 2014. Development and validation of a liquid chromatography-mass spectrometry assay for polymyxin B in bacterial growth media. J Pharm Biomed Anal 92:177–182. doi: 10.1016/j.jpba.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rennard S, Basset G, Lecossier D, O'Donnell K, Pinkston P, Martin P, Crystal R. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol 60:532–538. [DOI] [PubMed] [Google Scholar]
- 66.Bulitta JB, Landersdorfer CB. 2011. Performance and robustness of the Monte Carlo importance sampling algorithm using parallelized S-ADAPT for basic and complex mechanistic models. AAPS J 13:212–226. doi: 10.1208/s12248-011-9258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/A:1012299115260. [DOI] [PubMed] [Google Scholar]
- 68.Landersdorfer CB, Kirkpatrick CM, Kinzig-Schippers M, Bulitta JB, Holzgrabe U, Drusano GL, Sörgel F. 2007. Population pharmacokinetics at two dose levels and pharmacodynamic profiling of flucloxacillin. Antimicrob Agents Chemother 51:3290–3297. doi: 10.1128/AAC.01410-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bulitta JB, Zhao P, Arnold RD, Kessler DR, Daifuku R, Pratt J, Luciano G, Hanauske A-R, Gelderblom H, Awada A. 2009. Mechanistic population pharmacokinetics of total and unbound paclitaxel for a new nanodroplet formulation versus Taxol in cancer patients. Cancer Chemother Pharmacol 63:1049–1063. doi: 10.1007/s00280-008-0827-2. [DOI] [PubMed] [Google Scholar]
- 70.Tsuji BT, Okusanya OO, Bulitta JB, Forrest A, Bhavnani SM, Fernandez PB, Ambrose PG. 2011. Application of pharmacokinetic-pharmacodynamic modeling and the justification of a novel fusidic acid dosing regimen: raising Lazarus from the dead. Clin Infect Dis 52:S513–S519. doi: 10.1093/cid/cir166. [DOI] [PubMed] [Google Scholar]
- 71.Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


