ABSTRACT
The pathogenesis of Staphylococcus aureus is mediated by an array of important virulence factors, including the two-component leukocidin family of toxins. LukAB (also known as LukGH), the most recently discovered leukocidin, is potently lethal to phagocytes, produced during invasive human disease, and present in all known clinical isolates of S. aureus. Intravenous immunoglobulin (IVIg) is often used clinically in severe S. aureus infections. The primary aim of this study was to assess the binding and neutralization potential of IVIg against LukAB. A secondary aim was to examine the lot-to-lot variability of IVIg in the binding and neutralization of LukAB. We studied 24 distinct lots of IVIg and compared them to serum from children with invasive S. aureus infection (in the acute and convalescent phases) and from healthy, uninfected controls. We found that all lots of IVIg contained functional antibodies targeting LukAB. After adjusting for total antibody content per sample, we found that the amount of anti-LukAB antibody in IVIg was similar to that seen with healthy controls and less than that seen with patients with invasive S. aureus infection. IVIg samples had lower neutralization capacity than samples from healthy controls and children with invasive infection. IVIg had remarkably little lot-to-lot variation in LukAB binding but had significantly more variation in toxin neutralization. These results represent the first report of functional antibodies against the important S. aureus leukocidin LukAB in IVIg. Given the frequent clinical use of IVIg for severe S. aureus infections, improving our understanding of functional antibody properties exhibited by this therapeutic is essential.
KEYWORDS: LukAB, Staphylococcus aureus, immune response, intravenous immunoglobulin
INTRODUCTION
Staphylococcus aureus is the most common invasive pathogen in the United States and is a major cause of morbidity and mortality (1). The ability of S. aureus to elaborate a variety of potent cytolytic toxins and peptides is an essential mechanism to evade the host immune response and plays a critical role in pathogenesis (2). The bicomponent leukocidins—gamma-hemolysins (HIgAB and HIgCB), Panton-Valentine leukocidin (PVL), and LukED and LukAB (also known as LukGH)—are a group of pore-forming toxins capable of lysing human phagocytic cells (2, 3). LukAB, the most recently discovered staphylococcal leukocidin, has been shown to be the dominant toxin in vitro (4). Recent studies have shown that LukAB is abundantly produced by invasive disease-associated isolates, is recognized by the host response during invasive human infection, and is critical to the pathogen's ability to subvert the human innate immune response (2, 3, 5, 6, 7, 8).
The increasing prevalence of antibiotic-resistant S. aureus strains has emphasized the need for both active and passive immunization approaches (9). Intravenous immunoglobulin (IVIg) is a polyclonal antibody preparation that has been proposed as an adjunct therapy in severe staphylococcal and streptococcal infections (10, 11). Previous studies have investigated the ability of IVIg to neutralize S. aureus toxins, including Panton-Valentine leukocidin (PVL), hemolysin, and toxic shock syndrome toxin-1 (TSST-1) (12, 13). Diep et al. showed IVIg to have a protective effect against death in a rabbit model of necrotizing pneumonia and were able to characterize two specific IVIg antibodies that neutralized the toxic effects of α-hemolysin and PVL (14). No studies, however, have assessed the neutralization capacity of IVIg against LukAB, despite its ubiquitous presence in all clinical isolates tested to date (5, 15) and its clear role in pathogenesis in vivo (2, 7, 8). Thus, the primary aim of this study was to assess the binding and neutralization potential of commercially available IVIg against LukAB. A secondary aim of this study was to examine the lot-to-lot variability in the binding and neutralization of IVIg against LukAB.
(Preliminary results from this study were presented at IDWeek in October 2016 in New Orleans, LA.)
RESULTS
Sample characteristics.
We assessed LukAB toxin binding and neutralization of 24 distinct lots of commercially available IVIg preparations and compared them to 85 serum samples from pediatric subjects with invasive S. aureus infection (50 acute-phase samples and 35 convalescence samples) and to serum samples from 25 healthy, uninfected pediatric controls. The mean age of children with invasive infection was 6.7 years (standard deviation [SD], 4.8 years), and the mean age for healthy controls was 7.6 years (SD, 4.7 years).
Anti-LukAB antibody binding by ELISA.
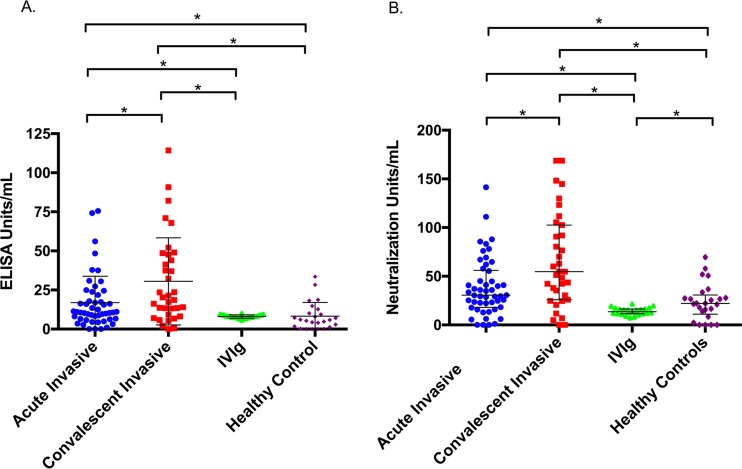
The median concentration determined by enzyme-linked immunosorbent assay (ELISA) of LukAB binding for IVIg was 8.1 U/ml (interquartile range [IQR], 7.3 to 8.9 U/ml). In comparison, pediatric patients with invasive infection had median LukAB antibody concentrations of 10.9 U/ml (IQR, 6.5 to 22.3 U/ml) in the acute phase and 20.3 U/ml (IQR, 9.2 to 47.7 U/ml) in convalescence, both of which were significantly higher than that seen with IVIg (P < 0.01 for both) (Fig. 1A). Healthy controls had a median LukAB antibody concentration of 5.9 U/ml (IQR, 1.6 to 11.5 U/ml), which was not significantly different from that seen with IVIg (P = 0.08). To account for the significantly larger overall IgG concentration in IVIg samples than in human serum, all samples were adjusted for the total IgG per sample prior to analysis.
FIG 1.
Binding and functional assessment (antibody-mediated toxin neutralization), adjusted for total IgG per sample. ELISA units and neutralization units were interpolated from a standard 5-PL curve using an anti-LukAB human monoclonal antibody. (A) Median concentration (determined by ELISA) of LukAB binding antibody in each group. Acutely infected subjects contained a median antibody concentration of 10.9 U/ml (IQR, 6.5 to 22.3 U/ml). Subjects in the convalescent phase had a median antibody concentration of 20.3 U/ml (IQR, 9.2 to 47.7 U/ml). IVIg had a median antibody concentration of 8.1 (IQR, 7.3 to 8.9 U/ml). Healthy controls had a median antibody concentration of 5.9 U/ml (IQR, 1.6 to 11.5 U/ml). (B) Median concentration of neutralizing antibody in each group. Sera obtained from acutely infected subjects contained 30.6 U/ml (IQR, 18.1 to 56 U/ml), convalescent-phase sera 54.7 U/ml (IQR, 26 to 102.6 U/ml), IVIg 13.5 U/ml (IQR, 11.6 to 16.5 U/ml), and sera from healthy controls 22.1 U/ml (IQR, 11.1 to 69.6 U/ml). *, P < 0.01.
Functional antibody assessment.
To assess the functionality of anti-LukAB antibodies, we performed toxin neutralization assays by measuring the cytoprotective effect of each sample incubated with purified toxin. We found that IVIg neutralized the cytotoxic activity of LukAB, with a median neutralization of 13.5 U/ml (IQR, 11.6 to 16.5 U/ml), which was significantly lower than the levels seen with the acute-phase samples (30.6 U/ml; IQR, 18.1 to 56 U/ml, P < 0.01), convalescent-phase samples (54.7 U/ml; IQR, 26 to 102.6 U/ml, P < 0.01), and samples from healthy controls (22.1 U/ml; IQR, 11.1 to 69.6 U/ml, P = 0.01) (Fig. 1B).
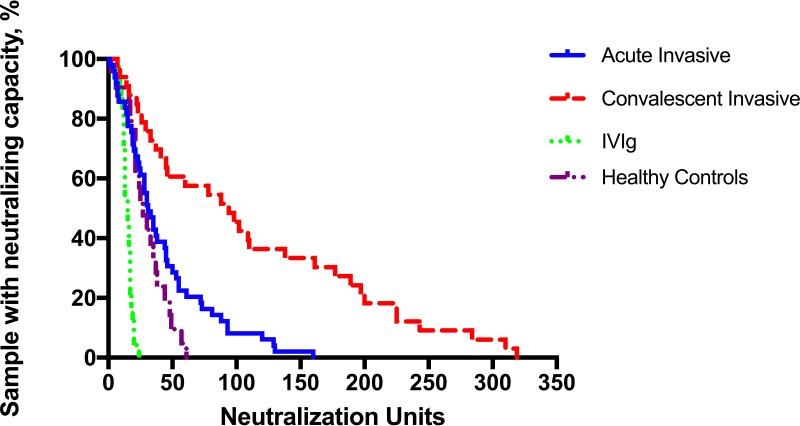
IVIg samples had lower neutralization capacity than the samples from healthy controls and children with invasive infection, with no lots showing values higher than 22 U/ml. Many subjects with invasive S. aureus in the acute phase had neutralizing antibody titers similar to the levels seen with healthy controls, while children with invasive S. aureus infection developed a much higher titer of neutralizing antibody in convalescence (Fig. 2).
FIG 2.
Reverse cumulative distribution plot of neutralization of LukAB in the presence of IVIg, sera from children with invasive infection, or sera from healthy controls. IVIg had a lower functional neutralization capacity per milliliter than was seen with children with invasive S. aureus infection and healthy controls. Samples were adjusted for total IgG per sample and tested independently in duplicate on separate days.
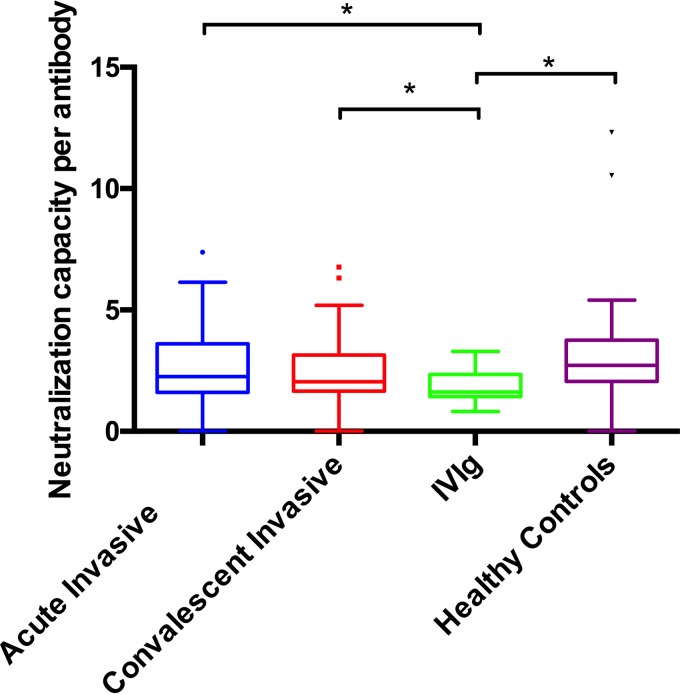
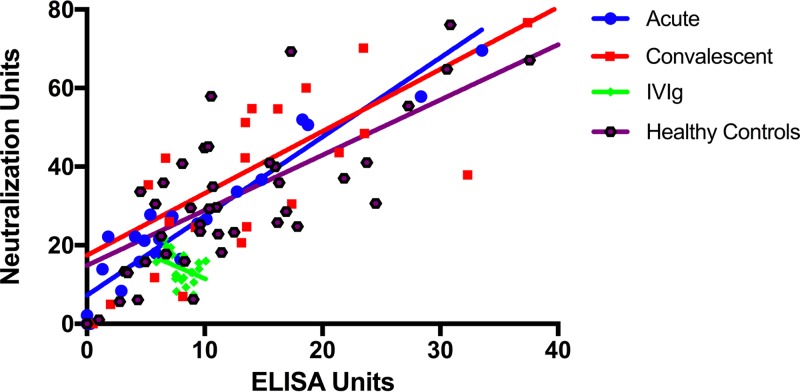
To investigate the functional neutralization capacity of each sample per binding antibody, we looked at the ratio of neutralization units to ELISA binding units (NU/EU). We observed that IVIg had a median NU/EU ratio of 1.6 (IQR, 1.4 to 2.3), which was significantly lower than the levels seen with acute (2.3; IQR 1.6 to 3.6, P = 0.01), convalescent (2.1, IQR 1.7 to 3.2, P = 0.02), and healthy control (2.7, 2.1 to 3.8, P < 0.01) samples (Fig. 3). Unlike the results seen with samples from patients with invasive (acute and convalescent) disease and healthy controls, toxin neutralization by IVIg samples did not correlate with the quantity of binding antibody present (Spearman's correlation coefficient [rs] = 0.14) (Fig. 4).
FIG 3.
Ratio of toxin neutralization capacity to binding antibody level (NU/EU) against LukAB. IVIg had the smallest amount of functional (neutralizing) antibody per binding antibody to LukAB.
FIG 4.
Neutralization of LukAB-mediated cytotoxicity as a function of the quantity of binding antibody. Samples were adjusted for total IgG per sample (slope of line represents all data; some data points are outside the axis limits). Toxin neutralization by IVIg samples did not correlate with the amount of antibody present (rs = 0.14), unlike that seen with samples from patients with invasive infection (acute-phase rs = 0.64; convalescent-phase rs = 0.83) and from healthy controls (rs = 0.90).
Lot-to-lot variability of IVIg binding and neutralization.
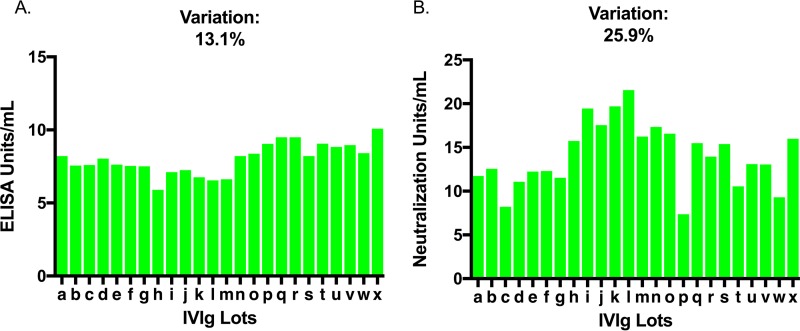
In order to determine the consistency of the presence and function of anti-LukAB antibody across distinct lots of commercial IVIg preparations, the coefficient of variation (CV) between samples was measured. We observed only minimal lot-to-lot variation in LukAB binding among IVIg samples, with a coefficient of variation of 13.1% (Fig. 5A). Moreover, the coefficient of variation of toxin neutralization between IVIg lots was 25.9%, which was significantly higher than the coefficient of variation of binding (P < 0.01, [95% CI, 1.32 to 2.64]) (Fig. 5B).
FIG 5.
Variation in binding and neutralization of 24 unique lots of IVIg against LukAB. (A) The coefficient of variation (CV) for IVIg binding LukAB was 13.1%. (B) The CV for IVIg neutralization was significantly higher at 25.9%. Samples were tested independently in duplicate on separate days.
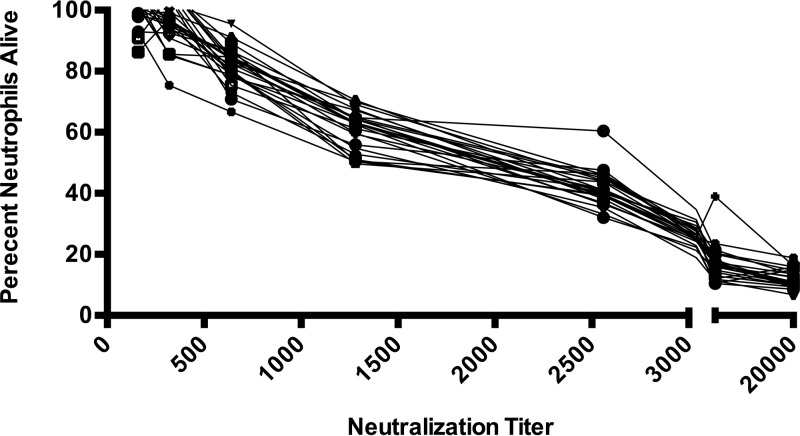
Unlike the results seen with individual patients with invasive S. aureus and healthy controls, there were no commercial lots of IVIg devoid of LukAB binding antibody. Similarly, analyzing the neutralization capacity of the individual lots of IVIg, we found that all IVIg lots showed protection at low (≤1:360) titers, though only 1 of 24 lots showed protection at titers of >1:1,280 (Fig. 6).
FIG 6.
LukAB cytotoxin neutralization of each lot of IVIg by titer. All IVIg lots showed protection at low (≤1:360) titers, while only 1 of 24 lots showed protection at titers of >1:1,280. Samples were tested independently in duplicate on separate days.
DISCUSSION
In this study, we found that commercially available lots of IVIg contain LukAB-specific antibodies capable of neutralizing the toxin-dependent lysis of phagocytes in vitro. Adjusted for total IgG per sample, the concentration of anti-LukAB binding antibodies in IVIg was similar to that seen with healthy, uninfected pediatric controls and significantly less than that seen with pediatric patients with invasive S. aureus infection. The functional neutralization capacity of IVIg was significantly lower than that seen with both pediatric patients with invasive S. aureus disease and healthy controls. One explanation for this may be that human serum, even after adjusting for total IgG, contains anti-LukAB IgA and IgM not present in IVIG. There was remarkably low variability between lots of IVIg in the amount of binding antibody present but significantly higher variability in LukAB neutralization.
Previous reports have shown that IVIg contains antibodies capable of neutralizing S. aureus enterotoxins A and B (13, 16), alpha- and beta-hemolysin, and TSST-1, as well as the leukocidin PVL (12). Our study was the first to show that commercial IVIg contains functional, LukAB-specific neutralizing antibodies. Although described only recently, there is considerable evidence that LukAB plays a critical role in the ability of S. aureus to evade the host innate immune response and is essential to virulence (2, 3, 6). Additionally, LukAB may play a unique role in bacterial survival during invasive infection as it has been shown to be required for escape from phagocytic killing by human neutrophils (2, 6, 7, 8). Our group has shown that children with invasive S. aureus infection produce a high titer of anti-LukAB antibodies under acute-phase conditions that increases in convalescence and that B cells obtained from children following invasive infection produce potently neutralizing anti-LukAB antibodies (5), strongly suggesting that the toxin is produced during invasive human disease. Additionally, all clinical isolates of S. aureus identified by our group to date harbored lukAB (5, 15). These characteristics of immunogenicity, and its universal presence in isolates causing human disease, make LukAB an interesting preventive or therapeutic target.
Despite a relative reemergence of methicillin-susceptible S. aureus strains in recent years, overall rates of resistance to antistaphylococcal antibiotics continue to increase (17, 18, 19). This, in combination with a paucity of novel antibiotics and high rates of morbidity and mortality associated with invasive S. aureus infections despite antibiotic susceptibility, has led to a renewed interest in the development of antibody-based therapeutics. Importantly, antibody-based therapeutics have the ability to neutralize toxin regardless of antibiotic susceptibility. To date, all antibody-based therapeutics against S. aureus have failed to show significant efficacy in clinical trials (9). Results of preclinical studies of leukocidin-specific monoclonal antibodies are promising, and important issues such as breadth of neutralization and potency in vivo warrant further investigation (20, 21). Passive immunization with an “antibody cocktail” against multiple different S. aureus virulence factors will likely be needed for a successful antibody-based therapeutic (9, 22).
IVIg, a polyclonal antibody preparation, is a commercially available “antibody cocktail” derived from plasma pooled from numerous donors. It has been well reported that commercial IVIg contains neutralizing antibodies to several streptococcal and staphylococcal exotoxins as well as other toxin-produced bacteria, including Clostridium difficile (10, 11, 23). Despite its wide use, however, many questions remain regarding the mechanism of action of IVIg in a variety of disease states. It has been proposed that IVIg works through several mechanisms, including both antigen-specific and nonspecific antibody binding and receptor blockade (12, 24). Additionally, it is believed that IVIg works through modulation of Fc-gamma receptors (FcγR), the complement pathway, cytokine expression, B cell differentiation, and T regulatory cell function (25, 26, 27). Our study results provide insight into direct toxin neutralization by naturally occurring antibodies pooled in IVIg. Previous data from our group have shown that functional antitoxin antibody responses following invasive human infection are diverse and that toxin neutralization can be achieved by interfering with multiple steps in the cytolysis pathway (20).
Randomized clinical trials investigating the effect of IVIg treatment in sepsis have led to conflicting conclusions (28, 29, 30); as a result, current guidelines do not recommend IVIg therapy for sepsis, despite anecdotal evidence to support its use. Our current study showed marked variation in the neutralizing capacity against LukAB of individual lots of IVIg. It is reasonable to hypothesize, then, that this variability of functional antibody exists for many antigens contained in commercial lots of IVIg. This may contribute to the conflicting results of studies assessing the efficacy of IVIg in sepsis.
Although we found that IVIg contained lower concentrations of binding and neutralizing antibodies than were seen with children with invasive S. aureus infection, commercially available IVIg contains roughly 10× more antibody per milliliter than human serum. In the doses used in clinical practice, one would expect the amount of LukAB-specific binding and neutralizing antibody to exceed that produced during infection.
One unexpected finding of the data analysis performed for this study was the observation that while the total anti-LukAB antibody concentration significantly increased as children progressed from acute disease to convalescence, the “pound-for-pound” neutralization capacity (ratio of neutralization units to ELISA binding units [NU/EU]) decreased slightly in convalescent-phase samples. One potential explanation for this may be the development of binding, nonneutralizing antibodies as a component of the humoral response to LukAB following invasive disease. Many of the IVIg lots that we assessed also appeared to have a large proportion of either lower-affinity or nonneutralizing antibodies (Fig. 3). The potential roles of nonneutralizing antibodies (e.g., enhancement of opsonophagocytosis, complement deposition mediated by the Fc fragment, or even inhibition of functional antibody activity by binding at locations near key functional epitopes) are a focus of ongoing work by our group. An additional ongoing focus of investigation in our laboratory is the difference in antibody responses to allelic variants of LukAB from distantly related S. aureus strains, as these differences may explain the distinctions between binding and neutralizing data for a given antibody profile, if key epitopes differ among toxin variants.
The results of our study represent the first report of functional antibodies against the important S. aureus leukocidin LukAB in IVIg. IVIg is used clinically in a variety of disease states, including invasive bacterial infections; however, a clear understanding of the mechanism of action and of the contents of the product is lacking. Further investigation of these mechanisms will be important to understand the clinical situations in which IVIg may be most useful.
MATERIALS AND METHODS
Patient samples.
Our group previously collected serum samples from children admitted to the Monroe Carell Jr. Children's Hospital at Vanderbilt (MCJCHV) with culture-confirmed invasive S. aureus infection identified within the first 5 days of hospitalization (5). Samples were obtained on the day of enrollment (acute) and 4 to 6 weeks following enrollment (convalescent). Invasive infections were defined as those in which S. aureus was identified in a typically sterile site (e.g., blood, bone, pleural fluid).
Healthy control samples were obtained from pediatric subjects undergoing outpatient procedures for noninfectious indications at MCJCHV and were selected if the subjects met the following eligibility criteria: age between 6 months and 18 years, no known history of S. aureus disease of any type, no known primary or secondary immune compromise (including long-term oral or parenteral corticosteroid therapies), no history of (or current) malignancy, and no receipt of IVIg or blood products in the previous 12 months. Blood samples were obtained from healthy control subjects at the time of enrollment.
Sera were obtained by centrifugation of unheparinized whole-blood samples, and sera were stored at −20°C until processing. Informed consent was obtained from the parents and, when possible, informed assent from the participant. The study was approved by the Vanderbilt University Medical Center Institutional Review Board (IRB).
IVIg.
Commercially available human polyclonal IVIg (Gammunex-c; Grifols, Research Triangle Park, NC, USA) was obtained from the MCJCHV pharmacy as a 10% protein/ml solution.
Antibody measurement by enzyme-linked immunosorbent assay (ELISA).
The binding antibody concentration of each sample (IVIg, infected-patient serum, healthy controls) was measured against dimerized, purified LukAB using indirect ELISA as previously described (31, 32). Briefly, LukAB (sequence variant from clonal complex 8, USA300 epidemic clone lineage) was expressed and purified in an Escherichia coli system as previously described (7), diluted in phosphate-buffered saline (PBS) to a concentration of 2.0 μg/ml, and bound to a 96-well ELISA plate for 1 h at room temperature (RT). Wells were then aspirated, washed, and blocked for 90 min with 5% nonfat dried milk–0.1% Tris-buffered saline. Serial 2-fold dilutions of sera were added to the plate and incubated for 1 h at RT. After plates were washed, horseradish peroxidase (HRP)-conjugated murine monoclonal antibodies recognizing human IgG were diluted 1:5,000 in the buffer and added to all wells, and the plates were incubated at RT for 1 h. Next, the plates were washed, substrate solution (3,3′,5,5′-tetramethylbenzidine) was added, and plates were incubated at RT in the dark. The reaction was stopped at 20 min with 2 M sulfuric acid, and plates were read spectrophotometrically at 450 nm. Serum depleted of IgG was used as a negative control. The antibody concentration (ELISA units/milliliter) in each sample was interpolated from a standard curve of a monoclonal antibody against LukAB (20) using a 5-parameter logistic regression (5PL) analysis. Samples were run independently in duplicate on separate days, and a third run was performed if the first two concentrations were discrepant.
Toxin neutralization assays.
To measure the neutralization capacity of each sample, a toxin neutralization assay was performed. Human promyelocytic HL-60 cells were cultured in RPMI 1640 (Gibco) plus 10% heat-inactivated fetal bovine serum (FBS, Gibco) plus 100 μg/ml penicillin/100 μg/ml streptomycin (Pen/Strep; Gibco) and allowed to differentiate to neutrophil-like cells (PMN-HL60) for 3 days with 1.5% dimethyl sulfoxide (DMSO) (33). Samples were diluted in RPMI media enriched with Casamino Acids (CAS) and bicarbonate, added to the plates, and serially diluted. Purified LukAB (1.25 μg/ml) was added at 10 μl/well, and wells were mixed by pipetting and incubated for 30 min at RT. Wells containing only RPMI plus CAS media and toxin alone served as an unintoxicated control (negative control) and a toxicity-positive control (positive control), respectively. PMN-HL60 cells were added to the plate at 80 μl/well (1.26 × 106 cells/ml) and mixed with the serum/toxin mixture. This mixture was incubated at 37°C with 5% CO2 for 1 h, and CellTiter (Promega) was added at 10 μl/well to measure cell viability/metabolism. After 2 h of incubation with CellTiter at 37°C plus 5% CO2, plates were read spectrophotometrically at 490 nm absorbance to measure cell survival.
The functional neutralization capacity of each sample was identified by interpolation of the 50% inhibitory concentration (IC50) from a standard curve using a 5PL analysis. Neutralization capacity was quantified by normalizing values to the neutralization capacity of a specific monoclonal antibody against LukAB (20) to allow the calculation of neutralization units per milliliter. The functional neutralization capacity was adjusted for the amount of total LukAB-binding antibody present by comparing the ratios of neutralization units to ELISA binding units (NU/EU). Analyzing toxin neutralization by titer, a titer was considered protective if ≥70% of the cells were alive at that dilution.
Total IgG quantification.
The total amount of IgG per sample was measured by an immunoturbidimetric assay performed by the Vanderbilt University Medical Center clinical laboratory.
Statistical analysis.
ELISA and neutralization units were adjusted for total IgG per sample in order to compare samples with differing amounts of IgG. Continuous variables that followed a normal distribution were analyzed by the use of a two-sample Student's t test and paired t tests for independent and paired variables, respectively. Continuous variables that did not follow a normal distribution were analyzed with a Mann-Whitney U test for independent samples and a Wilcoxon matched-pair signed-rank test for paired samples. The coefficient of variation, representing a ratio of standard deviation to the mean, was used to assess lot-to-lot variability among IVIg samples. Bootstrapping was performed to obtain confidence intervals around the coefficient of variation. Correlation was assessed by Spearman's correlation coefficient (rs). Two-sided P values of <0.05 were considered statistically significant. Statistical analysis was performed using GraphPad Prism for Mac OS X, version 6.0e.
ACKNOWLEDGMENTS
We are thankful for the funding support provided by the Vanderbilt Childhood Infections Research Program (ChIRP) T32 (NIAID 6T32AI095202-06) to J.B.W. and NIAID 1K23AI113150-01 to I.P.T. C.B.C. has joint research or grants with Pfizer and GSK and has served on scientific advisory boards for Theravance Pharmaceuticals and GSK Vaccines, each of which is outside the scope of the submitted work. I.P.T. serves as an investigator on studies funded by GlaxoSmithKline and Horizon Pharma. None of these studies conflict with the contents of the manuscript.
REFERENCES
- 1.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S. 2013. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med 173:1970–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumont AL, Nygaard TK, Watkins RL, Smith A, Kozhaya L, Kreiswirth BN, Shopsin B, Unutmaz D, Voyich JM, Torres VJ. 2011. Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis. Mol Microbiol 79:814–825. doi: 10.1111/j.1365-2958.2010.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ventura CL, Malachowa N, Hammer CH, Nardone GA, Robinson MA, Kobayashi SD, DeLeo FR. 2010. Identification of a novel Staphylococcus aureus two-component leukotoxin using cell surface proteomics. PLoS One 5:e11634. doi: 10.1371/journal.pone.0011634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janesch P, Rouha H, Weber S, Malafa S, Gross K, Maierhofer B, Badarau A, Visram ZC, Stulik L, Nagy E. 2017. Selective sensitization of human neutrophils to LukGH mediated cytotoxicity by Staphylococcus aureus and IL-8. J Infect 74:473–483. doi: 10.1016/j.jinf.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Thomsen IP, Dumont AL, James DB, Yoong P, Saville BR, Soper N, Torres VJ, Creech CB. 2014. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun 82:1234–1242. doi: 10.1128/IAI.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuMont AL, Yoong P, Day CJ, Alonzo F III, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DuMont AL, Yoong P, Surewaard BG, Benson MA, Nijland R, van Strijp JA, Torres VJ. 2013. Staphylococcus aureus elaborates leukocidin AB to mediate escape from within human neutrophils. Infect Immun 81:1830–1841. doi: 10.1128/IAI.00095-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. 2015. Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog 11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. 2016. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol Sci 37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cone LA, Stone RA, Schlievert PM, Sneider RA, Rubin AM, Jesser K, Renker SW. 2006. An early favorable outcome of streptococcal toxic shock syndrome may require a combination of antimicrobial and intravenous gamma globulin therapy together with activated protein C. Scand J Infect Dis 38:960–963. doi: 10.1080/00365540500373224. [DOI] [PubMed] [Google Scholar]
- 11.Schlievert PM. 2001. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J Allergy Clin Immunol 108:S107–S110. [DOI] [PubMed] [Google Scholar]
- 12.Gauduchon V, Cozon G, Vandenesch F, Genestier AL, Eyssade N, Peyrol S, Etienne J, Lina G. 2004. Neutralization of Staphylococcus aureus Panton Valentine leukocidin by intravenous immunoglobulin in vitro. J Infect Dis 189:346–353. doi: 10.1086/380909. [DOI] [PubMed] [Google Scholar]
- 13.Yanagisawa C, Hanaki H, Natae T, Sunakawa K. 2007. Neutralization of staphylococcal exotoxins in vitro by human-origin intravenous immunoglobulin. J Infect Chemother 13:368–372. doi: 10.1007/s10156-007-0551-6. [DOI] [PubMed] [Google Scholar]
- 14.Diep BA, Le VT, Badiou C, Le HN, Pinheiro MG, Duong AH, Wang X, Dip EC, Aguiar-Alves F, Basuino L, Marbach H, Mai TT, Sarda MN, Kajikawa O, Matute-Bello G, Tkaczyk C, Rasigade JP, Sellman BR, Chambers HF, Lina G. 2016. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med 8:357ra124. doi: 10.1126/scitranslmed.aag1153. [DOI] [PubMed] [Google Scholar]
- 15.Chadha AD, Thomsen IP, Jimenez-Truque N, Soper NR, Jones LS, Sokolow AG, Torres VJ, Creech CB. 2016. Host response to Staphylococcus aureus cytotoxins in children with cystic fibrosis. J Cyst Fibros 15:597–604. doi: 10.1016/j.jcf.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darville T, Milligan LB, Laffoon KK. 1997. Intravenous immunoglobulin inhibits staphylococcal toxin-induced human mononuclear phagocyte tumor necrosis factor alpha production. Infect Immun 65:366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas G, Moellering RC Jr. 2008. Increasing antibiotic resistance among methicillin-resistant Staphylococcus aureus strains. Clin Infect Dis 46:S360–367. doi: 10.1086/533592. [DOI] [PubMed] [Google Scholar]
- 18.Marty FM, Yeh WW, Wennersten CB, Venkataraman L, Albano E, Alyea EP, Gold HS, Baden LR, Pillai SK. 2006. Emergence of a clinical daptomycin resistant Staphylococcus aureus isolate during treatment of methicillin resistant Staphylococcus aureus bacteremia and osteomyelitis. J Clin Microbiol 44:595–597. doi: 10.1128/JCM.44.2.595-597.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, Perez KK, Musick WL, Rosato AE, Musser JM. 2014. PBP2a mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomsen IP, Sapparapu G, James DBA, Cassat JE, Nagarsheth M, Putnam N, Boguslawski KM, Jones LS, Wood JB, Creech CB, Torres VJ, Crowe JE. 2017. Monoclonal antibodies against the Staphylococcus aureus bicomponent leukotoxin AB (LukAB) isolated following invasive human infection reveal diverse binding and modes of action. J Infect Dis 215:1124–1131. doi: 10.1093/infdis/jix071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badarau A, Rouha H, Malafa S, Battles MB, Walker L, Nielson N, Dolezilkova I, Teubenbacher A, Banerjee S, Maierhofer B, Weber S, Stulik L, Logan DT, Welin M, Mirkina I, Pleban C, Zauner G, Gross K, Jägerhofer M, Magyarics Z, Nagy E. 2016. Context matters: the importance of dimerization-induced conformation of the LukGH leukocidin of Staphylococcus aureus for the generation of neutralizing antibodies. MAbs 8:1347–1360. doi: 10.1080/19420862.2016.1215791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung GY, Otto M. 2012. The potential use of toxin antibodies as a strategy for controlling acute Staphylococcus aureus infections. Expert Opin Ther Targets 16:601–612. doi: 10.1517/14728222.2012.682573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah PJ, Vakil N, Kabakov A. 2015. Role of intravenous immune globulin in streptococcal toxic shock syndrome and Clostridium difficile infection. Am J Health Syst Pharm 72:1013–1019. doi: 10.2146/ajhp140359. [DOI] [PubMed] [Google Scholar]
- 24.Takei S, Arora YK, Walker SM. 1993. Intravenous immunoglobulin contains specific antibodies inhibitory to activation of T cells by staphylococcal toxin superantigens. J Clin Invest 91:602–607. doi: 10.1172/JCI116240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seite JF, Shoenfeld Y, Youinou P, Hillion S. 2008. What is the contents of the magic draft IVIg? Autoimmun Rev 7:435–439. doi: 10.1016/j.autrev.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.de Grandmont MJ, Racine C, Roy A, Lemieux R, Néron S. 2003. Intravenous immunoglobulins induce the in vitro differentiation of human B lymphocytes and the secretion of IgG. Blood 101:3065–3073. doi: 10.1182/blood-2002-06-1684. [DOI] [PubMed] [Google Scholar]
- 27.Kessel A, Ammuri H, Peri R, Pavlotzky ER, Blank M, Shoenfeld Y, Toubi E. 2007. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J Immunol 179:5571–5575. doi: 10.4049/jimmunol.179.8.5571. [DOI] [PubMed] [Google Scholar]
- 28.Heming N, Lamothe L, Ambrosi X, Annane D. 2016. Emerging drugs for the treatment of sepsis. Expert Opin Emerg Drugs 21:27–37. doi: 10.1517/14728214.2016.1132700. [DOI] [PubMed] [Google Scholar]
- 29.Almansa R, Tamayo E, Andaluz-Ojeda D, Nogales L, Blanco J, Eiros JM, Gomez-Herreras JI, Bermejo-Martin JF. 2015. The original sins of clinical trials with intravenous immunoglobulins in sepsis. Crit Care 19:90. doi: 10.1186/s13054-015-0793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alejandria MM, Lansang MAD, Dans LF, Mantaring JB III. 2013. Intravenous immunoglobulin for treating sepsis, severe sepsis and septic shock. Cochrane Database Syst Rev 2013:CD001090. doi: 10.1002/14651858.CD001090.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi N, Hattori M, Miwa K, Nishida H. 2006. Antibodies against superantigenic exotoxins produced by Staphylococcus aureus in sera from mothers and their infants' cord blood. Am J Perinatol 23:413–419. doi: 10.1055/s-2006-951290. [DOI] [PubMed] [Google Scholar]
- 32.Ausubel FM. 1989. Current protocols in molecular biology. Greene Publishing Associates and Wiley-Interscience, New York, NY. [Google Scholar]
- 33.Ennaciri J, Girard D. 2009. Immune system: maturation of myeloid cells. Methods Mol Biol 550:195–203. doi: 10.1007/978-1-60327-009-0_12. [DOI] [PubMed] [Google Scholar]