ABSTRACT
We characterized the genetic environment of mcr-1 in colistin-resistant Escherichia coli strains isolated in Switzerland during 2014 to 2016 from humans (n = 3) and chicken meat (n = 6). Whole-genome and plasmid sequencing identified the mcr-1 gene integrated in IncX4 (of which, one strain carried the mcr-1.2 variant), IncI2, IncHI2, and novel IncK2 plasmids (overall, n = 7), as well as in the bacterial chromosome (n = 2) in single or duplicate copies. Our study supports the easy mobilization of mcr-1 across diverse genetic locations.
KEYWORDS: colistin, chromosome, E. coli, mcr-1, food, IncK2, Escherichia coli, animals, chromosome organization, humans, meat, plasmids
TEXT
In facing an era of multidrug-resistant Enterobacteriaceae, the recent discovery of plasmids carrying the colistin resistance mcr-1 gene has raised considerable health concern (1, 22). To date, mcr-1 has been identified in several plasmid types (IncI2, IncHI2, IncX4, IncP, IncY, and IncF plasmids) (2–5), as well as integrated in the bacterial chromosome primarily mobilized as ISApl1 composite transposon (6–8, 23). Herein, we investigated the mcr-1 genetic environment in colistin-resistant Escherichia coli strains isolated in Switzerland from humans and retail chicken meat between 2014 and 2016 (Table 1).
TABLE 1.
Overview of the plasmids, resistance genes, and colistin MICs of the mcr-1-positive Escherichia coli strains isolated in Switzerland included in this study
| E. coli strain | Sourceg | STa | Collection period | Antibiotic resistanceb | Colistin MIC (μg/ml)c | Plasmidd | Plasmidic resistance gene(s)e | mcr-1 location (plasmid length in kb) |
|---|---|---|---|---|---|---|---|---|
| 100R | Human feces (traveler) | 10 | 2015 | CST, PMB, LVX, CIP, DOX, SXT | ≥8 | IncHI2 | aadA1, aadA2, blaTEM-1, mcr-1, mph(A), sul3, tetA, dfrA15, dfrA14 | IncHI2 (256) |
| IncFII/FIB | blaTEM-1, aadA2, aadA24, aph(3)-Ia, qnrS1, cmlA1, sul3, tetA, dfrA12 | |||||||
| IncX1 | ||||||||
| ColRNAI | ||||||||
| 19-M12 | Human feces (traveler) | 5 | 2015 | CST, PMB, DOX, SXT | ≥8 | IncHI2 | aadA2, blaTEM-1, mcr-1, mph(A), cmlA1, sul3, tetA, dfrA12, dfrA14 | IncHI2 (232) |
| IncFII/FIB | blaTEM-1, aadA1, qnrS1, tetA, dfrA1 | |||||||
| 31349 | Human feces (HIV+ subject) | 5 | 2015 | CST, PMB | ≥8 | IncX4 | mcr-1.2 | IncX4 (33) |
| IncL/M | ||||||||
| IncFII | ||||||||
| IncFIB | ||||||||
| Mcp0271 | Retail chicken meat | 58 | 2016 | CST, AMP | 4 | IncX4 | mcr-1 | IncX4 (33) |
| IncFII/FIB/FIA | blaTEM-1 | |||||||
| Mcp0221 | Retail chicken meat | 1775 | 2016 | CST | 4 | IncI2 | mcr-1 | IncI2 (65) |
| Mbl488 | Retail chicken meat | 38 | 2014 | CST, SMX, TMP, NAL, CAZ, CTX, AMP, TET | 8 | IncK2 | blaTEM-1, mcr-1, sul2 | IncK2 (101) |
| IncFII/FIB | aadA1, blaTEM-1, sul1, tetA, dfrA1 | |||||||
| IncI1 | aadA5, blaCTX-M-1, sul2, dfrA17 | |||||||
| po111 | ||||||||
| Mbl536 | Retail chicken meat | 226 | 2014 | CST, SMX, TMP, CIP, TET, NAL, AMP | 4 | IncK2 | blaTEM-1, mcr-1, sul2 | IncK2 (101) |
| IncI1 | blaTEM-1, sul2, tetA, dfrA1 | |||||||
| IncX1 | blaTEM-52 | |||||||
| Mbl323 | Retail chicken meat | 38 | 2014 | CST, CAZ, AMP, NAL, CTX | 8 | IncK2 | blaCMY-2 | Chromosome |
| IncN | aadA22, lnuF | |||||||
| IncFII/FIBColRNAI | ||||||||
| Mbl506f | Retail chicken meat | 1049 | 2014 | CST, TET, NAL, CTX, CHL, SMX, TMP, CAZ, AMP | 8 | IncFII/FIB | tetA | Chromosomei |
| IncI1 | aadA1, erm(42) | |||||||
| IncI2 | aad24, blaTEM-1 | |||||||
| IncQ1h | aad24, strA, strB, mph(B), sul1, sul2, tetA, dfrA1h |
Sequence type (ST) based on multilocus sequence typing was determined with MLSTtool.
Only antibiotics with a nonsusceptible phenotype according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria, except for TET, SMX, DOX, and minocycline for which Clinical and Laboratory Standards Institute (CLSI) criteria were used (20, 21). CST, colistin; PMB, polymyxin B; LVX, levofloxacin; CIP, ciprofloxacin; DOX, doxycycline; SXT, trimethoprim-sulfamethoxazole; SMX, sulfamethoxazole; AMP, ampicillin; CAZ, ceftazidime; TMP, trimethoprim; TET, tetracycline; CTX, cefotaxime; NAL, nalidixic acid; CHL, chloramphenicol.
MIC values were obtained with microdilution Trek Diagnostic Systems panels GNX2F for human strains or EUVSEC panels for chicken meat strains; the two panels analyze different MIC ranges for colistin (0.25 to 4 μg/ml and 1 to 16 μg/ml, respectively).
Incompatibility groups were determined with PlasmidFinder.
Resistance genes were determined with ResFinder.
Strain harboring a chromosomally integrated blaCTX-M-32.
Retail chicken meat was imported from Germany.
Integrated into the chromosome based on whole-genome sequencing.
Two mcr-1 copies in different loci.
Three human isolates of sequence type (ST) 10 and ST5 were isolated from the feces of two Swiss travelers and an HIV-positive individual (from totals of 38 and 101 subjects, respectively), as part of two previous studies (Table 1) (9, 10). Strains of retail chicken meat origin (n = 6) were isolated from meat imported from Germany within the framework of the National Surveillance Program of Antibiotic Resistance in Switzerland (11). In particular, 4 out of 234 (1.7%) and 2 out of 302 (0.7%) samples in 2014 and 2016, respectively, harbored mcr-1-carrying E. coli strains belonging to ST38, ST58, ST226, ST1049, and ST1775. In addition to colistin, seven strains showed resistance to other antibiotics (Table 1).
To characterize the genetic location of mcr-1, whole-genome sequencing of the strains was performed with the MinION (Oxford Nanopore) (10). The scaffolds were corrected with mapped Illumina reads and further characterized with an online platform (www.genomicepidemiology.org/). In human E. coli strains 100R and 19-M12, mcr-1 was located on a multidrug resistance IncHI2 plasmid of 256 kb (p100R; KY689633) and 232 kb (p19-M12; KY689632), respectively. Both IncHI2 plasmids exhibited structural similarity with other previously described mcr-1-carrying IncHI2 plasmids (see Fig. S1 in the supplemental material). In particular, mcr-1 was flanked by two copies of ISApl1 in opposite orientation, in which the putative pap2 open reading frame (ORF) was disrupted (Fig. 1).
FIG 1.
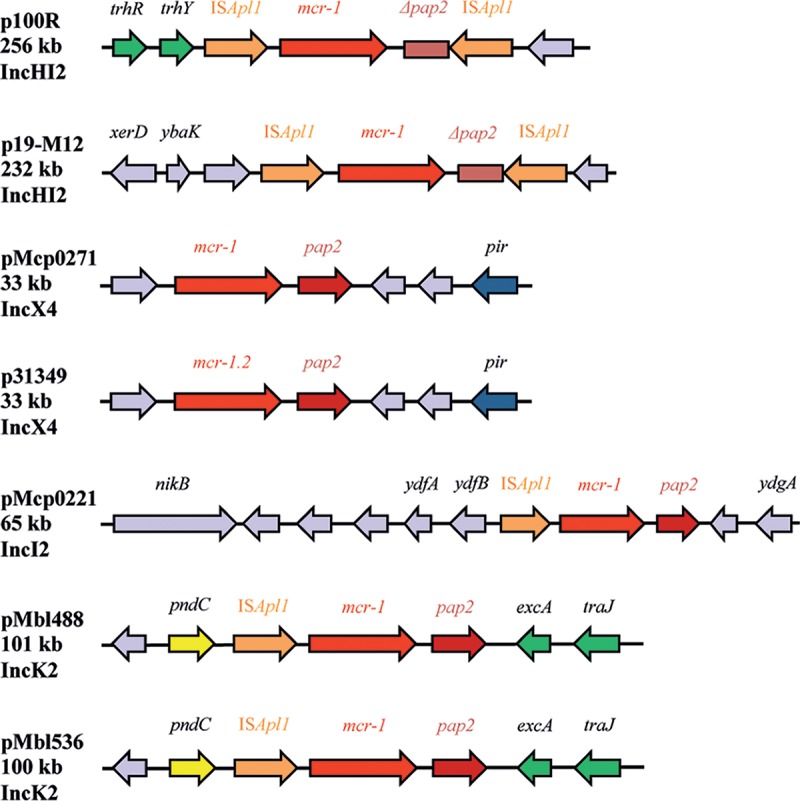
Schematic representation of the genetic environment of mcr-1 in the mcr-1-carrying plasmids. The open reading frames (ORFs) are illustrated by arrows pointing toward their respective orientation. The color code is as follows: the transfer-associated genes are in green, mcr-1 is in red, the putative pap2 ORF is in brown, insertion sequence (IS) elements are in orange, replication initiation protein-encoding gene (pir) is in dark blue, partitioning genes and toxin/anti-toxin and other stabilization systems are in yellow, and other genes are in lavender. Important gene names are indicated above the sequence. The indications on the left-hand side are the respective plasmid profiles. Genes are not drawn to scale.
Two strains from human and chicken meat carried the mcr-1 on 33 kb IncX4 plasmids (p31349 [KY689634] and pMcp0271 [KY565556], respectively) almost identical to others previously characterized, including the location of mcr-1 lacking the upstream ISApl1 inserted downstream of pir (Fig. 1; see also Fig. S2 in the supplemental material). Plasmid p31349 harbored the mcr-1.2 variant first detected in the IncX4 plasmid pMCR1.2-IT (KX236309) of a Klebsiella pneumoniae carbapenemase (KPC)-producing Klebsiella pneumoniae strain isolated in Italy (12).
Strain Mcp0221 from chicken meat harbored mcr-1 on a 65-kb IncI2 plasmid (pMcp0221; KY565557) showing structural similarity with other previously described mcr-1-carrying IncI2 plasmids (see Fig. S3 in the supplemental material). However, while mcr-1 (with or without an upstream ISApl1) was usually inserted immediately downstream of the nikB gene, the ISApl1-mcr-1 element in pMcp0221 was integrated five ORFs downstream of nikB (Fig. 1 and S3).
Sequence comparison based on average nucleotide identity (ANI) of pairwise sequence alignments performed with MUMmer (ANIm) (13) of complete sequences of IncHI2, IncX4, and IncI2 plasmids carrying the mcr-1 available in NCBI (see Fig. S4A in the supplemental material) confirmed the similarity with the respective plasmids found in the present study. Clustering of the dissimilarity matrices performed with the neighbor-joining algorithm (BIONJ algorithm) (14) showed that the three IncHI2 plasmids clustered together, appearing also to be closely related to plasmid pS38 (KX129782) from an E. coli strain isolated in Switzerland from poultry meat imported from Italy (Fig. S4A). The analysis further confirmed the high sequence similarity among all members of the IncX4 plasmids (ANIm, >97.89%), suggesting a dissemination of this plasmid type worldwide (Fig. S4B), whereas pMcp0221 did not closely cluster together with other IncI2 plasmid sequences (ANIm, ≤98.3%) (Fig. S4C).
Remarkably, in two strains from imported chicken meat (Mbl488 and Mbl536), mcr-1 was found on novel 100-kb IncK2 plasmids, which also harbored blaTEM-1 and sul2 (Table 1 and Fig. 2). Sequence comparison of both pMbl488 (KY565558) and pMbl536 (KY689635) with the recently described IncK2 blaCMY-2-carrying plasmid pDV45 (KR905384) showed high sequence similarity (Fig. 2) (15). As in pDV45, the ISApl1-mcr-1 element was found integrated in the tra region, but in a different location compared to the ISEcp1-blaCMY-2-blc-sugE1 element, namely, between pndC and excA (Fig. 1 and 2).
FIG 2.
Linear comparison of IncK2 plasmids. The open reading frames (ORFs) are illustrated in arrows pointing toward their respective orientations. The color code is as follows: the transfer-associated genes are in green, pilus genes are in light blue, mcr-1 is in red, the putative pap2 ORF is in brown, other antibiotic resistance genes are in pink, insertion sequence (IS) elements are in orange, replicase genes are in dark blue, partitioning genes and toxin/anti-toxin and other stabilization systems are in yellow, shufflon-associated genes are in violet, and other genes are in lavender. Important genes and loci are indicated above or below the plasmid sequence. The blue lines above the figure indicate different loci and the shufflon region. The indications on the left-hand side are the respective isolate/plasmid profiles. CH, Switzerland.
To assess transferability of the two IncK2 plasmids, conjugation experiments were performed by filter mating, using a rifampin-resistant derivative of the sodium azide-resistant E. coli strain J53. Transconjugants were selected on Luria-Bertani (LB) agar plus sodium azide (150 μg/ml) and colistin (2 μg/ml), and confirmed by counterselection on LB agar plus rifampin (50 μg/ml) and colistin (2 μg/ml) and mcr-1 real-time PCR (16). Conjugation efficiencies at 37°C were 2.6 × 10−4 for pMbl488 and 6.3 × 10−6 transconjugants/donor for pMbl536, respectively, with the lower frequency observed for pMbl536 possibly due to an adenine insertion in traE (confirmed by Sanger sequencing) leading to a premature stop codon.
To the best of our knowledge, this is the first report of IncK2 plasmids carrying mcr-1. These plasmids were structurally highly similar to IncK2 blaCMY-2/4-harboring plasmids that we recently described in E. coli (frequently of ST38) isolated in Switzerland from local chicken and chicken meat (15). Such E. coli strains carrying blaCMY-2-IncK2 plasmids, including strains belonging to ST38, were also associated with broiler production in other European countries (17, 18). Furthermore, a recent Norwegian report strongly supports the hypothesis that clonal transfer of these ST38 E. coli strains from chicken meat to humans may occur (19). Thus, the additional acquisition of mcr-1 in hyperepidemic extended-spectrum β-lactamase (ESBL)-producing ST38 E. coli (e.g., strain Mbl488 found in the present analysis) is concerning.
Notably, in the ST38 strain Mbl323 isolated from retail chicken meat, which also carried a 100-kb IncK2 plasmid harboring a blaCMY-2 gene, mcr-1 was found on a 2.85-Mbp contig (i.e., integrated in the genome; see Table 1). Thus, besides confirming the continuous ongoing spread of such plasmid-mediated AmpC-producing ST38 E. coli hyperepidemic clones in broiler production in Switzerland and other European countries, our report further suggests that these strains can also acquire additional mcr-1-mediated colistin resistance.
Interestingly, mcr-1 was also found on a 2.9-Mbp contig (i.e., in the chromosome of strain Mbl506) and sequence comparison of the mcr-1 surrounding genetic regions of Mbl323 (KY689636) and Mbl506 (KY689637) revealed that the ISApl1-mcr-1 element was integrated in the same location (Fig. S5A). Additionally, a second ISApl1-mcr-1 copy in Mbl506 (KY689638) was found on a 2 Mbp contig in a completely different genomic region (Fig. S5B).
In conclusion, mcr-1 was detected in a variety of plasmid types, including the newly identified IncK2-type plasmids, as well as integrated into the bacterial genome. Some of the plasmids were highly similar to others previously found in diverse E. coli lineages from different sources, suggesting horizontal spread. Nevertheless, direct colonization with mcr-1-carrying hyperepidemic E. coli clones from other reservoirs (e.g., food animals) cannot be excluded, as already suggested for ST38 E. coli carrying blaCMY-2-IncK2 plasmids (17–19). Therefore, adequate measures should be taken, particularly in broiler production, to limit the acquisition of the mcr-1 gene and further spread of such clones.
Accession number(s).
The complete nucleotide sequence of the seven plasmids and 40-kb sequences of the three chromosomal regions surrounding mcr-1 have been deposited into the GenBank database under accession numbers KY689633 (p100R), KY689632 (p19M12), KY565556 (pMcp0271), KY689634 (p31349), KY565557 (pMcp0221), KY565558 (pMbl488), KY689635 (pMbl536), KY689636 (Mbl323; chromosomal mcr-1 insertion region), KY689637 (Mbl506; chromosomal mcr-1 insertion region 1), and KY689638 (Mbl506; chromosomal mcr-1 insertion region 2).
Supplementary Material
ACKNOWLEDGMENTS
We thank Regula Tinguely, Sara Kasraian, and Alexandra Rossano for technical assistance.
This work was supported by the Swiss National Science Foundation (SNF; grant number 153377 to A.E.) and from the Federal Food Safety and Veterinary Office (FSVO; grant no. 1.15.07 to V.P.) within the framework of the Animal Health and Welfare (ANIHWA) ERA-Net project “Prevalence and optimized detection of resistance to antibiotics vital for animal and human health (PRAHAD).”
Odette J. Bernasconi is a PhD student (2015 to 2018) supported by the Hans Sigrist Foundation (Bern, Switzerland). João Pires is a PhD student (2014 to 2017) supported by SNF.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01245-17.
REFERENCES
- 1.Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 2.Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, Day M, Muller-Pebody B, Ellington MJ, de Pinna E, Johnson AP, Hopkins KL, Woodford N. 2016. Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother 71:2300–2305. doi: 10.1093/jac/dkw093. [DOI] [PubMed] [Google Scholar]
- 3.Zhao F, Feng Y, Lu X, McNally A, Zong Z. 2017. IncP Plasmid carrying colistin resistance gene mcr-1 in Klebsiella pneumoniae from hospital sewage. Antimicrob Agents Chemother 61:e02229-16. doi: 10.1128/AAC.02229-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. 2016. Escherichia coli harboring mcr-1 and blaCTX-M on a novel IncF plasmid: first report of mcr-1 in the United States. Antimicrob Agents Chemother 60:4420–4421. doi: 10.1128/AAC.01103-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang C, Feng Y, Liu F, Jiang H, Qu Z, Lei M, Wang J, Zhang B, Hu Y, Ding J, Zhu B. 2017. A phage-like incy plasmid carrying the mcr-1 gene in Escherichia coli from a pig farm in China. Antimicrob Agents Chemother 61:e02035–e02016. doi: 10.1128/AAC.02035-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zurfluh K, Tasara T, Poirel L, Nordmann P, Stephan R. 2016. Draft genome sequence of Escherichia coli S51, a chicken isolate harboring a chromosomally encoded mcr-1 gene. Genome Announc 4:e00796-16. doi: 10.1128/genomeA.00796-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CY, Ang GY, Chong TM, Chin PS, Ngeow YF, Yin WF, Chan KG. 2017. Complete genome sequencing revealed novel genetic contexts of the mcr-1 gene in Escherichia coli strains. J Antimicrob Chemother 72:1253–1255. doi: 10.1093/jac/dkw541. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Fang LX, Wu Z, Deng H, Yang RS, Li XP, Li SM, Liao XP, Feng Y, Liu YH. 2017. Genetic analysis of the IncX4 plasmids: implications for a unique pattern in the mcr-1 acquisition. Sci Rep 7:424. doi: 10.1038/s41598-017-00095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernasconi OJ, Kuenzli E, Pires J, Tinguely R, Carattoli A, Hatz C, Perreten V, Endimiani A. 2016. Travelers can import colistin-resistant Enterobacteriaceae, including those possessing the plasmid-mediated mcr-1 gene. Antimicrob Agents Chemother 60:5080–5084. doi: 10.1128/AAC.00731-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pires J, Bernasconi OJ, Hauser C, Tinguely R, Atkinson A, Perreten V, Dona V, Rauch A, Furrer H, Endimiani A. 2017. Intestinal colonisation with extended-spectrum cephalosporin- and colistin-resistant Enterobacteriaceae in HIV-positive individuals in Switzerland: molecular features and risk factors. Int J Antimicrob Agents 49:519–521. doi: 10.1016/j.ijantimicag.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Federal Office of Public Health and Federal Food Safety and Veterinary Office. 2016. Swiss antibiotic resistance report 2016. Usage of antibiotics and occurrence of antibiotic resistance in bacteria from humans and animals in Switzerland. FOPH publication number: 2016-OEG-30. [Google Scholar]
- 12.Di Pilato V, Arena F, Tascini C, Cannatelli A, Henrici De Angelis L, Fortunato S, Giani T, Menichetti F, Rossolini GM. 2016. mcr-1.2, a new mcr variant carried on a transferable plasmid from a colistin-resistant KPC carbapenemase-producing Klebsiella pneumoniae strain of sequence type 512. Antimicrob Agents Chemother 60:5612–5615. doi: 10.1128/AAC.01075-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richter M, Rossello-Mora R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci U S A 106:19126–19131. doi: 10.1073/pnas.0906412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gascuel O. 1997. BIONJ: an improved version of the NJ algorithm based on a simple model of sequence data. Mol Biol Evol 14:685–695. doi: 10.1093/oxfordjournals.molbev.a025808. [DOI] [PubMed] [Google Scholar]
- 15.Seiffert SN, Carattoli A, Schwendener S, Collaud A, Endimiani A, Perreten V. 2017. Plasmids carrying blaCMY-2/4 in Escherichia coli from poultry, poultry meat, and humans belong to a novel IncK subgroup designated IncK2. Front Microbiol 8:407. doi: 10.3389/fmicb.2017.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donà V, Bernasconi OJ, Kasraian S, Tinguely R, Endimiani A. 2017. A SYBR(R) Green-based real-time PCR method for improved detection of mcr-1-mediated colistin resistance in human stool samples. J Glob Antimicrob Resist 9:57–60. doi: 10.1016/j.jgar.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Voets GM, Fluit AC, Scharringa J, Schapendonk C, van den Munckhof T, Leverstein-van Hall MA, Stuart JC. 2013. Identical plasmid AmpC beta-lactamase genes and plasmid types in E. coli isolates from patients and poultry meat in the Netherlands. Int J Food Microbiol 167:359–362. doi: 10.1016/j.ijfoodmicro.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Mo SS, Slettemeas JS, Berg ES, Norstrom M, Sunde M. 2016. Plasmid and host strain characteristics of Escherichia coli resistant to extended-spectrum cephalosporins in the Norwegian broiler production. PLoS One 11:e0154019. doi: 10.1371/journal.pone.0154019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg ES, Wester AL, Ahrenfeldt J, Mo SS, Slettemeas JS, Steinbakk M, Samuelsen O, Grude N, Simonsen GS, Lohr IH, Jorgensen SB, Tofteland S, Lund O, Dahle UR, Sunde M. 2017. Norwegian patients and retail chicken meat share cephalosporin-resistant Escherichia coli and IncK/blaCMY-2 resistance plasmids. Clin Microbiol Infect 23:407.e9–407.e15. doi: 10.1016/j.cmi.2016.12.035. [DOI] [PubMed] [Google Scholar]
- 20.European Committee on Antimicrobial Susceptibility Testing. 2016. Breakpoint tables for interpretation of MICs and zone diameters. version 6.0 http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.pdf.
- 21.Clinical and Laboratory Standards Institute. 2016. Performance standards for antimicrobial susceptibility testing, 26th ed. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 22.Poirel L, Jayol A, Nordmann P. 2017. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30:557–596. doi: 10.1128/CMR.00064-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poirel L, Kieffer N, Nordmann P. 2017. In vitro study of ISApl1-mediated mobilization of the colistin resistance gene mcr-1. Antimicrob Agents Chemother 61:e00127-17. doi: 10.1128/AAC.00127-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



