ABSTRACT
We evaluated the in vitro and in vivo effects of nikkomycin Z combined with an echinocandin (anidulafungin or micafungin) against two Candida albicans isolates and their lab-derived echinocandin-resistant fks mutants with FKS1 S645Y and FKS1 S645P. Synergistic effects were observed in all tested strains (fractional inhibitory concentration index, <0.5). Enhanced survival was observed in an immunocompromised murine model (log-rank test, P < 0.02). Our study demonstrated the therapeutic potential of nikkomycin Z-echinocandin combinations in managing echinocandin resistance.
KEYWORDS: Candida albicans, FKS, echinocandin, nikkomycin Z
TEXT
Echinocandins are considered first-line treatment for invasive Candida infections (1). However, treatment failures associated with resistant isolates harboring fks hot-spot mutations have been reported (2, 3). Nikkomycin Z is a chitin synthase inhibitor with potential therapeutic effects against Candida infections (4). Moreover, in vitro synergistic effects were reported when nikkomycin Z was combined with echinocandins against Candida isolates (5, 6). However, the effects of the combination in vivo are not yet available. In this study, we evaluated the in vitro effects of nikkomycin Z combined with an echinocandin (anidulafungin or micafungin) against two Candida albicans isolates (ATCC 90028 and blood culture isolate CA 46503) and their lab-derived echinocandin-resistant fks mutants. The in vivo effects of the antifungal combinations were studied in an immunosuppressed murine model.
Anidulafungin (Pfizer, Inc., USA), micafungin (Astellas Pharma, Inc., Japan), and nikkomycin Z (Sigma, USA) were used throughout the study. Spontaneous fks mutants of the two C. albicans parent strains were isolated by plating 10 μl (∼108 cells) Sabouraud broth culture onto Sabouraud dextrose agar plates containing 8 μg/ml micafungin. Resistant isolates were reinoculated onto fresh plates containing 8 μg/ml micafungin to confirm the nonsusceptible phenotype. The isolates were characterized by fks hot-spot sequencing and antifungal susceptibility tests according to the CLSI broth microdilution method (7, 8). The nikkomycin Z MIC was the lowest drug concentration exhibiting 50% reduction in turbidity after 24 h of incubation. In vitro drug interactions were assessed by checkerboard assays with the fractional inhibitory concentration index (FICI) interpreted as follows: ≤0.5, synergistic; 0.5 to ≤4, indifferent; and >4, antagonistic (9). Tests were done in duplicate. C. albicans fks1 hot spot 1 was amplified with the forward primer BIO-1HS1F 5′-biotin-AATGGGCCGGTGCTCAACA-3′ and reverse (also sequencing) primer 1HS1-seq 5′-TTCACCATTACATCTCAT-3′. Corresponding primers for fks1 hot spot 2 were BIO-1HS2F 5′-biotin-AAGATTGGTGCTGGTATGGG-3′ and 1HS2-seq 5′-ACCTCTTTCAATCAATTCTTGAACAAC-3′ (10). The fks hot spots were examined by pyrosequencing (PyroMark Q24; Qiagen, CA).
Murine models of systemic candidiasis were established in ICR mice (weighing ∼20 g) by intravenous inoculation of 100 μl (in a 1-ml syringe; Terumo, USA) of the four C. albicans strains (2 parents and 2 fks mutants; 5 × 106 yeast cells) via tail vein (11). The mice were immunosuppressed by intraperitoneal injection of 100 mg/kg dexamethasone on days −3, 0, 7, and 14. Therapy began 1 day postinfection and continued for 12 days. A dose of 5 mg/kg of and echinocandin (anidulafungin or micafungin) was given subcutaneously once daily (12, 13). A dose of 10 mg/kg of nikkomycin Z was given subcutaneously twice daily (14). All mice were held for 17 days and monitored daily for mortalities. There were 10 mice per group. Kaplan-Meier survival plots were analyzed by a log-rank test (Prism, version 7.03; GraphPad Software, CA). P values were considered significant at the 0.05 level. All animal studies were approved by the Institutional Review Board.
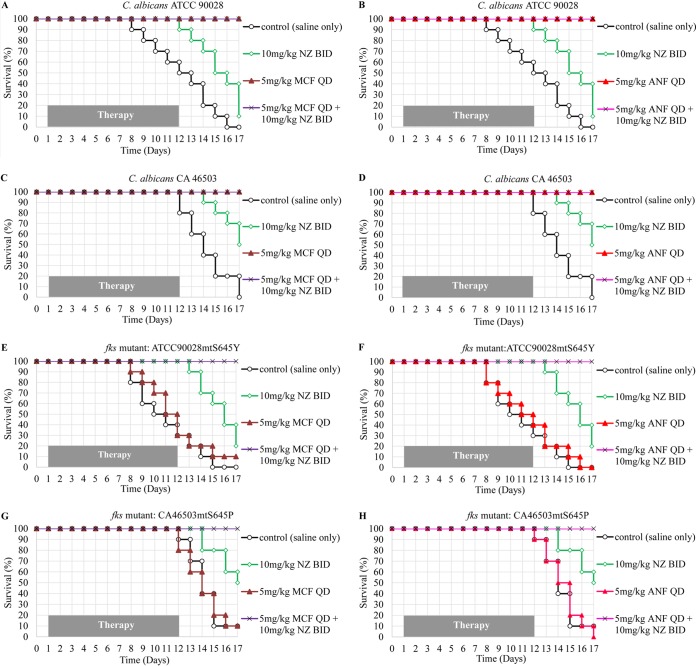
Two spontaneous fks mutants, ATCC90028fksmtS645Y and CA46503fksmtS645P, were derived from C. albicans ATCC 90028 and CA 46503, respectively. Both mutants harbored a single substitution mutation in the fks1 hot-spot region, and both were homozygous. The MIC results and FICIs are shown in Table 1. The fks mutants showed 32-fold elevations in MIC for anidulafungin and micafungin. Synergistic effects (nikkomycin Z and echinocandin) were observed in the parent strains and the fks mutants. Kaplan-Meier survival curves are shown in Fig. 1. In the saline treatment control group, C. albicans ATCC 90028 was more virulent than CA 46503. The killing rates of the parent strains and their derived fks mutants were similar. Monotherapy with nikkomycin Z prolonged the survival of all infected mice (log-rank test, P < 0.01), but the survival rates declined once the nikkomycin Z was discontinued. Treatment with either anidulafungin or micafungin improved the survival of mice infected with the parent strain but not in those infected with the fks mutants. Combination treatment with nikkomycin Z and either echinocandin significantly improved the survival rate of mice infected with the fks mutants compared with that of mice treated with nikkomycin Z or echinocandin monotherapy (log-rank test, P < 0.02).
TABLE 1.
MIC and FICI values of C. albicans parent strains and their lab-derived fks mutants
| C. albicans strain | FKS hot-spot region | MIC (μg/ml) ofa: |
Drug combinationb | MIC of combination (μg/ml) | FICIc | Interpretation | ||
|---|---|---|---|---|---|---|---|---|
| ANF | MCF | NZ | ||||||
| ATCC 90028 | Wild type | 0.03 (S) | 0.03 (S) | 4 | ANF + NZ | 0.004 + 1 | 0.38 | Synergy |
| MCF + NZ | 0.004 + 1 | 0.38 | Synergy | |||||
| ATCC 90028mtS645Y | FKS1 S645Y | 1 (R) | 1 (R) | 4 | ANF + NZ | 0.125 + 1 | 0.38 | Synergy |
| MCF + NZ | 0.125 + 1 | 0.38 | Synergy | |||||
| CA 46503 | Wild type | 0.03 (S) | 0.03 (S) | 4 | ANF + NZ | 0.004 + 1 | 0.38 | Synergy |
| MCF + NZ | 0.004 + 1 | 0.38 | Synergy | |||||
| CA 46503mtS645P | FKS1 S645P | 1 (R) | 1 (R) | 4 | ANF + NZ | 0.125 + 1 | 0.38 | Synergy |
| MCF + NZ | 0.125 + 1 | 0.38 | Synergy | |||||
S, sensitive; R, resistant.
ANF, anidulafungin; MCF, micafungin; NZ, nikkomycin Z.
FICI, fractional inhibitory concentration index.
FIG 1.
Survival curves of the immunosuppressed mice infected with C. albicans parent strains (ATCC 90028 and CA 46503) and their lab-derived fks mutants (ATCC90028mtS645Y and CA46503mtS645P). NZ, nikkomycin Z; MCF, micafungin; ANF, anidulafungin; QD, once daily; BID, twice daily.
In this study, spontaneous C. albicans fks mutants were derived to assess the effects of combinations of nikkomycin Z and echinocandins. The mutations, fks1 T1933C (FKS1 S645P) and fks1 C1934A (FKS1 S645Y), and their associated elevations in echinocandin MIC were also observed previously (15, 16). The maximum plasma concentrations of anidulafungin, micafungin, and nikkomycin Z were reported to be, respectively, 49.5, 53, and 49.5 μg/ml in murine (13, 17, 18) and 8, 16, and 6.42 μg/ml in human adults (19, 20). Our in vitro synergistic effects were observed at achievable plasma concentrations in murine and humans, suggesting that the effects are potentially useful in vivo. Although the mechanism of the synergy is not fully understood, it was reported that chitin synthesis was upregulated as a result of cell wall salvage pathways when C. albicans isolates were exposed to caspofungin (21). The simultaneous inhibition of chitin synthase and β-1,3-glucan synthase by nikkomycin Z and an echinocandin probably renders the salvage pathway useless and impairs construction of the cell wall.
The in vivo response in this study correlated well with the resistance phenotype. Monotherapy with echinocandin did not produce significant survival in fks mutant-infected mice. Their survival was enhanced by nikkomycin Z treatment; however, similar to a previous report, survival declined when treatment was discontinued (14). Combination treatment with nikkomycin Z and echinocandin prevented such a decline and significantly improved survival of the fks mutant-infected mice.
In contrast to previous reports that used immunocompetent murine models, presence of the fks mutations in C. albicans isolates was not associated with decreased virulence in our immunosuppressed murine model (15, 22). The use of dexamethasone as an immunosuppressant may have affected the virulence results. To the best of our knowledge, this is the first report to demonstrate the in vivo therapeutic effects of combined nikkomycin Z and echinocandin in treating fks mutation-associated echinocandin-resistant C. albicans infections. One limitation of this study was that we evaluated only one dosing regimen (nikkomycin Z at 10 mg/kg twice daily). Future studies are needed to determine the dose-dependent effect of nikkomycin Z.
ACKNOWLEDGMENT
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.El Zakhem A, Saad H, Tayyar R, Kanj SS. 2015. Controversies in Candida management. Int J Antimicrob Agents 46:S43–S46. doi: 10.1016/j.ijantimicag.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Baixench MT, Aoun N, Desnos-Ollivier M, Garcia-Hermoso D, Bretagne S, Ramires S, Piketty C, Dannaoui E. 2007. Acquired resistance to echinocandins in Candida albicans: case report and review. J Antimicrob Chemother 59:1076–1083. doi: 10.1093/jac/dkm095. [DOI] [PubMed] [Google Scholar]
- 3.Niimi K, Monk BC, Hirai A, Hatakenaka K, Umeyama T, Lamping E, Maki K, Tanabe K, Kamimura T, Ikeda F, Uehara Y, Kano R, Hasegawa A, Cannon RD, Niimi M. 2010. Clinically significant micafungin resistance in Candida albicans involves modification of a glucan synthase catalytic subunit GSC1 (FKS1) allele followed by loss of heterozygosity. J Antimicrob Chemother 65:842–852. doi: 10.1093/jac/dkq073. [DOI] [PubMed] [Google Scholar]
- 4.Chapman T, Kinsman O, Houston J. 1992. Chitin biosynthesis in Candida albicans grown in vitro and in vivo and its inhibition by nikkomycin Z. Antimicrob Agents Chemother 36:1909–1914. doi: 10.1128/AAC.36.9.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stevens DA. 2000. Drug interaction studies of a glucan synthase inhibitor (LY 303366) and a chitin synthase inhibitor (nikkomycin Z) for inhibition and killing of fungal pathogens. Antimicrob Agents Chemother 44:2547–2548. doi: 10.1128/AAC.44.9.2547-2548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sandovsky-Losica H, Shwartzman R, Lahat Y, Segal E. 2008. Antifungal activity against Candida albicans of nikkomycin Z in combination with caspofungin, voriconazole or amphotericin B. J Antimicrob Chemother 62:635–637. doi: 10.1093/jac/dkn216. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; 4th informational supplement. CLSI document M27-S4 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob Agents Chemother 48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother 52:2305–2312. doi: 10.1128/AAC.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luque JC, Clemons KV, Stevens DA. 2003. Efficacy of micafungin alone or in combination against systemic murine aspergillosis. Antimicrob Agents Chemother 47:1452–1455. doi: 10.1128/AAC.47.4.1452-1455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Patterson TF. 2012. Comparison of anidulafungin's and fluconazole's in vivo activity in neutropenic and non-neutropenic models of invasive candidiasis. Clin Microbiol Infect 18:E20–E23. doi: 10.1111/j.1469-0691.2011.03712.x. [DOI] [PubMed] [Google Scholar]
- 13.Andes DR, Diekema DJ, Pfaller MA, Marchillo K, Bohrmueller J. 2008. In vivo pharmacodynamic target investigation for micafungin against Candida albicans and C. glabrata in a neutropenic murine candidiasis model. Antimicrob Agents Chemother 52:3497–3503. doi: 10.1128/AAC.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker JM, Marcus S, Tallock J, Miller D, Krainer E, Khare RK, Naider F. 1988. Use of the chitin-synthesis inhibitor nikkomycin to treat disseminated candidiasis in mice. J Infect Dis 157:212–214. doi: 10.1093/infdis/157.1.212. [DOI] [PubMed] [Google Scholar]
- 15.Ben-Ami R, Garcia-Effron G, Lewis RE, Gamarra S, Leventakos K, Perlin DS, Kontoyiannis DP. 2011. Fitness and virulence costs of Candida albicans FKS1 hot spot mutations associated with echinocandin resistance. J Infect Dis 204:626–635. doi: 10.1093/infdis/jir351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castanheira M, Woosley LN, Diekema DJ, Messer SA, Jones RN, Pfaller MA. 2010. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob Agents Chemother 54:2655–2659. doi: 10.1128/AAC.01711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shubitz LF, Trinh HT, Perrill RH, Thompson CM, Hanan NJ, Galgiani JN, Nix DE. 2014. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J Infect Dis 209:1949–1954. doi: 10.1093/infdis/jiu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seyedmousavi S, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2013. Pharmacodynamics of anidulafungin against clinical Aspergillus fumigatus isolates in a nonneutropenic murine model of disseminated aspergillosis. Antimicrob Agents Chemother 57:303–308. doi: 10.1128/AAC.01430-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nix DE, Swezey RR, Hector R, Galgiani JN. 2009. Pharmacokinetics of nikkomycin Z after single rising oral doses. Antimicrob Agents Chemother 53:2517–2521. doi: 10.1128/AAC.01609-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cappelletty D, Eiselstein-McKitrick K. 2007. The echinocandins. Pharmacotherapy 27:369–388. doi: 10.1592/phco.27.3.369. [DOI] [PubMed] [Google Scholar]
- 21.Walker LA, Munro CA, de Bruijn I, Lenardon MD, McKinnon A, Gow NA. 2008. Stimulation of chitin synthesis rescues Candida albicans from echinocandins. PLoS Pathog 4:e1000040. doi: 10.1371/journal.ppat.1000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiederhold NP, Najvar LK, Bocanegra RA, Kirkpatrick WR, Patterson TF. 2011. Caspofungin dose escalation for invasive candidiasis due to resistant Candida albicans. Antimicrob Agents Chemother 55:3254–3260. doi: 10.1128/AAC.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]