ABSTRACT
In pharmacokinetic/pharmacodynamic models of pulmonary Mycobacterium abscessus complex, the recommended macrolide-containing combination therapy has poor kill rates. However, clinical outcomes are unknown. We searched the literature for studies published between 1990 and 2017 that reported microbial outcomes in patients treated for pulmonary M. abscessus disease. A good outcome was defined as sustained sputum culture conversion (SSCC) without relapse. Random effects models were used to pool studies and estimate proportions of patients with good outcomes. Odds ratios (OR) and 95% confidence intervals (CI) were computed. Sensitivity analyses and metaregression were used to assess the robustness of findings. In 19 studies of 1,533 patients, combination therapy was administered to 508 patients with M. abscessus subsp. abscessus, 204 with M. abscessus subsp. massiliense, and 301 with M. abscessus with no subspecies specified. Macrolide-containing regimens achieved SSCC in only 77/233 (34%) new M. abscessus subsp. abscessus patients versus 117/141 (54%) M. abscessus subsp. massiliense patients (OR, 0.108 [95% CI, 0.066 to 0.181]). In refractory disease, SSCC was achieved in 20% (95% CI, 7 to 36%) of patients, which was not significantly different across subspecies. The estimated recurrent rates per month were 1.835% (range, 1.667 to 3.196%) for M. abscessus subsp. abscessus versus 0.683% (range, 0.229 to 1.136%) for M. abscessus subsp. massiliense (OR, 6.189 [95% CI, 2.896 to 13.650]). The proportion of patients with good outcomes was 52/223 (23%) with M. abscessus subsp. abscessus versus 118/141 (84%) with M. abscessus subsp. massiliense disease (OR, 0.059 [95% CI, 0.034 to 0.101]). M. abscessus subsp. abscessus pulmonary disease outcomes with the currently recommended regimens are atrocious, with outcomes similar to those for extensively drug-resistant tuberculosis. Therapeutically, the concept of nontuberculous mycobacteria is misguided. There is an urgent need to craft entirely new treatment regimens.
KEYWORDS: macrolides, Mycobacterium abscessus, hollow-fiber model, medical outcomes, pulmonary infection
INTRODUCTION
Mycobacterium abscessus complex members are rapidly growing mycobacteria associated with a wide spectrum of disease in humans, of which pulmonary disease is the most recalcitrant (1). M. abscessus complex members are ubiquitous in the environment, including in household tap water and bioaerosols (2, 3). Hospital water supplies have been linked to M. abscessus complex disease outbreaks (4, 5). Whole-genome sequencing (WGS) has also suggested a potential for person-to-person transmission (4). Two groups of vulnerable patients are most affected by M. abscessus complex: nonsmoking women of European descent who are >60 years old and have no history of lung disease and younger men <40 years old with prior lung disease, such as α-1 antitrypsin deficiency and cystic fibrosis (6). In the latter group of patients, M. abscessus complex can be a coinfection with other mycobacteria, leading to a high rate of disease recurrence (7). This makes diagnosis and microbial killing of the individual mycobacterial species difficult, given the differences in susceptibility between species (8, 9). Fortunately, advances in medical therapy have increased, but this has had the effect of increasing the proportion of the population at risk; thus, the disease numbers from M. abscessus complex now surpass those for tuberculosis in some places (6). Greater efforts toward improving quality of life for those affected are now warranted (10, 11).
M. abscessus complex has three subspecies: M. abscessus subsp. abscessus, M. abscessus subsp. massiliense, and M. abscessus subsp. bolletii (12). M. abscessus complex subspecies are naturally resistant to many antibiotics and rapidly develop acquired drug resistance (ADR), leading to the moniker “the antibiotic nightmare” (1). There are also differences in susceptibility to macrolides (clarithromycin or azithromycin), aminoglycosides, quinolones, and tigecycline between the subspecies, with better susceptibility seen with M. abscessus subsp. massiliense than M. abscessus subsp. abscessus (8, 9, 13). Thus, treatment of M. abscessus subsp. abscessus disease has a disadvantage of poor MICs from the beginning. Treatment guidelines recommend a regimental backbone of a macrolide, a β-lactam (cefoxitin, imipenem, or meropenem), and an aminoglycoside given in the first 2 to 4 months (6). The optimal duration of therapy is undefined. These drugs and their doses were chosen based on their use in other bacterial infections. Except for clarithromycin, drug sensitivity testing (DST) for the antibiotics used against M. abscessus complex is known not to predict clinical outcomes (14, 15). Examination of the recommended antibiotics as monotherapy, or as combination therapy, in the novel hollow-fiber model of pulmonary M. abscessus subsp. abscessus disease identified a biphasic response that was universally terminated by emergence of ADR, even at optimized doses not tolerable in patients (9, 13, 16–18). This led us to ask what the real response rates of the recommended regimen in the clinic are. Is the regimen any good? On the other hand, if a drug such as amikacin or the three-drug combination is associated with therapy failure and ADR even at maximum dose or exposure, then it cannot be improved upon (drugs or combinations cannot kill any more than their maximum possible kill) even if we changed the administration routes. Therefore, we systematically reviewed the literature, rated risk of bias, and then determined the proportion of patients attaining sustained sputum culture conversion (SSCC) and disease recurrence in clinical studies that examined different therapy regimens for pulmonary M. abscessus complex.
RESULTS
Study selection and patient characteristics.
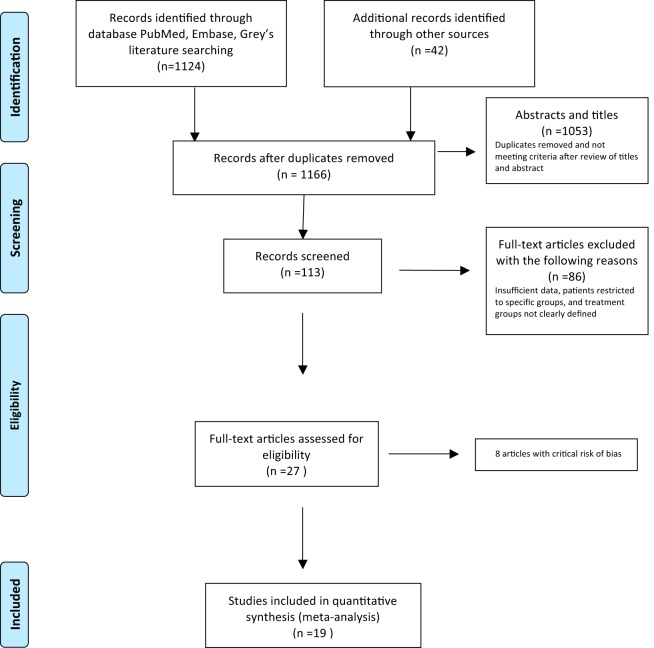
Of the 1,166 unique citations identified through systematic search, 27 were eligible for full review, and 19 of these were included in our analyses (Fig. 1) (4, 6, 7, 11, 12, 14–16, 19–36). We excluded 8 studies that we found to be at critical risk of bias: 4 studies did not clearly state therapy regimens, and the remaining 4 studies enrolled selected patient groups or the enrolled number of patients was <10, which was considered inadequate (see the supplemental material). Nonetheless, of the 19 selected studies, only 7 (37%) were at low risk of bias based on ROBINS-I (for risk of bias in nonrandomized studies–of interventions) criteria. The remainder, 12/19 (63%), were at moderate or serious risk of bias due to missing data across all domains and due to confounding (see Fig. S1 in the supplemental material). Eleven (58%) of the 19 studies were from Northeast Asia (2 from Japan, 8 from South Korea, and 1 from Taiwan), 7 (37%) were from North America, and one (5%) was from the Netherlands.
FIG 1.
Study enrollment.
The 19 selected papers reported 1,533 immunocompetent pulmonary M. abscessus complex patients, of whom 1,013 (66%) were started on antimicrobial combination chemotherapy (Table 1). Of the 1,013 treated patients, M. abscessus subsp. abscessus was isolated in 508 (50%) patients, M. abscessus subsp. massiliense in 204 (20%) patients, and M. abscessus subspecies bolletii in only 3 patients (<1%). The remainder, 301 (30%), did not have isolates identified by subspecies and were termed M. abscessus with no subspecies specified. Three patients had mixed infections, i.e., had more than one subspecies (Table 2).
TABLE 1.
Characteristics of pulmonary Mycobacterium abscessus complex studies identified through systematic review
| Reference | Enrollment period | Study location (referral) | Study designa | No. of patients | Age, yr (range) | Male/female ratio | Outcome(s) examinedb | No. (%) who died on therapyc,f |
|---|---|---|---|---|---|---|---|---|
| Macrolide-free regimens | ||||||||
| Griffith et al. (21) | 1976–1991 | Texas (southern USA) | RetroCS | 120 | 54 ± 19.6 | 42:78 | SCC, relapse, death | 20 (17) |
| Macrolide-containing regimens | ||||||||
| van Ingen et al. (32) | 1999–2005 | Netherlands | RetroCS | 30 | 53 (1–89) | 19:11 | SCC, death | 4 (8) |
| Jeon et al. (14) | 2000–2007 | Seoul, South Korea | RetroCS | 188 | 55 (43–67) | 48:140 | SCC, relapse, death (RSS) | 2 (1)d |
| Jarand et al. (23) | 2001–2004 | Colorado, USA (36 states) | RetroCS | 107 | 60.2 (20–85) | 18:89 | SCC, relapse, death | 17 (16) |
| Lyu et al. (26) | 2003–2008 | Seoul, South Korea | RetroCS | 112 | 53.2 (22–77) | 10:31 | SCC, relapse, death | 0 |
| Koh et al. (15) | 2004–2008 | Seoul, South Korea | RetroCS | 145 | 57.6 ± 13.0 | 37:108 | SCC, relapse (RSS) | 0 |
| Harada et al. (22) | 1990–2010 | Japan (12 centers) | RetroCS | 102 | 68 (27–94) | 39:58e | SCC, relapse (radiographic) | 0 |
| Tung et al. (31) | 2006–2012 | Kaohsiung, Taiwan | RetroCS | 106 | 64.56 ± 14.11 | 44:62 | SCC, relapse, death (radiographic) | 15 (14) |
| Griffith et al. (7) | 2000–2012 | Texas, USA | RetroCS | 21 | 75.5 ± 8.5 | 2:19 | SCC, relapse | 0 |
| Namkoong et al. (27) | 2004–2013 | Tokyo, Japan | RetroCS | 92 | 63.6 ± 8.5 | 2:11 | SCC (radiographic) | NA |
| Koh et al. (25) | 2007–2012 | Seoul, South Korea | ProspCS | 71 | 57 (50–64) | 10:61 | SCC (RSS) | NA |
| Czaja et al. (20) | 2009–2012 | Colorado, USA (southern states) | ProspCS | 53 | 65 ± 11 | 7:40 | SCC (RSS) | NA |
| Koh et al. (24) | 2002–2012 | Seoul, South Korea | ProspCS | 67 | 57 (48–64) | 15:52 | SCC (RSS) | NA |
| Park et al. (30) | 2006–2015 | Seoul, South Korea | RetroCS | 113 | 64 (52–71) | 39:71 | SCC (RSS) | NA |
| Macrolide-containing plus Investigational drugs in refractory diseaseg | ||||||||
| Olivier et al. (29) | 2003–2010 | Maryland, USA | RetroCS of inhaled amikacin | 23 | 56 ± 16 | 4:16 | SCC | NA |
| Wallace et al. (33) | 2002–2006 | Texas, USA | ProspCS of tigecycline | 36 | 35.2 ± 22.2 | 7:29 | SCC | NA |
| Yang et al. (34) | 2013–2015 | Seoul, South Korea | RetroCS of clofazimine | 42 | 60 (53–69) | 9:33 | SCC (RSS) | NA |
| Olivier et al. (28) | 2012–2015 | North America (Canada and USA) | RCT of liposomal amikacin (NCT01315236) | 90 | 58.5 ± 15.83 | 11:78 | Semiquantitative mycobacterial growth scale, SCC, 6-min walk, adverse events | 2 (2.2) |
| Choi et al. (19) | 2005–2015 | Seoul, South Korea | RetroCS | 15 | 57 (48–67) | 5:10 | SCC, death | 5 (33) |
RCT, randomized control trial; ProspCS, prospective cohort study; RetroCS, retrospective cohort study.
SCC, sputum culture conversion; RSS, radiographic, symptomatic response.
NA, data not available.
Studies that only reported deaths due to pulmonary Mycobacterium abscessus complex disease in treated patients.
Some data of patients with Mycobacterium abscessus subspecies bolletii are missing.
Data analyzed or available in text or tables only.
Refractory means failing initial therapy.
TABLE 2.
Combination antimycobacterial chemotherapy and other clinical interventions examined by selected studiesa
| Reference | No. of patients treated | Type of infectionc | erm(41) gene deletion (no. of patients/total no.) | No. of patients with CF, AAT, or CDb | Macrolide(s) used | Aminoglycoside(s) usedd | Other antibiotics used in combination therapy; no. of patients who received surgery | Duration, moe (range) |
|
|---|---|---|---|---|---|---|---|---|---|
| Therapy | Follow-up | ||||||||
| Macrolide-free regimens | |||||||||
| Griffith et al. (21) | 120 | 120 NSS | NA | 9 CF | None | i.v. AMK, daily | FOX, IPM, SXT, ERY, other anti-TB drugs; 7 | NA | 58.8 ± 4.8 |
| Macrolide-containing regimens | |||||||||
| van Ingen et al. (32) | 12 | 9 M. abscessus subsp. abscessus, 2 M. abscessus subsp. massiliense, 1 M. abscessus subsp. bolletii | NA | 4 CF | CLR | i.v. AMK, daily | FOX, IPM, SXT, LVX, and first-line anti-TB drugs; 1 | 13 | NA |
| Jeon et al. (14) | 86 | 86 NSS | NA | NA | CLR | 1/12 i.v. AMK, twice daily | FOX, IPM, CIP, DOX; 14 | 24.4 ± 0.2 | 12 (5–30) |
| Jarand et al. (23) | 107 | 107 M. abscessus subsp. abscessus | NA | 25 CF, 1 AAT | AZM, CLR | 3/12 i.v. AMK | FOX, IPM, LVX, SXT and others, individualized based on DST; 24 | 52 ± 40.6 | 34 ± 21.1 |
| Lyu et al. (26) | 41 | 41 NSS | NA | NA | AZM, CLR | 8/12 i.v. AMK, once daily | FOX, IPM, DOX, quinolones (CIP, MXF); 13 | 17.03 (5.46–41.63) | 14.83 (0–48.1) |
| Koh et al. (15) | 67 | 30 M. abscessus subsp. Abscessus, 37 M. abscessus subsp. massiliense | 0/19 M. abscessus subsp. abscessus, 28/28 M. abscessus subsp. massiliense | NA | CLR | 1/12 i.v., AMK twice daily | FOX, IPM, DOX, CIP; 0 | 23.1 ± 12.9 M. abscessus subsp. abscessus, 21.6 ± 7.7 M. abscessus subsp. massiliense | NA |
| Harada et al. (22) | 64 | 42 M. abscessus subsp. abscessus, 20 M. abscessus subsp. massiliense, 2 M. abscessus subsp. bolletii | NA | NA | AZM, CLR, ERY | i.v. STP, AMK, KAN | FOX, IPM, DOX, quinolones (CIP, MXF); anti-TB drugs; 6 | 33 (3–178) M. abscessus subsp. abscessus, 36 (1–122) M. abscessus subsp. massiliense, 36 (4–68) M. abscessus subsp. bolletii | 25 (1–120) M. abscessus subsp. abscessus, 18 (1–62) M. abscessus subsp. massiliense |
| Tung et al. (31) | 56 | 56 M. abscessus subsp. abscessus | NA | NA | CLR | i.v. AMK | FOX, IPM, MEM, DOX, quinolones (CIP, MXF); anti-TB drugs; 0 | 12 | NA |
| Griffith et al. (7) | 11 | 11 M. abscessus subsp. abscessus | NA | NA | AZM, CLR | i.v. AMK | FOX, IPM | NA | 48.3 ± 28.7 |
| Namkoong et al. (27) | 13 | 13 M. abscessus subsp. abscessus | NA | NA | CLR | i.v. AMK, thrice weekly | Faropenem, sitafloxacin, MIN, IPM; 0 | 21.31 ± 2.10 | 12 |
| Koh et al. (25) | 71 | 71 M. abscessus subsp. massiliense | 16/16 | NA | AZM, CLR | i.v. AMK | 2-wk regimen of FOX, IPM; 3; 4-wk regimen of FOX, IPM, quinolones (CIP, MXF); 2 | 2-wk regimen, 15.2 (12.7–18.1); 4-wk regimen, 23.9 (23.1–24.1) | 2-wk regimen, 14.7 (0.5–29.5); 4-wk regimen, 33.8 (12.3–50.3) |
| Czaja et al. (20) | 47 | 47 M. abscessus subsp. abscessus | NA | 9 CF, 5 AAT | AZM | 9/12 i.v. and inhalation AMK daily | FOX, IPM, quinolones, CFO; 16 | 17.3 ± 6.6 | 24.97 ± 1.40 |
| Koh et al. (24) | 67 | 67 M. abscessus subsp. abscessus | 7/44 | NA | AZM, CLR | i.v. AMK | FOX, IPM, quinolones (CIP, MXF), DOX; 9 | >12 mo | 11.8 (3.6–27) |
| Park et al. (30) | 113 | 56 M. abscessus subsp. abscessus, 54 M. abscessus subsp. massiliense, 3 mixed | 27/56 (M. abscessus subsp. abscessus), 3/54 (M. abscessus subsp. massiliense) | NA | AZM, CLR | i.v. AMK, 3–5 times weekly | FOX, IPM; 5 (3 M. abscessus subsp. abscessus, 2 M. abscessus subsp. massiliense) | 15.25 (7–29) M. abscessus subsp. Abscessus, 21.75 (16–30) M. abscessus subsp. massiliense | 42.13 ± 22.47 |
| Macrolide-containing regimen plus investigational drugs | |||||||||
| Olivier et al. (29) | 15 | 10 M. abscessus subsp. abscessus, 5 M. abscessus subsp. massiliense | 11/15 | 2 CF, 1 CD | CLR | Nebulized AMK | AMK nebulized 250 mg/ml daily; CLR given daily | 60 (6–190) | 19 (1–50) |
| Wallace et al. (33) | 34 | 34 NSS | 22 CF | AZM, CLR | AMK, tobramycin | FOX, IPM, MEM, quinolones (CIP, MXF), EMB, SXT; 3 | 8.5 ± 8.86 | NA | |
| Yang et al. (34) | 42 | 42 M. abscessus subsp. abscessus | 7/42 | NA | AZM | 4/52 i.v. AMK daily | Initial CLO therapy of FOX, IPM; 1; salvage CLO therapy added to existing regimen (quinolones [CIP, MXF], FOX, IPM); 2 | Both treatment groups, 48.0 (24.8–48.0) | 12.0 |
| Olivier et al. (28) | 32 | 32 NSS | NA | 14 CF | AZM, CLR | 3/12 liposomal AMK daily, Tobramycin | 15 patients on intervention regimen of FOX, IPM, MEM, quinolones (CIP, MXF, LVF), DOX, linezolid, CLO, anti-TB drugs; 0; 17 patients on placebo regimen of FOX, IPM, MEM, quinolone (CIP, MXF, LVF), DOX, linezolid, CLO, TGC, anti-TB drugs; 0 | >24 | 12 |
| Choi et al. (19) | 15 | 15 M. abscessus subsp. massiliense (all macrolide resistant) | 14/15 | NA | AZM, CLR | i.v. and inhalation AMK daily | FOX, IPM, quinolone (CIP, MXF), DOX, SXT; 3 surgery | Prior macrolide, 10 (IQR, 4–17) versus 18.7 (IQR, 11.2–39.8) | 38.7 (IQR, 11.4–41.9) |
Antibiotic or drug abbreviations: AMK, amikacin; AZM, azithromycin; CIP, ciprofloxacin; CLO, clofazimine; CLR, clarithromycin; DOX, doxycycline; EMB, ethambutol; ERY, erythromycin; FOX, cefoxitin; IPM, imipenem; LVX, levofloxacin; MEM, meropenem; MIN, minocycline; MXF, moxifloxacin; SXT, trimethoprim-sulfamethoxazole; TGC, tigecycline.
CF, cystic fibrosis; AAT, abnormal α-1 antitrypsin; CD, ciliary dyskinesia; NA, data not available.
NSS, M. abscessus subspecies not specified.
i.v., intravenous.
IQR, interquartile range.
Table 2 shows that while amikacin was given to patients in all studies examined, the other accompanying drugs in the combination therapy varied widely. In addition, amikacin duration and doses varied from as short as 2 weeks of daily parenteral doses to as long as a year given intermittently via either parenteral, inhalational, or liposomal delivery. Similarly, the duration of the combination therapy and follow-up also varied between studies and even between regimens examined in the same study. The number of patients receiving macrolide-free regimens in intent-to-treat analyses was 120, that for macrolide-containing regimens as initial therapy was 755, and that for patients with refractory disease was 138 (Table 2; see also the supplemental material).
Mortality outcome.
Death was inconsistently reported, and its effect size was considered a critical risk of bias (Table 1). Moreover, only 7 (37%) studies reported on this outcome. The pooled death incidence was 16.67% (95% confidence interval [CI], 10.49 to 24.56%) with macrolide-free regimens. With regard to macrolide-containing regimens as initial therapy, only 3 (16%) studies reported deaths, and the pooled incidence was 15% (95% CI, 11 to 20%) with an I2 value of 75%. For macrolide-containing regimens in refractory patients, only 2 (11%) studies reported deaths. The pooled incidence was 4% (95% CI, 0 to 9%) with an I2 value of 0%. Given the critical risk of bias, further analyses of death as an outcome were not pursued.
Sustained sputum culture conversion.
Only a single study reported use of macrolide-free regimens in 120 patients. Ten of 120 patients attained sustained sputum conversion. This translates to a sputum conversion rate of 8.33% (95% CI, 4.07 to 14.79%).
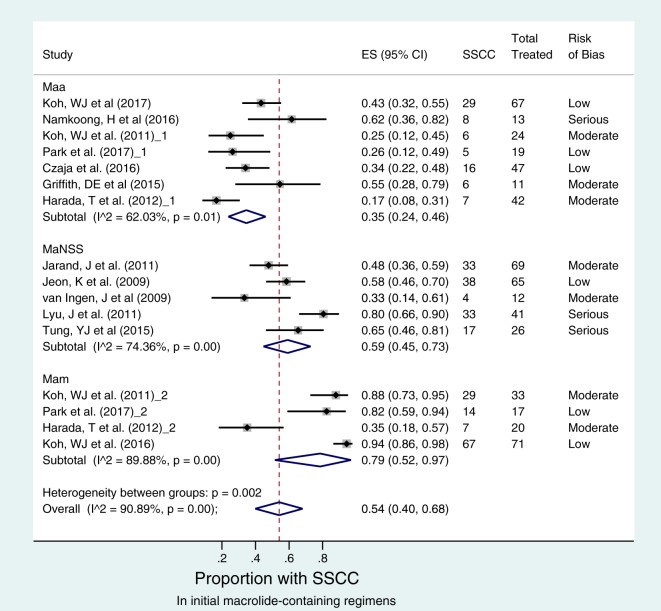
Regarding to macrolide-containing regimens as the initial therapy, there was significant heterogeneity among the 13 studies that examined sustained sputum culture (I2 = 91%; P < 0.001). However, there was lower heterogeneity among the seven M. abscessus subsp. abscessus disease studies (I2 = 62%; P = 0.01) (Fig. 2). There was no significant publication bias or small-study effects (P > 0.346 by Egger's test), suggesting that effect estimates from studies reflect true observations in study patients (Fig. S2). The forest plot in Fig. 2 shows that 77/223 patients with M. abscessus subsp. abscessus attained sustained sputum conversion, which was significantly lower than the 117/141 patients with M. abscessus subsp. massiliense disease. The sputum conversion rates were 35% (95% CI, 24 to 46%) in M. abscessus subsp. abscessus patients and 79% (95% CI, 52 to 97%) in M. abscessus subsp. massiliense patients. The odds ratio (OR) of sustained sputum conversion in M. abscessus subsp. abscessus versus M. abscessus subsp. massiliense diseases was 0.108 (95% CI, 0.066 to 0.181) (P < 0.001).
FIG 2.
Sustained sputum culture conversion (SSCC) with initial macrolide-containing regimens. The forest plot depicts 13 studies comprising 16 macrolide-containing regimens that were examined as initial therapy in 223 treatment-naive patients with M. abscessus subsp. abscessus (designated Maa), 141 treatment-naive patients with M. abscessus subsp. massiliense (designated Mam), and 213 treatment-naive patients with M. abscessus with no subspecies specified (designated MaNSS). Risk of bias assessed for each effect size (ES) estimate is shown in the extreme right column. Despite the marked heterogeneity between these regimens (overall I2 value of >90%), patients with M. abscessus subsp. abscessus were significantly less likely to have SSCC than patients with M. abscessus subsp. massiliense, as shown by noninterloping confidence intervals between the two subspecies.
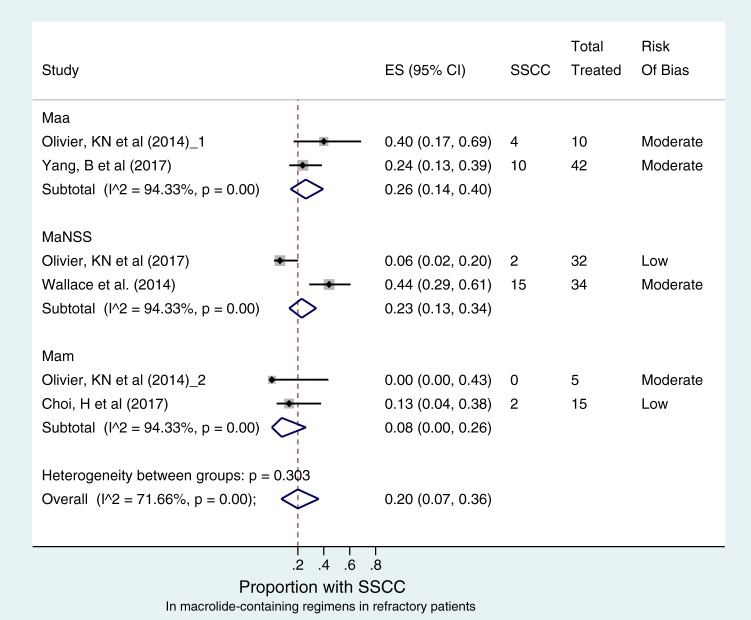
The I2 value among the 5 studies that examined sustained sputum culture of macrolide-containing regimens in refractory patients was 72% (P < 0.001), indicating significant heterogeneity. However, there was no significant publication bias or small-study effects (P > 0.399) (Fig. S3). Figure 3 shows that only 33/138 (23%) patients attained sustained sputum conversion across all M. abscessus species. The pooled culture conversion rate was 20% (95% CI, 7 to 36%), which on follow-up after stopping therapy for 12 months was not significantly different across the mycobacterial species. Comparison between M. abscessus subsp. abscessus- and M. abscessus subsp. massiliense-infected patients revealed no statistical difference in sputum culture conversion: the odds ratio was 3.316 (95% CI, 0.680 to 15.74), and the P value was 0.205. New investigational drugs and novel delivery systems were applied in these combination regimens. The pooled sputum conversion rates in patients with M. abscessus with no subspecies specified after liposomal aminoglycoside therapy versus parenteral macrolide-containing therapy were 13.33% versus 5.88% (P = 0.589). Nonetheless, these results revealed that sputum culture conversion in refractory patients was very poor across all species and statistically not different from each other, regardless of route of administration of aminoglycosides.
FIG 3.
Sustained sputum conversion (SSCC) with macrolide-containing regimens in refractory patients. The forest plot depicts 5 studies comprising 6 macrolide-containing regimens that were examined in 52 refractory patients with M. abscessus subsp. abscessus, 20 refractory patients with M. abscessus subsp. massiliense, and 66 treatment-naive patients with M. abscessus with no subspecies specified. Risk of bias assessed for each effect estimate is shown in the extreme right column. There was no significant difference in SSCC between the subspecies. There was also marked heterogeneity in SSCC estimate across the different regimens (overall I2 value of 72%; P < 0.001).
Recurrence after completing therapy.
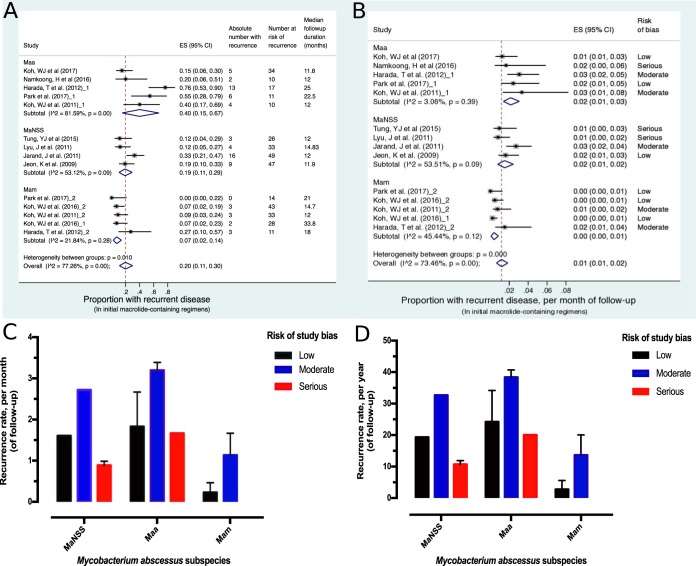
Figure 4 shows forest plots of disease recurrence on follow-up after completing initial macrolide-containing therapy; these patients had initially responded to therapy. The analyses is based on recurrence in only 73 (20%) patients who were monitored for a mean of 16.68 (standard deviation [SD], 6.44) months in the 10 studies that reported these data (Table 2). The I2 value was 77% (P < 0.001), indicating significant heterogeneity between the studies. There was also significant bias (P = 0.030 by Egger's test) observed with the recurrence outcome studies. However, this bias was expected, since fewer studies (10/19) with even fewer patients reported on this outcome. Overall, disease recurrence in M. abscessus subsp. abscessus-infected patients was 40% (95% CI, 15 to 67%) versus 7% (95% CI, 2 to 14%) in M. abscessus subsp. massiliense-infected patients (Fig. 4A). The odds ratio of recurrence in M. abscessus subsp. abscessus-infected versus M. abscessus subsp. massiliense-infected patients was 6.189 (95% CI, 2.317 to 8.046). Since the follow-up duration was variable between studies and the risk of recurrence was significantly different between the isolated organisms, we adjusted for both and then expressed disease recurrence per month and per year of follow-up, as shown in Fig. 4B to D. The estimated recurrence rates were 1.835% (range, 1.667 to 3.196%) per month in M. abscessus subsp. abscessus disease and 0.683% (range, 0.229 to 1.136%) per month in M. abscessus subsp. massiliense disease. Thus, recurrence was significantly higher with M. abscessus subsp. abscessus infection across all studies regardless of the risk of bias of each study.
FIG 4.
Recurrent pulmonary disease with confirmed M. abscessus complex. The forest plot depicts 10 studies comprising 14 macrolide-containing regimens that were examined after follow-up of 30 patients with M. abscessus subsp. abscessus, 11 patients with M. abscessus subsp. massiliense, and 32 patients with M. abscessus with no subspecies specified. Three hundred sixty-six patients were followed up and were at risk of recurrence; 73 suffered a recurrence. The median follow-up duration for each regimen is shown in the extreme right column in panel A, while the risk of bias is shown in the extreme right column in panel B. Panel A shows that, despite the marked heterogeneity between these regimens (overall I2 value of >77%), patients with M. abscessus subsp. abscessus were significantly more likely to have recurrent disease on follow-up, 40% (95% CI, 15 to 67%) compared to 7% (95% CI, 2 to 14%) in patients with M. abscessus subsp. massiliense, as shown by noninterloping confidence intervals between the two subspecies. The findings remain the same when the different follow-up durations are adjusted for, as shown in panel B. Panel C gives the average recurrence rate per month of follow-up, while panel D gives the same estimate per year of follow-up. Panels C and D also show that disease recurrences were significantly higher in studies with low/moderate risk of bias than those with some serious risk across the M. abscessus species.
Composite good versus poor outcomes.
Finally, in patients with appropriate follow-up, we defined good outcomes as sustained sputum conversion rate without relapse, a composite outcome, while the alternative was a poor outcome. The proportion of patients with good outcome was 52/223 (23%) with M. abscessus subsp. abscessus versus 118/141 (84%) with M. abscessus subsp. massiliense disease. The odds ratio of good outcomes was 0.059 (95% CI, 0.034 to 0.101) (P < 0.001).
Sensitivity analyses.
Sensitivity analysis revealed that pooled sustained sputum culture conversion in M. abscessus complex disease was 48% (95% CI, 36 to 59%) in North American patients versus 68% (95% CI, 53 to 81%) in the Northeast Asian patients. The odds ratio for sputum conversion rate among North America patients was 0.458 (95% CI, 0.249 to 0.831) compared to Northeast Asia patients (P = 0.015). However, when analysis was restricted to patients with M. abscessus subsp. abscessus infection, pooled sputum culture conversions were 37% (95% CI, 25 to 51%) in North America versus 33% (95% CI, 19 to 48%) in Northeast Asia. The odds ratio was 1.222 (95% CI, 0.648 to 2.312) and not significantly different (P = 0.526). This suggests that differences in sputum conversion rates between the two locales are partially driven by differences in prevalence of infection with different subspecies. Finally, we also examined the differential effect of macrolides (clarithromycin versus azithromycin) on sputum culture conversion and recurrence. There was no significant difference between the two drugs for either organism (Fig. S5). Risk of bias did not change our findings even after metaregression analyses, shown in Fig. S6.
DISCUSSION
In the treatment of drug-susceptible tuberculosis (TB), more than 80% of patients respond to therapy. For the much-feared multidrug-resistant TB (MDR-TB), microbial response rates are 50 to 90%; for the dreaded extensively drug-resistant TB (XDR-TB), favorable outcomes are found for 16% of patients at 24 months of follow-up (35). Here, we show that outcomes in patients with presumed drug-susceptible M. abscessus subsp. abscessus are dramatically worse than those for patients with MDR-TB and similar to those for patients with XDR-TB. Outcomes in patients with M. abscessus subsp. massiliense pulmonary disease were also poor but similar to those for patients with MDR-TB. In fact, we found outcomes are significantly worse than cure rates of >95% and 10% relapse over 10 years for the epic and ancient disease of leprosy (36). In a recent meta-analysis, we found that the cure rates for pulmonary Mycobacterium avium complex (MAC) were roughly 50%, which is very poor but still better than that for M. abscessus subsp. abscessus disease (37). Thus, the main finding is that we can say with confidence that M. abscessus complex pulmonary disease outcomes on modern chemotherapy are currently the worst for all mycobacterial species and are atrocious.
The general notion is that macrolides improve outcomes in M. abscessus complex, perhaps based on outcomes in MAC. We show that even with macrolide regimens, outcomes in pulmonary M. abscessus complex are very poor. Recently, in a hollow-fiber pharmacokinetics/pharmacodynamics (PK/PD) model of M. abscessus subsp. abscessus disease, we demonstrated that even when drug concentrations were at their most optimal, the standard macrolide-containing regimen had poor maximal microbial kill (Emax) (9, 13). At Emax, all antibiotic target sites are saturated or bound by antibiotic; thus, increasing the antibiotic concentration or dose will not result in increased kill (i.e., Emax is fixed for a drug or combination). Thus, it was not surprising that even inhalational therapy did not improve outcomes, since regimens cannot kill any more than their Emax (9, 13). Indeed, in the PK/PD studies ADR arose on clarithromycin, amikacin, and cefoxitin combination therapy even at the Emax of each drug in the regimen. Our meta-analysis findings are consistent with the PK/PD work and suggest that the currently recommended regimen for M. abscessus subsp. abscessus lung disease has limited to no value, regardless of method of delivery of the drugs.
We propose that the hope that macrolides could improve outcomes was partially based on the misguided idea of classifying highly virulent mycobacterial species as nontuberculous mycobacteria (NTM), equivalent to classifying lions and elephants in the African savanna as non-hyena animals. This led to conflation of improvement of different mycobacterial species (NTM) responses to macrolides with M. abscessus complex, which obviously do not respond. Our results show that there is no therapeutic benefit to conflating different mycobacterial species, even within M. abscessus complex itself: M. abscessus subsp. massiliense had dramatically different response rates than M. abscessus subsp. abscessus. Therefore, why conflate them or combine them in the same category? It is hoped that in the era of WGS and matrix-assisted laser desorption ionization–time-of-flight mass spectrometry, the designation of non-hyena animals will be ditched. No one ever calls a Shigella species a non-E. coli organism, even though Shigella species are considered to belong to the genus Escherichia in the Enterobacteriaceae family, a closer relationship than M. avium to M. abscessus subsp. abscessus. Therapies for M. abscessus subsp. abscessus and M. abscessus subsp. massiliense should be sought without reference to M. tuberculosis or even M. avium, which in any case have much more favorable outcomes than their cousins.
There are several strengths and limitations to our study. First, as stated throughout our results, there was much heterogeneity of studies. However, we used a validated instrument to assess the risk of bias and then applied random effects models with subgroup analyses in anticipation of much heterogeneity. Metaregression methods and sensitivity analyses supported this approach by consistently showing that our designated subgroups were homogenous, and the same estimates were obtained with these different approaches. This suggests that our estimates are robust and that our conclusions will be reproduced in future studies. A second important limitation is that the same authors from the same institutions performed a considerable number of the retrospective studies, albeit with different enrollment time frames and inclusion criterion but nonetheless drawing from the same databases. While we were able to exclude obvious duplicate studies of the same patient cohorts, in some instances we could have failed to decipher whether the same patients were reported in different studies. The third limitation relates to differential sputum sampling between patients within and between studies for both diagnosis and monitoring disease during therapy. Sputum sampling did not follow an identical schedule between studies. Fourth, the decision to treat pulmonary M. abscessus complex is based on the balance of potential risks and benefits for each individual. Patients who opted not to be treated even with severe disease were not included in the analyses, suggesting that the atrocious outcomes that were reported and we identified are more optimistic than findings in the clinic. As an example, our estimates of pooled mortality estimates are subject to critical risk of bias, while those for disease recurrence have moderate risk of bias.
MATERIALS AND METHODS
Search strategy.
We followed the PRISMA guidelines in performing and reporting the systematic review and meta-analyses (38). The following inclusion criteria were used for study selection: (i) American Thoracic Society (ATS) and Infectious Diseases Society of America (IDSA) bacteriological criteria for making diagnosis of pulmonary M. abscessus complex disease or infection, (ii) clear specification of combination therapy received by patients, and (iii) clear specification of microbial outcomes attained by patients after 12 months of treatment or at the end of treatment. Studies judged to be at (i) critical risk for bias or (ii) without adequate information upon which risk of bias for any of the outcomes evaluated in that study could be made were excluded from the meta-analyses. There is no consensus on definitions for treatment outcomes in nontuberculous mycobacterial pulmonary diseases, so we used the outcomes (including sputum culture conversion) stated by each study to define composite outcomes. Most studies defined sputum conversion as 2 to 3 or more consecutive negative cultures, consistent with ATS and IDSA criteria (6). If a patient failed to expectorate sputum, then the sputum was considered to have converted to negative. Sustained sputum culture conversion (SSCC) denotes patients who converted to negative and did not relapse during therapy. Sputum relapse and failure to convert sputum to culture negative with 12 months of therapy was considered treatment failure. Death from any cause was also considered treatment failure. Thus, in this study the term “recurrence” was used to define either disease relapse with the same isolates or disease reinfection with different isolates after sputum culture conversion, since earlier studies did not perform M. abscessus complex isolate identification and comparison.
The complete search strategy used with each database is included in the supplemental material. Briefly, we searched PubMed and Embase for reports published before 30 March 2017 that included MeSH terms or the free-text terms “nontuberculous mycobacteria,” “rapid growing mycobacteria,” “Mycobacterium abscessus,” “Mycobacterium abscessus complex,” “Mycobacterium massiliense,” or “Mycobacterium bolletii.” The search terms were combined with the MeSH terms “treatment” or “therapy” and “outcomes.” There were no language restrictions. The computer search was also supplemented by going through the list of references of systematic reviews on the subject and a search of the Grey Literature Database (http://www.greylit.org/).
Each study was examined for systematic bias using the ROBINS-I tool (version 1.0.0; www.riskofbias.info). The ROBIN-I tool addresses several weaknesses identified in previous instruments used to measure study quality in observational studies, is easy to use, is easy to interpret, and is highly reproducible (39–41). The ROBINS-I tool measures bias for each effect size in seven domains: confounding, selection of participants, classification of interventions, departure from intended interventions, missing data, measurement of outcomes, and selection of reported outcomes (40). Each study had an overall risk of bias judgment calculated across all seven domains and was graded into one of five levels of risk: low, moderate, serious, critical, and no information. Three effect sizes or microbial outcomes were assessed for risk of bias: death, therapy success (i.e., sustained sputum conversion), and disease recurrence. Critical risk of bias referred to serious risk of bias in two or more domains. Such high levels of bias were considered to lead to imprecision in effect size estimation. Therefore, studies with critical risk of bias or no information available to determine level of risk were excluded from the meta-analysis. Similarly, case reports or studies restricted to patients with specific pulmonary clinical conditions, such as pre- or posttransplant or cystic fibrosis, were excluded.
Data abstraction.
The following data were extracted from each study: (i) the author(s) and the year the study was conducted and published, (ii) criteria used to establish pulmonary M. abscessus complex disease, (iii) number of patients enrolled, receiving therapy, and had outcomes evaluated, (iv) combination therapy regimens examined and duration of therapy, and (v) number of patients with outcomes. Data were extracted from tables, text, or referred articles. Two reviewers (J.G.P. and D.O.) independently examined the studies for bias and extracted the data into a prespecified electronic database. The two databases were compared for consistency, and disagreements were settled after consultation with a third reviewer (T.G.). ATS/IDSA standard definitions and terms were used throughout (6).
Statistical analysis.
We used meta-analysis methods to estimate the proportion of patients with the following outcomes: SSCC and disease recurrence after at least 12 months of therapy. These definitions are consistent with ATS/IDSA therapy targets for microbiologic outcomes during treatment of nontuberculous mycobacterial infections. We computed odds ratios (OR) and their 95% confidence intervals (CI) across therapy regimens, stratified by organism and geographic locale. Since macrolides are considered essential for M. abscessus complex therapy (6), regimens were grouped into one of three categories: (i) macrolide-free regimens, (ii) macrolide-containing regimens used as the initial therapy for treatment-naive patients, or (iii) macrolide-containing regimens used in patients with refractory pulmonary disease. In the analyses, the term “Mycobacterium abscessus no species specified” was reserved for patients who did not have the subspecies characterized or had mixed infections.
The DerSimonian and Laird random effects model, which incorporates variation between studies in weighting, was used to pool estimates and performed with STATA software, version 14 (College Station, TX). Freeman and Tukey double arcsine transformation was used to stabilize the variance (42). This allowed identification of admissible 95% CI in events when sample sizes were small and/or proportions were near the margins. We used the I2 statistic to quantify heterogeneity of the effect size estimates between patient groups and between studies (39). Sensitivity analyses and metaregression were used to assess the veracity of findings (43). Egger's test was used to assess for publication bias and small-study effects.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Baylor Research Institute.
J.G.P. and T.G. designed the study. J.G.P., D.O., B.F., D.D., S.S., and T.G. interpreted the supervised literature search, identified additional studies, and reviewed the literature. J.G.P. analyzed data. J.G.P. and T.G. wrote the final version of the manuscript, which all authors approved for publication.
T.G. received funding from Baylor Research Institute, for which he works. All other authors have no competing interests to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.01206-17.
REFERENCES
- 1.Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. 2012. Mycobacterium abscessus: a new antibiotic nightmare. J Antimicrob Chemother 67:810–818. doi: 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- 2.Stout JE, Koh WJ, Yew WW. 2016. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Thomson R, Tolson C, Sidjabat H, Huygens F, Hargreaves M. 2013. Mycobacterium abscessus isolated from municipal water–a potential source of human infection. BMC Infect Dis 13:241. doi: 10.1186/1471-2334-13-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, Reacher M, Haworth CS, Curran MD, Harris SR, Peacock SJ, Parkhill J, Floto RA. 2013. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381:1551–1560. doi: 10.1016/S0140-6736(13)60632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker AW, Lewis SS, Alexander BD, Chen LF, Wallace RJ Jr, Brown-Elliott BA, Isaacs PJ, Pickett LC, Patel CB, Smith PK, Reynolds JM, Engel J, Wolfe CR, Milano CA, Schroder JN, Davis RD, Hartwig MG, Stout JE, Strittholt N, Maziarz EK, Saullo JH, Hazen KC, Walczak RJ Jr, Vasireddy R, Vasireddy S, McKnight CM, Anderson DJ, Sexton DJ. 2017. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 64:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 7.Griffith DE, Philley JV, Brown-Elliott BA, Benwill JL, Shepherd S, York D, Wallace RJ Jr. 2015. The significance of Mycobacterium abscessus subspecies abscessus isolation during Mycobacterium avium complex lung disease therapy. Chest 147:1369–1375. doi: 10.1378/chest.14-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nash KA, Andini N, Zhang Y, Brown-Elliott BA, Wallace RJ Jr. 2006. Intrinsic macrolide resistance in rapidly growing mycobacteria. Antimicrob Agents Chemother 50:3476–3478. doi: 10.1128/AAC.00402-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Lu J, Liu M, Wang Y, Zhao Y, Pang Y. 2017. In vitro activity of clarithromycin in combination with other antimicrobial agents against Mycobacterium abscessus and Mycobacterium massiliense. Int J Antimicrob Agents 49:383–386. doi: 10.1016/j.ijantimicag.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. 2012. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 185:881–886. doi: 10.1164/rccm.201111-2016OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim RD, Greenberg DE, Ehrmantraut ME, Guide SV, Ding L, Shea Y, Brown MR, Chernick M, Steagall WK, Glasgow CG, Lin J, Jolley C, Sorbara L, Raffeld M, Hill S, Avila N, Sachdev V, Barnhart LA, Anderson VL, Claypool R, Hilligoss DM, Garofalo M, Fitzgerald A, Anaya-O'Brien S, Darnell D, DeCastro R, Menning HM, Ricklefs SM, Porcella SF, Olivier KN, Moss J, Holland SM. 2008. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tortoli E, Kohl TA, Brown-Elliott BA, Trovato A, Leao SC, Garcia MJ, Vasireddy S, Turenne CY, Griffith DE, Philley JV, Baldan R, Campana S, Cariani L, Colombo C, Taccetti G, Teri A, Niemann S, Wallace RJ Jr, Cirillo DM. 2016. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int J Syst Evol Microbiol 66:4471–4479. doi: 10.1099/ijsem.0.001376. [DOI] [PubMed] [Google Scholar]
- 13.Chua KY, Bustamante A, Jelfs P, Chen SC, Sintchenko V. 2015. Antibiotic susceptibility of diverse Mycobacterium abscessus complex strains in New South Wales, Australia. Pathology 47:678–682. doi: 10.1097/PAT.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 14.Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Koh WJ. 2009. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180:896–902. doi: 10.1164/rccm.200905-0704OC. [DOI] [PubMed] [Google Scholar]
- 15.Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, Park YK, Kim CK, Shin SJ, Huitt GA, Daley CL, Kwon OJ. 2011. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med 183:405–410. doi: 10.1164/rccm.201003-0395OC. [DOI] [PubMed] [Google Scholar]
- 16.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, S van S D, Mouton JW, van Ingen J, Gumbo T. 2016. Failure of the amikacin, cefoxitin, and clarithromycin combination regimen for treating pulmonary Mycobacterium abscessus infection. Antimicrob Agents Chemother 60:6374–6376. doi: 10.1128/AAC.00990-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Moxifloxacin's limited efficacy in the hollow-fiber model of Mycobacterium abscessus disease. Antimicrob Agents Chemother 60:3779–3785. doi: 10.1128/AAC.02821-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferro BE, Srivastava S, Deshpande D, Pasipanodya JG, van Soolingen D, Mouton JW, van Ingen J, Gumbo T. 2016. Tigecycline is highly efficacious against Mycobacterium abscessus pulmonary disease. Antimicrob Agents Chemother 60:2895–2900. doi: 10.1128/AAC.03112-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi H, Kim SY, Lee H, Jhun BW, Park HY, Jeon K, Kim DH, Huh HJ, Ki CS, Lee NY, Lee SH, Shin SJ, Daley CL, Koh WJ. 2017. Clinical characteristics and treatment outcomes of patients with macrolide-resistant Mycobacterium massiliense lung disease. Antimicrob Agents Chemother 61:e02189-16. doi: 10.1128/AAC.02505-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czaja CA, Levin AR, Cox CW, Vargas D, Daley CL, Cott GR. 2016. Improvement in quality of life after therapy for Mycobacterium abscessus group lung infection. A prospective cohort study. Ann Am Thorac Soc 13:40–48. doi: 10.1513/AnnalsATS.201508-529OC. [DOI] [PubMed] [Google Scholar]
- 21.Griffith DE, Girard WM, Wallace RJ Jr. 1993. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis 147:1271–1278. doi: 10.1164/ajrccm/147.5.1271. [DOI] [PubMed] [Google Scholar]
- 22.Harada T, Akiyama Y, Kurashima A, Nagai H, Tsuyuguchi K, Fujii T, Yano S, Shigeto E, Kuraoka T, Kajiki A, Kobashi Y, Kokubu F, Sato A, Yoshida S, Iwamoto T, Saito H. 2012. Clinical and microbiological differences between Mycobacterium abscessus and Mycobacterium massiliense lung diseases. J Clin Microbiol 50:3556–3561. doi: 10.1128/JCM.01175-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. 2011. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52:565–571. doi: 10.1093/cid/ciq237. [DOI] [PubMed] [Google Scholar]
- 24.Koh WJ, Jeong BH, Kim SY, Jeon K, Park KU, Jhun BW, Lee H, Park HY, Kim DH, Huh HJ, Ki CS, Lee NY, Kim HK, Choi YS, Kim J, Lee SH, Kim CK, Shin SJ, Daley CL, Kim H, Kwon OJ. 2017. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin Infect Dis 64:309–316. doi: 10.1093/cid/ciw724. [DOI] [PubMed] [Google Scholar]
- 25.Koh WJ, Jeong BH, Jeon K, Kim SY, Park KU, Park HY, Huh HJ, Ki CS, Lee NY, Lee SH, Kim CK, Daley CL, Shin SJ, Kim H, Kwon OJ. 2016. Oral macrolide therapy following short-term combination antibiotic treatment of Mycobacterium massiliense lung disease. Chest 150:1211–1221. doi: 10.1016/j.chest.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Lyu J, Jang HJ, Song JW, Choi CM, Oh YM, Lee SD, Kim WS, Kim DS, Shim TS. 2011. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med 105:781–787. doi: 10.1016/j.rmed.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Namkoong H, Morimoto K, Nishimura T, Tanaka H, Sugiura H, Yamada Y, Kurosaki A, Asakura T, Suzuki S, Fujiwara H, Yagi K, Ishii M, Tasaka S, Betsuyaku T, Hoshino Y, Kurashima A, Hasegawa N. 2016. Clinical efficacy and safety of multidrug therapy including thrice weekly intravenous amikacin administration for Mycobacterium abscessus pulmonary disease in outpatient settings: a case series. BMC Infect Dis 16:396. doi: 10.1186/s12879-016-1689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier KN, Griffith DE, Eagle G, McGinnis JP, Micioni L, Liu K, Daley CL, Winthrop KL, Ruoss S, Addrizzo-Harris DJ, Flume PA, Dorgan D, Salathe M, Brown-Elliott BA, Gupta R, Wallace RJ Jr. 2017. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med 195:814–823. doi: 10.1164/rccm.201604-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, Zalewski CK, Folio LR, Siegelman JR, Shallom S, Park IK, Sampaio EP, Zelazny AM, Holland SM, Prevots DR. 2014. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc 11:30–35. doi: 10.1513/AnnalsATS.201307-231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park J, Cho J, Lee CH, Han SK, Yim JJ. 2017. Progression and treatment outcomes of lung disease caused by Mycobacterium abscessus and Mycobacterium massiliense. Clin Infect Dis 64:301–308. doi: 10.1093/cid/ciw723. [DOI] [PubMed] [Google Scholar]
- 31.Tung YJ, Bittaye SO, Tsai JR, Lin CY, Huang CH, Chen TC, Lin WR, Chang K, Lai CC, Lu PL, Chen YH. 2015. Risk factors for microbiologic failure among Taiwanese adults with Mycobacterium abscessus complex pulmonary disease. J Microbiol Immunol Infect 48:437–445. doi: 10.1016/j.jmii.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 32.van Ingen J, de Zwaan ZR, Dekhuijzen RP, Boeree MJ, van Soolingen SD. 2009. Clinical relevance of Mycobacterium chelonae-abscessus group isolation in 95 patients. J Infect 59:324–331. doi: 10.1016/j.jinf.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Wallace RJ Jr, Dukart G, Brown-Elliott BA, Griffith DE, Scerpella EG, Marshall B. 2014. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J Antimicrob Chemother 69:1945–1953. doi: 10.1093/jac/dku062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Jhun BW, Moon SM, Lee H, Park HY, Jeon K, Kim DH, Kim SY, Shin SJ, Daley CL, Koh WJ. 2017. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob Agents Chemother 61:e02052-16. doi: 10.1128/AAC.02052-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, Badri M, Lesosky M, van Helden P, Sirgel FA, Warren R, Dheda K. 2014. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet 383:1230–1239. doi: 10.1016/S0140-6736(13)62675-6. [DOI] [PubMed] [Google Scholar]
- 36.Gumbo T. 2010. General principals of antimicrobial therapy. In Brunton LL, Chabner BA, Knollmann BC (ed), Goodman and Gilman's the pharmacological basis of therapeutics. McGraw Hill Medical, New York, NY. [Google Scholar]
- 37.Pasipanodya JG, Ogbonna D, Deshpande D, Srivastava S, Gumbo T. 2017. Meta-analyses and the evidence-base for microbial outcomes in the treatment of pulmonary Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother 72(Suppl 2):ii3–ii19. doi: 10.1093/jac/dkx311. [DOI] [PubMed] [Google Scholar]
- 38.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med 3:e123–e130. [PMC free article] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG. 2004. Controlling the risk of spurious findings from meta-regression. Stat Med 23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 40.Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hrobjartsson A, Kirkham J, Juni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schunemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP. 2016. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanderson S, Tatt ID, Higgins JP. 2007. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 36:666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 42.Nyaga VN, Arbyn M, Aerts M. 2014. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juni P, Witschi A, Bloch R, Egger M. 1999. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA 282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.