ABSTRACT
Posaconazole is extensively used for prophylaxis for invasive fungal infections. The gastro-resistant tablet formulation has allowed the bioavailability issues encountered with the oral suspension to be overcome. However, overexposure is now frequent. This study aimed to (i) describe the pharmacokinetics of posaconazole tablets in a real-life cohort of patients with hematological malignancies and (ii) perform Monte Carlo simulations to assess the possibility that the daily dose can be reduced while keeping a sufficient exposure. Forty-nine consecutive inpatients were prospectively included in the study. Posaconazole trough concentrations (TC) were measured once a week, and biological and demographic data were collected. The concentrations were analyzed by compartmental modeling, and Monte Carlo simulations were performed using estimated parameters to assess the rate of attainment of the target TC after dose reduction. The pharmacokinetics of posaconazole were well described using a one-compartment model with first-order absorption and elimination. The values of the parameters (interindividual variabilities) were as follows: the absorption constant (ka) was 0.588 h−1 (fixed), the volume of distribution (V/F) was 420 liters (28.2%), and clearance (CL/F) was 7.3 liters/h (24.2%) with 31.9% interoccasion variability. Forty-nine percent of the simulated patients had TC at steady state of ≥1.5 μg/ml and maintained a TC above 1 μg/ml after a reduction of the dose to 200 mg daily. A third of these patients eligible for a dose reduction had TC of ≥1.5 μg/ml as soon as 48 h of treatment. Though posaconazole tablets were less impacted by bioavailability issues than the oral suspension, the pharmacokinetics of posaconazole tablets remain highly variable. Simulations showed that approximately half of the patients would benefit from a reduction of the dose from 300 mg to 200 mg while keeping the TC above the minimal recommended target of 0.7 μg/ml, resulting in a 33% savings in the cost of this very expensive drug.
KEYWORDS: posaconazole, pharmacokinetics, therapeutic drug monitoring, compartmental modeling, Monte Carlo simulations, antifungal agents, population pharmacokinetics
INTRODUCTION
Posaconazole is a broad-spectrum triazole antifungal approved for use for the prophylaxis of invasive fungal infections (IFI) in severely immunocompromised patients. To overcome the limitations with the poor bioavailability of the oral suspension, a delayed-release tablet maximizing systemic absorption was designed. Pharmacokinetic data showed a reduced interpatient variability and a more favorable absorption profile compared with the oral suspension (1–6) and a relative independence of dosing from food intake and the concomitant administration of medications altering the gastric pH (7, 8). The optimization of bioavailability appeared to be a major challenge, as several studies reported the existence of a concentration-effect relationship of posaconazole (9–12). This was confirmed by the analysis of data issued from large clinical trials (13, 14), on the basis of which the 6th European Conference on Infections in Leukemia (ECIL-6) and the British Society for Medical Mycology recommended a minimal trough concentration (TC) of 0.7 μg/ml for the prophylaxis of IFI (15). However, it is noticeable that if the goal of avoiding underexposure to posaconazole seems to be reached with the tablet formulation, there are now many patients in whom TC are largely above 0.7 μg/ml (16). In a phase 3 pharmacokinetic and safety study in 186 patients, Cornely et al. reported that 65% of the measured TC at steady state were above 1.25 μg/ml and 13% were above 2.5 μg/ml (4). Though no concentration-toxicity relationship has been established to date, it seems reasonable to think that a dose reduction might be considered in those patients, as long as it does not increase the risk of IFI. This is particularly relevant considering the substantial costs associated with the extended use of this highly expensive drug. However, to our knowledge, this issue has not been explored to date and a dose reduction in routine practice is currently an off-label use of the drug. Thus, we conducted this study, which aimed to (i) describe the pharmacokinetics of posaconazole by compartmental modeling in a real-life cohort of patients with hematological malignancies and (ii) explore, using Monte Carlo simulations, whether a dose reduction might be considered in highly exposed patients while keeping the TC above the recommended threshold of 0.7 μg/ml.
RESULTS
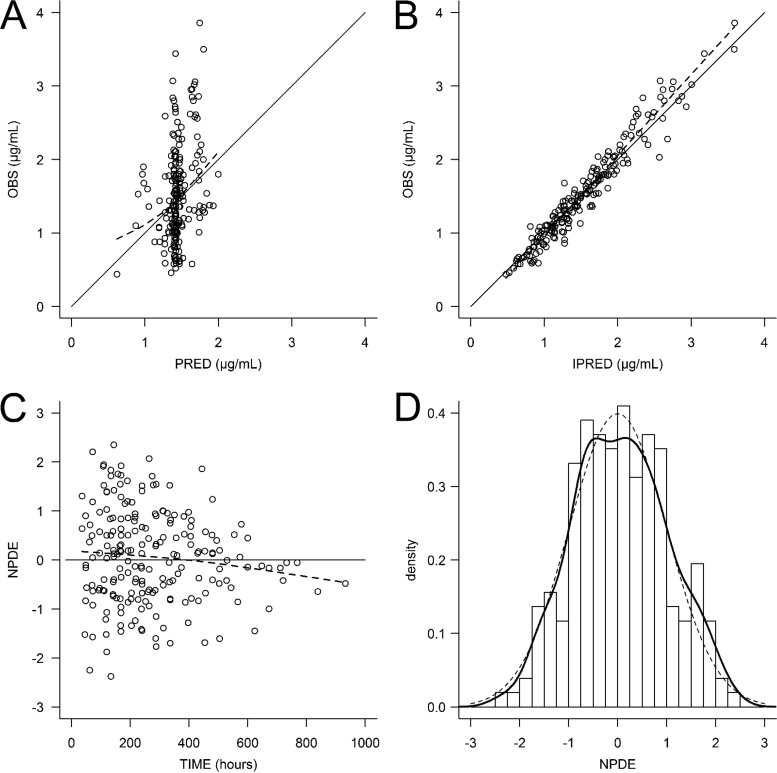
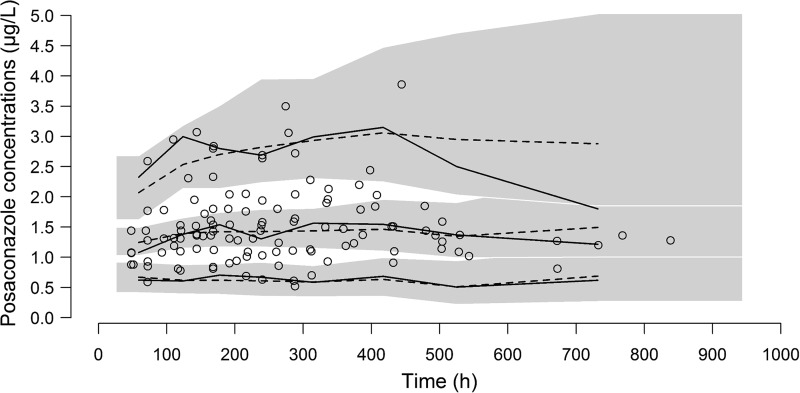
The patients' characteristics are summarized in Table 1. A total of 205 posaconazole concentrations were used to build the pharmacokinetic model, and 139 (67.8%) of these were collected 3 h before or after the time of the trough concentration. The other concentrations were drawn mostly before the trough concentration (60 concentrations; range, 9.0 to 20.8 h after posaconazole intake) or after the trough concentration (6 concentrations; range, 27.2 to 33.2 h after posaconazole intake) was reached. A one-compartment model best fitted the data. The pharmacokinetic parameters were accurately estimated, though the interindividual variability of the absorption rate constant (ka) could not be correctly estimated and was therefore fixed to 0. The addition of an interoccasion variability to account for intraindividual variations of posaconazole clearance from one stay to another greatly improved the predictive performance (P < 0.0001, likelihood ratio test [LRT]). The typical values (interindividual variability) of the pharmacokinetic parameters were as follows: the first-order absorption constant (ka) was 0.588 h−1 (fixed), the apparent central volume of distribution (V/F) was 420 liters (28.2%), and the apparent elimination clearance (CL/F) was 7.3 liters/h (24.2%) with 31.9% interoccasion variability (Table 2). Clearance slightly decreased with an increase in the baseline alanine aminotransferase (ALT) level (P = 0.022, LRT), and V/F was lower in women (P = 0.022, LRT). However, the addition of these covariates in the model altered the accuracy of the parameter estimation and barely improved the predictive performance; thus, we chose to remove them from the final model (Table 3). The inspection of the diagnostic plots did not reveal any obvious model misspecification or bias (Fig. 1 and 2).
TABLE 1.
Patients characteristics and study dataa
| Characteristic | Median | Range |
|---|---|---|
| Demographics | ||
| Age (yr) | 53 | 19–73 |
| % of male patients | 59.2 | NA |
| Body wt (kg) | 72 | 50–125 |
| Body mass index (kg/m2) | 26.4 | 17.7–40.4 |
| Biologics (baseline) | ||
| AST concn (IU/liter) | 25 | 4–64 |
| ALT concn (IU/liter) | 33 | 12–287 |
| Conjugated bilirubin concn (mmol/liter) | 4 | 2–37 |
| Total bilirubin concn (mmol/liter) | 8 | 3–40 |
| ALK concn (IU/liter) | 68 | 22–279 |
| GGT concn (IU/liter) | 37 | 9–602 |
| Serum creatinine concn (μmol/liter) | 69 | 41–155 |
| Study data | ||
| Total no. of stays | 91 | NA |
| No. of stays per patient | 2 | 1–5 |
| Follow-up length per stay (days) | 13 | 3–39 |
| No. of posaconazole concn | 205 | NA |
| Per patient | 3 | 1–14 |
| Per stay | 2 | 1–5 |
| Posaconazole concn (μg/ml) | 1.43 | 0.44–3.86 |
| TC (%) | 67.8 | NA |
| TC (μg/ml) | 1.36 | 0.46–3.44 |
Data are for 49 patients. AST, asparagine aminotransferase; ALT, alanine aminotransferase; ALK, alkaline phosphatase; GGT, gamma-glutamyltransferase; TC, trough concentration, defined as a concentration measured 24 ± 3 h after posaconazole intake; NA, not applicable.
TABLE 2.
Results of final modela
| Parameter | Value | RSE (%) |
|---|---|---|
| Fixed effects | ||
| ka (h−1) | 0.588 | 15 |
| V/F (liters) | 420 | 10 |
| CL/F (liters · h−1) | 7.3 | 5 |
| Random effects | ||
| IIV on ka (%) | 0 | Fixed |
| IIV on V/F (%) | 28.2 | 32 |
| IIV on CL/F (%) | 24.2 | 30 |
| IOV on CL/F (%) | 31.9 | 14 |
| Proportional residual error (%) | 14.8 | 4 |
RSE, relative standard error; IIV, interindividual variability; IOV, interoccasion variability.
TABLE 3.
Results of model selectiona
| Parameter | Model | OFV | ΔOFV | Reference model | P valueb (LRT) |
|---|---|---|---|---|---|
| Structural model | |||||
| Base model (no IOV), one compartment | 1 | 281.6 | |||
| One-compartment model with IOV on CL | 2 | 195.43 | 86.17 | 1 | <0.0001 |
| Covariate model | |||||
| BMI on CL | 4 | 195.79 | 0.36 | 2 | 0.55 |
| Sex on V | 5 | 190.22 | 5.21 | 2 | 0.022 |
| Disease on CL | 7 | 191.59 | 3.84 | 2 | 0.050 |
| Baseline ALT concn on CL | 8 | 190.23 | 5.20 | 2 | 0.023 |
| Baseline AST concn on CL | 9 | 195.5 | 0.07 | 2 | 0.79 |
| Baseline total bilirubin concn on CL | 10 | 195.01 | 0.42 | 2 | 0.52 |
| Baseline conjugated bilirubin concn on CL | 11 | 194.00 | 1.43 | 2 | 0.23 |
| Baseline ALK concn on CL | 12 | 195.82 | 0.39 | 2 | 0.53 |
| Baseline GGT concn on CL | 13 | 196.16 | 0.73 | 2 | 0.39 |
| Longitudinal ALT concn on CL | 14 | 193.19 | 2.24 | 2 | 0.13 |
| Longitudinal AST concn on CL | 15 | 195.24 | 0.19 | 2 | 0.66 |
| Longitudinal total bilirubin concn on CL | 16 | 195.37 | 0.06 | 2 | 0.81 |
| Longitudinal conjugated bilirubin concn on CL | 17 | 195.26 | 0.17 | 2 | 0.68 |
| Longitudinal ALK concn on CL | 18 | 191.82 | 3.61 | 2 | 0.057 |
| Longitudinal GGT concn on CL | 19 | 193.92 | 1.51 | 2 | 0.22 |
OFV, objective function value; ΔOFV, difference in OFV; LRT, likelihood ratio test; IOV, interoccasion variability; longitudinal, the use of dynamic values changing within the same period of observation.
P values in bold indicate statistically significant differences.
FIG 1.
Diagnostic plots. (A) Observed concentrations (OBS) versus population predicted concentrations (PRED); (B) observed concentrations versus individual predicted concentrations (IPRED); (C) normalized prediction distribution error (NPDE) versus time; (D) distribution of the NPDE.
FIG 2.
Visual predictive check (VPC). The figure shows the empirical median and 5th and 95th empirical percentiles (solid lines), the theoretical median and the 5th and 95th theoretical percentiles (dashed lines), the 95% confidence interval of the theoretical median and percentiles (shaded areas), and the observed concentrations (open circles). The 95% confidence interval of the theoretical 95th percentile is very large, and the theoretical and empirical 95th percentiles separate from each other at times later than 500 h because there is no observed high concentration after this point.
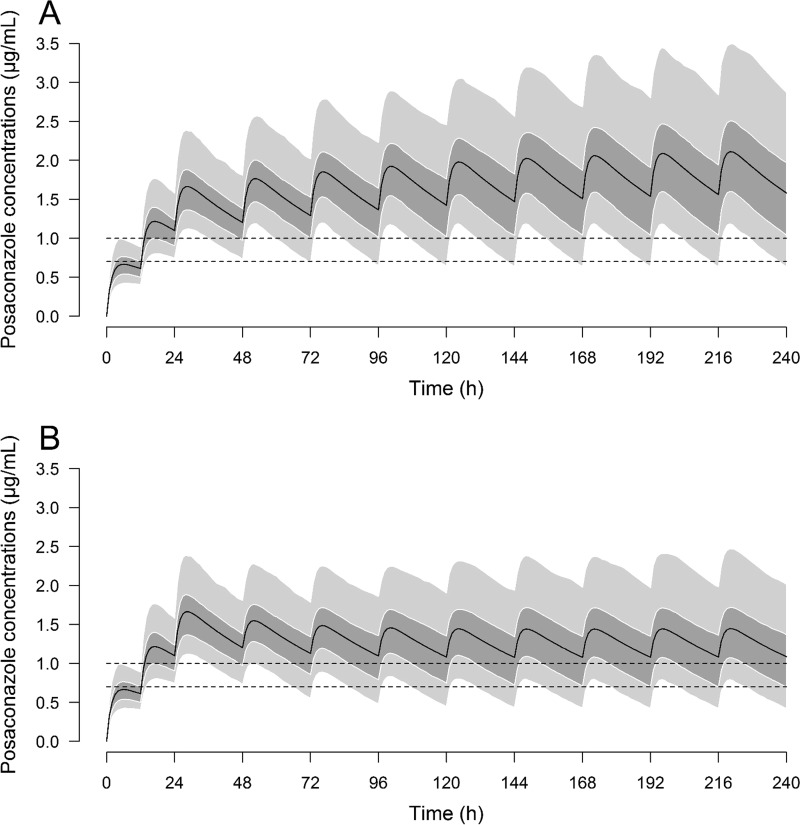
Simulations were performed according to the estimated pharmacokinetic parameters. Following the standard loading dose of 300 mg twice a day (BID) on day 1 and 300 mg once a day (QD) on day 2, 95.6% and 72.6% of simulated patients reached a TC of ≥0.7 μg/ml and ≥1 μg/ml at 48 h, respectively. With the standard regimen of 300 mg QD, the rates at which TC of ≥0.7 μg/ml and ≥1 μg/ml were reached were 93.0% and 76.8% at day 10, respectively. These rates fell to 74.4% and 50.8%, respectively, with the lowered-dose regimen (200 mg QD from day 3). The rates at which patients reached a TC of ≥2 μg/ml at day 10 were 24.6% and 5.2% with the standard and lowered-dose regimens, respectively. According to the simulations, we calculated that 100% of patients with a TC of ≥1.5 μg/ml at 48 h would keep a TC of ≥1 μg/ml at day 10 after lowering of the dose to 200 mg QD from day 3 (Fig. 3 and Table 4).
FIG 3.
Results of the Monte Carlo simulations. (A) Standard regimen (300 mg BID on day 1 and 300 mg thereafter); (B) lowered-dose regimen (300 mg BID on day 1, 300 mg QD on day 2, and 200 mg QD thereafter). Solid lines represent the mean concentration, the dark gray area is from the 25th to the 75th percentile, and the light gray areas are from the 5th to the 25th percentiles and from the 75th to the 95th percentiles. Dashed lines denote concentrations at 0.7 μg/ml and 1 μg/ml.
TABLE 4.
Results of Monte Carlo simulations
| Regimen and time of concn determination | % of patients achieving the following concn: |
||||
|---|---|---|---|---|---|
| <0.5 μg/ml | ≥0.7 μg/ml | ≥1.0 μg/ml | ≥1.5 μg/ml | ≥2.0 μg/ml | |
| Standard regimen (300 mg BID and then 300 mg QD) | |||||
| 48 h | 1.0 | 95.6 | 72.6 | 16.0 | 2.6 |
| Day 10 | 2.6 | 93.0 | 76.8 | 49.2 | 24.6 |
| Lowered-dose regimen of 300 mg BID, 300 mg on day 2, and then 200 mg QD | |||||
| 48 h | 1.0 | 95.6 | 72.6 | 16.0 | 2.6 |
| Day 10 | 9.2 | 74.4 | 50.8 | 18.4 | 5.2 |
| Lowered-dose regimen of 200 mg BID and then 200 mg QD | |||||
| 48 h | 6.2 | 66.8 | 16.0 | 0.6 | 0.0 |
| Day 10 | 9.4 | 74.4 | 49.2 | 15.4 | 4.4 |
| Lowered-dose regimen of 300 mg BID, 300 mg on day 2, and then 100 mg QD | |||||
| 48 h | 1.0 | 95.6 | 72.6 | 16.0 | 2.6 |
| Day 10 | 44.4 | 29.0 | 11.0 | 1.0 | 0.2 |
The additional simulations showed that only 66.8% and 16.0% of simulated patients reached TC of ≥0.7 μg/ml and ≥1 μg/ml at 48 h, respectively, following a reduced loading dose of 200 mg BID on day 1 and then 200 mg QD starting on day 2. Twenty-nine percent and 11.0% of the simulated patients had TC of ≥0.7 μg/ml and ≥1 μg/m at day 10, respectively, after a standard loading dose followed by a dose reduction to 100 mg QD from day 3 (Table 4).
DISCUSSION
To the best of our knowledge, this study is the first to describe the pharmacokinetics of posaconazole gastro-resistant tablets in a real-life cohort of patients using a population approach and the first to explore the potential impact of dosing adaptations using pharmacokinetic simulations.
The values of the TC in the present study are in accordance with data from phase 3 studies (4) and indicate a still important interpatient variability of posaconazole concentrations, with a range of from 0.46 μg/ml to 3.44 μg/ml and a coefficient of variation of 40.5%. Moreover, we also found an important intrapatient variability which was already reported before with the oral suspension (17) but was never investigated by compartmental modeling with the tablet formulation.
Posaconazole pharmacokinetics were well described by a one-compartment model. This was expected because the data set included a majority of concentrations measured at trough. The lack of data during the absorption phase did not allow either the interindividual variability of the first-order absorption constant to be properly estimated or other absorption models to be tested. However, we previously reported that a first-order absorption model described well the absorption of posaconazole tablets (18). The values of V/F and CL/F that we estimated are in accordance with those determined in patients by noncompartmental analysis with the tablet formulation (1, 4), though they are much smaller than those reported from studies with the oral suspension because of the enhanced bioavailability of the tablets (17, 19). These parameters were accurately estimated, as attested to by the values of the relative standard error (RSE; ≤10%) because the data set included about a third of the concentrations that were not drawn at the time of the trough concentration, which allowed the elimination phase to be described much more precisely than was possible with only trough concentrations. Noticeably, the addition of an interoccasion variability of posaconazole clearance greatly improved the model, indicating that the elimination of posaconazole is susceptible to variation in the same patient from one stay to another, though it is relatively stable in the time course of a stay. This further supports the necessity of therapeutic drug monitoring of posaconazole. Posaconazole pharmacokinetics were not influenced or were influenced at only a minimal level by the demographic and biological covariates that we tested, probably indicating that the variability of the concentrations mainly results from a variability of the bioavailability. In particular, baseline ALT levels were found to be negatively correlated to posaconazole clearance, suggesting that impaired liver function could be associated with a lower clearance of posaconazole. However, this effect was modest, with a 5-fold increase in ALT levels resulting in only a 25% decrease in posaconazole clearance. Moreover, because of a lack of data, the effect of this covariate was poorly estimated, and thus, this covariate was not retained in the final model.
The results of our simulations concur very closely with those for patients reported in a previous study: we calculated that 63.6% and 10.6% of the simulated patients receiving the standard regimen would have TC at steady state of >1.25 μg/ml and >2.5 μg/ml, respectively; in comparison, 65% and 13% of 186 patients with hematological malignancies in a phase 3 clinical study would have TC at steady state of >1.25 μg/ml and >2.5 μg/ml, respectively (4). On a smaller scale, the concentration-time profiles and concentrations of the simulations were also very close to those reported in a phase 1b study in 32 patients with hematological malignancies (3).
The results of the simulations showed that with the recommended regimen, almost all the patients would achieve a TC of ≥0.7 μg/ml at 48 h following the loading dose of 300 mg BID on day 1 followed by 300 mg QD on day 2, thus ensuring the efficacy of the prophylaxis. Conversely, only two-thirds of the simulated patients would reach a TC of >0.7 μg/ml at 48 h with a reduced loading dose of 200 mg BID on day 1 followed by 200 mg QD on day 2. On the basis of these results, there is no argument in favor of reducing the loading dose.
Nevertheless, regarding the maintenance dose, with the standard regimen of 300 mg QD, the TC kept increasing slowly in a proportion of patients, bringing to a quarter (24.6%; Table 4) the proportion of patients with a TC of ≥2 μg/ml at day 10. The simulations showed that following a dose reduction to 200 mg QD from day 3, half of the patients would keep a TC of >1 μg/ml thereafter. Additional simulations also showed that up to 11% of patients would maintain a TC of ≥1 μg/ml after a dose reduction to 100 mg QD.
However, the extended volume of distribution and the low elimination clearance of posaconazole render difficult the identification of patients eligible for a reduction of the maintenance dose because the pharmacokinetic steady state was not reached at 48 h, despite the use of the loading dose. Indeed, the median half-life calculated from the simulated pharmacokinetic parameters was 39.8 h (range, 15.4 to 127.8 h), which means that the median time to get 97% of the pharmacokinetic steady state (i.e., 5 half-lives) is approximately 200 h (8.3 days). We also calculated that at day 7, 95.0% of the simulated patients would have no more than a 5% variation in their subsequent TC.
Eventually, 16% of the simulated patients would have a TC of ≥1.5 μg/ml at 48 h, and all these patients would have a TC of ≥1.0 μg/ml at day 10 after a dose reduction to 200 mg QD from day 3. This means that approximately a third of the patients that could benefit from a dose reduction can be identified as soon as the second day of treatment. Thus, a dose reduction to 200 mg QD can be considered in patients with a TC of ≥1.5 μg/ml at 48 h, but monitoring of the TC at day 7 or 8 is also mandatory to identify other patients eligible for a dose reduction once they have reached pharmacokinetic steady state. Our results are, however, suitable only if the recommended trough concentration of 0.7 μg/ml for prophylaxis of IFI is targeted and are not suitable in the case of curative treatment or in certain particular situations, such as suspicion of a lowered susceptibility to triazoles.
Conclusion.
Posaconazole tablets show less but still important pharmacokinetic variability compared with the oral suspension. With the currently recommended dose regimen, trough concentrations are not likely to fall below the recommended target of 0.7 μg/ml, but many patients are overdosed with no evidence of enhanced efficacy. According to the pharmacokinetic simulations, half of the patients could benefit from a dose reduction. Early therapeutic drug monitoring allows identification of a third of the patients eligible for a dose reduction as soon as the second day of treatment. The others can be identified after a week, once pharmacokinetic steady state has been reached. Lowering of the dose to 200 mg QD in patients with a TC of ≥1.5 μg/ml at 48 h or at day 7 or 8 would allow the TC to be maintained above 1.0 μg/ml, thus ensuring the retention of prophylactic efficacy with a security margin and with savings of 33% of the daily treatment cost. These findings, however, need to be confirmed, and prospective clinical trials to assess the safety, efficacy, and cost-effectiveness of such a dose reduction are warranted.
MATERIALS AND METHODS
Ethics.
This was a fully noninterventional, observational study with no modification of patient management. This study was conducted in accordance with the Declaration of Helsinki and national and institutional standards. It was approved by the Rennes University Hospital Ethics Committee (approval no. 15.114). Patients were informed of their eligibility prior to their inclusion and could refuse to participate.
Patients and study design.
The study prospectively included 49 consecutive inpatients hospitalized between October 2015 and October 2016 in the Clinical Hematology Department of Rennes University Hospital and treated with posaconazole tablets for prophylaxis of IFI. Posaconazole was administered, following recommendations, as a loading dose of 300 mg twice a day (BID) on the first day of treatment and a maintenance dose of 300 mg once a day (QD) thereafter. Demographic and biological data were recorded at the baseline and throughout the hospitalization. Patients could be followed during several stays in the department. Blood samples for determination of posaconazole TC were to be drawn 7 days after the beginning of treatment and once a week thereafter until discharge or the discontinuation of posaconazole. As the concentrations used in the pharmacokinetic analysis were part of routine therapeutic drug monitoring of the posaconazole concentration, some samples were not drawn precisely 24 h after posaconazole intake. Therefore, details about the doses administered as well as the precise intake and sampling times were thoroughly recorded throughout the study for the purpose of pharmacokinetic modeling. Concentrations from patients with digestive disorders (such as diarrhea or vomiting), which might have altered posaconazole absorption and biased estimation of the values of the pharmacokinetic parameters, were not included in the data set for model building. Posaconazole concentrations were determined using a fully validated tandem mass spectrometry method (20). If needed, additional blood samples could be drawn and the posaconazole dose could be adapted at the clinician's discretion. No additional blood samples were drawn for the purpose of the study.
Pharmacokinetic analysis.
Population pharmacokinetic compartmental modeling was performed using Monolix (version 4.2.3) software (Lixsoft, Orsay, France).
(i) Structural model.
One- and two-compartment structural models with first-order absorption, distribution, and elimination were tested, using exponential interindividual and interoccasion variability models, as follows: θi = θTV · eηi/, ηi ∼ N(0, ω2), and θik = θi · eκi/, κi ∼ N(0, γ2), where θi is the estimated individual parameter for the ith patient at the first occasion (i.e., the first stay), θik is the estimated individual parameter for the ith patient at the kth occasion, θTV is the typical value of the parameter, and ηi and κi are the interindividual and interoccasion random effects for the ith patient, respectively. The values of ηi and κi are supposed to be normally distributed (N) with a mean of 0 and variances of ω2 and γ2, respectively. For each parameter, variabilities were fixed to 0 if the variances could not be estimated properly.
(ii) Error model.
Additive, proportional, and mixed additive-proportional residual error models were tested. The proportional error model was implemented as follows: YO,ij = YP,ij · (1 + εprop,ij), εprop,ij ∼ N(0, ), where YO,ij and YP,ij are the observed and predicted jth measurements for the ith patient, respectively, and εprop,ij is the proportional residual error with a mean of 0 and a variance of .
Covariate model.
The influence of relevant demographic and biological covariates on posaconazole pharmacokinetics was tested. The influence of covariates was implemented as described below.
(a) Continuous covariates.
Age, body weight (BW), body mass index (BMI), and the serum creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALK), and gamma-glutamyltransferase (GGT) concentrations were tested. Continuous covariates were centered on their median as follows: θTV = θ0 · [COV/med(COV)]βCOV, where θ0 is the value of θ for a median subject, βCOV quantifies the influence of the covariate on θ, and med(COV) is the median value of the covariate in the study population. As an initial approach, we tested the influence of the baseline values of the continuous covariates. However, considering the length of the hospital stay, we postulated that values for biological parameters could change over time, especially those reflecting liver function that could be altered by posaconazole, so we also tested ALT, AST, ALK, and GGT concentrations as time-varying covariates implemented in the model as described above.
(b) Categorical covariates.
The patients' gender and disease state were tested. The influence of categorical covariates on θTV was implemented as follows: ln(θTV) = ln(θCAT = 0) + (βCAT = i), where θCAT = 0 is the value of θTV in an arbitrary reference category and βCAT = i quantifies the influence of the ith category on the value of θTV.
(iii) Model comparison and covariate selection.
Structural, interindividual, interoccasion, residual error, and covariate models were compared using the likelihood ratio test (LRT) at a risk α value of 5% for the nested models or a reduction of Akaike's information criterion (AIC) value otherwise.
(iv) Evaluation of the goodness of fit and final model selection.
The goodness of fit for each model was assessed by plotting the population predicted (PRED) and individual predicted (IPRED) concentrations versus the observed concentrations (OBS) and by evaluating the residuals by graphical inspection of the normalized prediction distribution errors (NPDE) versus time and the NPDE distribution. The precision of parameter estimation, as determined by the relative standard errors (RSE), was taken into account for model selection and selection of the final model. Stochastic approximation was used for RSE estimation and the correlation matrix for determination of the estimates. Individual fits were also inspected. The model offering the greater reduction of the objective function value (or AIC) together with an acceptable precision of the estimates of the parameters and goodness of fit was selected. A visual predictive check (VPC) figure was built to ensure the predictive performance of the model.
Monte Carlo simulations.
Monte Carlo simulations were performed using SimulX (version 1.0.0) software (Lixoft, Orsay, France). The values of the pharmacokinetic parameters previously estimated were used to simulate the concentration profiles of 500 patients following two dosing regimens: (i) 300 mg BID on day 1 and then 300 mg QD (standard regimen, used as a reference) and (ii) 300 mg BID on day 1 and 300 mg QD on day 2, followed by 200 mg QD (lowered-dose regimen). The mean concentration and 90% confidence interval were determined for each regimen. Simulation endpoints were the proportion of patients achieving a TC of ≥0.7 μg/ml (the minimal recommended concentration for prophylaxis of IFI) and a TC of ≥1 μg/ml (the proposed TC to be targeted in clinical practice to ensure maintenance of a TC of ≥0.7 μg/ml with a safety margin of 0.3 μg/ml) at 48 h and at day 10. Additional simulations at a dose of 200 mg BID on day 1 and 200 mg QD thereafter, to evaluate the proportion of patients achieving the targeted TC with a reduced loading dose, and at a dose of 300 mg BID on day 1, 300 mg on day 2, and 100 mg QD thereafter, to assess the proportion of patients that reached a sufficient TC with only a third of the recommended daily dose, were then performed.
ACKNOWLEDGMENTS
We sincerely thank all physicians and nurses who were involved in patient care, as well as the patients themselves.
This work received no financial support.
We have no conflicts of interest to declare.
C.B.-K. and A.P. designed the study. S.N. provided medical care to the patients and was in charge of their recruitment into the study and blood sample collection. C.B.-K., F.L., and M.-C.V. were in charge of posaconazole concentration measurements. A.P. performed the statistical and pharmacokinetic analyses. A.P. drafted the manuscript. C.T., S.L., C.B.-K., F.L., E.B., and M.-C.V. revised the draft and participated in the writing of the final manuscript, the interpretation of the data, and discussion of the results. All authors revised the manuscript for important intellectual content and approved the manuscript in its submitted form.
REFERENCES
- 1.Krishna G, Ma L, Martinho M, Preston RA, O'Mara E. 2012. A new solid oral tablet formulation of posaconazole: a randomized clinical trial to investigate rising single- and multiple-dose pharmacokinetics and safety in healthy volunteers. J Antimicrob Chemother 67:2725–2730. doi: 10.1093/jac/dks268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krishna G, Ma L, Martinho M, O'Mara E. 2012. Single-dose phase I study to evaluate the pharmacokinetics of posaconazole in new tablet and capsule formulations relative to oral suspension. Antimicrob Agents Chemother 56:4196–4201. doi: 10.1128/AAC.00222-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duarte RF, Lopez-Jimenez J, Cornely OA, Laverdiere M, Helfgott D, Haider S, Chandrasekar P, Langston A, Perfect J, Ma L, van Iersel ML, Connelly N, Kartsonis N, Waskin H. 2014. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob Agents Chemother 58:5758–5765. doi: 10.1128/AAC.03050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cornely OA, Duarte RF, Haider S, Chandrasekar P, Helfgott D, Jimenez JL, Candoni A, Raad I, Laverdiere M, Langston A, Kartsonis N, Van Iersel M, Connelly N, Waskin H. 2016. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J Antimicrob Chemother 71:1747. doi: 10.1093/jac/dkw079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durani U, Tosh PK, Barreto JN, Estes LL, Jannetto PJ, Tande AJ. 2015. Retrospective comparison of posaconazole levels in patients taking the delayed-release tablet versus the oral suspension. Antimicrob Agents Chemother 59:4914–4918. doi: 10.1128/AAC.00496-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung DS, Tverdek FP, Kontoyiannis DP. 2014. Switching from posaconazole suspension to tablets increases serum drug levels in leukemia patients without clinically relevant hepatotoxicity. Antimicrob Agents Chemother 58:6993–6995. doi: 10.1128/AAC.04035-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kraft WK, Chang PS, van Iersel ML, Waskin H, Krishna G, Kersemaekers WM. 2014. Posaconazole tablet pharmacokinetics: lack of effect of concomitant medications altering gastric pH and gastric motility in healthy subjects. Antimicrob Agents Chemother 58:4020–4025. doi: 10.1128/AAC.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kersemaekers WM, Dogterom P, Xu J, Marcantonio EE, de Greef R, Waskin H, van Iersel ML. 2015. Effect of a high-fat meal on the pharmacokinetics of 300-milligram posaconazole in a solid oral tablet formulation. Antimicrob Agents Chemother 59:3385–3389. doi: 10.1128/AAC.05000-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jang SH, Colangelo PM, Gobburu JV. 2010. Exposure-response of posaconazole used for prophylaxis against invasive fungal infections: evaluating the need to adjust doses based on drug concentrations in plasma. Clin Pharmacol Ther 88:115–119. doi: 10.1038/clpt.2010.64. [DOI] [PubMed] [Google Scholar]
- 10.Dolton MJ, Ray JE, Chen SC, Ng K, Pont L, McLachlan AJ. 2012. Multicenter study of posaconazole therapeutic drug monitoring: exposure-response relationship and factors affecting concentration. Antimicrob Agents Chemother 56:5503–5510. doi: 10.1128/AAC.00802-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields RK, Clancy CJ, Vadnerkar A, Kwak EJ, Silveira FP, Massih RC, Pilewski JM, Crespo M, Toyoda Y, Bhama JK, Bermudez C, Nguyen MH. 2011. Posaconazole serum concentrations among cardiothoracic transplant recipients: factors impacting trough levels and correlation with clinical response to therapy. Antimicrob Agents Chemother 55:1308–1311. doi: 10.1128/AAC.01325-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cattaneo C, Panzali A, Passi A, Borlenghi E, Lamorgese C, Petulla M, Re A, Caimi L, Rossi G. 2015. Serum posaconazole levels during acute myeloid leukaemia induction therapy: correlations with breakthrough invasive fungal infections. Mycoses 58:362–367. doi: 10.1111/myc.12326. [DOI] [PubMed] [Google Scholar]
- 13.Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 14.Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR, Greinix H, Morais de Azevedo W, Reddy V, Boparai N, Pedicone L, Patino H, Durrant S. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 356:335–347. doi: 10.1056/NEJMoa061098. [DOI] [PubMed] [Google Scholar]
- 15.Ashbee HR, Barnes RA, Johnson EM, Richardson MD, Gorton R, Hope WW. 2014. Therapeutic drug monitoring (TDM) of antifungal agents: guidelines from the British Society for Medical Mycology. J Antimicrob Chemother 69:1162–1176. doi: 10.1093/jac/dkt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekkers BG, Bakker M, van der Elst KC, Sturkenboom MG, Veringa A, Span LF, Alffenaar JC. 2016. Therapeutic drug monitoring of posaconazole: an update. Curr Fungal Infect Rep 10:51–61. doi: 10.1007/s12281-016-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dolton MJ, Bruggemann RJ, Burger DM, McLachlan AJ. 2014. Understanding variability in posaconazole exposure using an integrated population pharmacokinetic analysis. Antimicrob Agents Chemother 58:6879–6885. doi: 10.1128/AAC.03777-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petitcollin A, Crochette R, Tron C, Verdier MC, Boglione-Kerrien C, Vigneau C, Bellissant E, Lemaitre F. 2016. Increased inhibition of cytochrome P450 3A4 with the tablet formulation of posaconazole. Drug Metab Pharmacokinet 31:389–393. doi: 10.1016/j.dmpk.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 19.AbuTarif MA, Krishna G, Statkevich P. 2010. Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr Med Res Opin 26:397–405. doi: 10.1185/03007990903485056. [DOI] [PubMed] [Google Scholar]
- 20.Verdier MC, Bentue-Ferrer D, Tribut O, Bellissant E. 2010. Liquid chromatography-tandem mass spectrometry method for simultaneous quantification of four triazole antifungal agents in human plasma. Clin Chem Lab Med 48:1515–1522. doi: 10.1515/CCLM.2010.252. [DOI] [PubMed] [Google Scholar]