ABSTRACT
This study investigated the effects of subinhibitory doses of the lipoglycopeptide antibiotic dalbavancin on Staphylococcus aureus toxin production in vitro. S. aureus toxin production levels were compared to those seen with the natural glycopeptide antibiotic vancomycin and with representative beta-lactam and oxazolidinone antibiotics. While neither dalbavancin nor vancomycin adversely affected toxin production, of these glycopeptide antibiotics, only dalbavancin significantly attenuated toxin production at subinhibitory concentrations. These findings support the recent success of dalbavancin for treatment of staphylococcal infections.
KEYWORDS: Staphylococcus aureus, subinhibitory antibiotics, toxin production
TEXT
Invasive Staphylococcus aureus infections are mediated by potent extracellular bacterial toxins, including Panton-Valentine leukocidin (PVL), toxic shock syndrome toxin 1 (TSST-1), and alpha-hemolysin (AH). Vancomycin, a glycopeptide antibiotic, is recommended for treatment of severe S. aureus infections (1), but increased resistance and poor clinical outcomes remain very serious concerns. Further, vancomycin-induced inhibition of peptidoglycan synthesis results in slow bacterial killing (2, 3), potentially allowing continued synthesis and accumulation of toxins. Dalbavancin, a semisynthetic lipoglycopeptide in the same class as vancomycin, has recently been approved by the FDA for treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by Gram-positive bacteria, including S. aureus. Compared to vancomycin, dalbavancin has improved stability in bacterial membranes that is attributed to its extended lipophilic side chain (4, 5). With respect to staphylococcal infection, dalbavancin possesses several key advantages, including potent and rapid bactericidal activity, a long half-life, and slow clearance from the body, allowing a once-per-week dosing schedule (6–8). While dalbavancin offers superior and extended antibacterial activity, its effects on bacterial toxin production have not been directly studied.
Our group and others have shown that subinhibitory doses of cell wall synthesis inhibitors, mainly beta-lactams, often fail in infections caused by toxin-producing organisms due to their intrinsic inability to suppress toxin protein synthesis and potential to trigger an SOS response resulting in increased toxin production (9–11). In contrast, protein synthesis inhibitors that suppress toxin production (e.g., linezolid) are associated with better clinical outcomes (12–14). These findings have formed the basis of current recommendations for treatment of severe soft tissue infections caused by toxin-producing pathogens (1). Since suppression of bacterial toxin synthesis is an important therapeutic goal in the treatment of staphylococcal infections, the current study investigated how subinhibitory concentrations of dalbavancin impact the production of PVL, TSST-1, and AH from both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus (MSSA).
To determine subinhibitory doses of antibiotics for the current study, the MIC of dalbavancin and the MIC of vancomycin were established for each of four S. aureus clinical isolates by a microdilution broth method per Clinical and Laboratory Standards Institute (CLSI) guidelines (Table 1). Nafcillin, a beta-lactam antibiotic previously shown to induce toxin expression, and linezolid, a protein synthesis inhibitor, were included as comparator agents. In agreement with previous reports (5, 15, 16), dalbavancin was highly potent and each S. aureus strain tested was more susceptible to dalbavancin than to vancomycin, linezolid, or nafcillin.
TABLE 1.
Toxin production profiles and MICs of strains used in the present study
| Toxin profile (PVL/TSST/AH) | S. aureus strain | MIC (mg/liter) |
|||
|---|---|---|---|---|---|
| Dalbavancin | Vancomycin | Nafcillin | Linezolid | ||
| +/−/+ | MRSA 1560 USA400 | 0.06 | 1.0 | 12.5 | 4.0 |
| +/−/+ | MRSA FPR3757 USA300 | 0.12 | 1.0 | 6.25 | 2.0 |
| −/+/+ | MRSA 04-014 (CDC strain 368-04) | 0.03 | 1.0 | 12.5 | 4.0 |
| −/+/+ | MSSA 04-002 | 0.12 | 1.0 | 0.76 | 2.0 |
Next, a comprehensive growth curve analysis was completed for each S. aureus strain to define subinhibitory MIC doses for each agent. Mueller-Hinton II broth was inoculated with 5 × 105 CFU/ml, and antibiotics were added at time zero to final concentrations of 1, 1/2, 1/4, 1/8, or 1/16 the MIC. Polysorbate 80 (0.002%) served as a vehicle control for dalbavancin. All cultures were incubated at 37°C with shaking (200 rpm), and 5-ml samples were collected at 0, 3, 6, 9, 12, and 24 h for quantitative dilution plating and analysis of production of toxins (PVL-LukS, TSST-1, and AH). Subinhibitory concentrations for each antibiotic were defined as any drug concentration causing no more than a 0.5 log decrease in the total population throughout the duration (24 h) of the experiment, relative to the starting bacterial concentration. For the glycopeptide antibiotics, concentrations of ≤1/4 the MIC were subinhibitory (data not shown). In all cases, administration of 1/4 the MIC of dalbavancin increased the length of the lag phase; however, bacteria reached levels comparable to those seen with the vehicle control by 12 h and 24 h. Nafcillin was subinhibitory at ≤1/8 the MIC for all strains, and linezolid was subinhibitory (bacteriostatic) at ≤1/4 the MIC (data not shown). Only subinhibitory antibiotic concentrations were tested for effects on S. aureus toxin production.
The effects of subinhibitory antibiotics on LukS-PVL and TSST-1 production were determined for each strain at 9, 12, and 24 h by enzyme-linked immunosorbent assay (ELISA) (17, 18). Briefly, enzyme immunoassay/radioimmunoassay (EIA/RIA) plates were coated with 1 μg/ml anti-PVL monoclonal antibody (MAb) 1D9 (IBT Bioservices, Gaithersburg, MD) or 5 μg/ml anti-TSST-1 affinity-purified sheep antisera (Toxin Technology, Sarasota, FL). ELISA plates were blocked, and samples were applied for 2 h at 37°C using the following combinations: LukS-PV standard (IBT Bioservices) (0.8 to 50 ng/ml) and culture supernatants (1:200 to 1:800) or TSST-1 standard (Toxin Technology) (0.15 to 20 ng/ml) and culture supernatants (1:2,000 to 1:8,000). Plates were washed with phosphate-buffered saline (PBS-Tween) (0.05%) and incubated with 0.25 μg/ml rabbit polyclonal anti-PVL (LukS) (IBT Bioservices) and horseradish peroxidase (HRP)-linked anti-rabbit IgG (H+L) (Cell Signaling, Danvers, MA) or a 1:1,200 dilution of HRP-conjugated anti-TSST-1 sheep antisera (Toxin Technology). Assays were developed with 1-step Ultra TMB (Life Technologies, Grand Island, NY). Recombinant LukF-PV (IBT Bioservices) was undetectable by these methods (not shown); however, we cannot rule out the possibility of cross-reaction with other S components. Toxin concentrations falling below the linear range of the standard were reported as half the lowest detectable limit so as to not overestimate or underestimate the actual value. S. aureus protein A did not interfere with either immunoassay at concentrations of ≤500 ng/ml. AH activity was assayed by a standard rabbit erythrocyte lysis assay, as described previously (11). In brief, sterile-filtered culture supernatants were diluted in Dulbecco's PBS (DPBS) (1:10 to 1:80) and an equal volume of washed rabbit erythrocytes (2% in DPBS) was added. Sterile deionized water was included as a 100% hemolysis control. After 1 h of incubation at 37°C, supernatants were transferred to a new microtiter plate and A550 was measured. Activity (in hemolytic units per milliliter) was defined as the inverse of the dilution causing 50% hemolysis, multiplied by 2. For each experimental condition, three independent samples were tested. The log of the toxin response was modeled using analysis of variance (ANOVA) in a mixed-model framework, with separate models for each bacterium/toxin combination. Fixed effects were dose, time, and antibiotic, and random effects were experimental batch and day. Pairwise comparisons were made between antibacterial and control treatments for each subinhibitory dose and among individual S. aureus strains, with P values (≤0.05) controlled for multiple comparisons with false-discovery-rate adjustments (19).
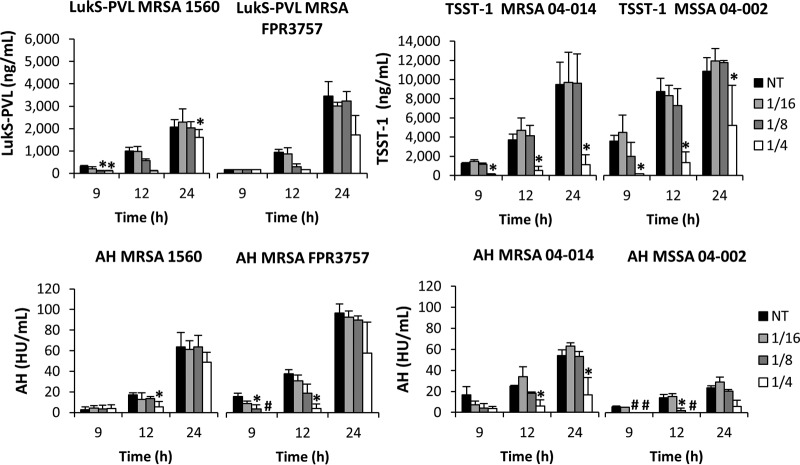
Dalbavancin administration did not increase toxin production in any S. aureus strain and, at 1/4 the MIC, significantly suppressed toxin production compared to control treatment (Fig. 1). Notably, toxin suppression was not observed with 1/4 the MIC of vancomycin but was equivalent to that seen with the no-treatment control (Fig. 2). Cultures treated with 1/16 and 1/8 the MIC of dalbavancin (Fig. 1) or vancomycin (data not shown) also showed no effect on toxin production. Given that the two antibiotics are from the same antibiotic class, it is somewhat remarkable that dalbavancin demonstrated toxin suppression whereas vancomycin did not; this can likely be credited to dalbavancin's greater stability and lower MIC (Table 1) (5, 20–22). In agreement with previous work (11), subinhibitory levels of nafcillin significantly increased maximal toxin production whereas linezolid exhibited clear dose-dependent suppression of toxin production in all strains. Ultimately, the subinhibitory effects on toxin production were most apparent in MRSA 04-014 (Fig. 3) and MSSA 04-002, where dalbavancin significantly decreased toxin production at 1/4 the MIC throughout the time course, suggesting that the relationship between antibiotic and toxin production may reflect a strain-dependent response rather than a universal event.
FIG 1.
Effects of subinhibitory dalbavancin concentrations on S. aureus extracellular toxin production. S. aureus strains were cultured in the presence of 1/16, 1/8, or 1/4 the MIC of dalbavancin, and results were compared to those seen with a no-treatment (NT) control. Levels of PVL and TSST-1 (in nanograms per milliliter) were determined by ELISA (top panel). Alpha-hemolysin (AH) was quantitated by rabbit erythrocyte lysis assay (in hemolytic units [HU] per ml) (bottom panel). Error bars represent the standard errors of results between biological replicates (n = 3). A hash mark (#) indicates that no toxin was detected, and an asterisk (*) indicates that data are significantly different from those determined with the NT control (P ≤ 0.05) at each corresponding time point based on an ANOVA with false-discovery-rate adjustments.
FIG 2.
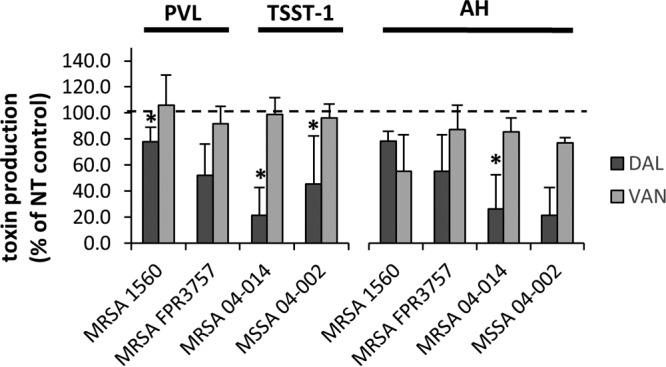
Comparison of toxin protein production levels at 24 h following exposure to 1/4 the MIC of dalbavancin or vancomycin. S. aureus strains were cultured for 24 h in the presence of 1/4 the MIC of dalbavancin (DAL) or vancomycin (VAN). Bars represent the percentages of toxin production relative to the NT control, set at 100% (dashed line). Shown are PVL and TSST-1 levels (left) and AH levels (right) ± standard errors of three biological replicates. An asterisk (*) indicates that data are significantly different from those determined with the NT control (P ≤ 0.05) based on an ANOVA with false-discovery-rate adjustments.
FIG 3.
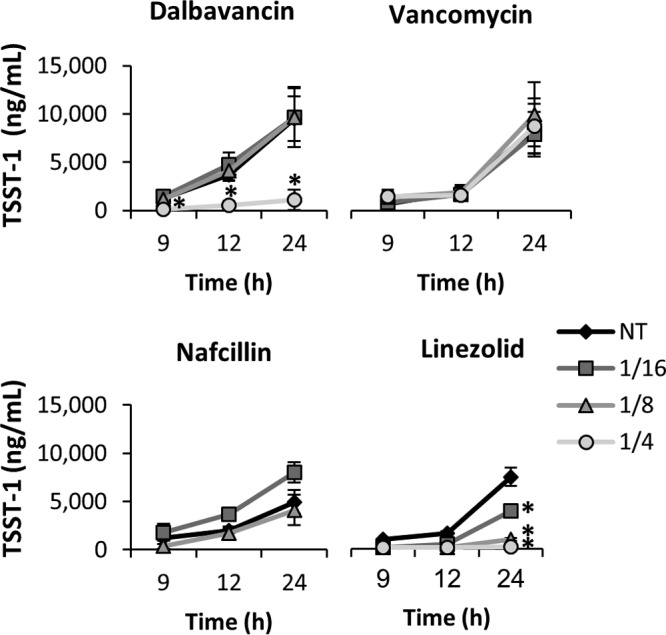
Effects of subinhibitory antibiotics on MRSA 04-014 TSST-1 production. MRSA 04-014 was cultured in the presence of subinhibitory doses (1/16, 1/8, or 1/4 the MIC) of antibiotic, and results were compared to those seen with a no-treatment (NT) control. TSST-1 production (in nanograms per milliliter) was determined by ELISA. Error bars represent the standard errors of results of comparisons between biological replicates (n = 3). An asterisk (*) indicates that data are significantly different from those determined with the NT control (P ≤ 0.05) based on an ANOVA with false-discovery-rate adjustments.
Extracellular protein toxins such as PVL, AH, and TSST-1 are key virulence factors in the pathogenesis of a variety of S. aureus infections. Although they are somewhat controversial in their role in staphylococcal SSSIs, particularly PVL (23), several studies have highlighted that suppression or neutralization of such toxins improves outcomes in both animal models and human cases of severe S. aureus infection (12, 14, 24–29). While the relationship between therapeutic effect and toxin production with respect to glycopeptide is not known, it is possible that a glycopeptide antibiotic that can suppress toxin production at subinhibitory levels may provide treatment superior to that provided by those that do not. Finally, given its long half-life (∼14.5 days in humans) (22), low MIC (Table 1) (5), and ability to suppress key toxins in vitro (Fig. 1), dalbavancin may offer physicians a treatment for staphylococcal SSSI that is more effective than that seen with other cell wall-active antibiotics.
ACKNOWLEDGMENTS
This material is based on work supported in part by the Forest Research Institute, Inc. (DAL-IT-02); NIH—National Institute of General Medical Sciences, Centers of Biomedical Research Excellence (P20GM109007 and P20GM103408); Idaho IDeA Network of Biomedical Research Excellence (P20 GM103408); NIH General Medical Sciences (U54GM104944), U.S. Department of Veterans Affairs—Office of Research and Development, the Idaho Veterans Research and Education Foundation PAVER fellowship, the MJ Murdock Charitable Trust, and the Idaho State Board of Education. The funding sources played no role in the design, execution, analysis, or reporting of this research.
REFERENCES
- 1.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, Hirschmann JV, Kaplan SL, Montoya JG, Wade JC. 2014. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 59:e10–e52. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 2.Moise PA, Forrest A, Bayer AS, Xiong YQ, Yeaman MR, Sakoulas G. 2010. Factors influencing time to vancomycin-induced clearance of nonendocarditis methicillin-resistant Staphylococcus aureus bacteremia: role of platelet microbicidal protein killing and agr genotypes. J Infect Dis 201:233–240. doi: 10.1086/649429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moise PA, Sakoulas G, Forrest A, Schentag JJ. 2007. Vancomycin in vitro bactericidal activity and its relationship to efficacy in clearance of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 51:2582–2586. doi: 10.1128/AAC.00939-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dash RP, Babu RJ, Srinivas NR. 2017. Review of the pharmacokinetics of dalbavancin, a recently approved lipoglycopeptide antibiotic. Infect Dis (Lond) 49:483–492. doi: 10.1080/23744235.2017.1296968. [DOI] [PubMed] [Google Scholar]
- 5.Streit JM, Fritsche TR, Sader HS, Jones RN. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn Microbiol Infect Dis 48:137–143. doi: 10.1016/j.diagmicrobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JW, Lewis JS, Ellis MW. 2008. Dalbavancin in the treatment of complicated skin and soft-tissue infections: a review. Ther Clin Risk Manag 4:31–40. doi: 10.2147/TCRM.S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavaleri M, Riva S, Valagussa A, Guanci M, Colombo L, Dowell J, Stogniew M. 2005. Pharmacokinetics and excretion of dalbavancin in the rat. J Antimicrob Chemother 55(Suppl 2):ii31–ii35. doi: 10.1093/jac/dki006. [DOI] [PubMed] [Google Scholar]
- 8.Leighton A, Gottlieb AB, Dorr MB, Jabes D, Mosconi G, VanSaders C, Mroszczak EJ, Campbell KC, Kelly E. 2004. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother 48:940–945. doi: 10.1128/AAC.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kernodle DS, McGraw PA, Barg NL, Menzies BE, Voladri RK, Harshman S. 1995. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis 172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 10.Ohlsen K, Ziebuhr W, Koller KP, Hell W, Wichelhaus TA, Hacker J. 1998. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother 42:2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens DL, Ma Y, Salmi DB, McIndoo E, Wallace RJ, Bryant AE. 2007. Impact of antibiotics on expression of virulence-associated exotoxin genes in methicillin-sensitive and methicillin-resistant Staphylococcus aureus. J Infect Dis 195:202–211. doi: 10.1086/510396. [DOI] [PubMed] [Google Scholar]
- 12.Stevens DL, Maier KA, Laine BM, Mitten JE. 1987. Comparison of clindamycin, rifampin, tetracycline, metronidazole, and penicillin for efficacy in prevention of experimental gas gangrene due to Clostridium perfringens. J Infect Dis 155:220–228. doi: 10.1093/infdis/155.2.220. [DOI] [PubMed] [Google Scholar]
- 13.Stevens DL, Wallace RJ, Hamilton SM, Bryant AE. 2006. Successful treatment of staphylococcal toxic shock syndrome with linezolid: a case report and in vitro evaluation of the production of toxic shock syndrome toxin type 1 in the presence of antibiotics. Clin Infect Dis 42:729–730. doi: 10.1086/500265. [DOI] [PubMed] [Google Scholar]
- 14.Zimbelman J, Palmer A, Todd J. 1999. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J 18:1096–1100. doi: 10.1097/00006454-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Candiani G, Abbondi M, Borgonovi M, Romano G, Parenti F. 1999. In-vitro and in-vivo antibacterial activity of BI 397, a new semi-synthetic glycopeptide antibiotic. J Antimicrob Chemother 44:179–192. doi: 10.1093/jac/44.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Mushtaq S, Warner M, Johnson AP, Livermore DM. 2004. Activity of dalbavancin against staphylococci and streptococci, assessed by BSAC and NCCLS agar dilution methods. J Antimicrob Chemother 54:617–620. doi: 10.1093/jac/dkh401. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton SM, Bryant AE, Carroll KC, Lockary V, Ma Y, McIndoo E, Miller LG, Perdreau-Remington F, Pullman J, Risi GF, Salmi DB, Stevens DL. 2007. In vitro production of Panton-Valentine leukocidin among strains of methicillin-resistant Staphylococcus aureus causing diverse infections. Clin Infect Dis 45:1550–1558. doi: 10.1086/523581. [DOI] [PubMed] [Google Scholar]
- 18.van Langevelde P, van Dissel JT, Meurs CJ, Renz J, Groeneveld PH. 1997. Combination of flucloxacillin and gentamicin inhibits toxic shock syndrome toxin 1 production by Staphylococcus aureus in both logarithmic and stationary phases of growth. Antimicrob Agents Chemother 41:1682–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc 57:289–300. [Google Scholar]
- 20.Jones RN, Flamm RK, Sader HS. 2013. Surveillance of dalbavancin potency and spectrum in the United States (2012). Diagn Microbiol Infect Dis 76:122–123. doi: 10.1016/j.diagmicrobio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Malabarba A, Ciabatti R. 2001. Glycopeptide derivatives. Curr Med Chem 8:1759–1773. doi: 10.2174/0929867013371716. [DOI] [PubMed] [Google Scholar]
- 22.Smith JR, Roberts KD, Rybak MJ. 2015. Dalbavancin: a novel lipoglycopeptide antibiotic with extended activity against Gram-positive infections. Infect Dis Ther 4:245–258. doi: 10.1007/s40121-015-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shallcross LJ, Fragaszy E, Johnson AM, Hayward AC. 2013. The role of the Panton-Valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect Dis 13:43–54. doi: 10.1016/S1473-3099(12)70238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adhikari RP, Karauzum H, Sarwar J, Abaandou L, Mahmoudieh M, Boroun AR, Vu H, Nguyen T, Devi VS, Shulenin S, Warfield KL, Aman MJ. 2012. Novel structurally designed vaccine for S. aureus alpha-hemolysin: protection against bacteremia and pneumonia. PLoS One 7:e38567. doi: 10.1371/journal.pone.0038567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy AD, Bubeck WJ, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. 2010. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis 202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menzies BE, Kernodle DS. 1996. Passive immunization with antiserum to a nontoxic alpha-toxin mutant from Staphylococcus aureus is protective in a murine model. Infect Immun 64:1839–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mocca CP, Brady RA, Burns DL. 2014. Role of antibodies in protection elicited by active vaccination with genetically inactivated alpha hemolysin in a mouse model of staphylococcus aureus skin and soft tissue infections. Clin Vaccine Immunol 21:622–627. doi: 10.1128/CVI.00051-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaulding AR, Lin YC, Merriman JA, Brosnahan AJ, Peterson ML, Schlievert PM. 2012. Immunity to Staphylococcus aureus secreted proteins protects rabbits from serious illnesses. Vaccine 30:5099–5109. doi: 10.1016/j.vaccine.2012.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stevens DL, Gibbons AE, Bergstrom R, Winn V. 1988. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis 158:23–28. doi: 10.1093/infdis/158.1.23. [DOI] [PubMed] [Google Scholar]